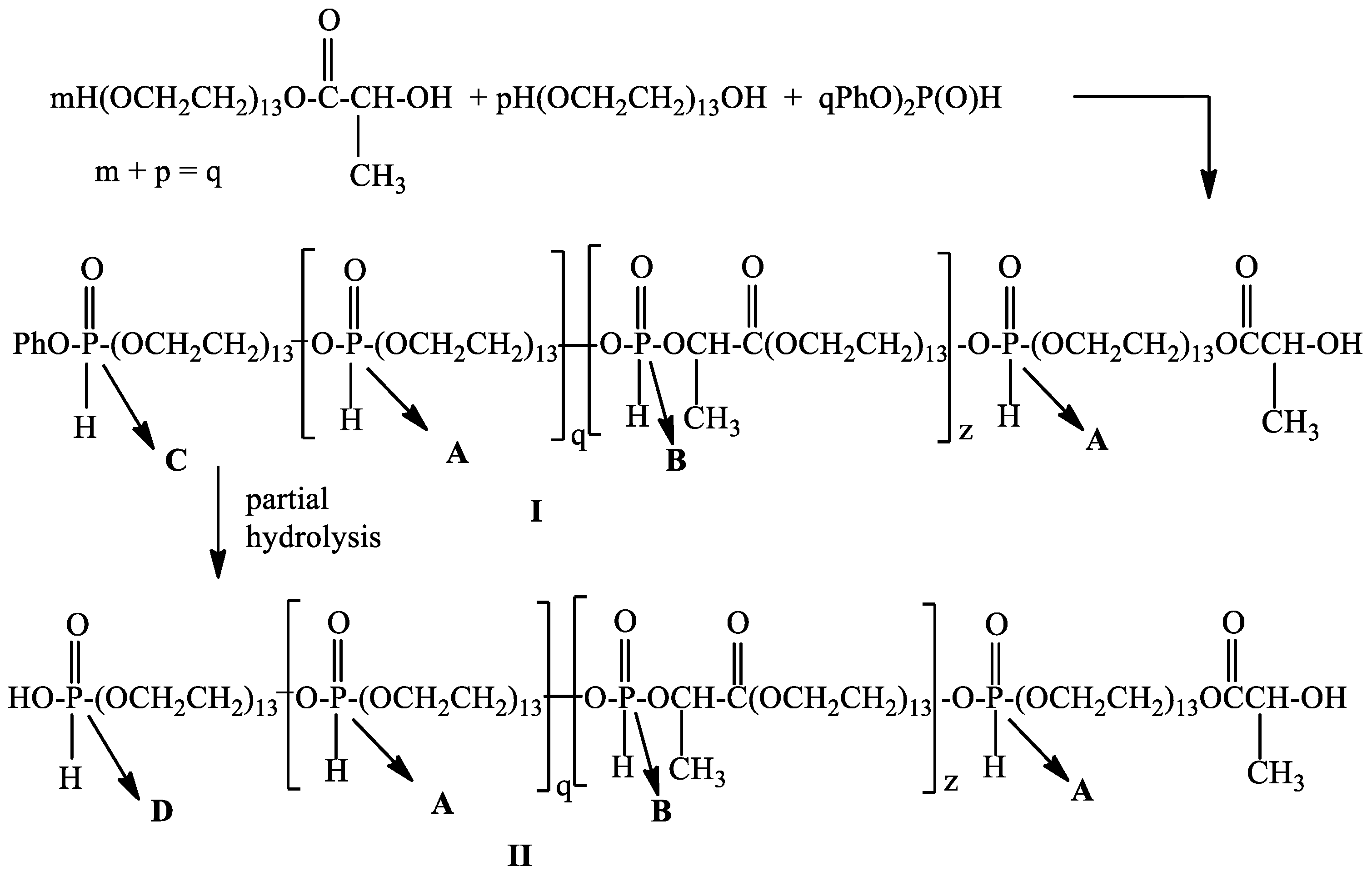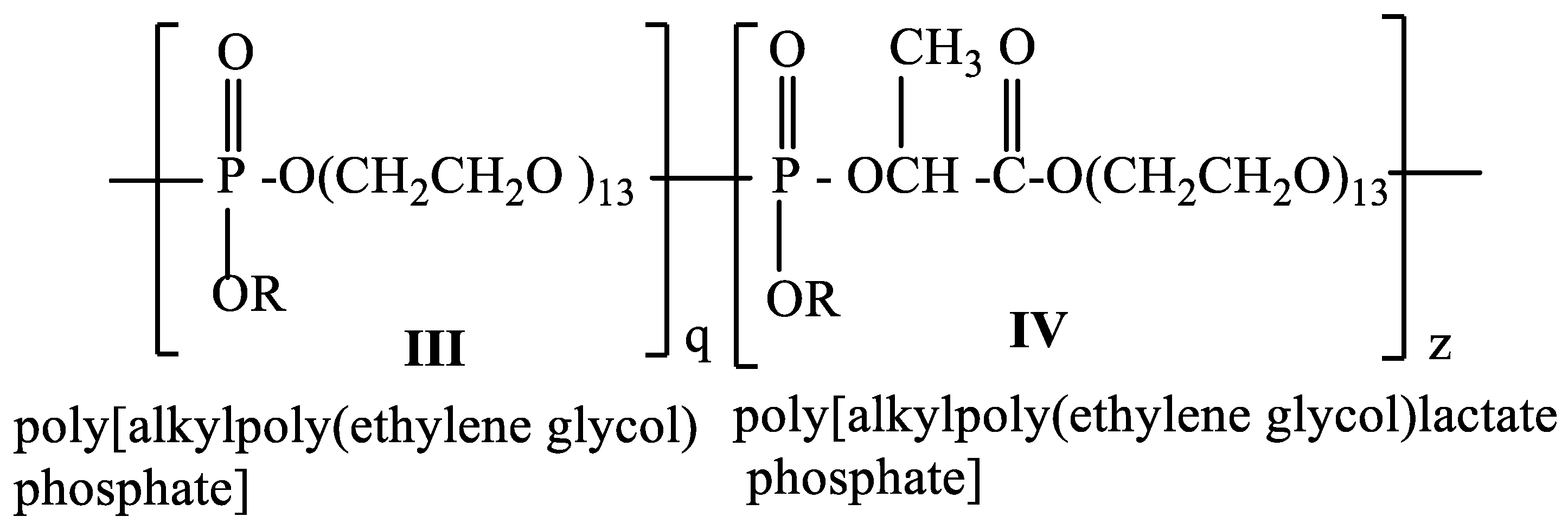Abstract
Multistep one-pot polycondensation reactions synthesized amphiphilic diblock polyphosphoesters containing lactic acid units in the polymer backbone. At the first step was synthesized poly[poly(ethylene glycol) H-phosphonate–b-poly(ethylene glycol)lactate H-phosphonate] was converted through one pot oxidation into poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s. They were characterized by 1H, 13C {H},31P NMR, and size exclusion chromatography (SEC). The effects of the polymer composition on micelle formation and stability, and micelle size were studied via dynamic light scattering (DLS). The hydrophilic/hydrophobic balance of these polymers can be controlled by changing the chain lengths of hydrophobic alcohols. Drug loading and encapsulation efficiency tests using Sudan III and doxorubicin revealed that hydrophobic substances can be incorporated inside the hydrophobic core of polymer micelles. The micelle size was 72–108 nm when encapsulating Sudan III and 89–116 nm when encapsulating doxorubicin. Loading capacity and encapsulation efficiency depend on the length of alkyl side chains. Changing the alkyl side chain from 8 to 16 carbon atoms increased micelle-encapsulated Sudan III and doxorubicin by 1.6- and 1.1-fold, respectively. The results obtained indicate that these diblock copolymers have the potential as drug carriers.
1. Introduction
A well-known problem with biodegradable polymers for medical applications is connected with the safety of their degradation products. In this respect, polyphosphoesters, based on poly(ethylene glycol) (PEG) are promising because they are degraded into small compounds: PEG and phosphoric acid, that are known to be non-toxic and are small enough for natural clearance mechanisms. Poly(alkylene H-phosphonate)s with phosphoester repeating units in the backbone are particularly interesting due to their high reactivity, biocompatibility, biodegradability, low toxicity, and structural resemblance to natural biomacromolecules such as nucleic acids. They are especially attractive advanced reactive and functional polymers due to the following advantages [1]: (i) they are highly reactive; (ii) they are water soluble; (iii) the drug-carrying capacity is determined by the highly reactive P-H group in each repeating units; (iv) the chemical immobilization of drugs proceeds under mild conditions; (v) the presence of highly polar P=O group in the repeating units affords the possibility for physical immobilization of drugs; (vi) possibility of hydrophilic/hydrophobic balance control; (vii) they can be regarded as biodegradable (enzymes the dominant component in the degradation process) and biocompatible synthetic polymers; (viii) they can be designed to have nontoxic building blocks; (ix) they can be administrated over a wider molecular weight range because, after hydrolysis, the low molecular PEG and phosphoric acid will be safely excreted; (x) easy to prepare in industrial scale; (xi) they are low toxic (IC50 1000 mg/kg). They are one of the most interesting class polyphosphoesters because both the polymer backbone and phosphorus substituents can be modified. Poly(alkylene H-phosphonate)s offers a unique opportunity to introduce various modifications at the phosphorus center through different reaction schemes [2,3,4,5,6,7,8,9,10,11,12,13,14]. They are being actively investigated for pharmaceutical and biomedical applications such as carriers of drugs [15,16,17,18,19,20,21] and genes [22,23,24,25,26,27,28]. We recently published the synthesis of thermoresponsive amphiphilic polyphosphoesters based on poly(oxyethylene H phosphonate)s with different lengths of poly(ethylene glycol) segments and aliphatic alcohols with different alkyl chain lengths, using polycondensation reactions. They have the potential as carriers of hydrophobic drugs [29].
Lactic acid (LA) is a naturally occurring and renewable monomer that is used for the synthesis of biodegradable polymers. LA-based polymers are important biomaterials for pharmaceutical, medical, and environmental applications because of their biocompatibility and environmental degradability [30,31,32,33,34]. The common way to prepare LA-based polymers is by ring-opening polymerization (ROP) of lactones and lactides [35,36,37]. However, the catalysts used to initiate ROP are difficult to remove from the resulting polymer [38], and the polymer’s purity is quite important for biomaterial applications [39]. In addition, the preparation of lactide is complicated and expensive [40]. Thus, it is of significant merit to develop a new route for preparing LA-based polymers while retaining their degradability. So far, there are no reports that describe LA-based polymers that contain single LA units. Recently, studies on smart pellets for controlled delivery [41] and potential smart colonic drug delivery system [42] were reported. The polymers synthesized in this study are also expected to contribute to the realization of a more advanced drug delivery system.
Herein we report a multistep one pot of synthesis of amphiphilic diblock copolymers poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol) lactate-phosphate]s containing a lactic acid unit in the polymer backbone via polycondensation and oxidation reactions. Micelle formation and drug loading and encapsulation efficiency of copolymers were studied.
