Arylcyanomethylenequinone Oximes: An Overview of Synthesis, Chemical Transformations, and Biological Activity
Abstract
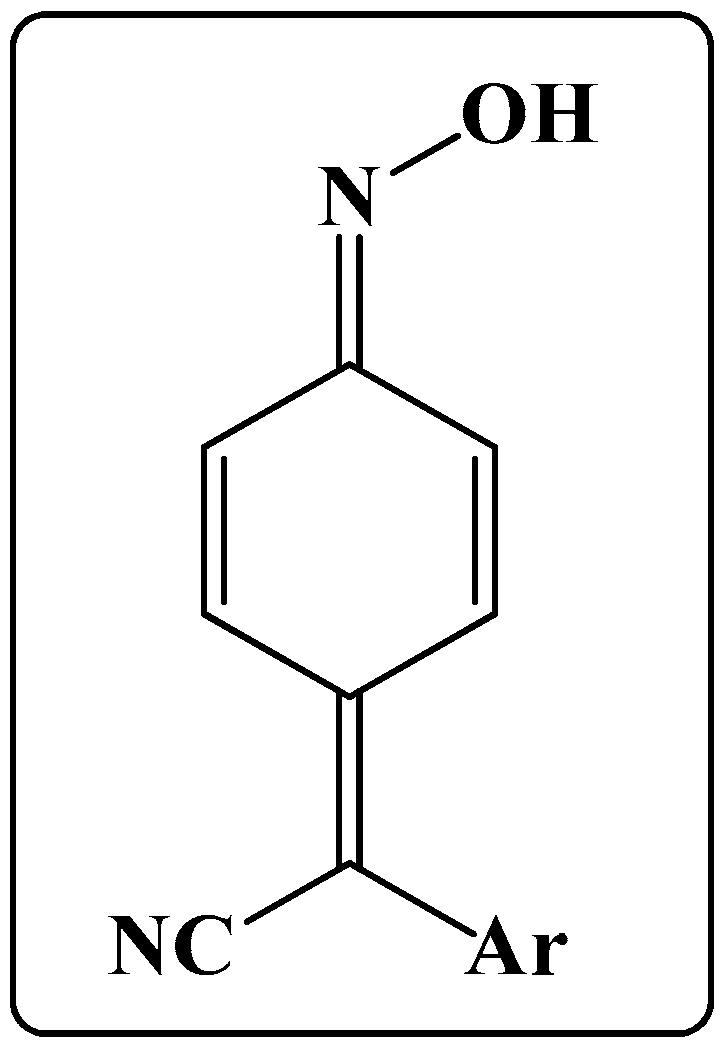
1. Introduction
2. Synthesis of Arylcyanomethylenequinone Oximes and Some of Their Derivatives
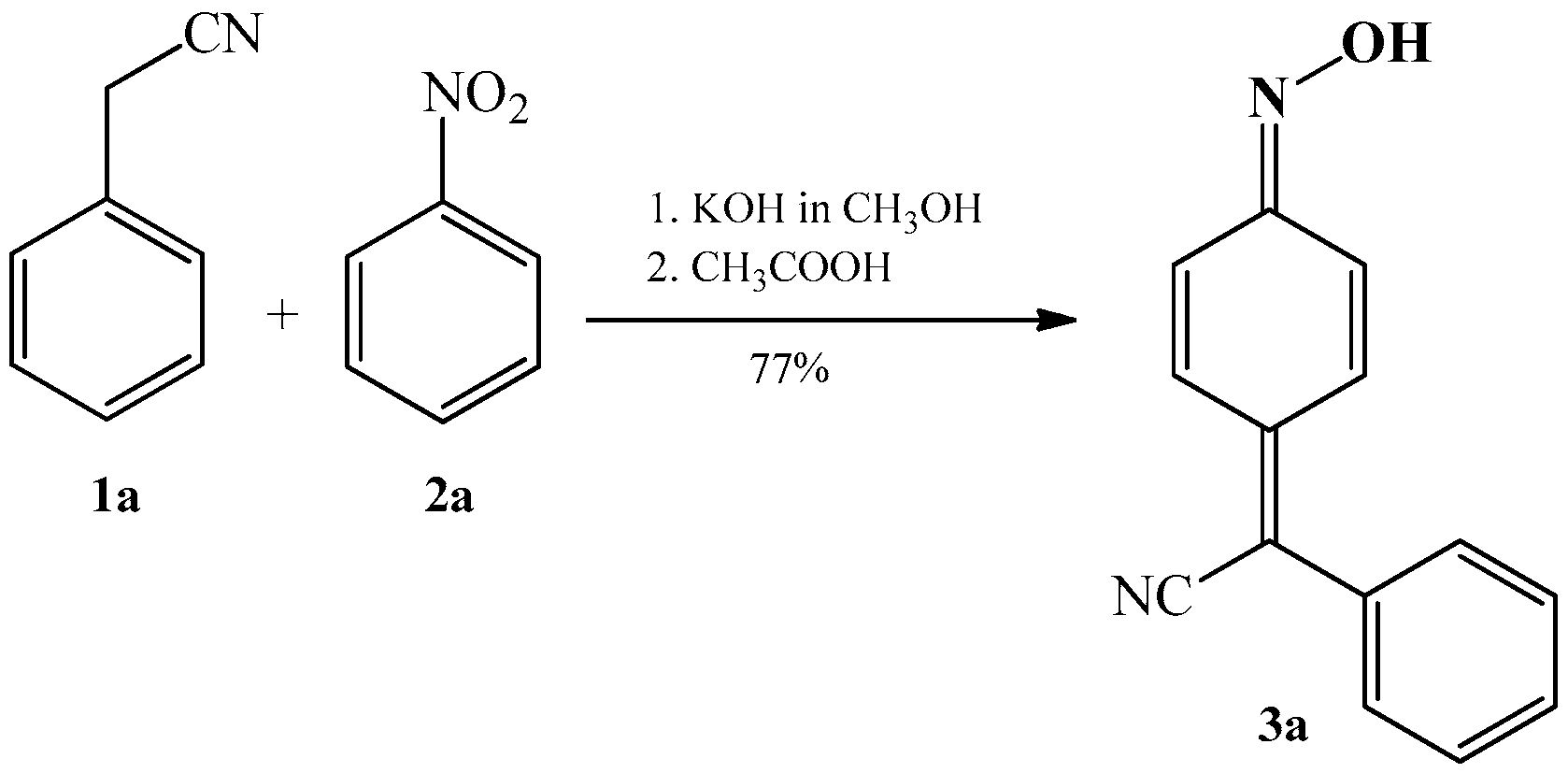
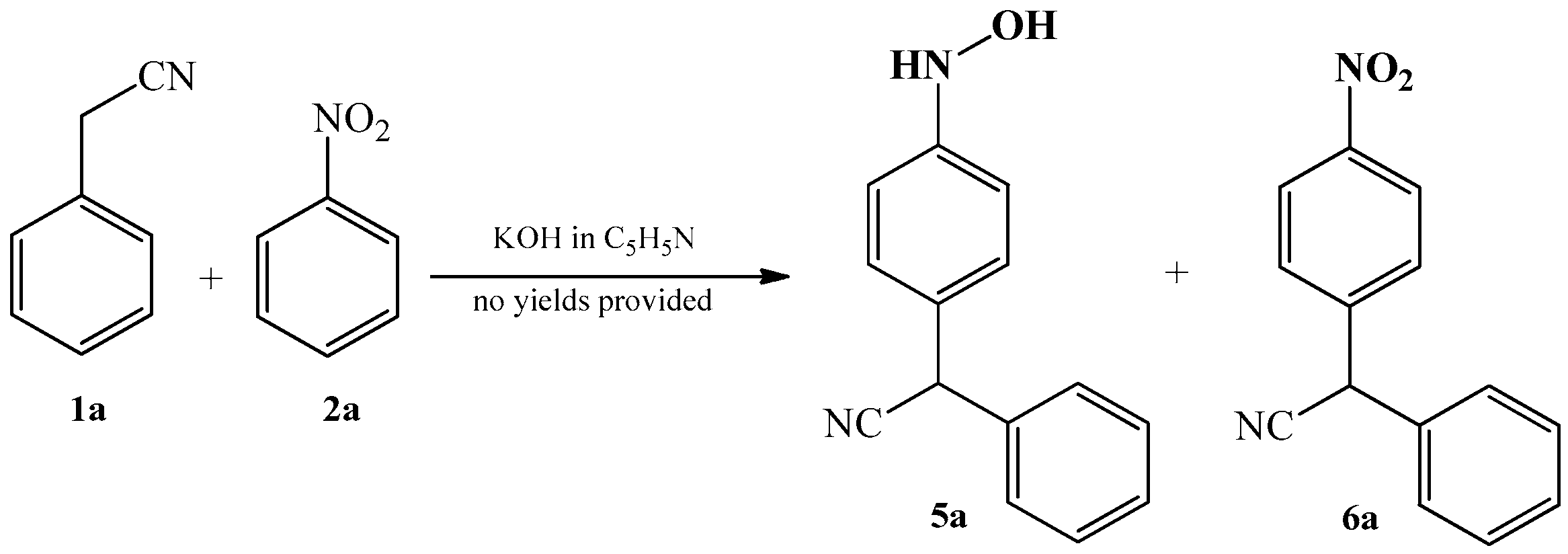
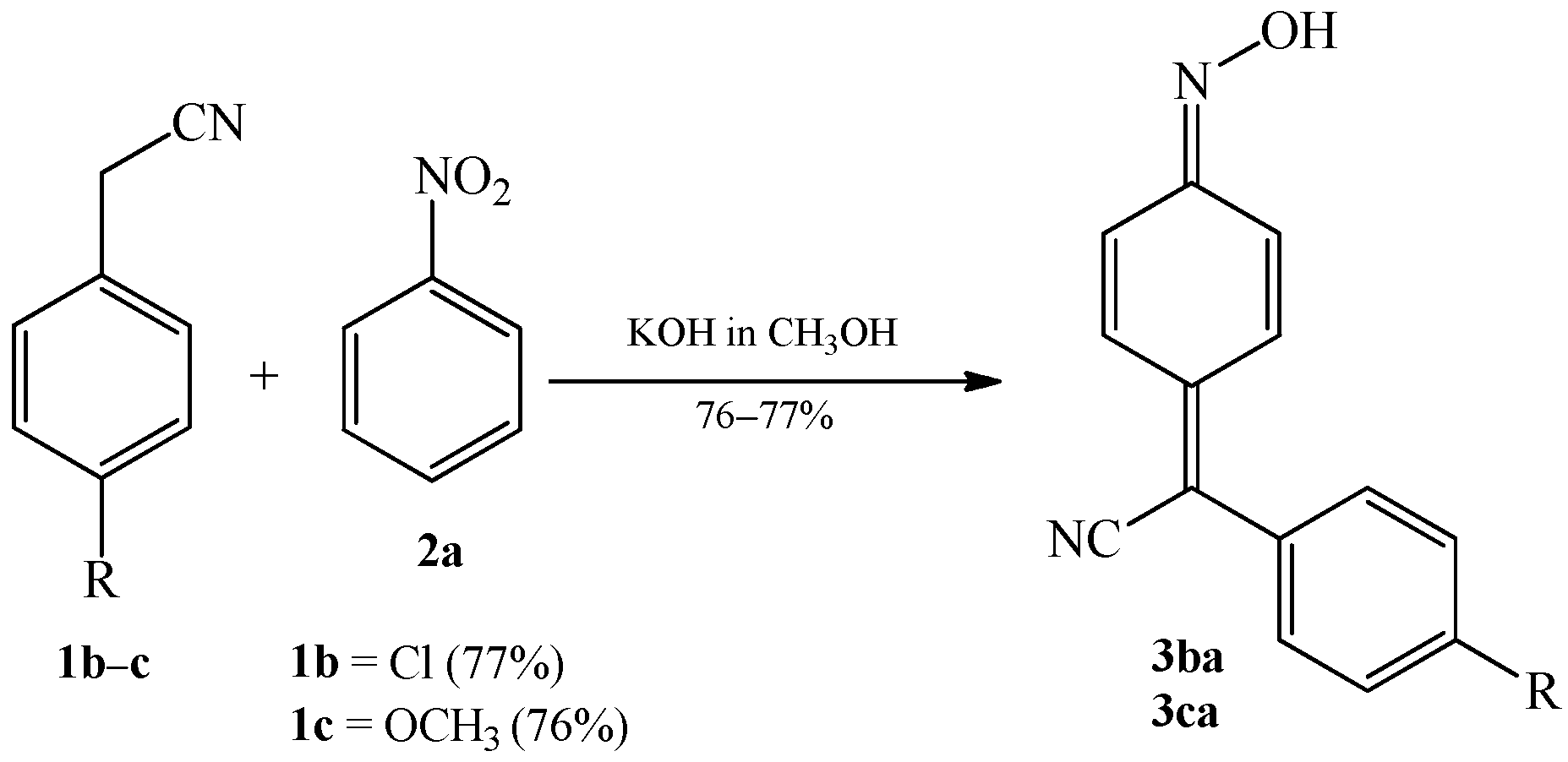
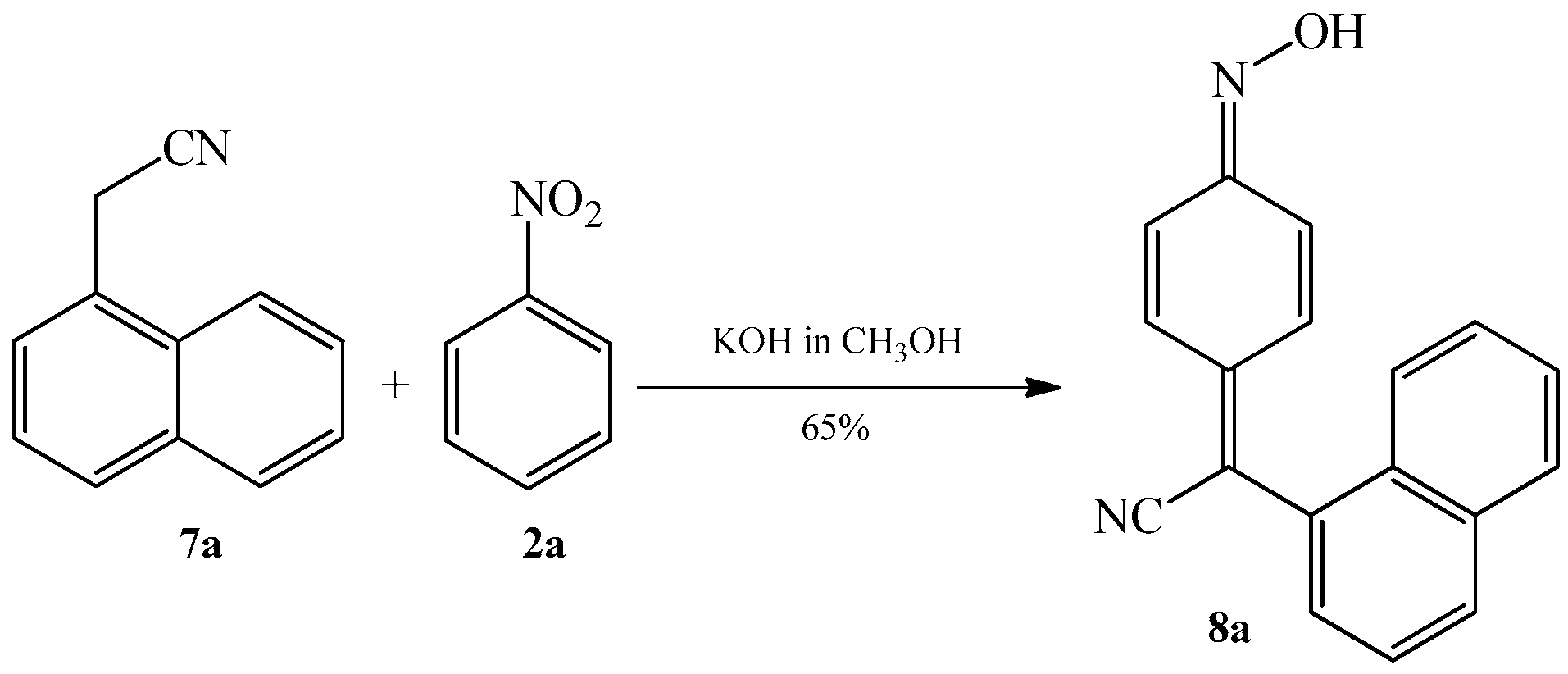
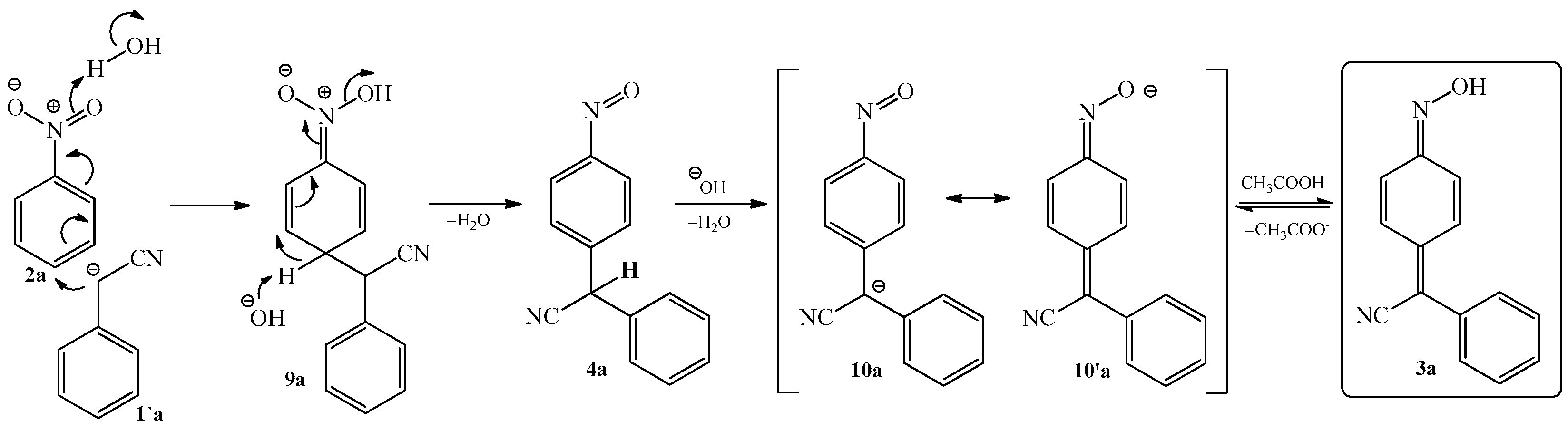
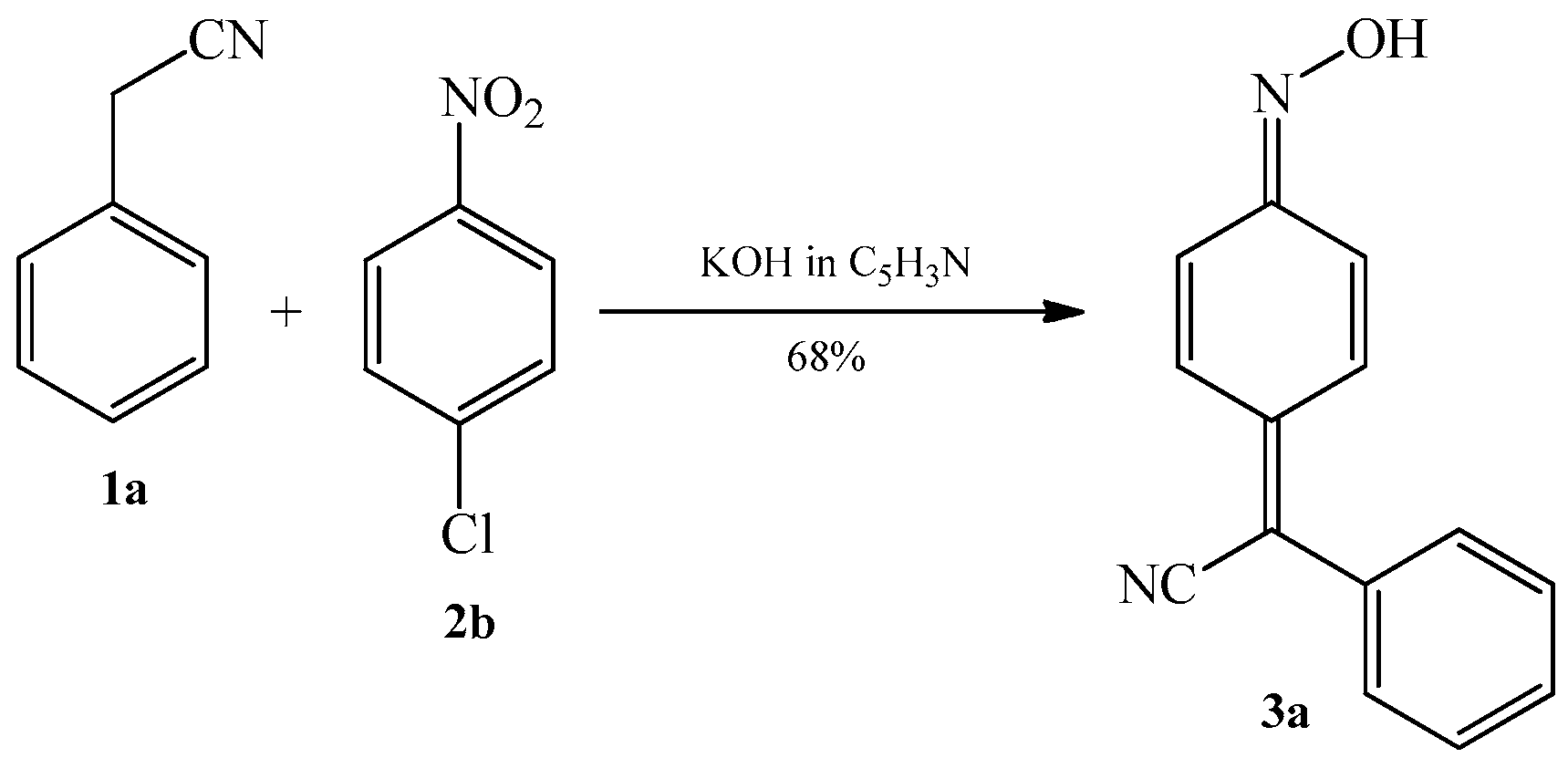
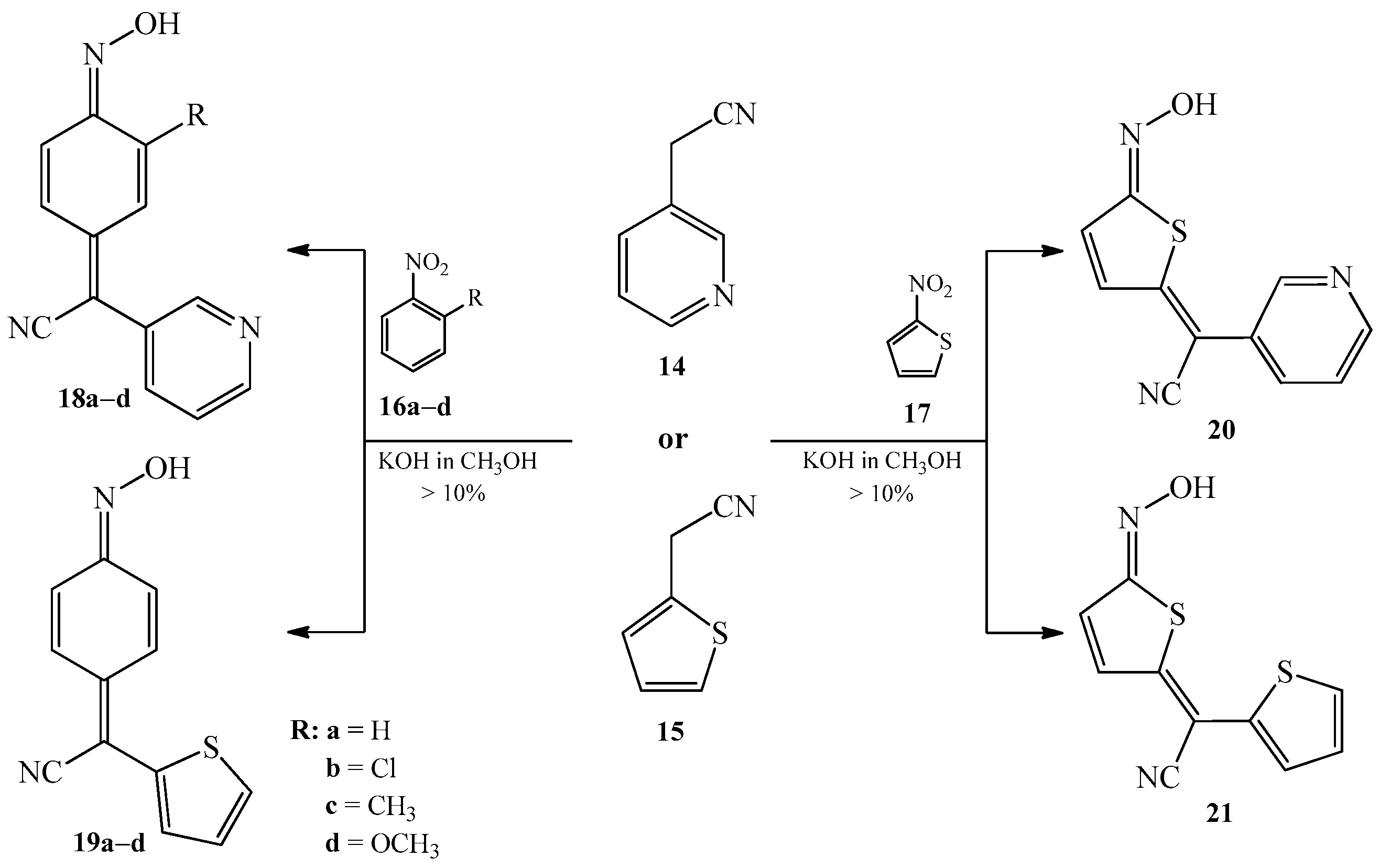
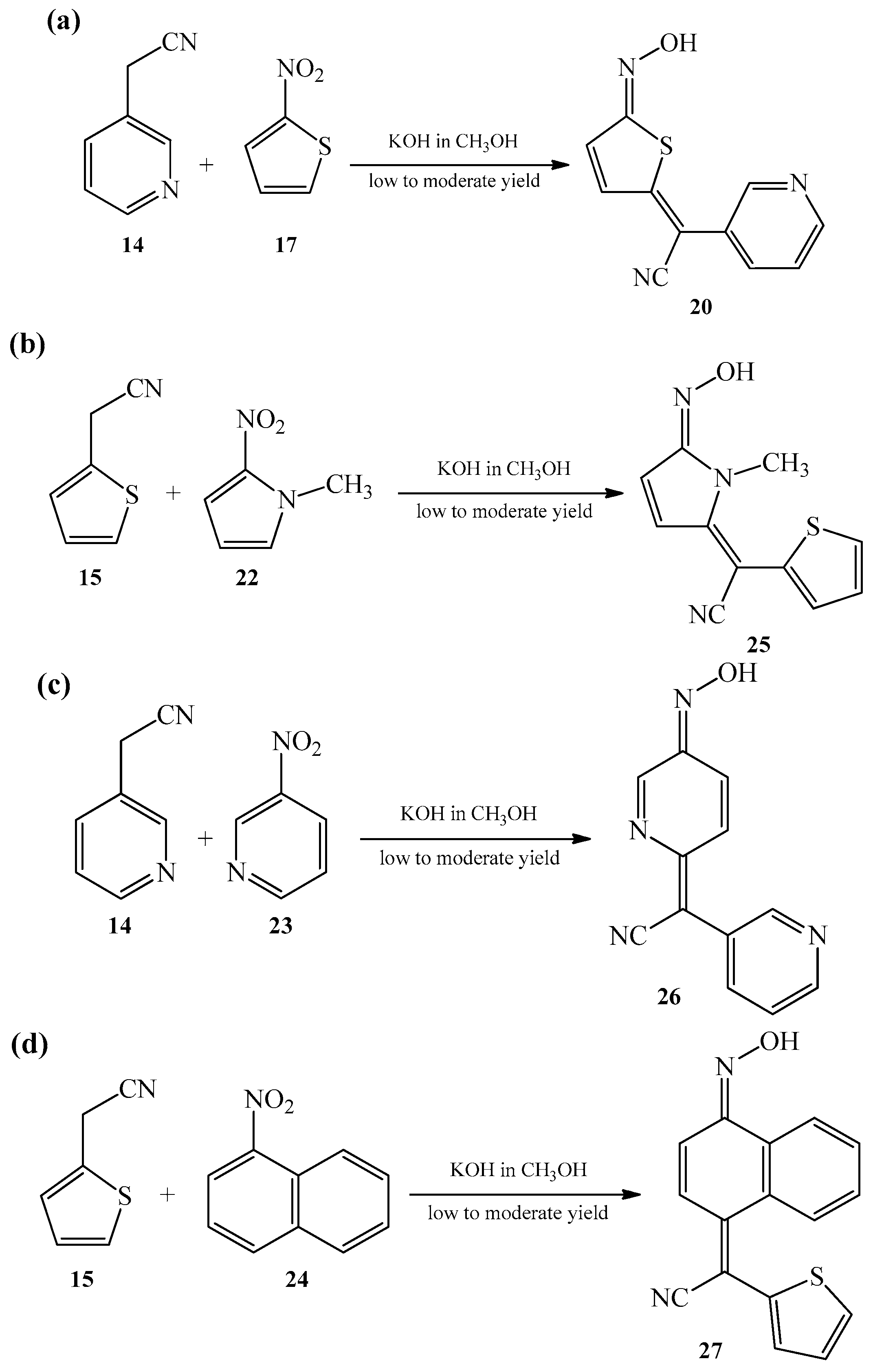
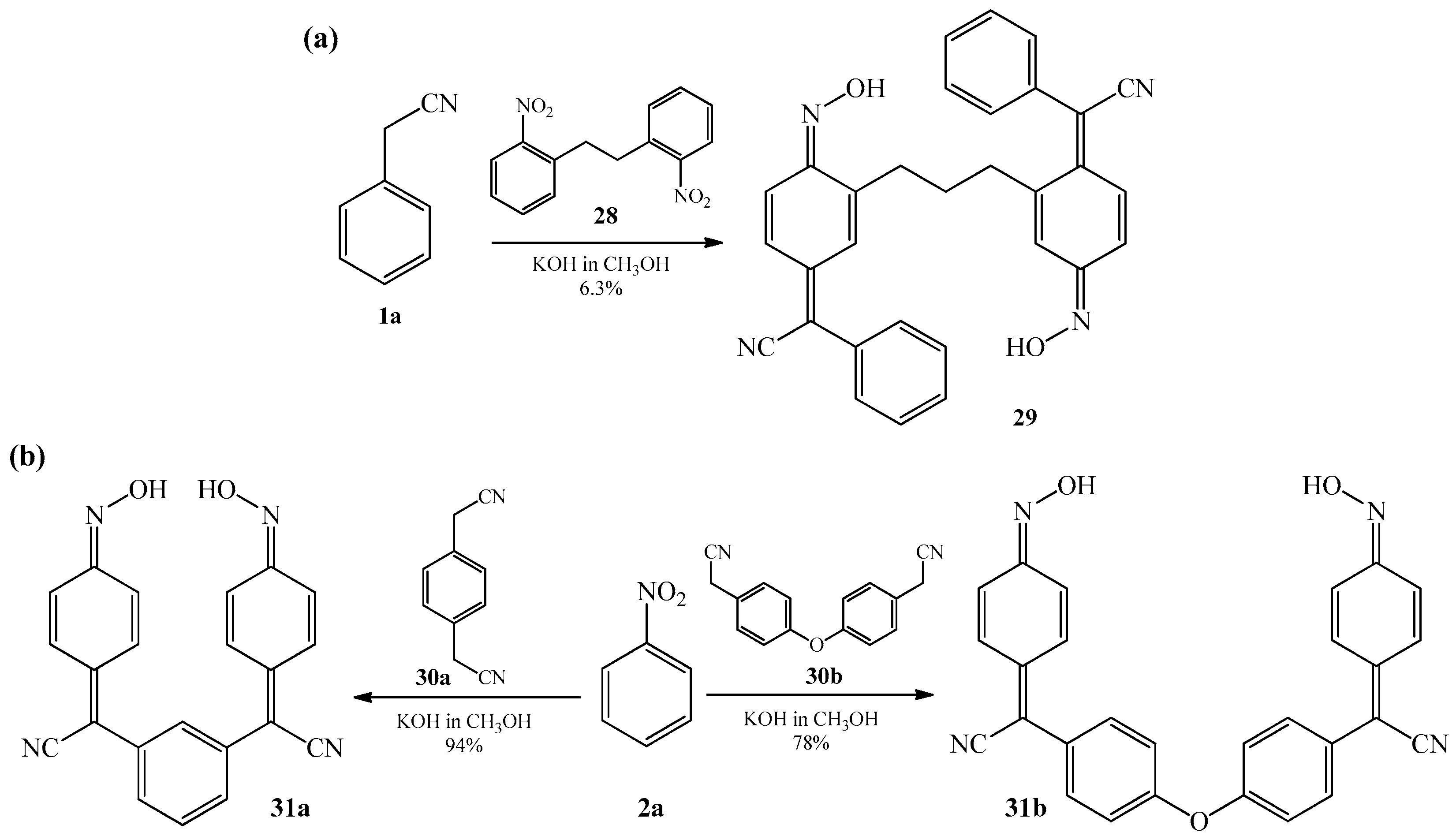
2.1. The Condensation of (Hetero) Arylacetonitriles with Nitro (Hetero) Arenes
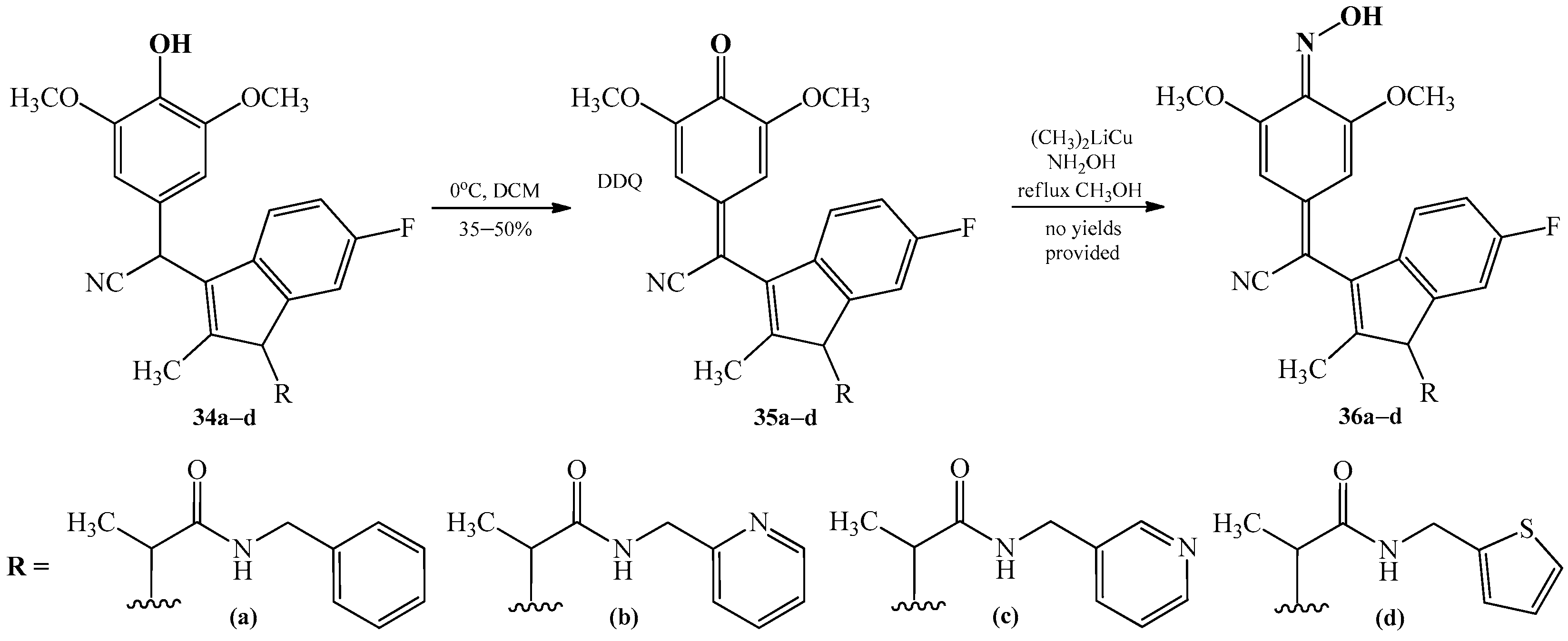
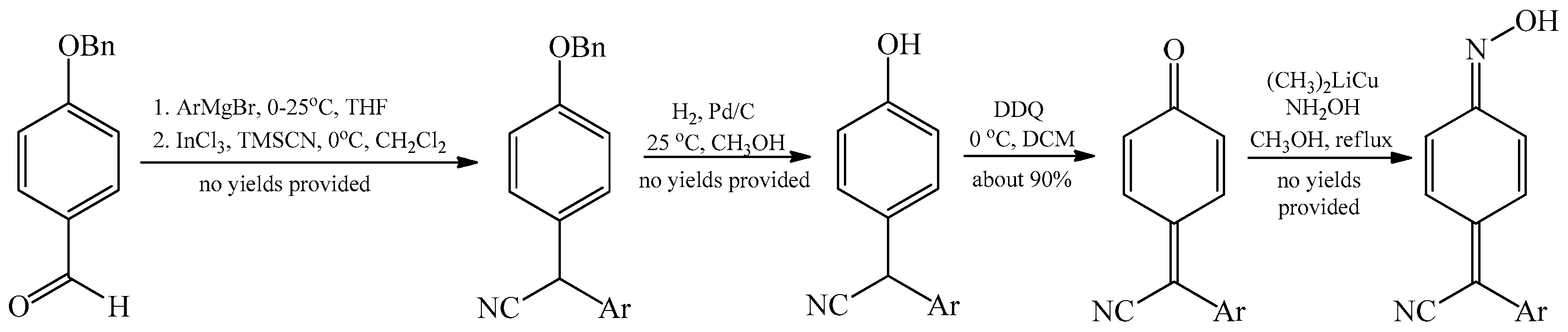
2.2. The Conversion of Quinone Methide Derivatives Using Hydroxylamine
3. Chemical Transformation of Arylcyanomethylenequinone Oximes
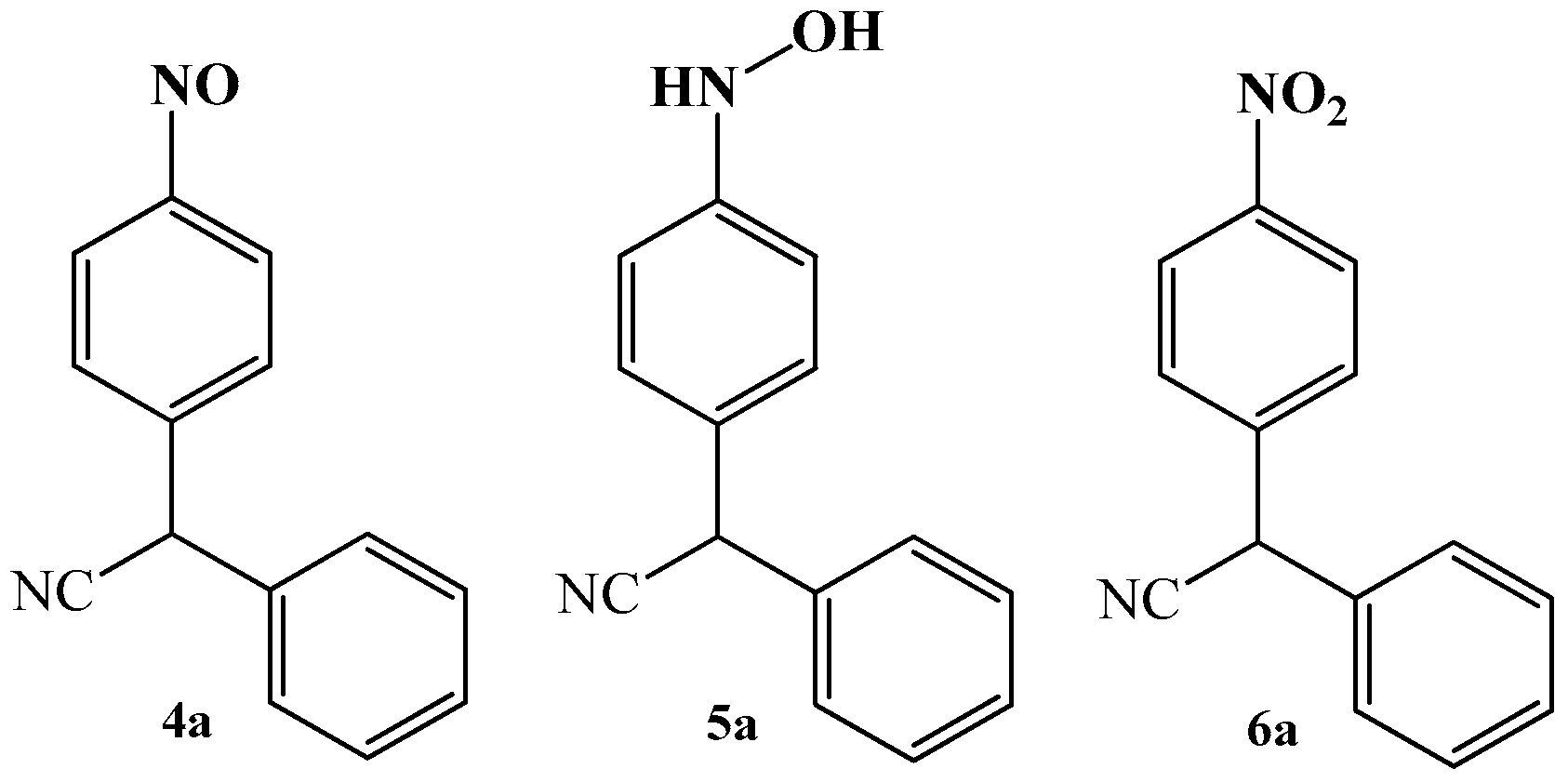
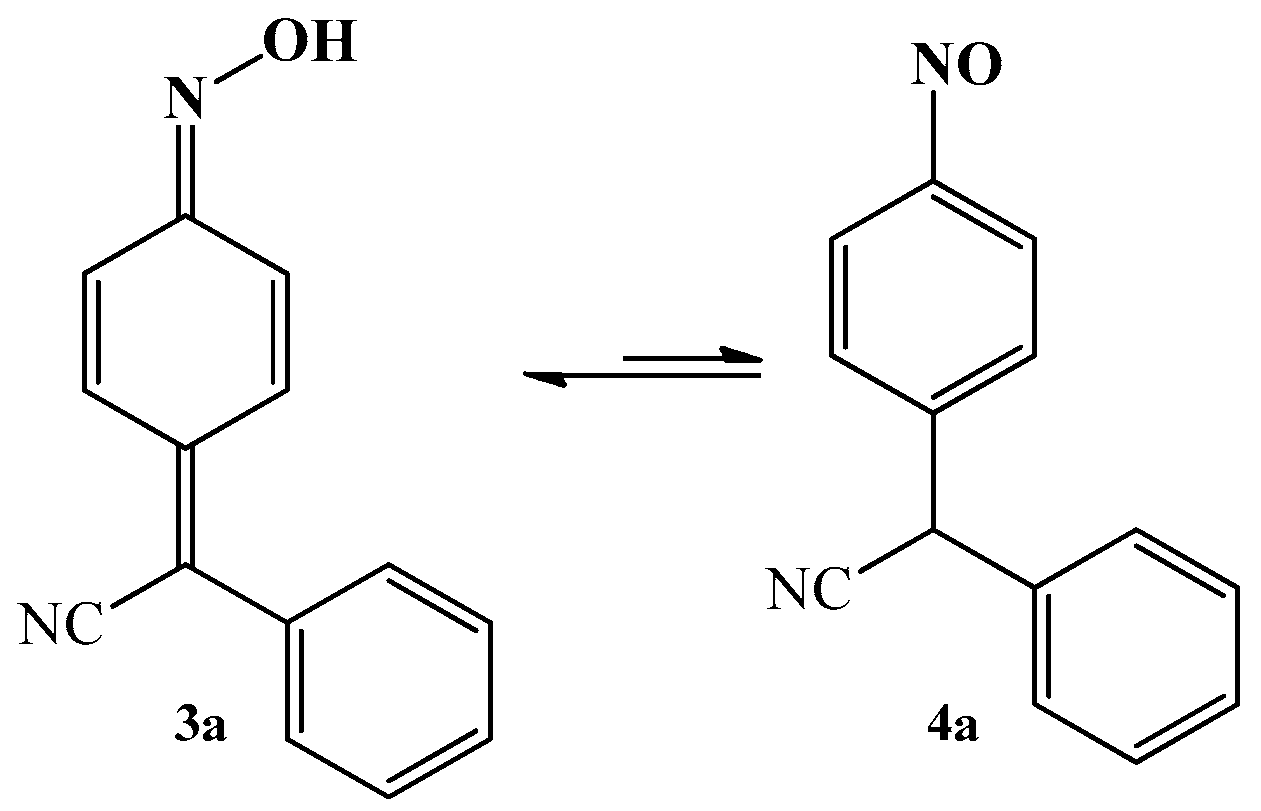
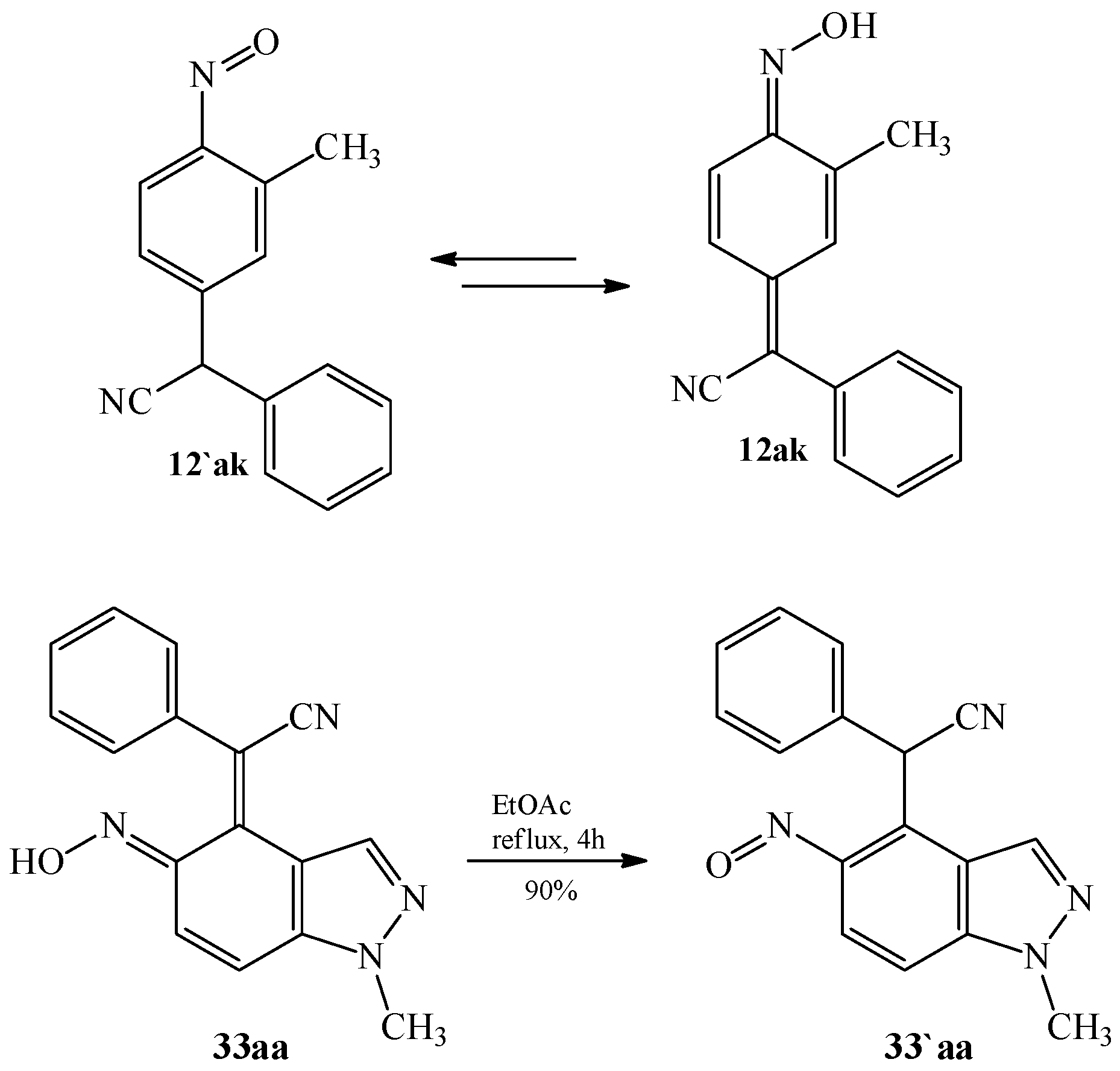
3.1. The Oxime–Nitroso Tautomerism Equilibrium in Arylcyanomethylenequinone Oxime
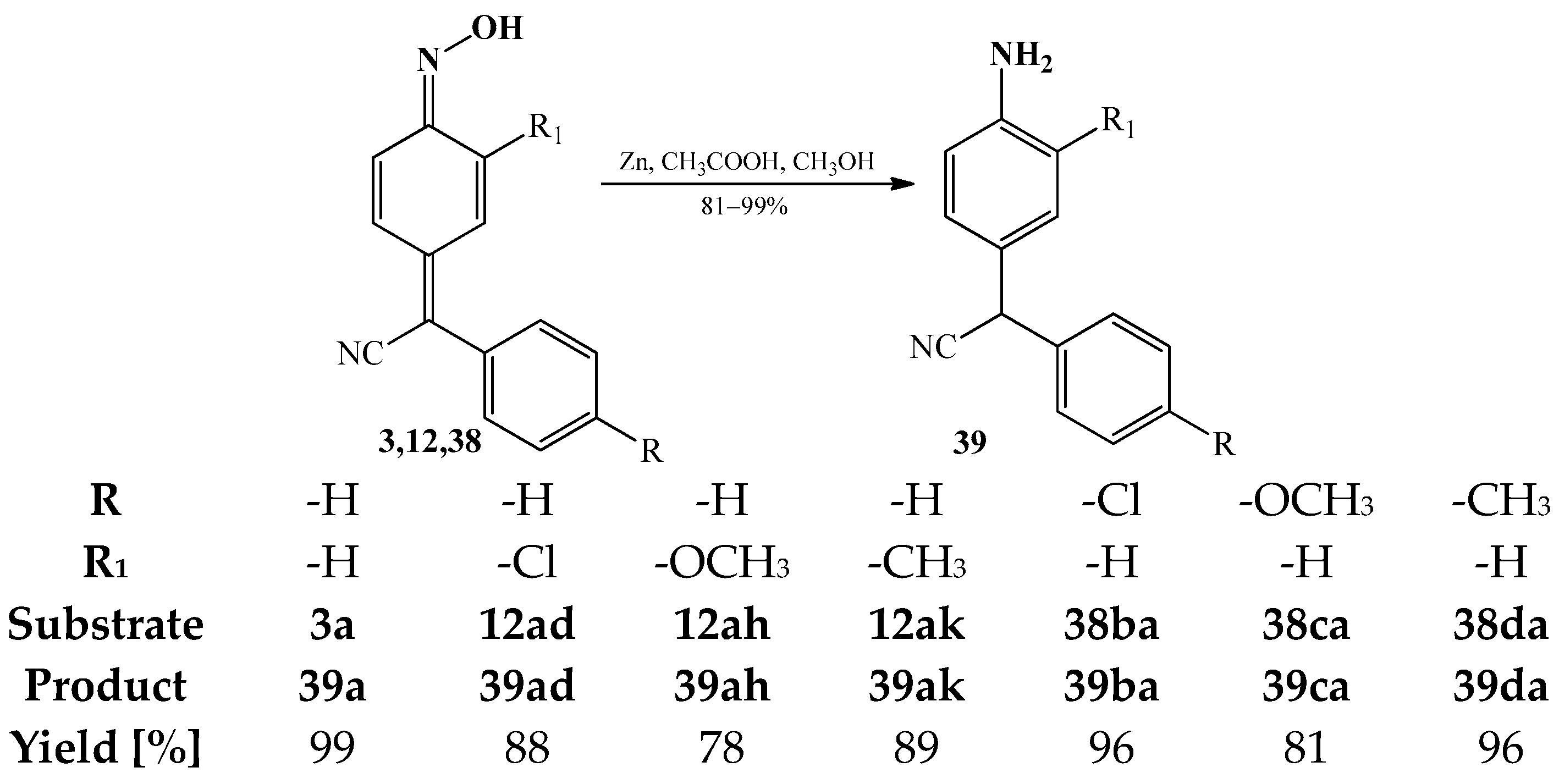
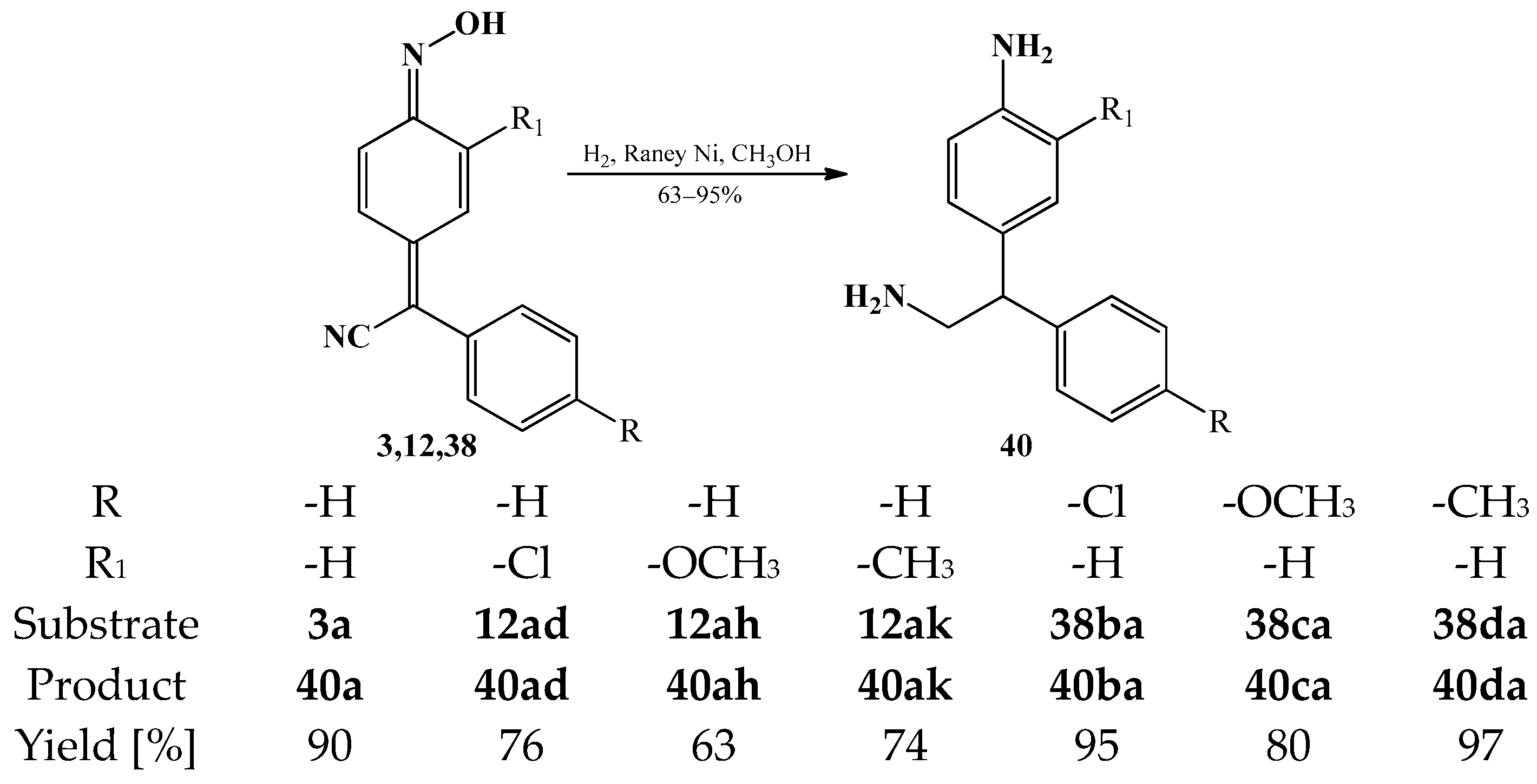
3.2. The Reduction Reactions of Arylcyanomethylenequinone Oxime
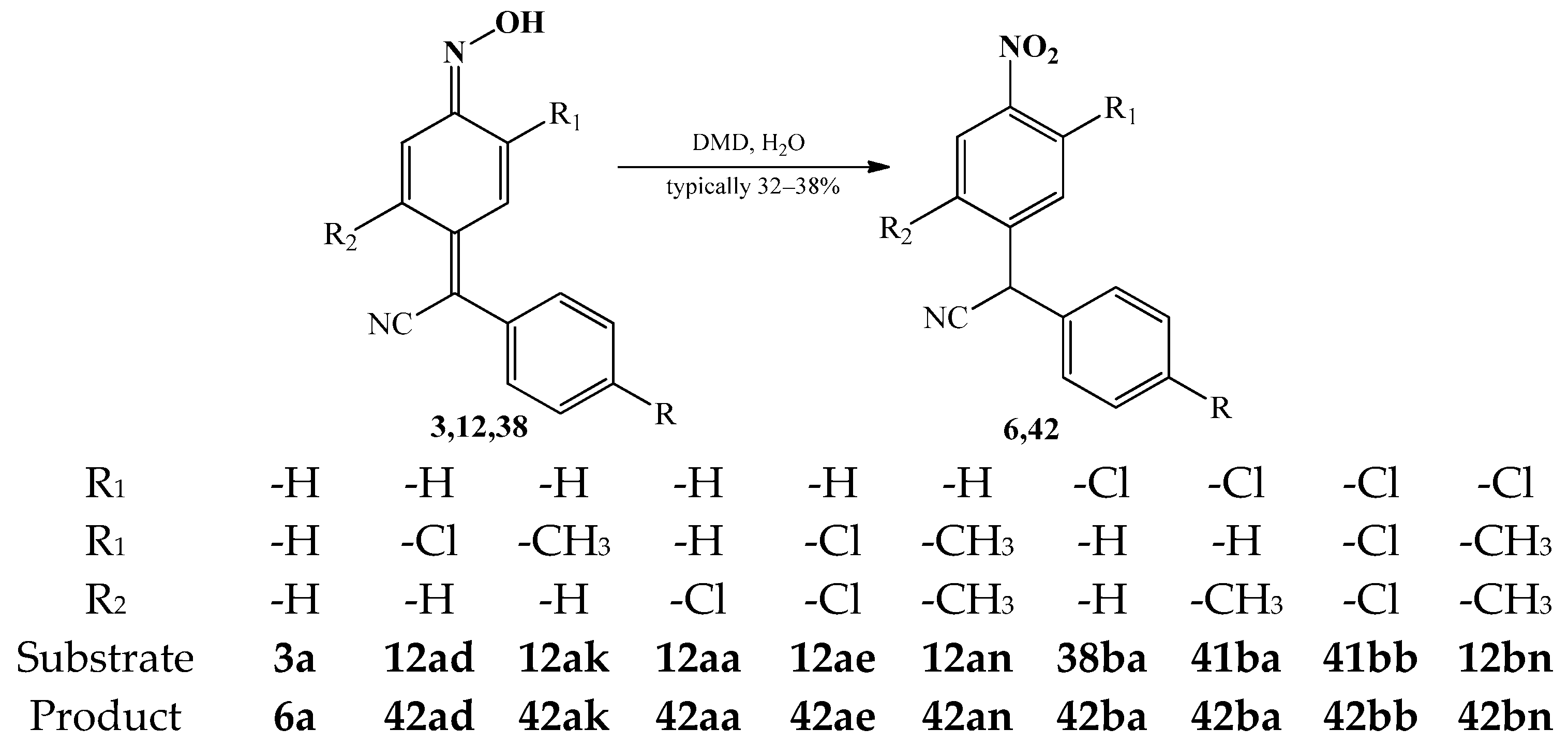
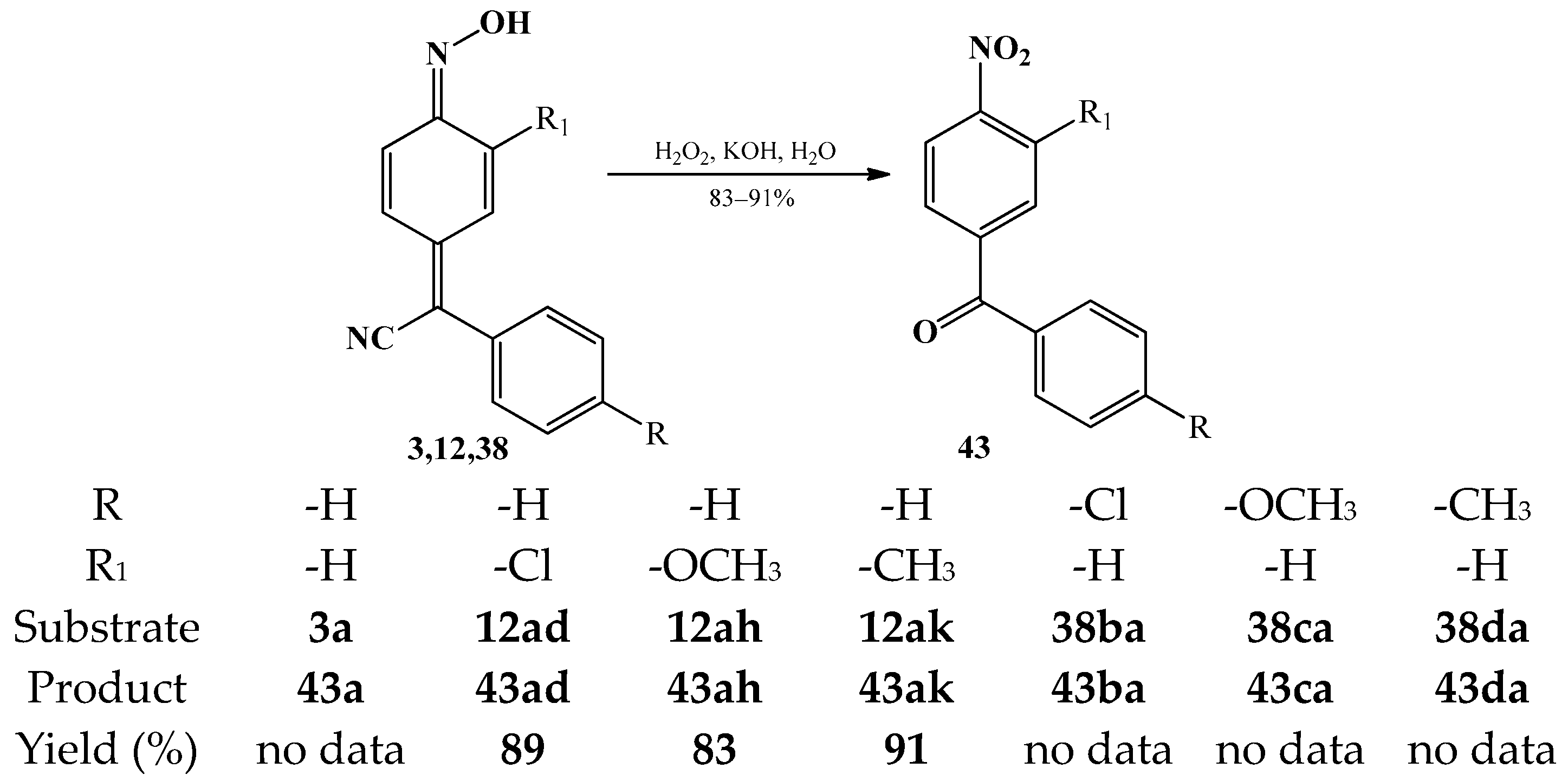
3.3. The Oxidation Reactions of Arylcyanomethylenequinone Oxime
3.4. The Substitution of a Hydrogen Atom at the Oxime Group in Arylcyanomethylenequinone Oxime
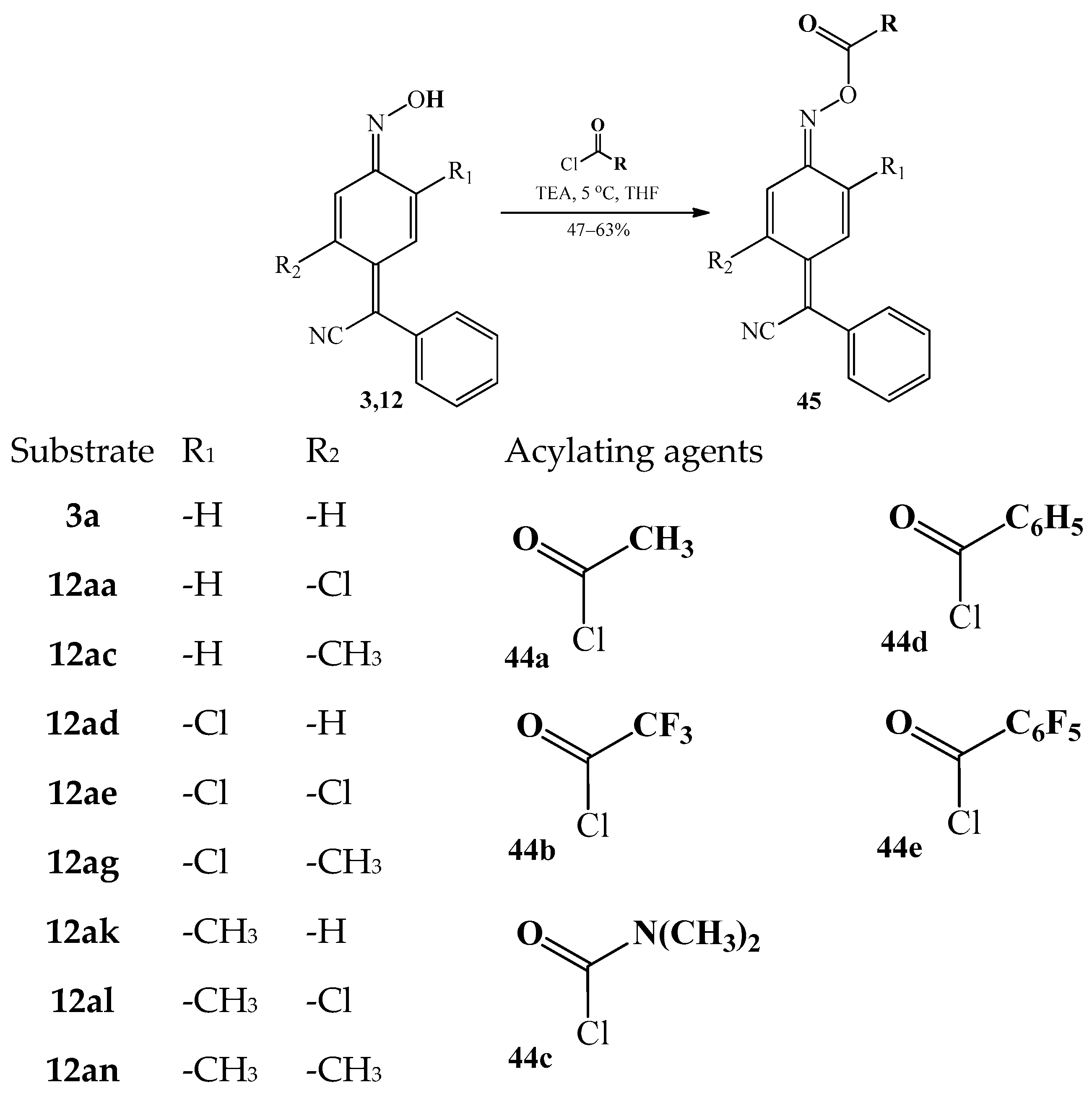
3.4.1. The Acylation of a Hydrogen Atom at the Oxime Group
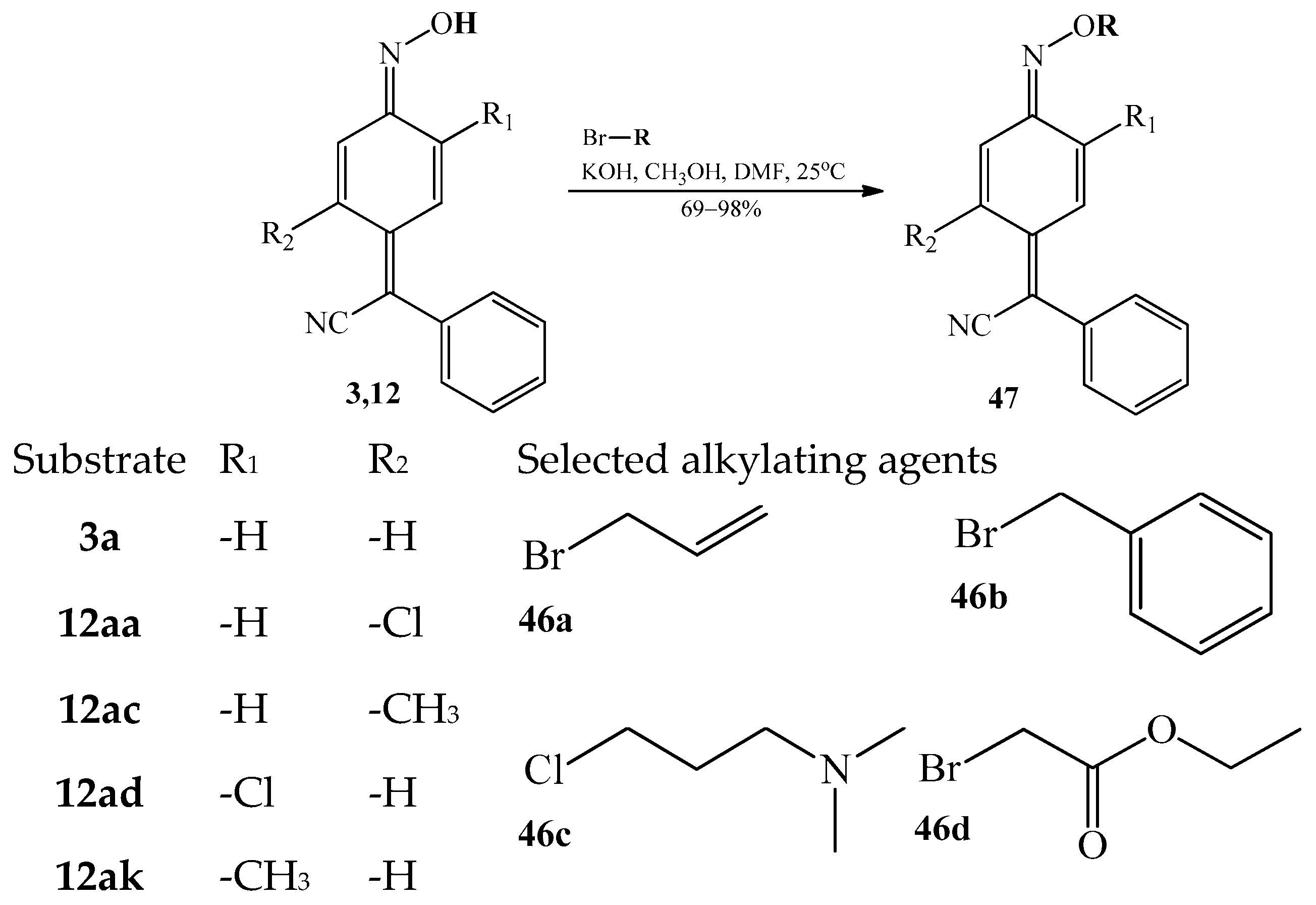
3.4.2. The Alkylation of a Hydrogen Atom at the Oxime Group
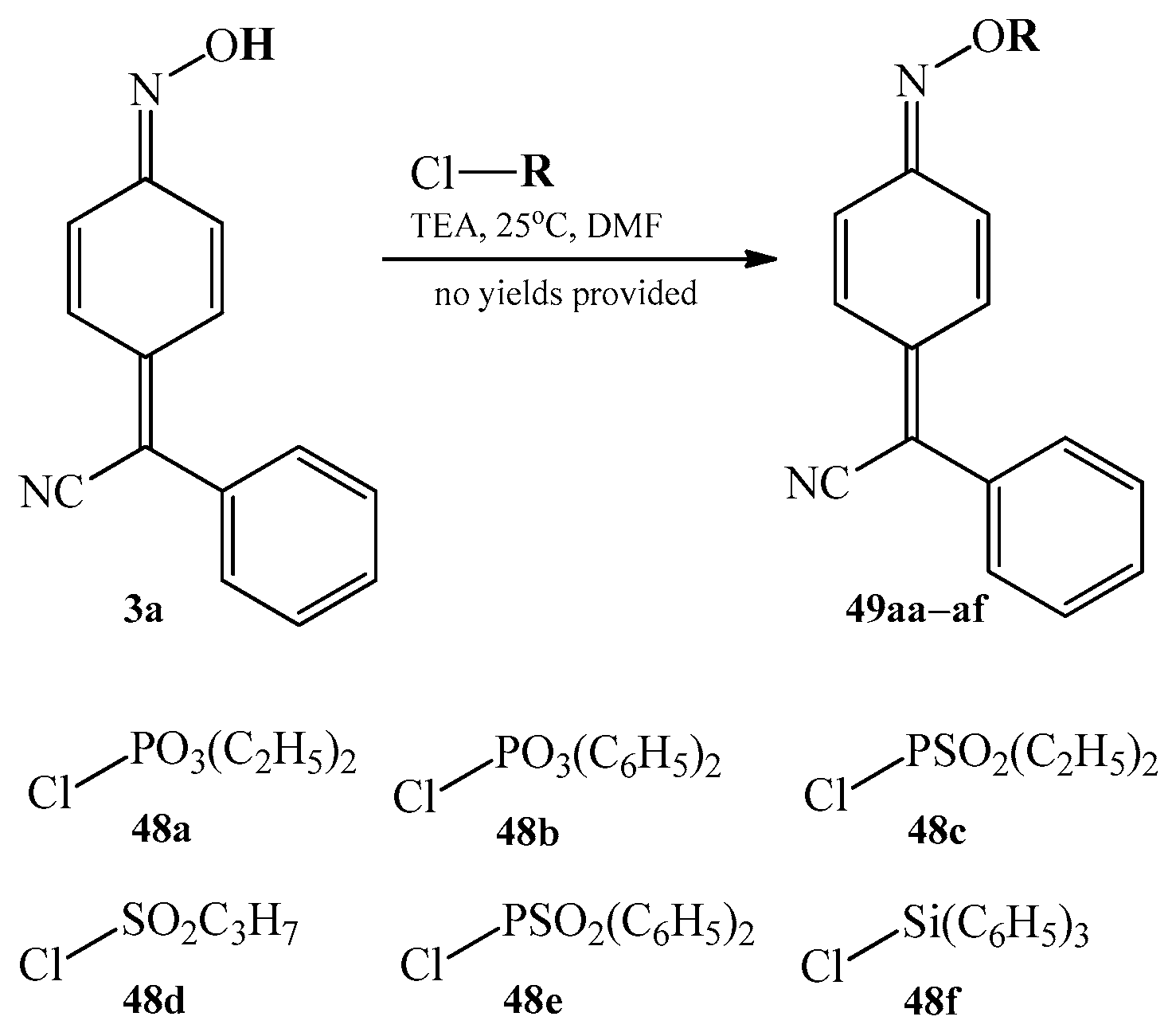
3.4.3. Other Substitution Reactions at Oxygen Atom of the Oxime Group
4. Biological Properties of Arylcyanomethylenequinone Oximes
4.1. Antiallergic Activity
4.2. Antifungal Activity
4.3. Anti-Inflammatory Activity
4.4. Anticancer Activity
4.5. Positive Inotropic and Lusitropic Effects
4.6. Potential Application of Arylcyanomethylenequinone Oximes in Veterinary Medicine
4.7. Potential Application of Arylcyanomethylenequinone Oximes as Plant Protection Products
5. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Peter, R.; Bendall, D.S. A mechanism for the reduction of cytochromes by quinols in solution and its relevance to biological electron transfer reactions. FEBS Lett. 1979, 105, 189–194. [Google Scholar] [CrossRef]
- Rich, P.R. Electron and proton transfers in chemical and biological quinone systems. Faraday Discuss. Chem. Soc. 1982, 74, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, E.; Wraight, C.A. A crucial role for AspL213 in the proton transfer pathway to the secondary quinone of reaction centers from Rhodobacter sphaeroides. Biochim. Biophys. Acta Bioenerg. 1990, 1020, 107–111. [Google Scholar] [CrossRef]
- Bolton, J.L.; Trush, M.A.; Penning, T.M. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef]
- Monks, T.J.; Jones, D.C. The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr. Drug. Metab. 2002, 3, 425–438. [Google Scholar] [CrossRef]
- Ralph, S.J.; Moreno-Sanchez, R.; Neuzil, J.; Rodriguez-Enriquez, S. Inhibitors of succinate: Quinone reductase/complex ii regulate production of mitochondrial reactive oxygen species and protect normal cells from ischemic damage but induce specific cancer cell death. Pharm. Res. 2011, 28, 2695–2730. [Google Scholar] [CrossRef]
- Janeczko, M.; Demchuk, O.M.; Strzelecka, D.; Kubiński, K.; Masłyk, M. New family of antimicrobial agents derived from 1,4-naphthoquinone. Eur. J. Med. Chem. 2016, 124, 1019–1025. [Google Scholar] [CrossRef]
- Campanini-Salinas, J.; Andrades-Lagos, J.; Hinojosa, N.; Moreno, F.; Alarcón, P.; González-Rocha, G.; Burbulis, I.E.; Vásquez-Velásquez, D. New quinone antibiotics against methicillin-resistant S. aureus. Antibiotics 2021, 10, 614. [Google Scholar] [CrossRef]
- Green, D.E.; Isler, C.M.; Morton, R.A. Nomenclature of quinones with isoprenoid side-chains recommendations (1973). Arch. Biochem. Biophys. 1974, 165, 1–5. [Google Scholar] [CrossRef]
