Design and Synthesis of New Sulfonic Acid Functionalized Ionic Liquids as Catalysts for Esterification of Fatty Acids with Bioethanol
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of SAILs
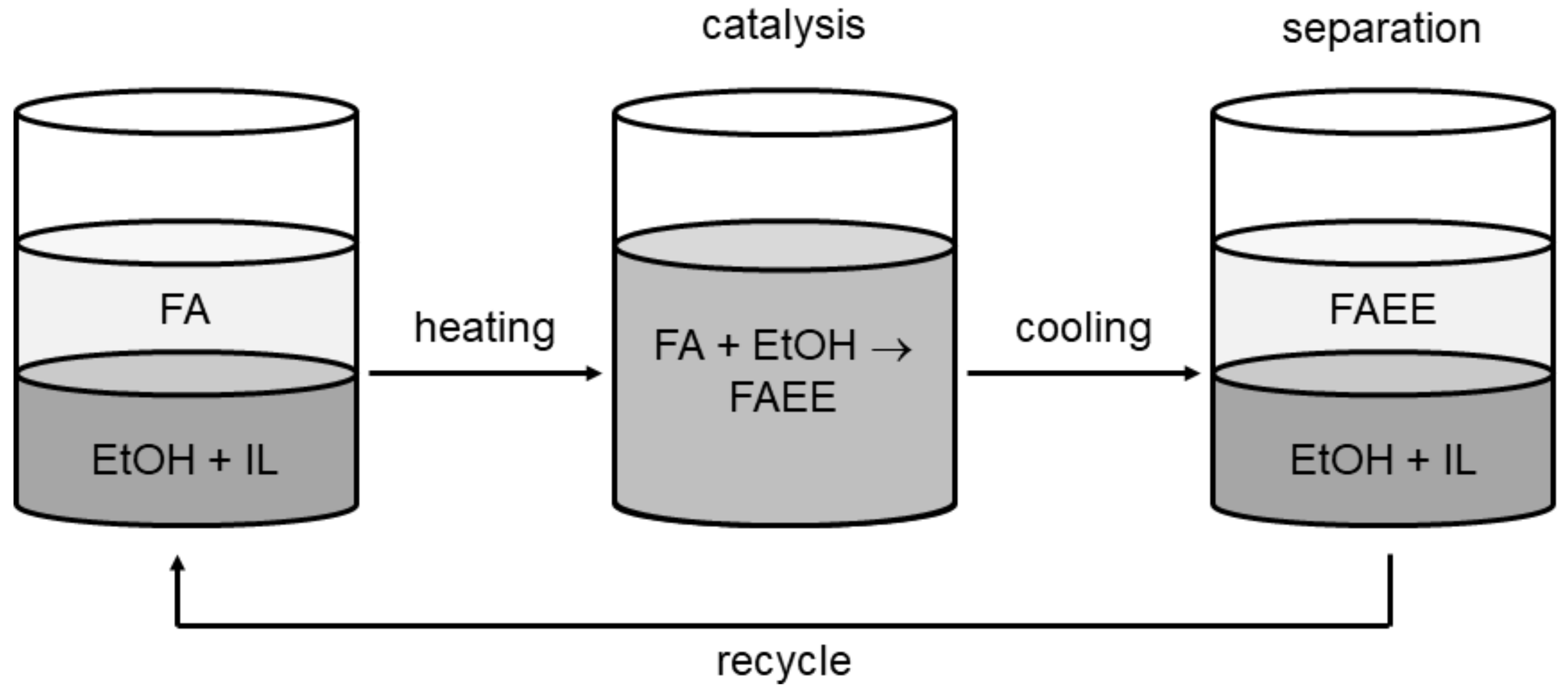
2.2. Catalyst Evaluation for Oleic Acid Esterification with Ethanol
3. Materials and Methods
3.1. Materials and Reagents
3.2. Characterization Methods
3.3. Synthesis of the Catalysts
3.3.1. Brønsted Acidic Imidazolium-Based IL
3.3.2. Brønsted Acidic Benzimidazolium-Based IL
3.4. Esterification of Oleic Acid with Ethanol Catalyzed by Ionic Liquids and Analysis
3.4.1. General Procedure for the Microwave Esterification Reaction
3.4.2. Esterification of Fatty Acids with Ethanol with Conventional Heating
3.4.3. The Recycling Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Borges, M.E.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Yu, H.; Niu, S.; Lu, C.; Li, J.; Yang, Y. Preparation and esterification performance of sulfonated coal-based heterogeneous acid catalyst for methyl oleate production. Energy Convers. Manag. 2016, 126, 488–496. [Google Scholar] [CrossRef]
- Esmaeili, H. A critical review on the economic aspects and life cycle assessment of biodiesel production using heterogeneous nanocatalysts. Fuel Process. Technol. 2022, 230, 107224. [Google Scholar] [CrossRef]
- Vitiello, R.; Taddeo, F.; Russo, V.; Turco, R.; Buonerba, A.; Grassi, A.; Di Serio, M.; Tesser, R. Production of Sustainable Biochemicals by Means of Esterification Reaction and Heterogeneous Acid Catalysts. Chemengineering 2021, 5, 46. [Google Scholar] [CrossRef]
- Lawer-Yolar, G.; Dawson-Andoh, B.; Atta-Obeng, E. Synthesis of Biodiesel from Tall Oil Fatty Acids by Homogeneous and Heterogeneous Catalysis. Sustain. Chem. 2021, 2, 206–221. [Google Scholar] [CrossRef]
- Munyentwali, A.; Li, H.; Yang, Q. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Appl. Catal. A Gen. 2022, 633, 118525. [Google Scholar] [CrossRef]
- Hardacre, C.; Parvulescu, V. Catalysis in Ionic Liquids: From Catalyst Synthesis to Application; RSC: London, UK, 2014; ISBN 978-1-84973-603-9. [Google Scholar] [CrossRef]
- Keskin, S.; Kayrak-Talay, D.; Akman, U.; Hortaçsu, Ö. A review of ionic liquids towards supercritical fluid applications. J. Supercrit. Fluids 2007, 43, 150–180. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Đokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Fauzi, A.H.M.; Amin, N.A.S. Optimization of oleic acid esterification catalyzed by ionic liquid for green biodiesel synthesis. Energy Convers. Manag. 2013, 76, 818–882. [Google Scholar] [CrossRef]
- Roman, F.F.; Ribeiro, A.E.; Queiroz, A.; Lenzi, G.G.; Chaves, E.S.; Brito, P. Optimization and kinetic study of biodiesel production through esterification of oleic acid applying ionic liquids as catalysts. Fuel 2018, 239, 1231–1239. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Zhang, H.; Wang, A.; Yang, S. Acidic ionic liquid-functionalized mesoporous melamine-formaldehyde polymer as heterogeneous catalyst for biodiesel production. Fuel 2019, 239, 886–895. [Google Scholar] [CrossRef]