2. Results and Discussion
2.1. Synthesis and Characterization of Poly(ethylene glycol)lactate (PEG-LA)
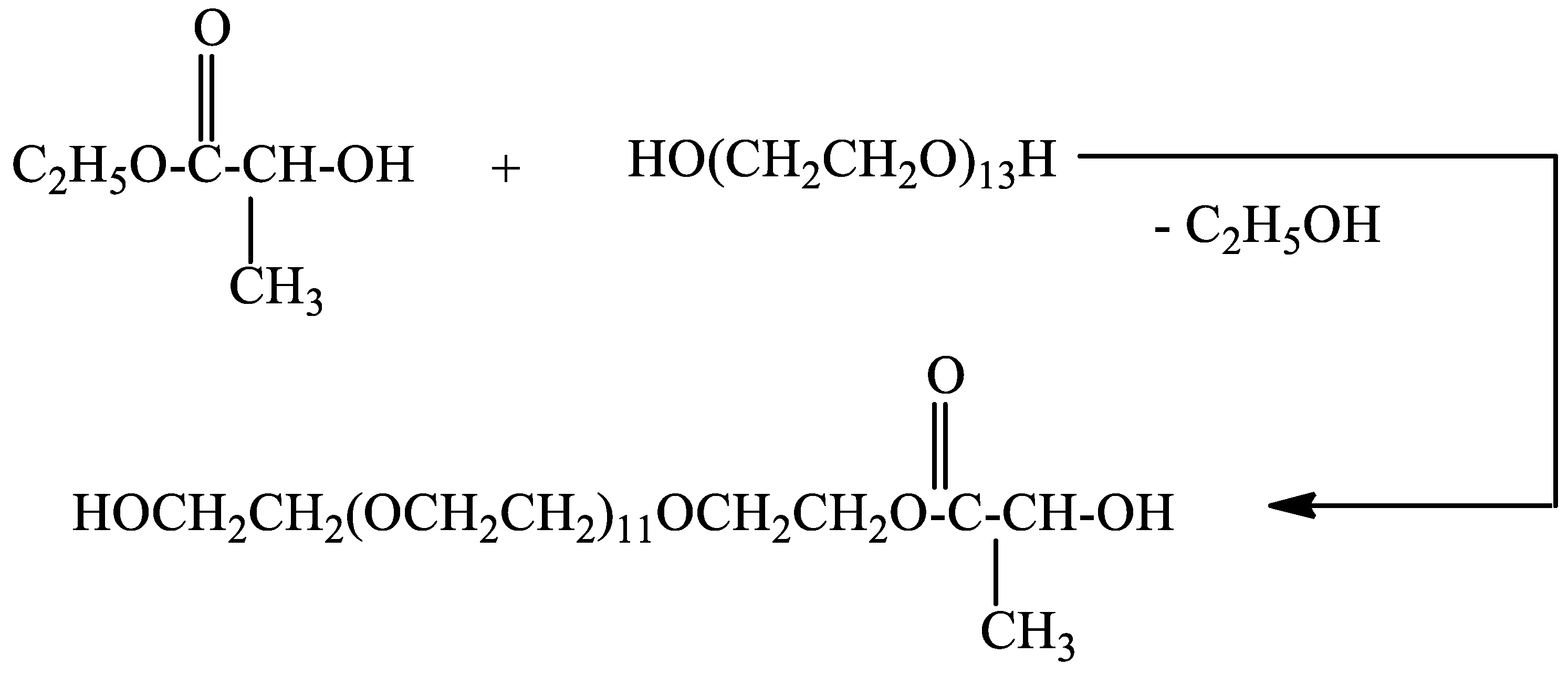
Poly(ethylene glycol)lactate was prepared via transesterification of ethyl lactate with poly(ethylene glycol 600) (Scheme 1). The reaction was carried out at a molar ratio of 1:1 at 120 °C.
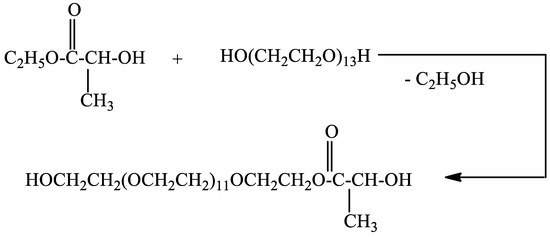
Scheme 1.
Synthesis of poly(ethylene glycol 600)lactate.
The structure and composition of the reaction product were confirmed by 1H and 13C{H}NMR spectroscopy. In the 1H NMR spectrum (Figure S1) after 18 h of heating there is no signal for CH3CH2O protons at 1.32 ppm. This revealed that the reaction product did not contain free ethyl lactate. The two doublets at 1.43 and 1.428 ppm in the 1H NMR spectrum can be assigned to CH3CH-protons. Poly(ethylene glycol) lactate consists of the racemic mixture of levo and dextro forms. The multiple ranging from 4.28 to 4.34 ppm can be assigned to CH3CH-and CH2CH2OC(O) protons. This signal for CH3CH-proton has to be a quartet, the one for CH2CH2OC(O) protons is a triplet but as the result of overlapping the signal appears as a multiplet. In the 13C{H}NMR spectrum (Figure S4) the signal at 14.21 ppm for the CH3CH2O carbon atom disappears. The signals at δ ppm: 20.34, 61.64, 64.40, 66.72, 68.83, 70.28, 72.48, and 175.51 can be assigned to the carbon atoms of methyl group CH3CH-, HOCH2-, CH3CH-, C(O)OCH2-;-C(O)OCH2CH2O-,HOCH2CH2OCH2-, -(OCH2CH2O)-, HOCH2CH2O-, C=O, respectively. Data from 13C{H} NMR spectroscopy confirm the structure of the reaction product. The yield of poly(ethylene glycol 600)lactate is 70.82%, 41.72 g (based on 1H NMR spectrum see (Figure S2); PEG 600-17.76 g (0.03 mol). The proposed method for the synthesis of poly(ethylene glycol)lactate allows varying the molar ratio between ethyl lactate and PEG to synthesize copolymers with different lactic acid content.
2.2. Synthesis of Poly[poly(ethylene glycol) H-phosphonate-b-poly(ethylene glycol)lactate H-phosphonate]
It is known that the oxygen atom of the secondary hydroxyl group is a weaker nucleophile compared to the oxygen atom of the primary hydroxyl group. On the other hand, it was known that diphenyl H-phosphonate is more reactive compared to dialkyl esters of H-phosphonic acid in transesterification reactions [1]. In this connection, we decided to use diphenyl H-phosphonate as a starting monomer for the preparation of poly[poly(ethylene glycol) H–phosphonate-b-poly(ethylene glycol)lactate H-phosphonate] using poly(ethylene glycol 600) and poly(ethylene glycol)lactate as a dihydroxy compound. To the reaction mixture (see Section 2.3) was added diphenyl H-phosphonate. Transesterification was carried out at a temperature of 145 °C in an inert atmosphere without the use of a catalyst, a vacuum of 0.6 mm Hg, and a reaction time of 10 h. In the 1H NMR spectrum of the reaction product after 10 h heating (see Figure S5) there are signals at δ: 1.42 ppm, d, 3J(H,H) = 7.07 Hz for CH3-protons in C(O)CH(CH3)-OH; 1.60 ppm, d, 3J(H,H) = 6.83 Hz, -POCH(CH3)C(O)-; 1.58 ppm, 3J(H,H) = 6.83 Hz, -POCH(CH3)C(O)-(two diastereoisomers); 3.64, s, -CH2OCH2; 4.99–5.06 ppm, m, POCH(CH3)-protons; 6.83–7.21 for aromatic protons; there are four types of P-H protons at δ = 6.95 ppm, d, 1J(P,H) = 717.69 Hz. This doublet can be assigned to a phosphorus atom with the following structure: OCH2OP(O)(H)OCH2 in the repeating units [43]; δ = 7.02 ppm, d, 1J(P,H) = 729.40 Hz for OCH2OP(O)(H)OCH(CH3)-; δ = 7.13 ppm, d, 1J(P,H) = 734.8 Hz for OCH2OP(O)(H)OCH(CH3)-; δ = 7.16 ppm, d, 1J(P,H) = 724.76 Hz for PhOP(O)(H)OCH2-; 4.13–4.37 ppm, m, OCH2CH2OP(O)(H)OCH2CH2. The data from 1H NMR spectroscopy confirm that the secondary hydroxyl group of poly(ethylene glycol)lactate participated in the transesterification reaction and a diblock copolymer is formed. The 31P{H}NMR spectrum (Figure S6). of the reaction product shows five types of phosphorus atom at δ = 9.98 ppm with integral intensity (II) = 1.00; 9.30 ppm, II = 0.34; 8.54 ppm, II = 0.02; 7.90 ppm, II = 0.38; 7.34 ppm, II = 0.05; 5.99 ppm, II = 0.06. The signal at δ = 9.98 ppm in the 31P NMR spectrum (Figure S7) is a doublet of quintets with 1J(P,H) = 717.78 Hz, and 3J(P,H) = 9.7 Hz. This signal can be assigned to the phosphorus atom with the following substituents -CH2OP(O)(H)OCH2-(type A); the signal at 9.30 ppm appears as a doublet of quartets with 1J(P,H) = 734.76 Hz, and 3J(P,H) = 8.57 and 9.05 Hz. This signal can be assigned to the phosphorus atom with the following substituents -CH2OP(O)(H)OCH(CH3)C(O)-(type B). The second doublet of quartets at 7.90 ppm with 1J(P,H) = 729.26 Hz, and 3J(P,H) = 9.7 and 10.35 Hz. can be assigned to the phosphorus atom with the same substituents as in the case of the first doublet of quartets. These data revealed that poly(ethylene glycol)lactate exists as a racemic mixture of D- and L-lactides [44]. It is a two-diastereoisomer. The ratio between the integral intensity of the signals at 9.98 ppm, 9.30 ppm, and 7.90 ppm is 1:0.72. The signal at 5.99 ppm which appears as a doublet of triplets with 1J(P,H) = 721.41 Hz can be assigned to the phosphorus atom in the end group PhO-P(O)(H)-OCH2 (type C). The signals at 8.54 ppm with very low intensity 0.02 represents a doublet of triplets and can be assigned to the phosphorus atom with the following substituents -HO-P(O)(H)-OCH2-(type D), which occurs as a result of partial hydrolysis of the terminal phenoxy group (20% of the terminal PhO group is hydrolyzed). Based on the data from 1H and 31P NMR spectroscopy, we assume that the reaction of poly(ethylene glycol)lactate and poly(ethylene glycol) with diphenyl H-phosphonate resulted in the formation of diblock copolymer with the following structure (Scheme 2).
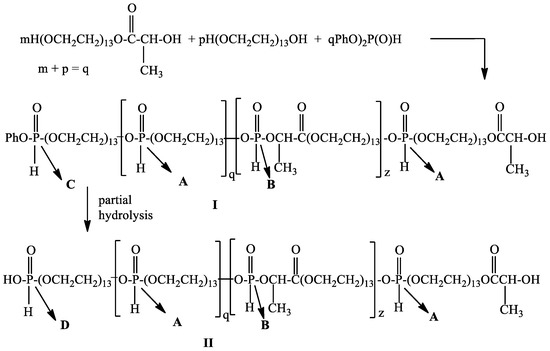
Scheme 2.
General scheme of the interaction of poly(ethylene glycol)lactate and poly(ethylene glycol) with diphenyl H-phosphonate. Subfigures I: Poly[poly(ethylene Glycol) H-phosphonate-b-poly(ethylene Glycol)lactate H-phosphonate] with phenoxy end groups; II: Poly[poly(ethylene Glycol) H-phosphonate-b-poly(ethylene Glycol)lactate H-phosphonate] with hydroxy end groups.
Based on the 31P{H}NMR spectrum (see Figure S1) the value of q = 12, z = 9, the average molecular weight of the reaction product is 14,124 Da, the content of polymer I is 80%, that of polymer II is 20%. SEC analysis showed that the average number molecular weight of the reaction product after 10 h heating is 13,475 with PDI = 1.64.
The hydrophilicity of the poly[alkylpoly(ethylene glycol) phosphate] III is determined by the poly(ethylene glycol) and the phosphoryl group, which is a strong proton acceptor [45]. The hydrophilicity of the poly[alkylpoly(ethylene glycol)lactate phosphate] IV will be determined not only by polyethylene glycol and the phosphoryl group but also by the carbonyl group of lactic acid, which is a proton acceptor, too. By varying the ratio between q and z we can control the hydrophilicity of the copolymer and control the hydrophilic/hydrophobic balance of the copolymer (Scheme 3).
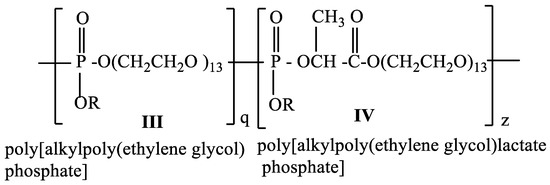
Scheme 3.