- Cavitt, S.B.; Sarrafizadeh, H.; Gardner, P.D. The structure of o-quinone methide trimer. J. Org. Chem. 1962, 27, 1211–1216. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, K.J.; Seo, W.D.; Jang, S.Y.; Kim, M.; Lee, B.W.; Kim, J.Y.; Kang, S.; Park, K.H.; Lee, Y.-S. Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. Int. J. Mol. Med. 2011, 27, 441–446. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.W.; Cai, T.Y.; Cao, J.; Tu, C.X.; Lu, W.; He, Q.J.; Yang, B. Celastrol acts as a potent antimetastatic agent targeting beta 1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway. J. Pharmacol. Exp. Ther. 2010, 334, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, A.M.; Gohar, A.A.; Naiem, Z.; Bar, F.M.A. Zeitschrift für naturforschung. C. Taxodione, a DNA-binding compound from Taxodium distichum L. (Rich.). Z. Naturforsch. C 2008, 63, 355–360. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kang, S.C. Antibacterial abietane-type diterpenoid, taxodone from Metasequoia glyptostroboides Miki ex Hu. J. Biosci. 2010, 35, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Kurabe, J.; Yoshida, T.; Ohkanda, T.; Matsumoto, Y. Syntheses and antibacterial activities of diterpene catechol derivatives with abietane, totarane and podocarpane skeletons against methicillin-resistant Staphylococcus aureus and Propionibacterium acnes. Chem. Pharm. Bull. 2010, 58, 818–824. [Google Scholar] [CrossRef]
- Jansen, R.; Gerth, K.; Steinmetz, H.; Reinecke, S.; Kessler, W.; Kirschning, A.; Müller, R. Elansolid A3, a unique p-quinone methide antibiotic from Chitinophaga sancti. Chem. Eur. J. 2011, 17, 7739–7744. [Google Scholar] [CrossRef]
- Kusumoto, N.; Ashitani, T.; Murayama, T.; Ogiyama, K.; Takahashi, K. Antifungal abietane-type diterpenes from the cones of Taxodium distichum Rich. J. Chem. Ecol. 2010, 36, 1381–1386. [Google Scholar] [CrossRef]
- Wang, P.; Song, Y.; Zhang, L.; He, H.; Zhou, X. Quinone methide derivatives: Important intermediates to DNA alkylating and DNA cross-linking actions. Curr. Med. Chem. 2005, 12, 2893–2913. [Google Scholar] [CrossRef]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.; Alvarez, X.A.; Vigo, C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 1341–1357. [Google Scholar] [CrossRef] [PubMed]
- Kolak, U.; Kabouche, A.; Öztürk, M.; Kabouche, Z.; Topçu, G.; Ulubelen, A. Antioxidant diterpenoids from the roots of Salvia barrelieri. Phytochem. Anal. 2009, 20, 320–327. [Google Scholar] [CrossRef]