- Zhang, W.; Li, M.; Wang, J.; Zhao, Y.; Zhou, S.; Xing, W. Heterogeneous poly(ionic liquids) catalyst on nanofiber-like palygorskite supports for biodiesel production. Appl. Clay Sci. 2017, 146, 167–175. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, Y.; Li, S.; Zhang, M.; Wang, Y.; Wang, Z.; Peng, Y.; Wang, M.; Li, X.; Pan, H. Recent advances in supported acid/base ionic liquids as catalysts for biodiesel production. Front. Chem. 2022, 10, 999607. [Google Scholar] [CrossRef] [PubMed]
- Panchal, B.; Bian, K.; Chang, T.; Zhu, Z.; Wang, J.; Qin, S.; Zhao, C.; Sun, Y. Synthesis of Generation-2 polyamidoamine based ionic liquid: Efficient dendrimer based catalytic green fuel production from yellow grease. Energy 2020, 219, 119637. [Google Scholar] [CrossRef]
- Tankov, I.; Mustafa, Z.; Nikolova, R.; Veli, A.; Yankova, R. Biodiesel (methyl oleate) synthesis in the presence of pyridinium and aminotriazolium acidic ionic liquids: Kinetic, thermodynamic studies. Fuel 2021, 307, 121876. [Google Scholar] [CrossRef]
- Panchal, B.; Zhu, Z.; Qin, S.; Chang, T.; Zhao, Q.; Sun, Y.; Zhao, C.; Wang, J.; Bian, K.; Rankhamb, S. The current state applications of ethyl carbonate with ionic liquid in sustainable biodiesel production: A review. Renew. Energy 2021, 181, 341–354. [Google Scholar] [CrossRef]
- Deshpande, G.; Shrikhande, S.; Sawarkar, A.N.; Patle, D.S. Multiobjective optimization of ultrasound intensified and ionic liquid catalyzed in situ algal biodiesel production considering economic, environmental and safety indicators. Chem. Eng. Res. Des. 2022, 180, 134–152. [Google Scholar] [CrossRef]
- Zhang, L.; Xian, M.; He, Y.; Li, L.; Yang, J.; Yu, S.; Xu, X. A Brønsted acidic ionic liquid as an efficient and environmentally benign catalyst for biodiesel synthesis from free fatty acids and alcohols. Bioresour. Technol. 2009, 100, 4368–4373. [Google Scholar] [CrossRef]
- Zhao, Y.; Long, J.; Deng, F.; Liu, X.; Li, Z.; Xia, C.; Peng, J. Catalytic amounts of Brønsted acidic ionic liquids promoted esterification: Study of acidity–activity relationship. Catal. Commun. 2009, 10, 732–736. [Google Scholar] [CrossRef]
- Fan, P.; Xing, S.; Wang, J.; Fu, J.; Yang, L.; Yang, G.; Miao, C.; Lv, P. Sulfonated imidazolium ionic liquid-catalyzed transesterification for biodiesel synthesis. Fuel 2017, 188, 483–488. [Google Scholar] [CrossRef]
- Fang, D.; Yang, J.; Jiao, C. Dicationic Ionic Liquids as Environmentally Benign Catalysts for Biodiesel Synthesis. ACS Catal. 2010, 1, 42–47. [Google Scholar] [CrossRef]
- Aghabarari, B.; Dorostkar, N.; Ghiaci, M.; Amini, S.G.; Rahimi, E.; Martinez-Huerta, M.V. Esterification of fatty acids by new ionic liquids as acid catalysts. J. Taiwan Inst. Chem. Eng. 2014, 45, 431–435. [Google Scholar] [CrossRef]