Structure of a copolymer consisting of poly[alkylpoly(ethylene glycol) phosphate] (III) and poly[alkylpoly(ethylene glycol)lactate phosphate] (IV).
2.3. One-Pot Synthesis of Poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s
One-Pot Synthesis of Poly[hexadecylpoly(ethylene glycol) phosphate)-b-hexadecylpoly(ethylene glycol)lactate phosphate]
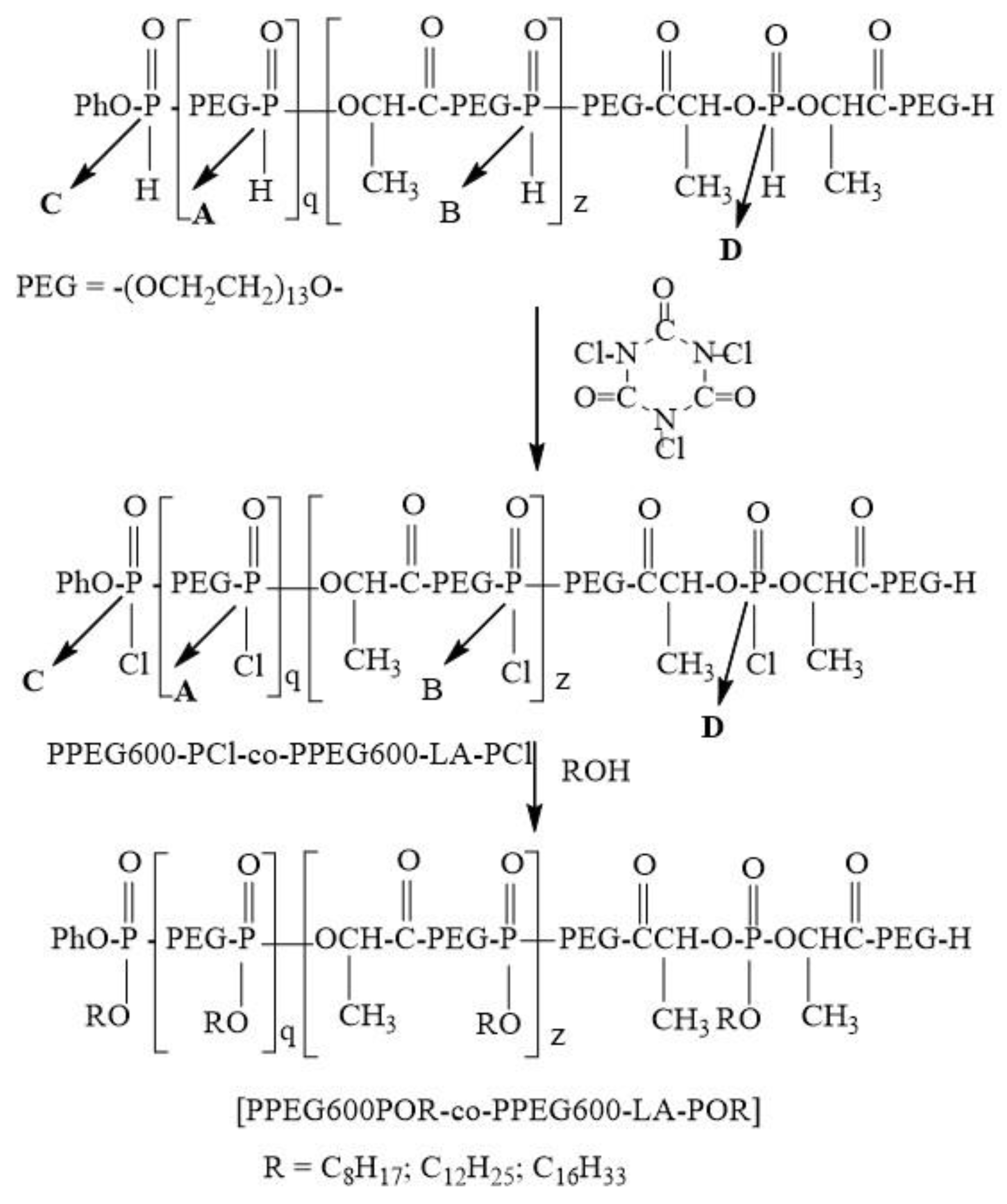
Poly[(alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate] was obtained via one-pot synthesis avoiding a lengthy separation process and purification of the intermediate chemical compound. For this purpose, poly[poly(ethylene glycol H-phosphonate-b-poly(ethylene glycol)lactate H-phosphonate] was converted into the corresponding poly[poly(ethylene glycol) chlorophosphate-b-poly(ethylene glycol)lactate chlorophosphate] via treatment with trichloroisocyanuric acid (Scheme 4).
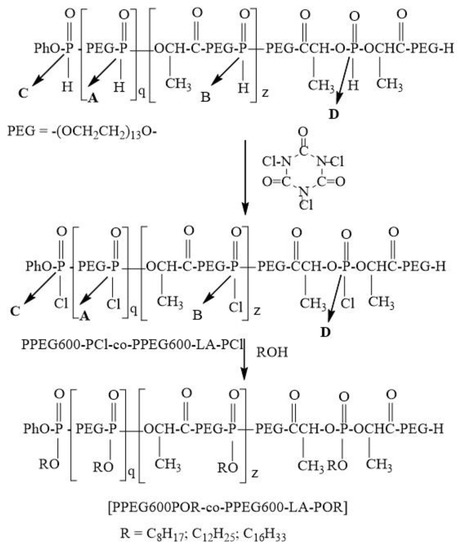
Scheme 4.
Synthesis of poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s.
The conversion was monitored by 31P{H}NMR spectroscopy. 31P{H}NMR spectrum of the reaction product (Figure S8) after 14 h heating revealed that the signals for the phosphorus atoms of poly[(ethylene glycol H-phosphonate-b-poly(ethylene glycol)lactate H-phosphonate] disappear and new three signals at δ = 5.89 ppm; δ = 5.48 ppm and δ = 4.76 ppm appear. These signals can be assigned to the phosphorus atoms of the poly[poly(ethylene glycol) chlorophosphate-b-poly(ethylene glycol)lactate chlorophosphate]s. The signal at δ = 5.89 ppm can be assigned to the phosphorus atom in the following structure -CH2OP(O)(Cl)OCH2-. Signals at δ = 5.48 ppm and δ = 4.76 can be assigned to phosphorus atoms with the following structure -CH2O-P(O)(Cl)OCH(CH3)-. The chirality of the CH carbon atom is a reason for appearing of two signals. The ratio between the integral intensity of the signals at 5.89 ppm and (5.49 ppm + 4.76 ppm) is 1: 0.74. This ratio for the integral intensities of the phosphorus atoms of poly[poly(ethylene glycol) H-phosphonate-b-poly(ethylene glycol)lactate H-phosphonate] was 1:0.72. This result indicates that the reaction is not accompanied by the occurrence of side reactions. After completion of the oxidation reaction solution of 1-hexadecanol in diethyl ether was added to the reaction product. The formation of poly[hexadecylpoly(ethylene glycol) phosphate-b-hexadecylpoly(ethylene glycol)lactate phosphate] was controlled by 31P{H} NMR spectroscopy. The reaction is stopped when signals for phosphorus atoms of poly[poly(ethylene glycol) chlorophosphate-b-poly(ethylene glycol)lactate chlorophosphate] disappear in the 31P{H}NMR spectrum, and new signals at 0.24 ppm, −0.26 ppm and −1.32 ppm (Figure S9) appear, which are characteristic for phosphate structures—-CH2O-P(O)(OR)OCH2O- and –CH2O-P(O)(OR)-OCH(CH3)-. Using a one-pot synthesis strategy we synthesized poly[ocylpoly(ethylene glycol) phosphate-b-ocylpoly(ethylene glycol)lactate phosphate] and poly[dodecylpoly(ethylene glycol) phosphate-b-dodecylpoly(ethylene glycol)lactate phosphate]. The application of a one-pot synthesis strategy for the preparation of poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s has a big advantage because poly[poly(ethylene glycol) chlorophosphate-b-poly(ethylene glycol)lactate chlorophosphate]s are highly reactive polymers and extremely sensitive to moisture, so purification requires an absolutely dry atmosphere and dry chemicals. Using one-pot synthesis strategy poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s were obtained in situ, without isolation of the corresponding poly[poly(ethylene glycol) chlorophosphate-b-poly(ethylene glycol)lactate chlorophosphate]s.
Table 1 shows the results of molecular weight measurements of the novel polymers using SEC. PDI values ranged from 1.56 to 1.65. The molecular weight of the polymer also increased with increasing side chain length. Therefore, it was suggested that the molecular weight of the polymer can be controlled by the type of side chain used.

Table 1.
Molecular weight measurement results of polymers using SEC.
2.4. Self-Assembly of Poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s
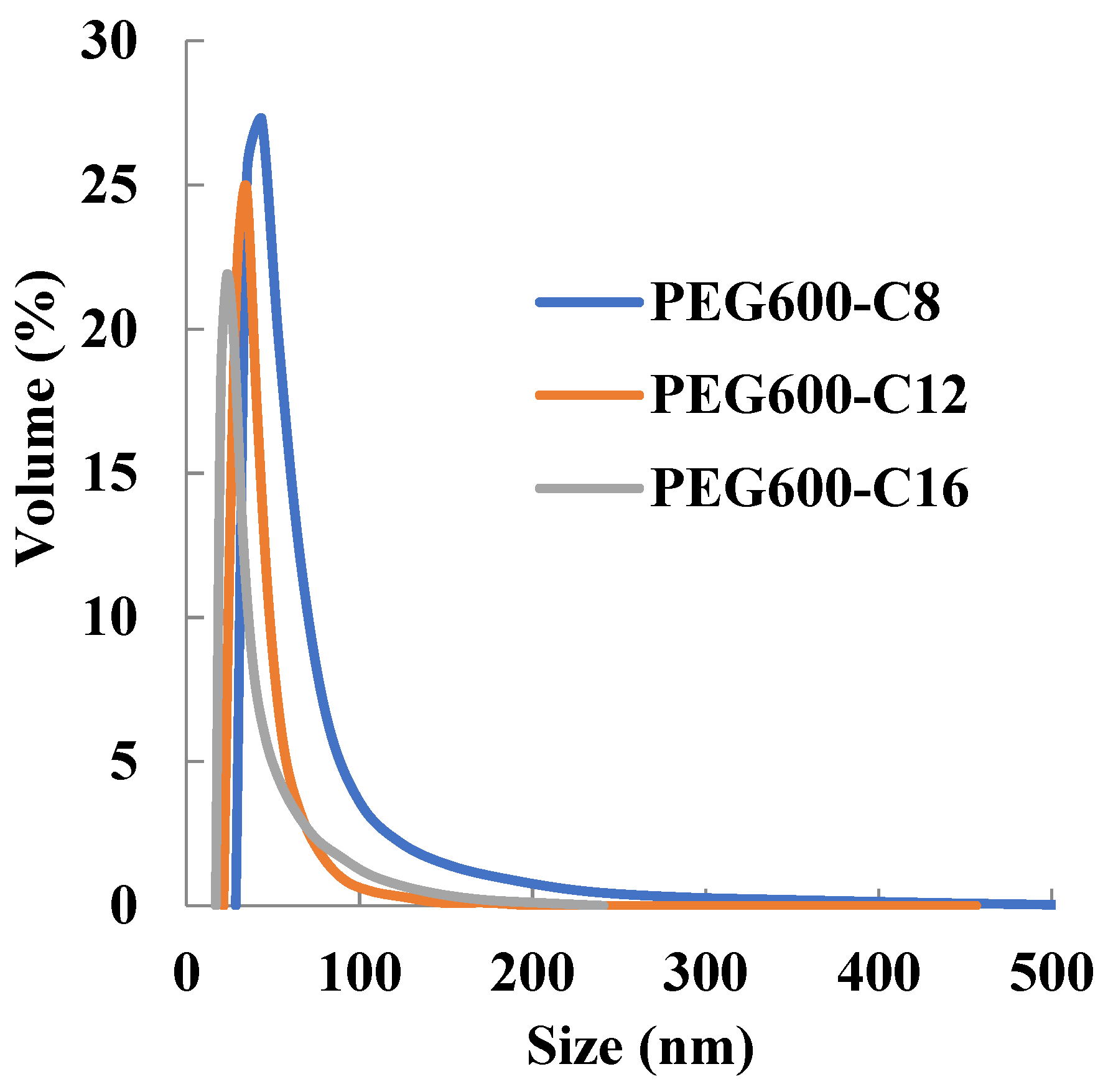
The results obtained revealed that the newly synthesized polymers self-assembled into the micellar structure in an aqueous solution and micelles show monodisperse peaks (Figure 1). It was found that the particle size is inversely proportional to the increase in the number of hydrophobic side chains (Table 2). This is probably because the increased hydrophobic interaction increased the cohesive force in the inner core of the micelle and reduced the particle size. Therefore, it was suggested that the particle size can be controlled by changing the hydrophobic side chain. In addition, the micelles prepared in this study were dispersed in a size of 10–200 nm, which shows the EPR effect.