- Kim, D.H.; Shin, E.K.; Kim, Y.H.; Lee, B.W.; Jun, J.G.; Park, J.H.; Kim, J.K. Suppression of inflammatory responses by celastrol, a quinone methide triterpenoid isolated from Celastrus regelii. Eur. J. Clin. Investig. 2009, 39, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Messina, P.; Labbé, E.; Buriez, O.; Hillard, E.A.; Vessières, A.; Hamels, D.; Top, S.; Jaouen, G.; Frapart, Y.M.; Mansuy, D.; et al. Deciphering the activation sequence of ferrociphenol anticancer drug candidates. Chem. Eur. J. 2012, 18, 6581–6587. [Google Scholar] [CrossRef] [PubMed]
- Yamato, H.; Bleier, H.; Birbaum, J.L.; Kunz, M.; Dietliker, K.; De Leo, C.; Asakura, T. New Oxime Sulfonates and Use Thereof as Latent Sulfonic Acids for Photoresists. International Patent Publication No. WO9901429A1, 14 January 1999. [Google Scholar]
- Yamato, H.; Asakura, T.; Birbaum, J.L.; Kurt, D. Oximes and Use Thereof as Latent Acids for Photoresists. International Patent Publication No. WO2000026219A1, 11 May 2000. [Google Scholar]
- Suzuki, H.; Miyazawa, T.; Kikuchi, T. Dye-Containing Resist Composition Containing Photoacid Generator, and Cyclohexadiene-Type Oxime Sulfonate Compound as the Photoacid Generator for Forming Color Filter. International Patent Publication No. WO2,008,032,736, 20 March 2008. [Google Scholar]
- Arai, T.; Hirata, H. Fiber-Reinforced Rubber Laminated Materials. Japan Patent Publication No. JP44,026,549B, 6 November 1969. [Google Scholar]
- Takaba, S.; Takehara, T. Manufacture of Bonded Unit of Ethylene-α-olefin Rubber Compositions and Fibers and Their Transmission Belt. Japan Patent Publication No. JP2006124484A, 18 May 2006. [Google Scholar]
- Yamaguchi, Y.; Takagi, K. Polyolefin Foams. Japan Patent Publication No. JP51045170A, 17 April 1976. [Google Scholar]
- Foghel, S.; Banta, M.; Pirvulescu, S.; Nichitovici, D.; Dobrescu, D.; Pietris, V. Material for Anticorrosive Films. Romania Patent Publication No. RO59038A3, 15 January 1976. [Google Scholar]
- Yoshida, N.; Toko, M. Bonding Method of Outer Panel and Reinforcing Member in Assembling Vehicle by Using Mastic Adhesive. Japan Patent Publication No. JP2018135472A, 30 August 2018. [Google Scholar]
- Yuan, R. Method for Catalytic Synthesis of p-benzoquinone Dioxime. China Patent Publication No. CN101712635A, 26 May 2010. [Google Scholar]