- Chang, T.; He, L.; Zhang, X.; Yuan, M.; Qin, S.; Zhao, J. Brönsted acid surfactant-combined dicationic ionic liquids as green catalysts for biodiesel synthesis from free fatty acids and alcohols. Chin. J. Catal. 2015, 36, 982–986. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, Y.; Liu, W.; Jiang, S.; Zhang, J.; Ma, W. Hydrogen Bonds in Disulfonic-Functionalized Acid Ionic Liquids for Efficient Biodiesel Synthesis. ACS Omega 2020, 5, 12110–12118. [Google Scholar] [CrossRef]
- Lin, X.; Ling, X.; Chen, J.; Li, M.; Xu, T.; Qiu, T. Self-solidification ionic liquids as heterogeneous catalysts for biodiesel production. Green Chem. 2019, 21, 3182–3189. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Li, L.; Ye, C.; Lin, X.; Qiu, T. Novel multi–SO3H functionalized ionic liquids as highly efficient catalyst for synthesis of biodiesel. Green Energy Environ. 2020, 6, 271–282. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Li, M.; Lin, X.; Qiu, T. Self-Solidifying Quaternary Phosphonium-Containing Ionic Liquids as Efficient and Reusable Catalysts for Biodiesel Production. ACS Sustain. Chem. Eng. 2020, 8, 6956–6963. [Google Scholar] [CrossRef]
- He, L.; Qin, S.; Chang, T.; Sun, Y.; Gao, X. Biodiesel synthesis from the esterification of free fatty acids and alcohol catalyzed by long-chain Brønsted acid ionic liquid. Catal. Sci. Technol. 2012, 3, 1102–1107. [Google Scholar] [CrossRef]
- Cai, D.; Zhan, G.; Xiao, J.; Zhou, S.-F.; Qiu, T. Design and synthesis of novel amphipathic ionic liquids for biodiesel production from soapberry oil. Renew. Energy 2020, 168, 779–790. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Gómez, C.; Álvarez, E.; Markiv, B.; García-Verdugo, E.; Luis, S.V. Green biocatalytic synthesis of biodiesel from microalgae in one-pot systems based on sponge-like ionic liquids. Catal. Today 2019, 346, 87–92. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Yusof, N.M.; Kamis, N.H.H.; Shaleh, S.R.M. Optimization of the ionic liquid-microwave assisted one-step biodiesel production process from wet microalgal biomass. Energy Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. Ionic liquid as a promising biobased green solvent in combination with microwave irradiation for direct biodiesel production. Bioresour. Technol. 2016, 206, 150–154. [Google Scholar] [CrossRef] [PubMed]
- El Sherbiny, S.A.; Refaat, A.A.; El Sheltawy, S.T. Production of biodiesel using the microwave technique. J. Adv. Res. 2010, 1, 309–314. [Google Scholar] [CrossRef]
- Motasemi, F.; Ani, F.N. A review on microwave-assisted production of biodiesel. Renew. Sustain. Energy Rev. 2012, 16, 4719–4733. [Google Scholar] [CrossRef]
- Quitain, A.T.; Sumigawa, Y.; Mission, E.G.; Sasaki, M.; Assabumrungrat, S.; Kida, T. Graphene Oxide and Microwave Synergism for Efficient Esterification of Fatty Acids. Energy Fuels 2018, 32, 3599–3607. [Google Scholar] [CrossRef]