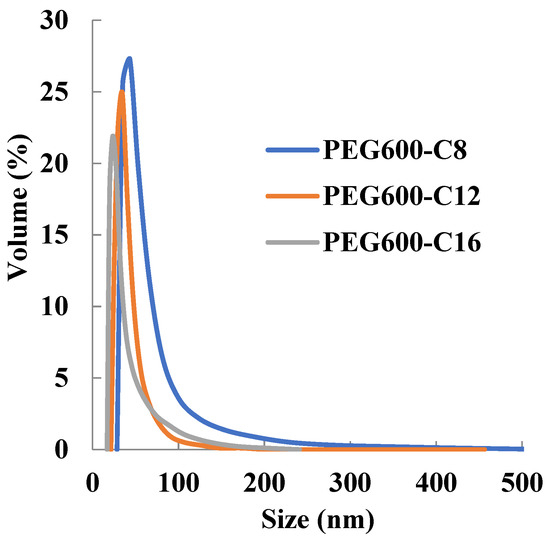
Figure 1.
Dependence of micelle size of poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s from alkyl chain length in PBS at 37 °C (n = 3).

Table 2.
Particle size of polymer micelles in PBS at 37 °C.
2.5. Particle Size Distribution and Loading Rate and Encapsulation Efficiency of Sudan III Encapsulating Micelles
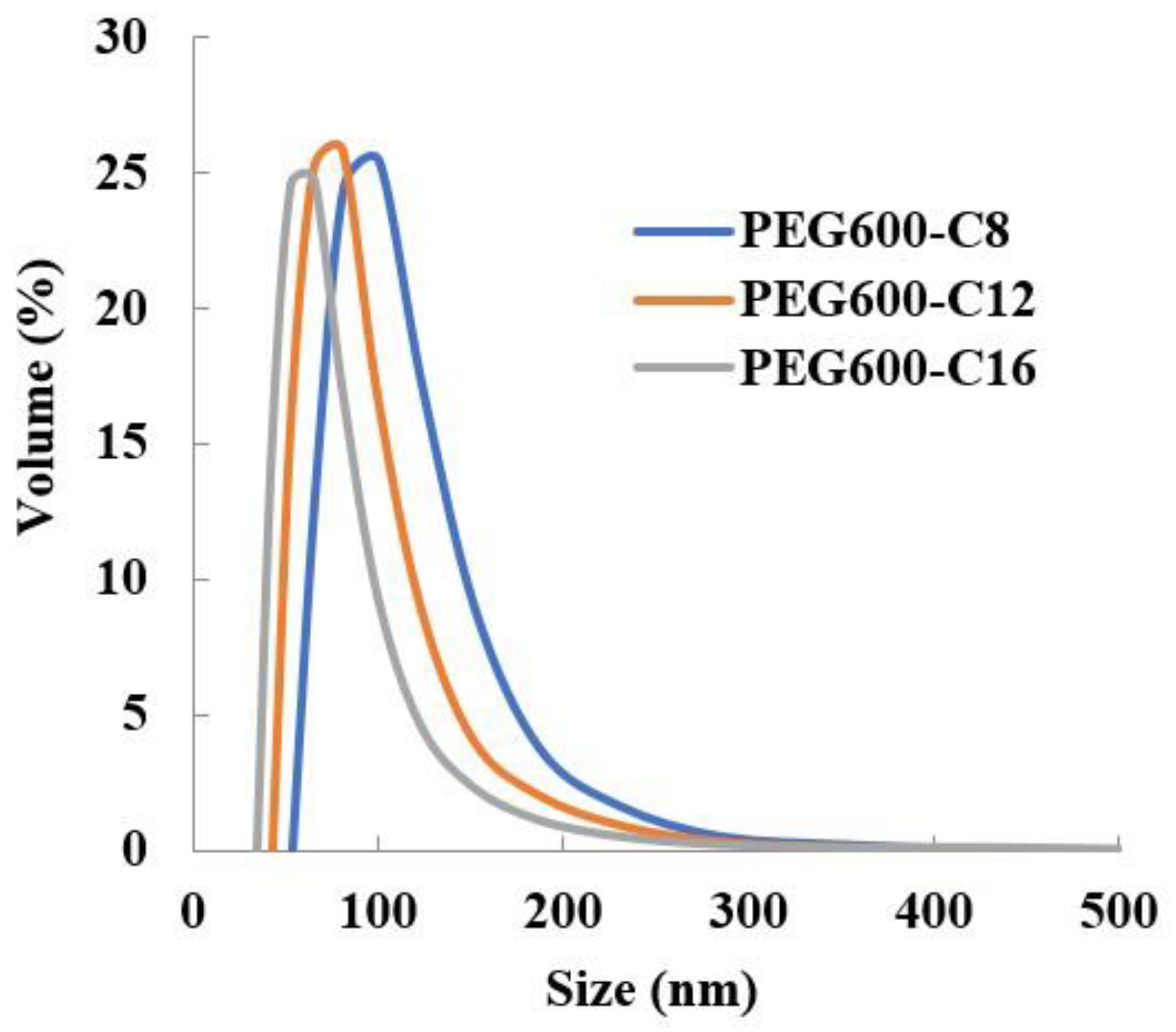
Results revealed that the newly synthesized polymers can encapsulate Sudan III (Table 3) and the particle size distribution is monodispersed (Figure 2). It was found that the encapsulation of Sudan III increased in particle size. As in the case of the free micelles, the increase in the number of hydrophobic side chains leads to a reduction in the size of the micelles.

Table 3.
The particle size of micelles with encapsulated Sudan III in PBS at 37 °C.
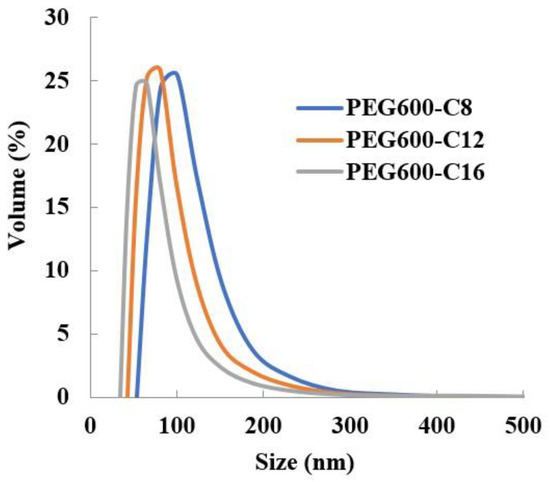
Figure 2.
Dependence of Sudan III micelle size of poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate)]s from alkyl chain length in PBS at 37 °C (n = 3).
Drug loading and encapsulation efficiency of the newly synthesized polymers relative to Sudan III are low (Table 4). The highest drug loading and encapsulation efficiency revealed poly[hexadecylpoly(ethylene glycol) phosphate-b-hexadecylpoly(ethylene glycol)lactate phosphate] 0.75% and 8.16%, respectively. The results obtained revealed that the drug loading and encapsulation efficiency can be improved by introducing a more hydrophobic side chain.

Table 4.
Drug loading and encapsulation efficiency of poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate)]s for Sudan III (mean ± SD, n = 3).
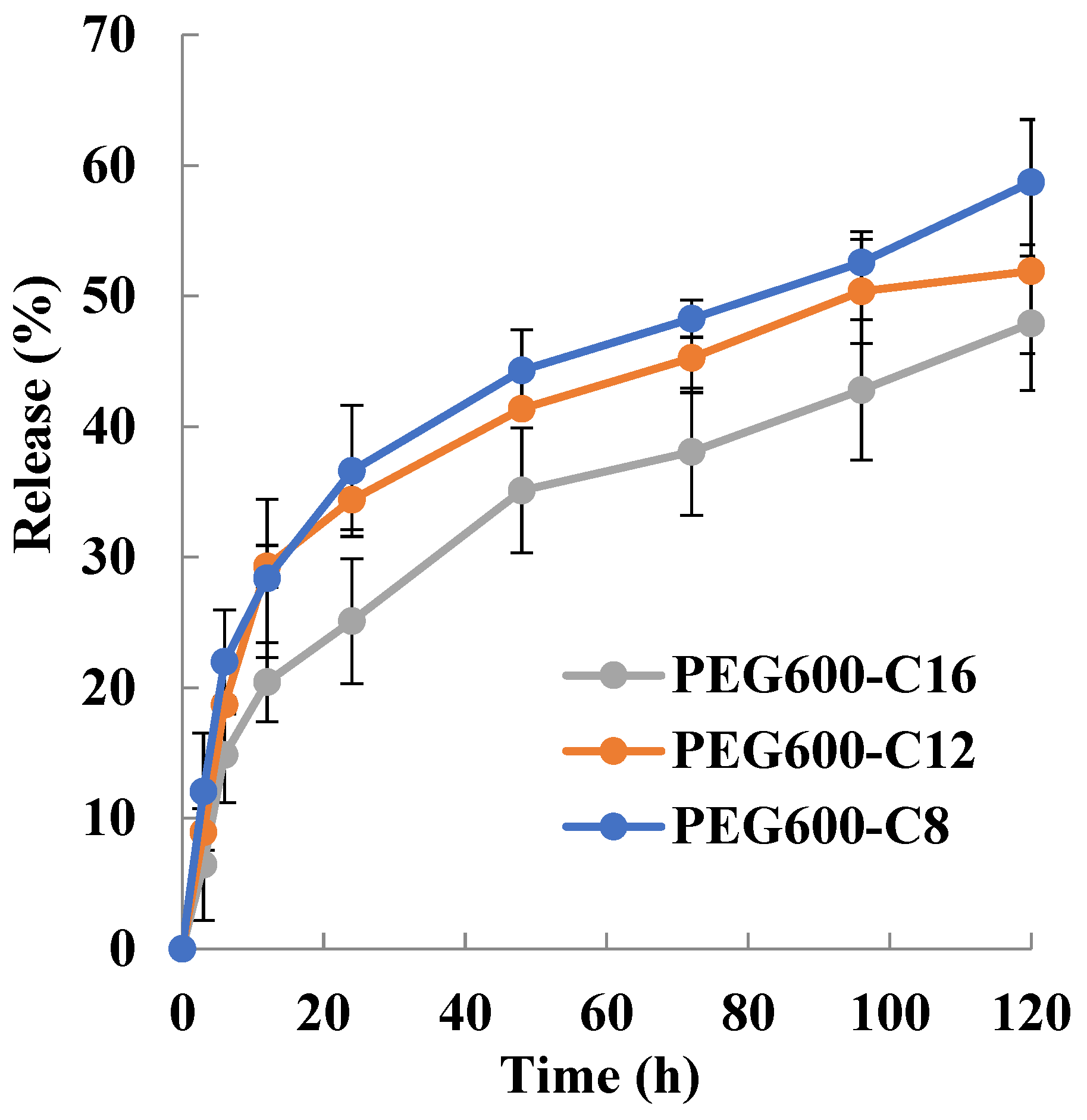
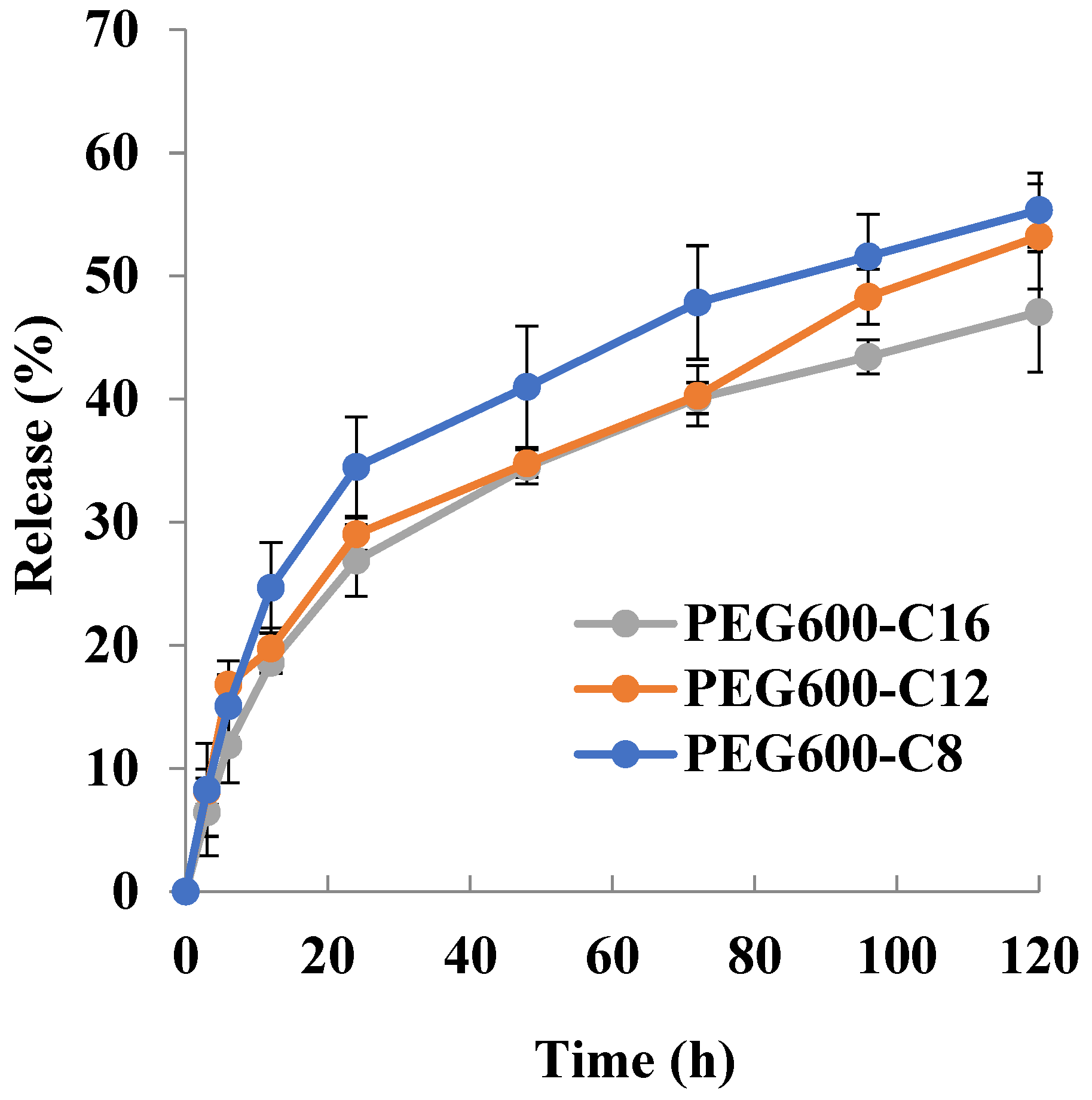
The release of drugs from block copolymer micelles depends upon the rate of diffusion of the drug from the micelles, micelle stability, and the rate of biodegradation of the copolymer. The release rate is mostly influenced by the following factors: the strength of the interactions between the drug and the core-forming block [46], the physical state of the micelle core [47], the amount of drug loaded [46], the molecular volume of the drug, the length of the core-forming block, the molecular weight of copolymer [48] and the localization of the drug within the micelle [49]. Drug release is highly influenced by where the