- Dixon, W.D. 4-oxo-(α-cyanobenzylidene)-2,5-cyclohexadiene-4-one O-(methylcarbamoyl)oximes. Herbicidal and Plant Growth-Regulating Federal Republic of. Germany Patent Publication No. DE2257527A1, 30 May 1973. [Google Scholar]
- Hong, Z.; Chen, G.; Jiang, H.J.; Chen, R.E.; Su, W.K. Crystal structure of α-(4-(hydroxyimino)-2, 5-dichloro-2, 5-cyclohexadien-1-ylidene)-benzeneacetonitrile, C14H8Cl2N2O. Z. Kristallogr.-New Cryst. Struct. 2015, 230, 71–72. [Google Scholar] [CrossRef]
- Worek, F.; Wille, T.; Koller, M.; Thiermann, H. Structural requirements for effective oximes—Evaluation of kinetic in vitro data with phosphylated human AChE and structurally different oximes. Chem. Biol. Interact. 2013, 203, 125–128. [Google Scholar] [CrossRef]
- Buehler, S.; Ott, M.; Pfleiderer, W. Novel photolabile protective groups for improved processes to prepare oligonucleotide arrays. International Patent Publication No. WO2,004,074,300A2, 2 September 2004. [Google Scholar]
- Cao, X.; Song, M.; Sui, Z. Reaction mechanism of protonated benzidine oxidation with chlorine dioxide in water. J. Text. Res. 2013, 34, 106–112. [Google Scholar]
- Soukup, O.; Jun, D.; Tobin, G.; Kuca, K. The summary on non-reactivation cholinergic properties of oxime reactivators: The interaction with muscarinic and nicotinic receptors. Arch. Toxicol. 2013, 87, 711–719. [Google Scholar] [CrossRef]
- Malatesta, P.; Quaglia, G.; La Floresta, E. Acetylcholinesterase reactivators. I. Oxime of quinoliniumphenacyl chloride. Farmaco Sci. 1968, 23, 757–764. [Google Scholar]
- Freyne, E.J.E.; Venet, M.G.; Raeymaekers, A.H.M.; Sanz, G.C. Preparation of (1H-azol-1-ylmethyl)quinolines, -quinazolines, and -quinoxalines as Drugs. European Patent Publication No. EP371,564A2, 6 June 1990. [Google Scholar]
- Piazza, G.A.; Chen, X.; Keeton, A.B.; Boyd, M.R. Compounds, Compositions and Methods of Treating Cancer. International Patent Publication No. WO2017106520A1, 22 June 2017. [Google Scholar]
- Masłyk, M.; Janeczko, M.; Demchuk, O.M.; Boguszewska-Czubara, A.; Golczyk, H.; Sierosławska, A.; Rymuszka, A.; Martyna, A.; Kubiński, K. A representative of arylcyanomethylenequinone oximes effectively inhibits growth and formation of hyphae in Candida albicans and Influences the Activity of Protein Kinases in vitro. Saudi Pharm. J. 2018, 26, 244–252. [Google Scholar] [CrossRef]