- Ding, H.; Ye, W.; Wang, Y.; Wang, X.; Li, L.; Liu, D.; Gui, J.; Song, C.; Ji, N. Process intensification of transesterification for biodiesel production from palm oil: Microwave irradiation on transesterification reaction catalyzed by acidic imidazolium ionic liquids. Energy 2018, 144, 957–967. [Google Scholar] [CrossRef]
- Bölük, S.; Sönmez, Ö. Microwave-Assisted Esterification of Oleic Acid Using an Ionic Liquid Catalyst. Chem. Eng. Technol. 2020, 43, 1792–1801. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Li, K.; Zhang, H.; Fan, L.; Lu, Z. Efficient production of biodiesel from ionic liquid catalyzed esterification using ultrasonic-microwave combined intensification. Chem. Eng. Process. Process Intensif. 2020, 149, 107870. [Google Scholar] [CrossRef]

| Catalyst Loading | SAIL 2 0.1 eq | SAIL 2 0.2 eq | SAIL 2 0.3 eq | SAIL 5 0.1 eq | SAIL 5 0.2 eq | SAIL 5 0.3 eq | SAIL 8 0.1 eq |
|---|---|---|---|---|---|---|---|
| Conversion FAEE [%] | 86.8 | 86.6 | 92.2 | 90.8 | 94.9 | 94.5 | 4.3 |
| Catalyst | Conversion of Fatty Acids to FAEE (%) | ||||
|---|---|---|---|---|---|
| Experiment 1 | Recycling 1 | Recycling 2 | Recycling 3 | Recycling 4 | |
| SAIL 2 | 99.3 | 98.2 | 95.7 | 78.0 | 66.7 |
| SAIL 5 | 98.4 | 97.2 | 98.8 | 96.1 | 81.4 |
| SAIL 8 | 99.7 | 98.1 | 96.0 | 99.7 | 99.1 |
| Catalyst | Loading (mol %) | Ethanol/Acid | Temperature (°C) | Reaction Time (h) | Conversion (%) | Ref. | TOF (h−1) |
|---|---|---|---|---|---|---|---|
| [C12Sb][Tos] | 2 | 1.5/1 | 60 | 4.5 | 95.7 | 24 | 10.6 |
| [DDPA][Tos] | 10 | 1.5/1 | 60 | 3 | 94.6 | 29 | 3.2 |
| [NMP][CH3SO3] | 34 | 2/1 | 70 | 8 | 96.5 a | 19 | 0.35 |
| [TMEDAPS][HSO4] | 20 | 1.8/1 | 70 | 6 | 96 a | 22 | 0.8 |
| IL8B ionic liquid | 7.5 | 2/1 | 50 | 6 | 95.4 b | 23 | 2.1 |
| SAIL 8 | 30 | 16.5/1 | 100 c | 2 | 99.7 a | This work | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen Thi, T.H.; Koutecká, J.; Kaule, P.; Vrtoch, L.; Šícha, V.; Čermák, J. Design and Synthesis of New Sulfonic Acid Functionalized Ionic Liquids as Catalysts for Esterification of Fatty Acids with Bioethanol. Molecules 2023, 28, 5231. https://doi.org/10.3390/molecules28135231
Nguyen Thi TH, Koutecká J, Kaule P, Vrtoch L, Šícha V, Čermák J. Design and Synthesis of New Sulfonic Acid Functionalized Ionic Liquids as Catalysts for Esterification of Fatty Acids with Bioethanol. Molecules. 2023; 28(13):5231. https://doi.org/10.3390/molecules28135231
Chicago/Turabian StyleNguyen Thi, Thu Huong, Jiřina Koutecká, Pavel Kaule, Luboš Vrtoch, Václav Šícha, and Jan Čermák. 2023. "Design and Synthesis of New Sulfonic Acid Functionalized Ionic Liquids as Catalysts for Esterification of Fatty Acids with Bioethanol" Molecules 28, no. 13: 5231. https://doi.org/10.3390/molecules28135231
APA StyleNguyen Thi, T. H., Koutecká, J., Kaule, P., Vrtoch, L., Šícha, V., & Čermák, J. (2023). Design and Synthesis of New Sulfonic Acid Functionalized Ionic Liquids as Catalysts for Esterification of Fatty Acids with Bioethanol. Molecules, 28(13), 5231. https://doi.org/10.3390/molecules28135231