drug molecules are located. If the drug is located predominantly in the corona, then the length of the core-forming block, the micelle size, and the molecular volume of the drug are less important in determining the release rate. On the other hand, the amount of drug loaded in the micelle core; the higher the concentration of drug, the slower the release rate [50,51]. It has been found that the more soluble compounds are localized in the inner corona or the core–corona interface while the more hydrophobic compounds are situated mostly in the micelle core [49]. The release rate of the drug is a function of its localization within the micelle. The outer corona region of the micelle is quite mobile; as a result, release from this area is rapid. The release of drugs localized in the corona or at the interface is said to account for “burst release” from the micelle [49]. The release behavior of Sudan III is shown in Figure 3. The release rate decreases with increasing the alkyl chain length. The micelles release for 12 h 28%, 28%, and 20% Sudan III for poly[octylpoly(ethylene glycol) phosphate-b-octylpoly(ethylene glycol)lactate phosphate], poly[dodecylpoly(ethylene glycol) phosphate-b-dodecylpoly(ethylene glycol)lactate phosphate)], and poly[hexadecylpoly(ethylene glycol) phosphate-b-hexadecylpoly(ethylene glycol)lactate phosphate)], respectively. The release rate of Sudan III strongly decreases after 12 h. For 120 h the released amount of Sudan III was 58.7%, 51.9, and 47.0% for C8, C12, and C16, respectively.
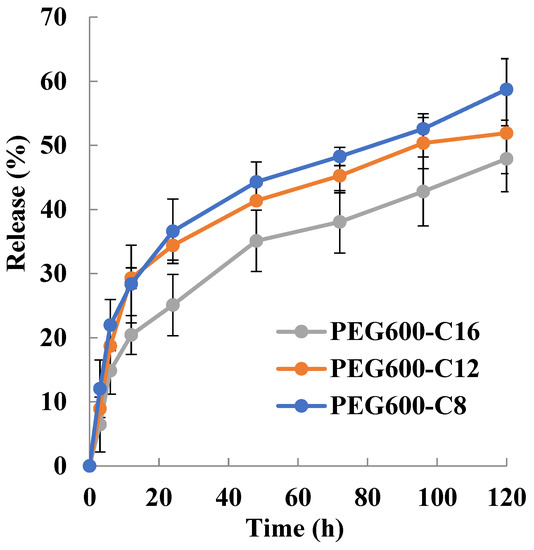
Figure 3.
Sudan III release profile from micelle in PBS at 37 °C (PEG600) (n = 3).
2.6. Particle Size Distribution and Drug Loading and Encapsulation Efficiency for Doxorubicin
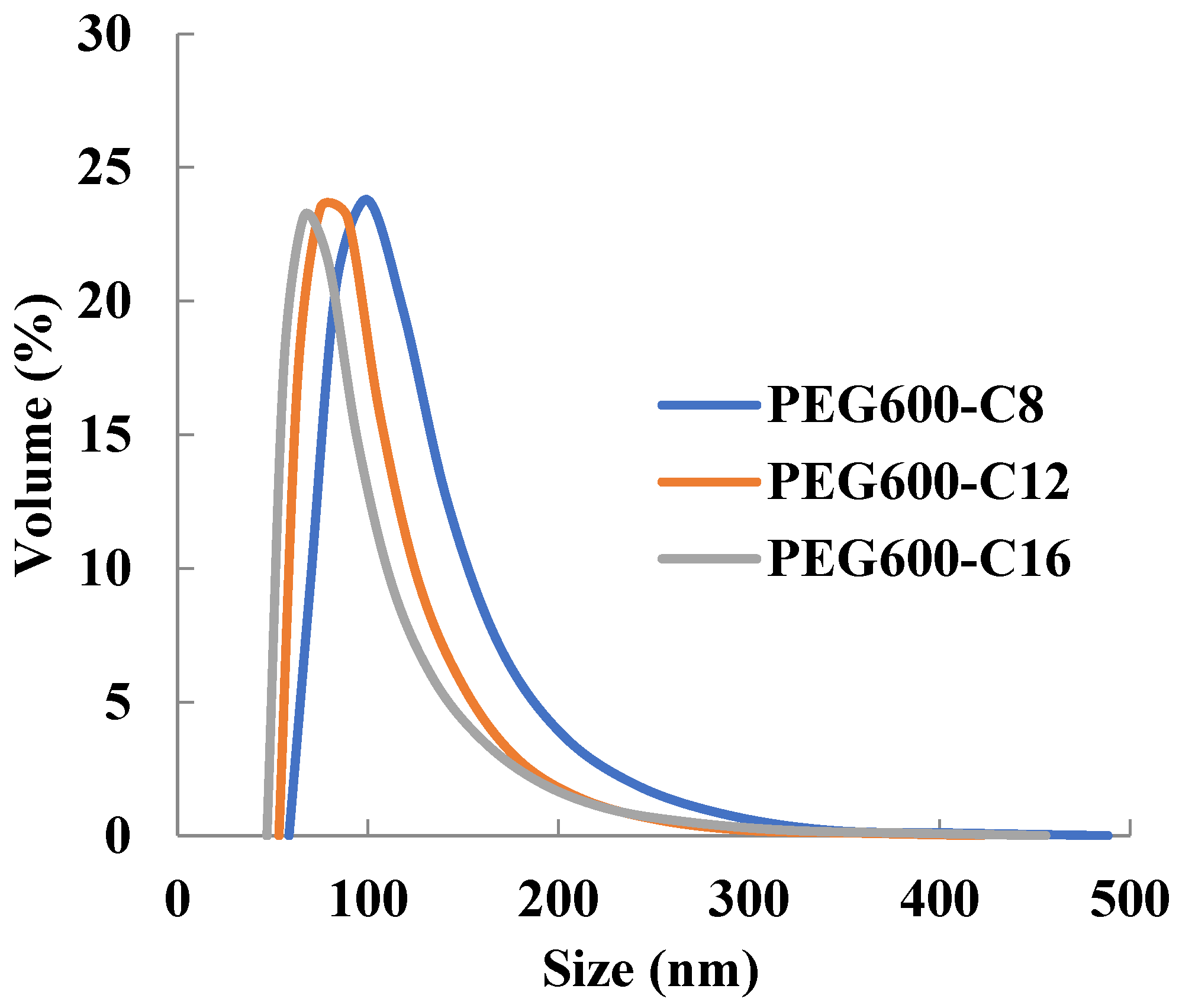
Micelles of newly synthesized polymers can encapsulate doxorubicin in PBS solution (Table 5). The diameter of the micelles with encapsulated doxorubicin increases compared to the free micelles and those with encapsulated Sudan III. The particle size distribution was monodispersed (Figure 4) and the size was sufficient to exhibit the EPR effect. It can be assumed that selective delivery to tumors is possible as a micelle formulation.

Table 5.
The particle size of micelles with encapsulated doxorubicin in PBS at 37 °C.
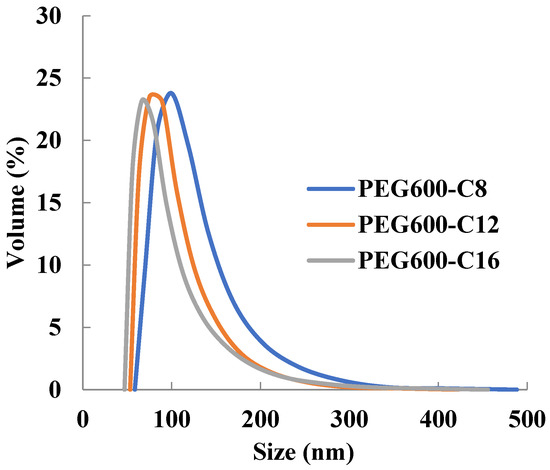
Figure 4.