- Seidl, G.; Nesemann, G. Phenylcyanomethylene Quinone Oxime Derivatives. Federal Republic of. Germany Patent Publication No. DE1194845B, 16 June 1965. [Google Scholar]
- Dore, C.J.; Viel, C. Cytotoxic activity of molecules possessing an ethylenic double bond substituted in α and β positions by an electron-attracting group. Eur. J. Med. Chem. 1975, 10, 47–54. [Google Scholar]
- Poorhaji, S.; Pordel, M.; Ramezani, S. New heterocyclic green, blue and orange dyes from indazole: Synthesis, tautomerism, alkylation studies, spectroscopic characterization and DFT/TD-DFT calculations. J. Mol. Struc. 2016, 1119, 151–156. [Google Scholar] [CrossRef]
- Davis, R.B.; Pizzini, L.C.; Benigni, J.D. The condensation of aromatic nitro compounds with arylacetonitriles. I. Nitrobenzene. J. Am. Chem. Soc. 1960, 82, 2913–2915. [Google Scholar] [CrossRef]
- Toteva, M.M.; Richard, J.P. The generation and reactions of quinone methides. Adv. Phys. Org. Chem. 2011, 45, 39–91. [Google Scholar] [CrossRef]
- Caruana, L.; Fochi, M.; Bernardi, L. The emergence of quinone methides in asymmetric organocatalysis. Molecules 2015, 20, 11733–11764. [Google Scholar] [CrossRef]
- Sugumaran, M. Reactivities of Quinone Methides versus o-Quinones in Catecholamine Metabolism and Eumelanin Biosynthesis. Int. J. Mol. Sci. 2016, 17, 1576. [Google Scholar] [CrossRef]
- Ito, S.; Sugumaran, M.; Wakamatsu, K. Chemical Reactivities of ortho-Quinones Produced in Living Organisms: Fate of Quinonoid Products Formed by Tyrosinase and Phenoloxidase Action on Phenols and Catechols. Int. J. Mol. Sci. 2020, 21, 6080. [Google Scholar] [CrossRef]
- Kern, D.; Kormos, A. Self-Immobilizing Quinone Methides for the Fluorescent Sensing of Enzyme Activity. Chemosensors 2023, 11, 155. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Goncalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Dicken, R. Synthesis of Pyridine and Pyridinium Quinone Methide Precursors: Studies Towards the Realkylation of Aged Acetylcholinesterase. Available online: https://kb.osu.edu/handle/1811/69265 (accessed on 26 April 2023).
- Ma, D.; Lyu, X.I.; Yu, L.; Han, T.; Zhang, G.; Wu, C. Preparation of p-Benzoquinone Dioxime. China Patent Publication No. CN113735737A, 3 December 2021. [Google Scholar]
- Hong, Z.; Jiang, H.; Cui, H.; Zhang, B.; Lu, C.; Chen, X.; Yao, P.; Li, S.; Yao, Z. Ultrasonic-Accelerated Catalytic Transfer Hydrogenation Reduction of 4-chloro-α-(2-chloro-4-(hydroxyimino)-5-methyl-2,5-cyclo-hexadienylidene)phenylacetonitril. China Patent Publication No. CN104945282A, 30 September 2015. [Google Scholar]
- Marey, T.; Person, M. Mechanism of the polarographic reduction of the products of condensation of acetonitriles, substituted with an aromatic or heterocyclic group, with aromatic or heterocyclic nitro-derivatives. Bull. Soc. Chim. Fr. 1970, 7, 2756–2762. [Google Scholar]
- Zhao, G.; Yang, H.; Tan, W. Preparation Method of Sodium Closantel Injection. China Patent Publication No. CN109091454A, 28 December 2018. [Google Scholar]
- Davis, R.B.; Weber, J.D. Condensation of aromatic nitro compounds with arylacetonitriles. IV. Some reactions of the arylcyanomethylenequinone oximes. J. Org. Chem. 1962, 27, 1605–1608. [Google Scholar] [CrossRef]
- Yamada, K.; Shigehiro, K.; Tomizawa, T.; Iida, H. The effect of surfactants and β-cyclodextrin on the photooxidation of stable carbanions. Bull. Chem. Soc. Jpn. 1978, 51, 3302–3306. [Google Scholar] [CrossRef]
- Kouakou, A.; Abbassi, N.; Chicha, H.; Ammari, L.E.; Saadi, M.; Rakib, E.M. Synthesis of novel substituted indazoles via nucleophilic substitution of hydrogen (SNH). Heteroat. Chem. 2015, 26, 374–381. [Google Scholar] [CrossRef]
- Hong, Z.; Li, J.J.; Chen, G.; Jiang, H.J.; Yang, X.F.; Pan, H.; Su, W.K. Solvent-free mechanochemical synthesis of arylcyanomethylenequinone oximes from phenylacetonitriles and 4-unsubstituted nitroaromatic compounds using KF/nano-gamma-Al2O3 as catalyst. RSC Adv. 2016, 6, 13581–13588. [Google Scholar] [CrossRef]
- Anssen, M.A.C.; Offenwert, T.T.; Sanczuk, S. Anthelmintic and Coccidiostatic 4’-benzoyl- and 4’-benzylsalicylanilides. Federal Republic of. Germany Patent Publication No. DE2311229A1, 13 September 1973. [Google Scholar]
- Martyna, A.; Masłyk, M.; Janeczko, M.; Kochanowicz, E.; Gielniewski, B.; Świercz, A.; Demchuk, O.M.; Kubiński, K. Antifungal agent 4-an changes the genome-wide expression profile, downregulates virulence-associated genes and induces necrosis in Candida albicans cells. Molecules 2020, 25, 2928. [Google Scholar] [CrossRef] [PubMed]
- Gleason, M.M.; Rojas, C.J.; Learn, K.S.; Perrone, M.H.; Bilder, G.E. Characterization and Inhibition of 15-lipoxygenase in human monocytes: Comparison with soybean 15-lipoxygenase. Am. J. Physiol. Physiol. 1995, 268, C1301–C1307. [Google Scholar] [CrossRef]
- Michel, F.; Mercklein, L.; De Paulet, A.C.; Dore, J.C.; Gilbert, J.; Miquel, J.F. The effect of various acrylonitriles and related compounds on prostaglandin biosynthesis. Prostaglandins 1984, 27, 69–84. [Google Scholar] [CrossRef]
- Dixon, W.D. Herbicidal 4-oxo-α-phenyl-2,5-cyclohexadiene-Δ’, α-acetonitrile o-(alkylcarbamoyl)oximes. Africa Patent Publication No. ZA7208311A, 29 August 1973. [Google Scholar]
- Shimada, T.; Yoshida, K. Phytotoxicity test of futharide, a newly developed fungicide for rice-blast. Tokyo Agr. Chem. Insp. Sta. Bull. 1971, 11, 141–142. [Google Scholar]
- D’Silva, T.D. Pesticidal α-cyanobenzyl phenyl benzoyl Urea Compounds. U.S. Patent Publication No. US4578402A, 25 March 1986. [Google Scholar]
- Lichtenberger, J.; Weiss, F. Derivatives of phenylfiuoroform. I. Trifiuoromethylbanzophenones. Bull. Soc. Chim. Fr. 1962, 1, 587–593. [Google Scholar]
- Davis, R.B.; Pizzini, L.C.; Bara, E.J. The condensation of aromatic nitro compounds with arylacetonitriles. III. Some ortho- and meta-substituted nitrobenzenes. J. Org. Chem. 1961, 26, 4270–4274. [Google Scholar] [CrossRef]
- Davis, R.B. Arylcyanomethylenequinone Oximes. U.S. Patent Publication No. US3156704A, 10 November 1964. [Google Scholar]
- Marey, T.; Fournari, P.; Tirouflet, J. Condensation of nitriles with aromatic and heterocyclic nitro derivatives. Compt. Rend. 1965, 260, 4340–4342. [Google Scholar]
- Fournari, P.; Marey, T. Synthesis and spectroscopic results of the products resulting from the condensation of aromatic or heterocyclic nitro-derivatives with acetonitrile substituted with an aromatic or a heterocyclic group. Bull. Soc. Chim. Fr. 1968, 8, 3223–3233. [Google Scholar]
- Mitchell, C.D. Phenylacetonitrile Oximes. U.S. Patent Publication No. US3374248A, 19 March 1968. [Google Scholar]
- Mitchell, C.D. Bis(phenylacetonitrile oximes) as Ultraviolet Light Absorbers in Plastics. U.S. Patent Publication No. US3374249A, 19 March 1968. [Google Scholar]
- Takahashi, K.; Tsuboi, T.; Kazutoshi, Y.; Hirotada, I. Studies on the base-catalyzed reaction. VIII. Reactions of cyanophenylmethanide ion with nitrobenzene. Nippon Kagaku Kaishi 1976, 1, 144–147. [Google Scholar] [CrossRef]
- Arseniyadis, S.; Kyler, K.S.; Watt, D.S. Addition and substitution reactions of nitrile-stabilized carbanions. Org. React. 1984, 31, 1–364. [Google Scholar] [CrossRef]
- Freyne, E.J.E.; Raeymaekers, A.H.M. Positive Inotropic and Lusitropic 3,5-dihydroimidazo[2,1-b]quinazolin-2(1H)-one Derivatives, Compositions and Use. U.S. Patent Publication No. US5043327A, 27 August 1991. [Google Scholar]
- Freyne, E.J.E.; Raeymaekers, A.H.M.; De Chaffoy, D.C.D. 1,3-Dihydro-2H-imidazo[4,5-b]quinolin-2-one Derivatives as Inhibitors of Phosphodiesterase III and IV. U.S. Patent Publication No. US5521187A, 28 May 1996. [Google Scholar]
- Makosza, M.; Wróbel, Z. Conversion of 1-(o-nitroaryl) methyl p-tolyl sulfones into anthranilic ester analogues. Acta Chem. Scand. 1996, 50, 646–648. [Google Scholar] [CrossRef]
- Wróbel, Z. Synthesis of 4-arylsulfonylquinolines by double condensation of allyl aryl sulfones with nitroarenes. Eur. J. Org. Chem. 2000, 3, 521–525. [Google Scholar] [CrossRef]
- Suwiński, J.; Świerczek, K.; Wagner, P.; Kubicki, M.; Borowiak, T.; Stowikowska, J. Oximes as intermediates or final products in reactions of nitroheteroarenes with nucleophiles in the presence of sodium methoxide-methanol system. J. Heterocycl. Chem. 2003, 40, 523–528. [Google Scholar] [CrossRef]
- Orlov, V.Y.; Bazlov, D.A.; Ganzha, V.V.; Kotov, A.D.; Rusakov, A.I. Direction of the nucleophilic substitution reaction of hydrogen in nitrobenzene by arylacetonitrile carbanion. Bashk. Khim. Zh. 2007, 14, 22–25. [Google Scholar]