Dependence of micelle size of poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s with encapsulated doxorubicin from alkyl chain length in PBS at 37 °C (n = 3).
The drug loading efficiency increased with the ratio of drug to polymer and hydrophobic chain lengths of a block copolymer (Table 6). The highest drug loading and encapsulation efficiency were obtained for poly[hexadecylpoly(ethylene glycol) phosphate-b-hexadecylpoly(ethylene glycol)lactate phosphate]—1.63% and 45.8%, respectively. Drug encapsulation efficiency is an important index for drug delivery systems, especially for an expensive drug. Drug loading and encapsulation efficiency were higher than those of Sudan III.

Table 6.
Drug loading and encapsulation efficiency for doxorubicin (mean ± SD, n = 3).
Doxorubicin release profiles in PBS at 37 °C (Figure 5) revealed that the release rate of the encapsulated doxorubicin decreases with increasing alkyl chain length and amount of encapsulated doxorubicin. The obtained results are in line with what has been found in the literature that micelles with longer hydrophobic blocks showed slower drug release rates compared with micelles with shorter hydrophobic blocks, as a consequence of increased hydrophobic interaction between the drug molecules and hydrophobic blocks [52].
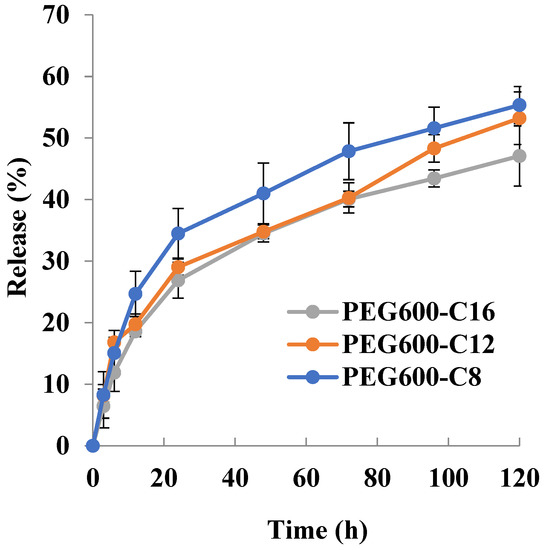
Figure 5.
Doxorubicin release profile from micelle in PBS at 37 °C (PEG600) (n = 3).
The doxorubicin release profile revealed that for 12 h the release amount of doxorubicin is: 24.6% for C8; 19.7% for C12 and 18.5% for C16; for 120 h is 55.3% for C8; 53.2% for C12 and 47% for C16. These results obtained showed the influence of the molecular weight of the polymer on the release rate of doxorubicin from micelles, which matches with the established by Kim and coworkers [48] that increase in the molecular weight of copolymer resulted in a decrease in the release rate. The release profile suggests the good stability of DOX-loaded micelles under physiological conditions.
Release profiles of Sudan III and doxorubicin revealed that release proceeds in two stages: first stage—with a higher release rate from 0 h to 12 h; second stage—with a lower rate from 12 h to 120 h. It can be assumed that the decrease in the release rate of Sudan III and doxorubicin is due to their location in the micelle. A higher release rate implies that they are located in the core–corona interface or the periphery of the core, while a lower release rate means they are located in the center of the core. When drug molecules are predominantly located in the core the higher the concentration of the drug, the slower the release rate [48]. The micelles prepared from poly[alkylpoly(ethylene glycol) phosphate]-b-poly[alkylpoly(ethylene glycol)lactate phosphate]s entrap doxorubicin and disperse to a size exhibiting the EPR effect. The drug loading was as high as 1.4–1.6% for 3.8% preparation. Based on the above results, the new micelles prepared are considered to be useful as micellar preparations, not only because they are degradable but also because they have a certain degree of sustained release.
3. Experimental Part
3.1. Materials
Poly(ethylene glycol)s with a number-average molecular weight of 600 g/mol was purchased from Wako Pure Chemical Industries, Ltd. (current FUJIFILM Wako Pure Chemical Co., Osaka, Japan). It was dried before use by a two-stage process: an azeotropic distillation with toluene and a subsequent 4 h heating at 120 °C under a dynamic vacuum. Ethyl lactate was purchased from Wako Pure Chemical Industries, Ltd. It was distilled before use. Sodium methoxide was purchased from Wako Pure Chemical Industries, Ltd. and was used as received. Diphenyl H-phosphonate was purchased from Wako Pure Chemical Industries, Ltd. with, a purity of 85%, containing < 15% phenol. Phenol was removed by distillation before use. 1-octanol, 1-dodecanol, and 1-hexadecanol were purchased from Wako Pure Chemical Industries, Ltd. Trichloroisocianuric acid, 97% (TCIA) Sigma-Aldrich (St. Louis, MO, USA).
3.2. Characterization Methods and Instruments
All 1H, 13C NMR spectra were recorded on a Bruker NMR spectrometer operating at 600 MHz at 37 °C in CDCl3. The molecular weights and polydispersity index (PDI) of polymers were determined by size exclusion chromatography (SEC) system (SIL-20A, RID-10A, LC-20AD, CTO-20A, and DGU-20A3; Shimadzu Corp., Kyoto, Japan) with columns (GPC KF-804Lx2; Showa Denko K. K., Tokyo, Japan) using the PEG standard. THF, 10 mM at 40 °C was used as eluent (flow rate: 1.0 mL/min).
3.3. Synthesis of Poly(ethylene glycol)lactate
Poly(ethylene glycol) with molecular weight 600 Da (52.57 g, 0.088 mol) and ethyl lactate (10.4 g, 0.088 mol) and NaOCH3 (1.9 × 10−3 mol%) were stirred under Ar-atmosphere with a magnetic stirrer in a round-bottom two-necked flask, equipped with a condenser, attached to the vacuum line. The reaction took place at temperatures between 100 and 120 °C for 18 h. The byproduct (ethanol) was removed under normal pressure. Reaction progress was controlled by 1H NMR spectroscopy (Figure S1). The end of the reaction was subjected to a vacuum to remove unreacted ethyl lactate. The catalyst was removed by extraction. Reaction products are soluble in water, ethanol, dichloromethane, chloroform, and THF. The product is lightly brown. The yield of poly(ethylene glycol 600)lactate was 70.82%, 41.72 g (based on 1H NMR spectrum see Figure S2); PEG 600 −17.76 g (0.03 mol).
1HNMR (CDCl3), δ (ppm) (Figure S3):1.43 (d, Ha, CH3CH, 3J(H,H) = 7.07 Hz); 1.428 (d,Ha, CH3CH, 3J(H,H) = 6.83 Hz); 3.47 (d, Hb, CH3CH(OH), 3J(H,H) = 4.6 Hz); 3.64, Hc s, -CH2OCH2-; 3.72 (t, Hd, HOCH2-, 3J(H,H) = 4.6 Hz); 4.28 to 4.34 area for Hf, CH3CH(OH)C-, quartet, and He, CH2CH2OC(O)-, triplet, overlapping of the signals;
13C{H}NMR (CDCl3), δ (ppm) (Figure S4): 20.34, CH3CH; 61.64, HOCH2-; 64.40, CH3CH-; 66.72, -C(O)OCH2-; 68.83, -C(O)OCH2CH2O-; 70.28, HOCH2CH2OCH2-; 70.51, -(OCH2CH2O)-; 72.48, HOCH2CH2O-; 175.51, C=O.
3.4. Synthesis of Poly[poly(ethylene glycol) H-phosphonate-b-poly(ethylene glycol)lactate H-phosphonate]
To 58.92 g reaction product from 2.2 containing: 41.72 g. An amount of 0.042 mol poly(ethyleneglycol600)lactate and 17.76 g 0.022 mol PEG600 were added to 17.61 g, 0.066 mol diphenyl H-phosphonate under Ar-atmosphere in a round-bottom two-necked flask, equipped with a magnetic stirrer, condenser attached to the vacuum line. The reaction was carried out at temperature of 130 °C for 6 h and at 145 °C for 4 h, vacuum 0.6 mm Hg. The progress of the reaction was monitored by the amount of phenol that evolved. When the evolution of phenol stopped, the system was cooled under argon flow. The product was obtained as a waxy solid. Yield, 40.9 g, 92.2%. Molecular weight based on the 31P{H}NMR spectrum (see Figure S1) 13,424 Da. 1H NMR (CDCl3) (Figure S5) δ, ppm, reaction product after 10 h heating: δ = 1.42 ppm, d, 3J(H,H) = 7.07 Hz for -C(O)CH(CH3)-OH protons; 1.60 ppm, d, 3J(H,H) = 6.83 Hz, -POCH(CH3)C(O)-; 1.58 ppm, 3J(H,H) = 6.83 Hz, -POCH(CH3)C(O)-; 3.64, s, -CH2OCH2; 4.99- 5.06 ppm, m, POCH(CH3)-protons; 6.83–7.21 for aromatic protons; δ = 6.95 ppm, d, 1J(P,H) = 717.69 Hz. OCH2OP(O)(H)OCH2; δ = 7.02 ppm, d, 1J(P,H) = 729.40 Hz for OCH2OP(O)(H)OCH(CH3); δ = 7.13 ppm, d, 1J(P,H) = 734.8 Hz for OCH2OP(O)(H)OCH(CH3); δ = 7.16 ppm, d, 1J(P,H) = 724.76 Hz for PhOP(O)(H)OCH2-; 4.13–4.37 ppm, m, OCH2CH2OP(O)(H)OCH2CH2-.
31P{H} NMR (Figure S6) (CDCl3), δ ppm, reaction product after 6 h heating: 9.98 ppm with integral intensity (II) = 1.00; 9.30 ppm, II = 0.34; 8.54 ppm, II = 0.02; 7.90 ppm, II = 0.38; 7.34 ppm, II = 0.05; 5.99 ppm, II = 0.06 and 5.68 ppm, II = 0.02. The ratio between the integral intensity of the signals at 9.98 ppm and (9.30 ppm and 7.90 ppm) is 1: 0.72 = 1.38.
31P NMR (CDCl3) (Figure S7), δ ppm: 9.98 ppm, doublet of quintets with 1J(P,H) = 717.78 Hz, and 3J(P,H) = 9.7 Hz, CH2OP(O)(H)OCH2; 9.30 ppm, dq, with 1J(P,H) = 734.76 Hz, and 3J(P,H) = 8.57 and 9.05 Hz, CH2OP(O)(H)OCH(CH3)-. Further, 7.90 ppm, dq, with 1J(P,H) = 729.26 Hz, and 3J(P,H) = 9.7 and 10.35 Hz, CH2OP(O)(H)OCH(CH3)-.
3.5. Synthesis of Poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)-lactate phosphate]s
3.5.1. Synthesis of Poly[hexadecylpoly(ethylene glycol) phosphate-b-[hexadecylpoly(ethylene glycol)lactate phosphate]
Poly[hexadecylpoly(ethylene glycol) phosphate)-b-hexadecylpoly(ethylene glycol)lactate phosphate] was obtained from poly[poly(ethylene glycol) H-phosphonate]-co-poly(ethylene glycol)lactate H-phosphonate], trichloroisocyanuric acid and 1-hexadecanol (HD) in a one-pot synthesis. The entire synthesis was carried out under an inert atmosphere. A typical synthetic procedure is described below: poly[(ethylene glycol) H-phosphonate]-co-[poly(ethylene glycol)lactate H-phosphonate] 1.58 g, 2.4 × 10−3 mol; trichloroisocyanuric acid 0.57 g, 2.4 × 10−3 mol. To the stirred reaction mixture in acetonitrile (10 mL) at room temperature was added in one portion a solution of trichloroisocyanuric acid in acetonitrile 21 mL. The reaction mixture was kept for 5 h at 40 °C, 4h at 60 °C, and 5 h at 50 °C. The optimum reaction time of 14 h was determined by observing 31P{H} NMR spectroscopy.31P{H}NMR spectrum showed signals at 5.89 ppm; 5.48 ppm and 4.76 ppm (Figure S8) which are characteristic of chlorophosphate structures. The ratio between the integral intensity of the signals at 5.89 ppm, 5.49 ppm, and 4.76 ppm is 1: 0.74. To the reaction product poly[poly(ethylene glycol) chlorophosphate-b-poly(ethylene glycol)lactate chlorophosphate] without isolation, 1-hexadecanol 0.58 g (2.4 × 10−3 mol in 7 mL diethyl ether was added. The optimum reaction time was determined by observing 31P{H} NMR spectroscopy. Yield 96%, 2.64 g.
1H NMR (CD3OD), δ (ppm): 0.89, CH3CH2-(t, 3J(H,H) = 5.7 Hz); 1.22–1.26, CH3(CH2)14-CH2-(m); 1.48–1.53, CH3(CH2)8-(CH2)6-CH2-,m; 3.66−3.58 (m, CH2OCH2). 4.14−4.10 (m, -CH2OP(O)(OCH2(CH2)14-CH3)OCH2-).
31P{H}NMR (CDCl3), δ 0.13 ppm; −0.19 ppm, −0.94ppm, −1.2 ppm, and −12.15 ppm.
3.5.2. Synthesis of Poly[octylpoly(ethylene glycol) phosphate-b-octylpoly(ethylene glycol)lactate phosphate]
Using the same procedure was synthesized: poly[octylpoly(ethylene glycol) phosphate-b-octylpoly(ethylene glycol)lactate phosphate]. A typical synthetic procedure: poly[(ethylene glycol) H-phosphonate-b-poly(ethylene glycol)lactate H-phosphonate] 1.98 g, 3.0 × 10−3 mol; trichloroisocyanuric acid 0.70 g, 3.0 × 10−3 mol; 1-octanol-0.39 g, 3.0 × 10−3 mol. Yield 94%, 2.97 g.
1H NMR (CD3OD), δ (ppm): 0.89, CH3CH2- (t, 3J(H,H) = 5.7 Hz); 1.22–1.26, CH3(CH2)6-CH2-(m); 1.48–1.53, CH3(CH2)4-(CH2)2-CH2-,m; 3.66−3.58 (m, -CH2OCH2-); 4.14−4.10 (m, -CH2OP(O)(OCH2(CH2)6-CH3)OCH2-).
31P{H}NMR (CDCl3), δ 0.11 ppm; −0.20 ppm, −0.93ppm, −1.2 ppm, and −11.15 ppm.
3.5.3. Synthesis of Poly[dodecylpoly(ethylene glycol) phosphate)-b-dodecylpoly(ethylene glycol)lactate phosphate]
Using the same procedure was synthesized poly[dodecyl poly(ethylene glycol) phosphate-b-dodecylpoly(ethylene glycol)lactate phosphate].
A typical synthetic procedure: poly[(ethylene glycol) H-phosphonate-b-poly(ethylene glycol)lactate H-phosphonate] 2.08 g, 3.1 × 10−3 mol; trichloroisocyanuric acid 0.74 g, 3.1 × 10−3 mol; 1-dodecanol-0.58 g, 3.1 × 10−3 mol. Yield 94%, 3.29 g.
1H NMR (CD3OD), δ (ppm): 0.89, CH3CH2-(t, 3J(H,H) = 5.7 Hz); 1.22–1.26, CH3(CH2)10-CH2-(m); 1.48–1.53, CH3(CH2)8-(CH2)2-CH2-,m; 3.66−3.58 (m, -CH2OCH2-). 4.14−4.10 (m, -CH2OP(O)(OCH2(CH2)10-CH3)OCH2-).
31P{H}NMR (CDCl3), δ 0.11 ppm; −0.18 ppm, −0.93ppm, −1.2 ppm, and −11.15 ppm.
3.6. Measurement of Polymeric Micelle Size
Add 50 mg of the synthesized poly[alkylpoly(ethylene glycol) phosphate-b-alkylpoly(ethylene glycol)lactate phosphate]s (referred to as sample) and 20 mL of acetone to the flask and dissolve completely. The tin film was formed. Then, the remaining acetone was completely removed by drying under reduced pressure. A PBS solution of pH 7.4 was added thereto. Then, after ultrasonic irradiation, it was filtered through a 0.2 μm filter. Furthermore, the particle size was measured at 37 °C by dynamic light scattering (DLS) system, ELS-Z2 (Otsuka Electronics Co., Ltd., Hirakata, Japan), to evaluate dispersibility.
3.7. Solubilizing Test
3.7.1. Preparation of Sudan III Encapsulating Micelles
Acetone 20 mL was added to 50 mg of the sample and 5 mg of Sudan III to dissolve completely. Thereafter, evaporation was performed to form a thin film, and acetone was completely removed by drying under reduced pressure. Then, a PBS solution was added, followed by ultrasonic irradiation, followed by filtration through a 0.2 μm filter to remove liberated Sudan III. An amount of 1 mL of the Sudan III-encapsulating micelle solution was freeze-dried. Then, it was dissolved in 10 mL of acetone and filtered through a 0.45 μm filter to remove precipitated salts. The absorbance of micellar acetone solution was measured using UV–vis, and Sudan III was quantified from a calibration curve prepared separately. The absorbance of the Sudan III-encapsulating micelle solution was measured with UV–Vis at an absorption wavelength of 500 nm, and the value was defined as the absorbance on day 0 (A0). Thereafter, the mixture was shaken in a hot water bath for predetermined hours (3, 6, 12, 24, 48, 72, 96, 120 h), and released Sudan III was removed with a 0.2 μm syringe filter. After that, the absorbance (An) was measured, and the release rate was calculated from the degree of decrease in absorbance by applying the following Formula (1).
where
- A0: Absorbance of Sudan III encapsulating micelle on day 0
- An: Absorbance of Sudan III encapsulating micelle on day n.
3.7.2. Preparation of Doxorubicin-Encapsulating Micelles
Two milligrams of doxorubicin hydrochloride were weighed into a flask, and 0.1 mL of triethylamine was added for dehydrogenation. Amounts of 50 mg of sample and 20 mL of chloroform were added and completely dissolved. Evaporation was performed to form a thin film, and chloroform was completely removed by drying under reduced pressure. A PBS solution was added to it. Then, after ultrasonic irradiation, it was filtered through a 0.2 μm filter to remove liberated doxorubicin. One milligram of the doxorubicin-encapsulating micelle solution was freeze-dried. Then, it was dissolved in 10 mL of chloroform and filtered through a 0.45 μm filter to remove precipitated salts. Then, the absorbance of the micelle chloroform solution was measured using UV–Vis, and doxorubicin was quantified from a separately prepared calibration curve. The absorbance of the doxorubicin-encapsulating micelle solution was measured with UV–Vis at an absorption wavelength of 500 nm, and the value was defined as the absorbance on day 0. Thereafter, the mixture was shaken in a hot water bath for predetermined hours (3, 6, 12, 24, 48, 72, 96, 120 h), and freed doxorubicin was removed with a 0.2 μm syringe filter. After that, the absorbance was measured, and the release rate was calculated from the degree of decrease in absorbance by applying Formula (1) in the same manner as for Sudan III-encapsulating micelles.
4. Conclusions
Amphiphilic diblock polyphosphoesters containing lactic acid units in the polymer backbone were synthesized by multistep one-pot polycondensation reactions. One-pot synthesis avoids a lengthy separation process and purification of the intermediate chemical compound. The proposed method for the synthesis of poly(ethylene glycol)lactate allows varying the molar ratio between ethyl lactate and PEG to synthesize copolymers with different lactic acid content. The inclusion of lactic acid leads to an increase in the hydrophilicity of the copolymer due to the carbonyl group. The effects of the polymer composition on micelle formation and stability, and micelle size were studied via DLS. The hydrophilic/hydrophobic balance of these polymers can be controlled by changing the chain lengths of hydrophobic alcohols. Loading capacity and encapsulation efficiency depend on the length of alkyl side chains. Drug loading and encapsulation efficiency tests using Sudan III and doxorubicin revealed that hydrophobic can be delivered by copolymers. The highest drug loading and encapsulation efficiency were obtained when hydrophobic alcohol was used hexadecanol—1.63% and 45.8%, respectively. The results obtained indicate that amphiphilic diblock polyphosphoesters have the potential as drug carriers. In this study, we succeeded in synthesizing novel polymers that can form micelles. To evaluate the safety and usefulness of these polymers in vivo, it will be important to conduct cytotoxicity tests, cell uptake tests, bio-security tests, and pharmacokinetic tests in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28135243/s1, Figure S1: Reaction progress based on 1H NMR spectroscopy. Figure S2: Calculation of the content of the products in the reaction mixture based on the integral intensity. Figure S3: 1H NMR spectrum of the reaction product obtained from poly(ethylene glycol) 600 and ethyl lactate. Figure S4: 13C{H} NMR spectrum of the reaction product obtained from poly(ethylene glycol) 600 and ethyl lactate. Figure S5: 1HNMR spectrum of poly[(oxyethylene H-phosphonate)-co-poly(ethylene glycol)lactate H-phosphonate]. Figure S6: 31P{H} NMR spectrum of poly[poly(ethylene glycol H-phosphonate] co poly[poly(ethylene glycol)lactate H-phosphonate]. Figure S7: 31P NMR spectrum of poly[poly(ethylene glycol H-phosphonate] co poly[poly(ethylene glycol)lactate H-phosphonate]. Figure S8: 31P{H}NMR of poly[poly(ethylene glycol) chlorophosphate] co poly[poly(ethylene glycol)lactate chlorophosphate]. Figure S9: 31P{H}NMR of poly[polyalklyl(ethylene glycol) phosphate] co poly[polyalkyl(ethylene glycol)lactate phosphate].
Author Contributions
Conceptualization, K.M., H.T., I.T. and K.T.; data curation, T.S. and I.T.; formal analysis, T.S., I.T. and K.T.; funding acquisition, K.M.; investigation, T.S.; methodology, I.T., K.M., V.M. and K.T.; resources, K.M. and H.T.; supervision, K.M., H.T. and K.T.; validation, I.T. and K.T.; visualization, T.S., I.T. and K.T.; writing—original draft, T.S.; writing—review and editing, I.T., V.M. and K.T. The late H.T. read the original draft of the manuscript and agreed to prepare it for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2010–2014 (grant number: S1001019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Acknowledgments
We gratefully acknowledge the support of our laboratory members.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors upon request.
References
- Troev, K.D. Polyphosphoesters: Chemistry and Application, 1st ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA; Oxford, UK; Tokyo, Japan, 2012; p. 84. [Google Scholar]
- Tzevi, R.; Todorova, G.; Kossev, K.; Troev, K.; Georgiev, E.; Roundhill, D.M. Immobilization of bioactive substances on poly(a1kylenephosphate)s. Immobilization of 2-phenylethylamine. Macromol. Chem. 1993, 194, 3261–3269. [Google Scholar] [CrossRef]
- Troev, K.; Tsacheva, I.; Koseva, N.; Georgieva, R.; Gitsov, I. Immobilization of Aminothiols on Poly(oxyethylene H-phosphonate)s and Poly(oxyethylene phosphate)s—An Approach to Polymeric Protective Agents for Radiotherapy of Cancer. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1349–1363. [Google Scholar] [CrossRef]
- Myrex, R.D.; Farmer, B.; Gray, G.M.; Wright, Y.J.; Dees, J.; Bharara, P.C.; Byrd, H.; Branham, K.E. 31P and 1H NMR studies of the transesterification polymerization of polyphosphonate oligomers. Eur. Polym. J. 2003, 39, 1105–1115. [Google Scholar] [CrossRef]
- Oussadi, K.; Montemblault, V.; Fontaine, L. Synthesis of poly(oxyethylene phosphate)-g-poly(ethylene oxide) via the “grafting onto” approach by “click” chemistry. J. Polym. Sci. Part A Polymer Chem. 2011, 49, 5124–5128. [Google Scholar] [CrossRef]
- Oussadi, K.; Montemblault, V.; Belbachir, M.; Fontaine, L. Ring-opening bulk polymerization of five-and six-membered cyclic phosphonates using maghnite, a nontoxic proton exchanged montmorillonite clay. J. Appl. Polym. Sci. 2011, 122, 891–897. [Google Scholar] [CrossRef]
- Koseva, N.; Bogomilova, A.; Atkova, K.; Troev, K. New functional polyphosphoesters: Design and characterization. React. Funct. Polym. 2008, 68, 954–966. [Google Scholar] [CrossRef]
- Branham, K.E.; Mays, J.W.; Gray, G.M.; Bharara, P.C.; HByrd, H.; Bittenger, R.; Farmer, B. Polycondensations of dimethyl phosphonate with diols: SEC and 31P and 13C NMR spectroscopic studies. Polymer 2000, 41, 3371–3379. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. Poly(ethylene glycol) ionomers with phosphate diester linkages. Makromol. Chem. Rapid Commun. 1988, 9, 731–737. [Google Scholar] [CrossRef]
- Pretula, J.; Penczek, S. High-molecular-weight poly (alkylene phosphonate)s by condensation of dialkylphosphonates with diols. Makromol. Chem. 1990, 191, 671–680. [Google Scholar] [CrossRef]
- Penczek, S.; Pretula, J. High-molecular-weight poly (alkylene phosphates) and preparation of amphiphilic polymers thereof. Macromolecules 1993, 26, 2228–2233. [Google Scholar] [CrossRef]
- Gorjanova, B.; Gitsov, I.; Troev, K. Hydrolytic stability of poly (oxyethylene-H-phosphonate)s. In Proceedings of the 37th Middle Atlantic Regional Meeting of the American Chemical Society, New Brunswick, NJ, USA, 22–25 May 2005. [Google Scholar]
- Gitsov, I.; Johnson, F.E. Synthesis and hydrolytic stability of poly (oxyethylene-H-phosphonate)s. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4130–4139. [Google Scholar] [CrossRef]
- Pretula, J.; Kaluzynski, K.; Szymanski, R.; Penczek, S. Preparation of poly (alkylene H-phosphonate) s and their derivatives by polycondensation of diphenyl H-phosphonate with diols and subsequent transformations. Macromolecules 1997, 30, 8172–8176. [Google Scholar] [CrossRef]
- Georgieva, R.; Tsevi, R.; Kossev, K.; Kusheva, R.; Balgjiska, M.; Petrova, R.; Tenchova, V.; Gitsov, I.; Troev, K. Immobilization of Aminothiols on Poly(oxyalkylene phosphates). Formation of Poly(oxyethylene phosphates)/Cysteamine Complexes and Their Radioprotective Efficiency. J. Med. Chem. 2002, 45, 5797–5801. [Google Scholar] [CrossRef]
- Pencheva, I.; Bogomilova, A.; Koseva, N.; Obreshkova, D.; Troev, K. HPLC study on the stability of bendamustine hydrochloride immobilized onto polyphosphoesters. J. Pharm. Biomed. Anal. 2008, 48, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Kraicheva, I.; Bogomilova, A.; Tsacheva, I.; Momekov, G.; Momekova, D.; Troev, K. Synthesis, NMR characterization and in vitro cytotoxicity evaluation of new poly (oxyethylene aminophosphonate)s. Eur. J. Med. Chem. 2010, 45, 6039–6044. [Google Scholar] [CrossRef] [PubMed]
- Troev, K.; Mitova, V.; Ivanov, I. On the design of polymeric 5′-O-ester prodrugs of 3′-azido-2′, 3′-dideoxythymidine (AZT). Tetrahedron Lett. 2010, 51, 6123–6125. [Google Scholar] [CrossRef]
- Mitova, V.; Hristova, T.; Cherkezova, R.; Koseva, N.; Yusa, S.; Troev, K. Polyphosphoesters Based—Placlitaxel Complexes. Synthesis and Charcterization. J. Appl. Polym. Sci. 2015, 132, 42772. [Google Scholar] [CrossRef]
- Hirota, K.; Hristova, T.; Mitova, V.; Koda, T.; Fushimi, M.; Kuniya, M.; Makino, K.; Terada, H.; Cherkezova, R.; Yusa, S.-I.; et al. Polyphosphoesters Based—Placlitaxel Complexes. Biol. Eval. Anticancer. Res. 2016, 36, 1613–1620. [Google Scholar]
- Todorova, Z.; Koseva, N.; Ugrinova, I.K.; Troev, K. Synthesis of Poly(oxyethylene phosphoramidate)s and Glycopolymers via. Staudinger Reaction: Multivalent Binding Studies with Concanavalin A. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1730. [Google Scholar] [CrossRef]
- Wang, J.; Mao, H.Q.; Leong, K.W. A novel biodegradable gene carrier based on polyphosphoester. J. Am. Chem. Soc. 2001, 123, 9480–9481. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.C.; Mao, H.Q.; Leong, K.W. Enhanced gene expression in mouse muscle by sustained release of plasmid DNA using PPE-EA as a carrier. Gene Ther. 2002, 9, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.C.; Lu, H.F.; Ma, N.; Wang, S.; Mao, H.Q.; Leong, K.W. New polyphosphoramidate with a spermidine side chain as a gene carrier. J. Control. Release 2002, 83, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jiang, X.; Pan, D.; Mao, H.Q. Charge density and molecular weight of polyphosphoramidate gene carrier are key parameters influencing its DNA compaction ability and transfection efficiency. Biomacromolecules 2010, 11, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, S.J.; Zhang, P.C.; Wang, S.; Mao, H.Q.; Leong, K.W. Polyphosphoramidate gene carriers: Effect of charge group on gene transfer efficiency. Gene Ther. 2004, 11, 1001–1010. [Google Scholar] [CrossRef]
- Huang, S.W.; Wang, J.; Zhang, P.C.; Mao, H.Q.; Zhuo, R.X.; Leong, K.W. Water-soluble and nonionic polyphosphoester: Synthesis, degradation, biocompatibility and enhancement of gene expression in mouse muscle. Biomacromolecules 2004, 5, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dai, H.; Ke, C.Y.; Mo, X.; Torbenson, M.S.; Li, Z.; Mao, H.Q. PEG-b-PPA/DNA micelles improve transgene expression in rat liver through intrabiliary infusion. J. Control. Release 2007, 122, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Yamakita, Y.; Tekauchi, I.; Makino, K.; Terada, H.; Kikuchi, A.; Troev, K. Thermoresponsive Polyphosphoester via Polycondensation Reactions: Synthesis, Characterization, and Self-Assembly. Molecules 2022, 27, 6006. [Google Scholar] [CrossRef]
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Vert, M. Aliphatic polyesters: Great degradable polymers that cannot do everything. Biomacromolecules 2005, 6, 538–546. [Google Scholar] [CrossRef]
- Parrish, B.; Emrick, T. Aliphatic Polyesters with Pendant Cyclopentene Groups: Controlled Synthesis and Conversion to Polyester-graft-PEG Copolymers. Macromolecules 2004, 37, 5863–5865. [Google Scholar] [CrossRef]
- Langlois, V.; Vallee-Rehel, K.; Peron, J.J.; Borgne, A.; Walls, M.; Guerin, P. Synthesis and hydrolytic degradation of graft copolymers containing poly (lactic acid) side chains: In vitro release studies of bioactive molecules. Polym. Degrad. Stab. 2002, 76, 411–417. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyase, T.; Tezuka, Y.; Saha, S.K. Physical Properties, Crystallization, and Spherulite Growth of Linear and 3-Arm Poly(l-lactide)s. Biomacromolecules 2005, 6, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, J.; Kylma, J.; Seppala, J. Chain extending of lactic acid oligomers. 2. Increase of molecular weight with 1, 6-hexamethylene diisocyanate and 2, 2′-bis (2-oxazoline). Polymer 2002, 43, 3–10. [Google Scholar] [CrossRef]
- Andreopoulos, A.G.; Hatzi, G.E.; Doxastakis, M. Synthesis and properties. J. Mater. Sci. Mater. Med. 1999, 10, 29–33. [Google Scholar] [CrossRef]
- Stridsberg, K.; Albertsson, A.C. Controlled ring-opening polymerization of L-lactide and 1,5-dioxepan-2-one forming a triblock copolymer. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1774–1784. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Hile, D.D.; Pishko, M.V. Emulsion copolymerization of D,L-lactide and glycolide in supercritical carbon dioxide. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 562–570. [Google Scholar] [CrossRef]
- Tuominen, J.; Seppala, J.V. Synthesis and characterization of lactic acid based poly (ester−amide). Macromolecules 2000, 33, 3530–3535. [Google Scholar] [CrossRef]
- Bayan, M.F.; Jaradat, A.; Alyami, M.H.; Naser, A.Y. Smart pellets for controlled delivery of 5-fluorouracil. Molecules 2023, 28, 306. [Google Scholar] [CrossRef]
- Bayan, F.B.; Marji, S.M.; Salem, M.S.; Begum, M.Y.; Chidambaram, K.; Chandrasekaran, B. Development of polymeric-based formulation as potential smart colonic drug delivery system. Polymers 2022, 14, 3697. [Google Scholar] [CrossRef]
- Troev, K.; Naruoka, A.; Terada, H.; Kikuchi, A.; Makino, K. New Efficient Method of Oxidation of Poly(alkylene H-phosphonate)s: A Promising Route to Novel co-Polyphosphoesters. Macromolecules 2012, 45, 5698–5703. [Google Scholar] [CrossRef]
- Chaubal, M.V.; Wang, B.; Su, G.; Zhao, Z. Compositional analysis of biodegradable polyphosphoester copolymers using nmr spectroscopic methods. J. Appl. Polym. Sci. 2003, 90, 4021–4031. [Google Scholar] [CrossRef]
- Modro, A.M.; Modro, T.A. The phosphoryl and the carbonyl group as hydrogen bond acceptors. Phosphorus Sulfur Silicon 2002, 177, 2067. [Google Scholar] [CrossRef]
- La, S.B.; Okano, T.; Kataoka, K. Preparation and characterization of the micelle-forming polymeric drug indomethacin-incorporated poly(ethylene oxide)-poly(beta-benzyl L-aspartate) block copolymer micelles. J. Pharm. Sci. 1996, 85, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Block copolymer micelles for drug delivery: Loading and release of doxorubicin. J. Control. Release 1997, 48, 195–201. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, I.G.; Lee, Y.M.; Cho, C.S.; Sung, Y.K. Methoxy poly(ethylene glycol) and e -caprolactone amphiphilic block copolymeric micelle containing indomethacin. II. Micelle formation and drug release behaviors. J. Control. Release 1998, 51, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Maysinger, D. Adi Eisenberg Nano-engineering block copolymer aggregates for drug delivery. Colloids Surf. B Biointerfaces 1999, 16, 3–27. [Google Scholar] [CrossRef]
- De Jaeghere, F.; Allemann, E.; Leroux, J.C.; Stevels, W.; Feijen, J.; Doelker, E.; Gurny, R. Formulation and lyoprotection of poly (lactic acid-co-ethyleneoxide) nanoparticles: Influence on physical stability and in vitro cell uptake. Pharm. Res. 1999, 16, 859–866. [Google Scholar] [CrossRef]
- Hussein, Y.H.A.; Youssry, M. Polymeric Micelles of Biodegradable Diblock Copolymers: Enhanced Encapsulation of Hydrophobic Drugs. Materials 2018, 11, 688–704. [Google Scholar] [CrossRef]
- Jeong, Y.-I.; Cheon, J.-B.; Kim, S.-H.; Nah, J.-W.; Lee, Y.-M.; Sung, Y.-K.; Akaike, T.; Cho, C.-S. Clonazepam release from core-shell type nanoparticles in vitro. J. Control. Release 1998, 51, 169–178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).