- Konovalova, N.V. Aromatic nucleophilic substitution of hydrogen in nitroarenes by phenylacetonitrile carbanion. Bashk. Khim. Zh. 2008, 15, 103–104. [Google Scholar]
- Orlov, V.Y. Selection of optimal conditions for synthesis of 2[4-(hydroxyimino)-cyclohexa-2,5-dien-1-ylidene]arylacetonitriles. Khim. Tekhnolog. 2009, 10, 393–395. [Google Scholar]
- Buehler, S.; Ott, M.; Pfleiderer, W. Photolabile Protective Groups for Improved Processes to Prepare Oligonucleotide Arrays. U.S. Patent Publication No. US7759513B2, 20 July 2010. [Google Scholar]
- Orlov, V.Y.; Kotov, A.D.; Bazlov, D.A.; Ganzha, V.V.; Konovalova, N.V. Theoretical calculation of the equilibrium constant of nitroso-oxime tautomerization of phenylcyanomethylene-2,5-cyclohexadien-1-one monooxime. Izv. Vyss. Uchebnykh Zaved. 2010, 53, 124–125. [Google Scholar]
- Li, X.Z.; Jiang, S.Y.; Li, G.Q.; Jiang, Q.R.; Li, J.W.; Li, C.C.; Han, Y.Q.; Song, B.L.; Ma, X.R.; Qi, W.; et al. Synthesis of heterocyclic ring-fused analogs of HMG499 as novel degraders of HMG-CoA reductase that lower cholesterol. Eur. J. Med. Chem. 2022, 236, e114323. [Google Scholar] [CrossRef]
- Qiu, W.; Song, B.; Jiang, Q.; Jiang, S.; Shi, X.; Li, C. Cholic Acid Derivatives as HMG-CoA Reductase Inhibitors and Their Preparation, Pharmaceutical Compositions and Use in the Reducing Cholesterol. China Patent Publication No. CN113,563,405A, 29 October 2021. [Google Scholar]
- Zheng, X.; Zhang, Y.; Fu, C. Preparation of Antiviral Drug Molnupiravir Using Microchannel Reactor. China Patent Publication No. CN112608357A, 6 April 2021. [Google Scholar]
- Zhao, P.; Guo, Y.; Luan, X. Total synthesis of dalesconol a by Pd (0)/norbornene-catalyzed three-fold domino reaction and Pd (II)-catalyzed trihydroxylation. J. Am. Chem. Soc. 2021, 143, 21270–21274. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Qi, Y.; Sun, S.; Liu, X.; Li, W.; Liu, L. Synthesis of sterically hindered α-aminonitriles through 1,6-aza-conjugate addition of anilines to δ-cyano substituted para-quinone methides. Chin. J. Org. Chem. 2020, 40, 3934–3943. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, H.; Wang, G.; Wang, Z.; Liu, Z.; Liu, L. Enantioselective construction of single and vicinal all-carbon quaternary stereocenters through ion-pair-catalyzed 1,6-conjugate addition. Org. Lett. 2021, 23, 7248–7253. [Google Scholar] [CrossRef] [PubMed]
- Zsako, I.; Anghel, C.; Cohn, R. Acid-base equilibrium of the phenylcyanomethylene-p-quinone oxime. Stud. UBB Chem. 1971, 16, 5–8. [Google Scholar]
- Adam, W.; Makosza, M.; Zhao, C.G.; Surowiec, M. On the mechanism of the dimethyldioxirane oxidation of σH adducts (Meisenheimer complexes) generated from nitroarenes and carbanions. J. Org. Chem. 2000, 65, 1099–1101. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Panunzio, M.; Qin, W.; Bongini, A.; Monari, M. Efficient aldol-type reaction of o-protected α-hydroxy aldehydes and n-trimethylsilyl ketene imines: Synthesis of β,γ-dihydroxy-nitriles. Eur. J. Org. Chem. 2013, 23, 5127–5142. [Google Scholar] [CrossRef]
- Eddahmi, M.; Moura, N.M.M.; Bouissane, L.; Gamouh, A.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Paz, F.A.A.; Mendes, R.F.; Lodeiro, C.; Santos, S.M.; et al. New nitroindazolylacetonitriles: Efficient synthetic access via vicarious nucleophilic substitution and tautomeric switching mediated by anions. New J. Chem. 2019, 43, 14355–14367. [Google Scholar] [CrossRef]
- Davis, R.B.; Weber, J.D. Condensation of aromatic nitro compounds with arylacetonitriles. V–VI. Some reactions of the arylcyanomethylenequinone oximes. J. Chem. Eng. Data 1963, 8, 578–581. [Google Scholar] [CrossRef]
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2001, 14, 347–361. [Google Scholar] [CrossRef]
- Contreras, J.-M.; Bourguignon, J.-J. Identical and Non-Identical Twin Drugs. In The Practice of Medicinal Chemistry, 1st ed.; Wermouth, C.G., Ed.; Academic: San Diego, CA, USA, 2003; pp. 251–273. [Google Scholar]
- Stepan, A.F.; Walker, D.P.; Bauman, J.; Price, D.A.; Baillie, T.A.; Kalgutkar, A.S.; Aleo, M.D. Structural alert/reactive metabolite concept as applied in medicinal chemistry to mitigate the risk of idiosyncratic drug toxicity: A perspective based on the critical examination of trends in the top 200 drugs marketed in the United States. Chem. Res. Toxicol. 2011, 24, 1345–1410. [Google Scholar] [CrossRef]
- Zuccarello, W.A.; Blank, B.; Frishmuth, G.J.; Cohen, S.R.; Scaricaciottoli, D.; Owings, F.F. Synthesis and adrenocortical-inhibiting activity of substituted 2, 2-diphenethylamines. J. Med. Chem. 1969, 12, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Masłyk, M.; Janeczko, M.; Martyna, A.; Czernik, S.; Tokarska-Rodak, M.; Chwedczuk, M.; Foll-Josselin, B.; Ruchaud, S.; Bach, S. The anti-Candida albicans agent 4-AN inhibits multiple protein kinases. Molecules 2019, 24, e153. [Google Scholar] [CrossRef]
- Freyne, E.J.E.; Raeymaekers, A.H.M. Positive Inotropic and Lusitropic 3,5-dihydro-imidazo[2,1-b]quinazolin-2(1H)-one Derivatives. European Patent Publication No. EP406958A2, 9 January 1991. [Google Scholar]
- Janssen, M.A.C.; Sipido, V.K. Salicylanilide Derivatives. Federal Republic of. Germany Patent Publication No. DE2,610,837A1, 30 September 1976. [Google Scholar]
- Fang, B.; Xu, M.; Ke, J. Method for Preparing Closantel. China Patent Publication No. CN109,851,526A, 7 June 2019. [Google Scholar]
- Casanova, C.D. 2-(4-Amino-2-chloro-5-alkylphenyl)-2-(4-chlorophenyl)acetonitrile. Belgium Patent Publication No. BE895742A1, 16 May 1983. [Google Scholar]
- Foguet, A.R. Process for the Preparation of N-[5-chloro-4-{(4-chlorophenyl)cyanomethyl]-2-methylphenyl]-2-hydroxy-3,5-diiodobenzamide. Spain Patent Publication No. ES524551A1, 16 December 1984. [Google Scholar]
- Lei, S.; Meiju, X. Study on synthesis of closantel sodium. Guangdong Chem. Ind. 2013, 40, 14−15. [Google Scholar]
- Tabatabaei, S.A.; Soleimani, M.; Mansouri, M.R.; Mirshahi, A.; Inanlou, B.; Abrishami, M.; Pakrah, A.R.; Masarat, H. Closantel: A veterinary drug with potential severe morbidity in humans. BMC Ophthalmol. 2016, 16, e207. [Google Scholar] [CrossRef] [PubMed]
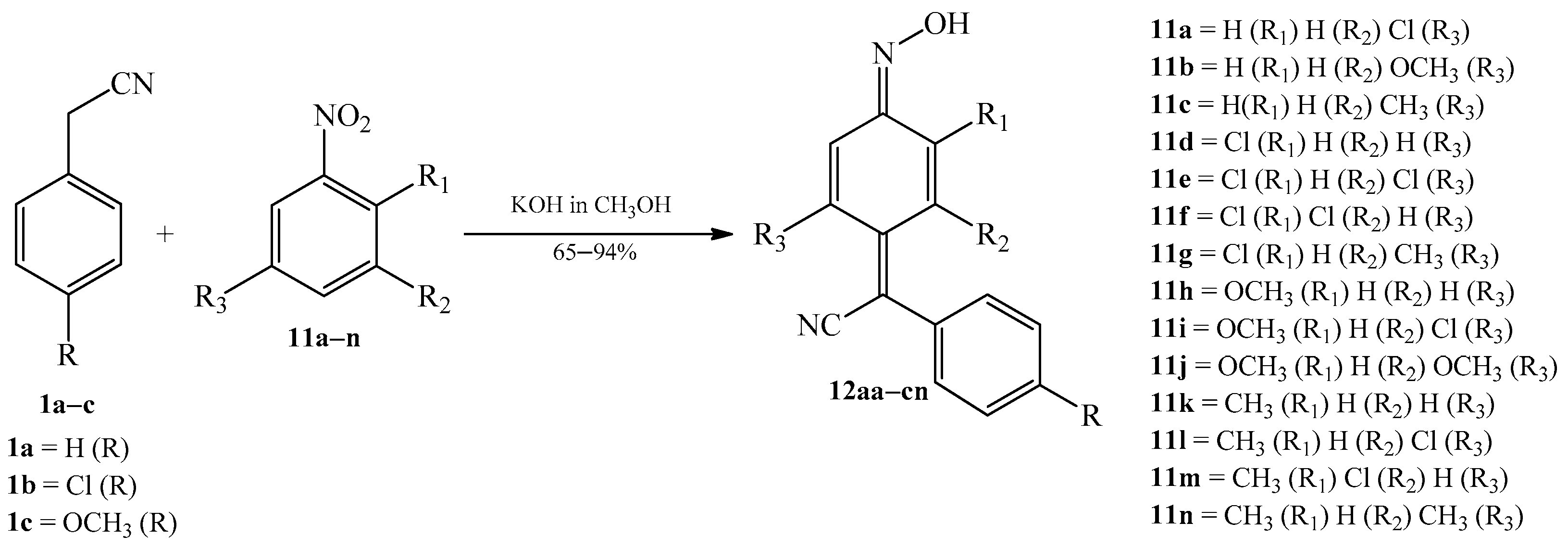

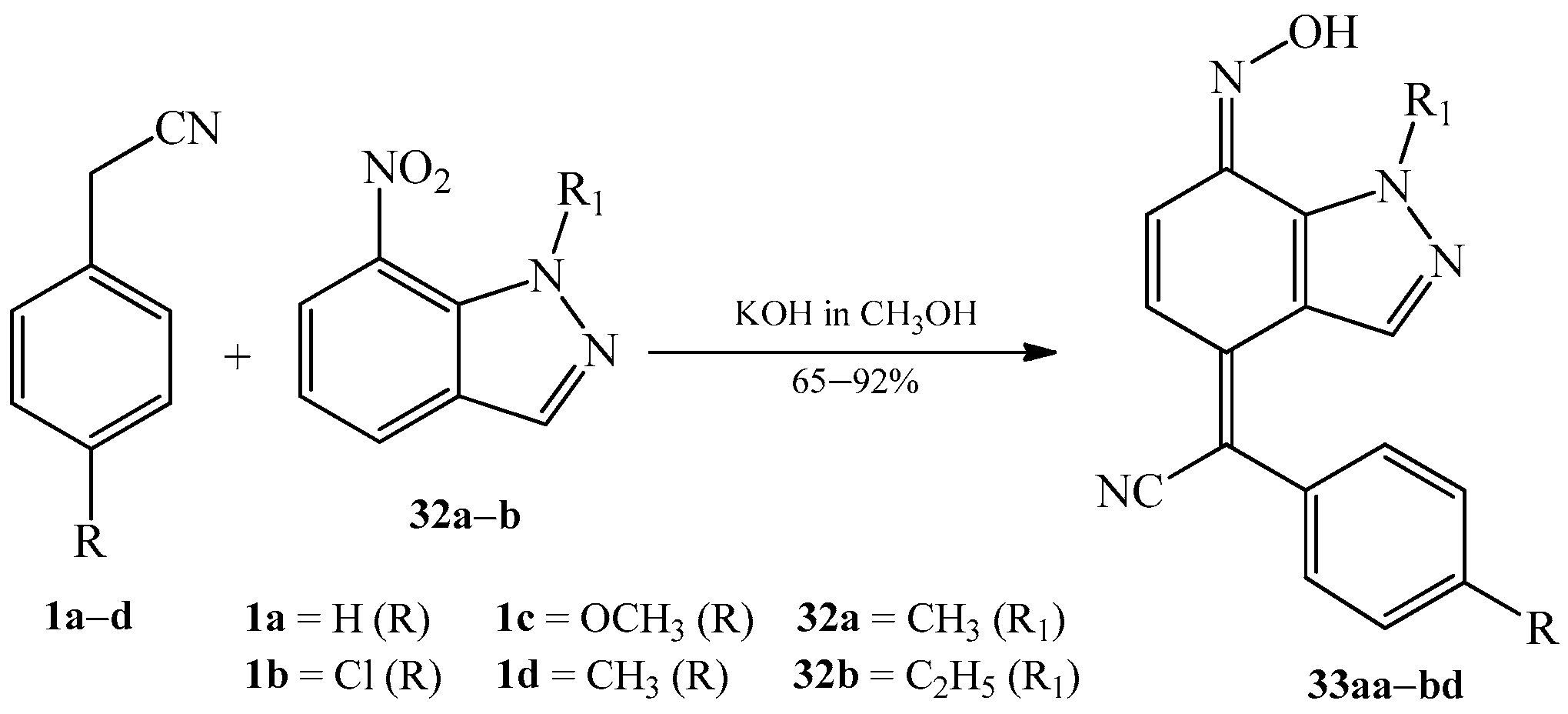

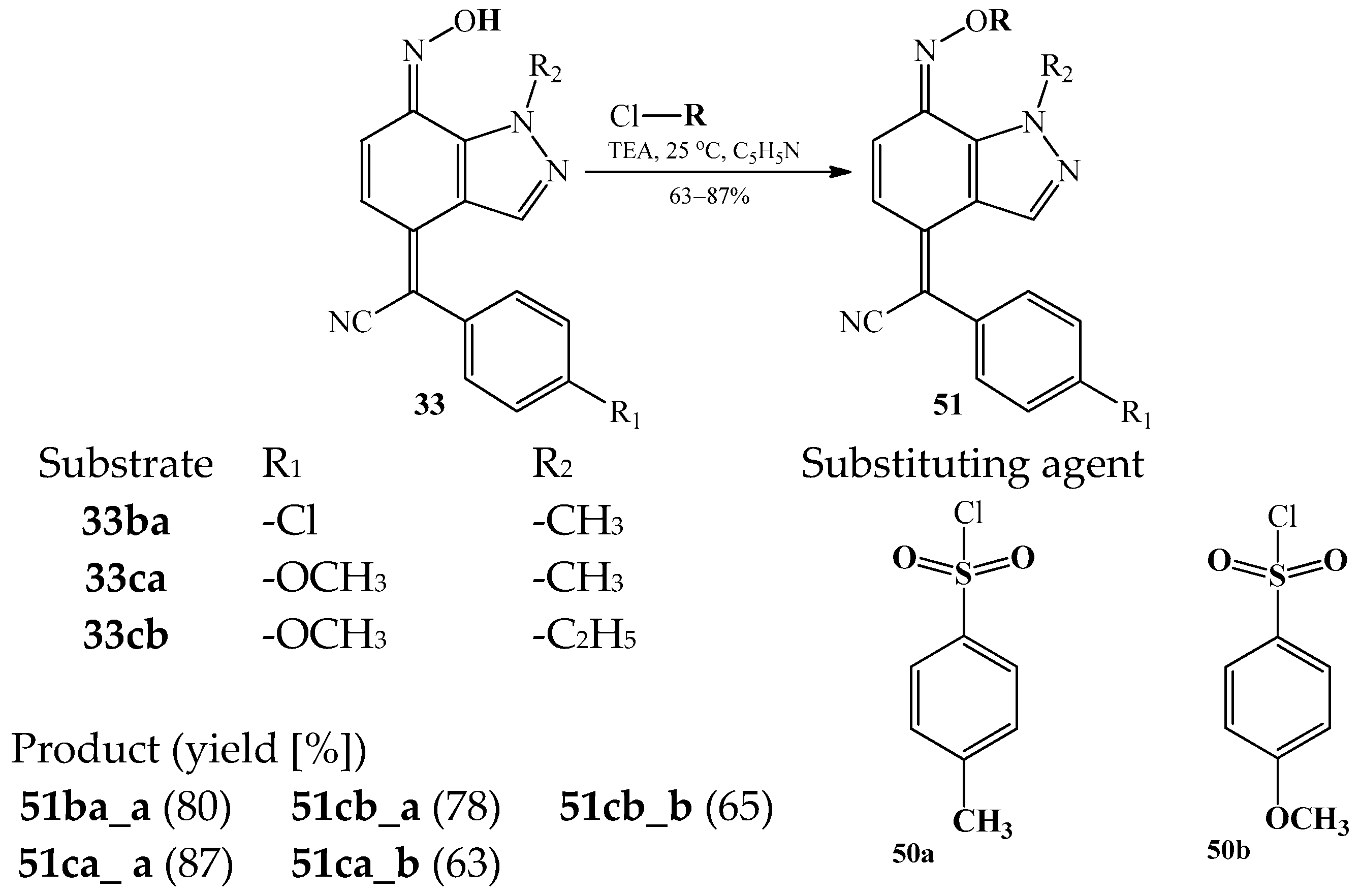

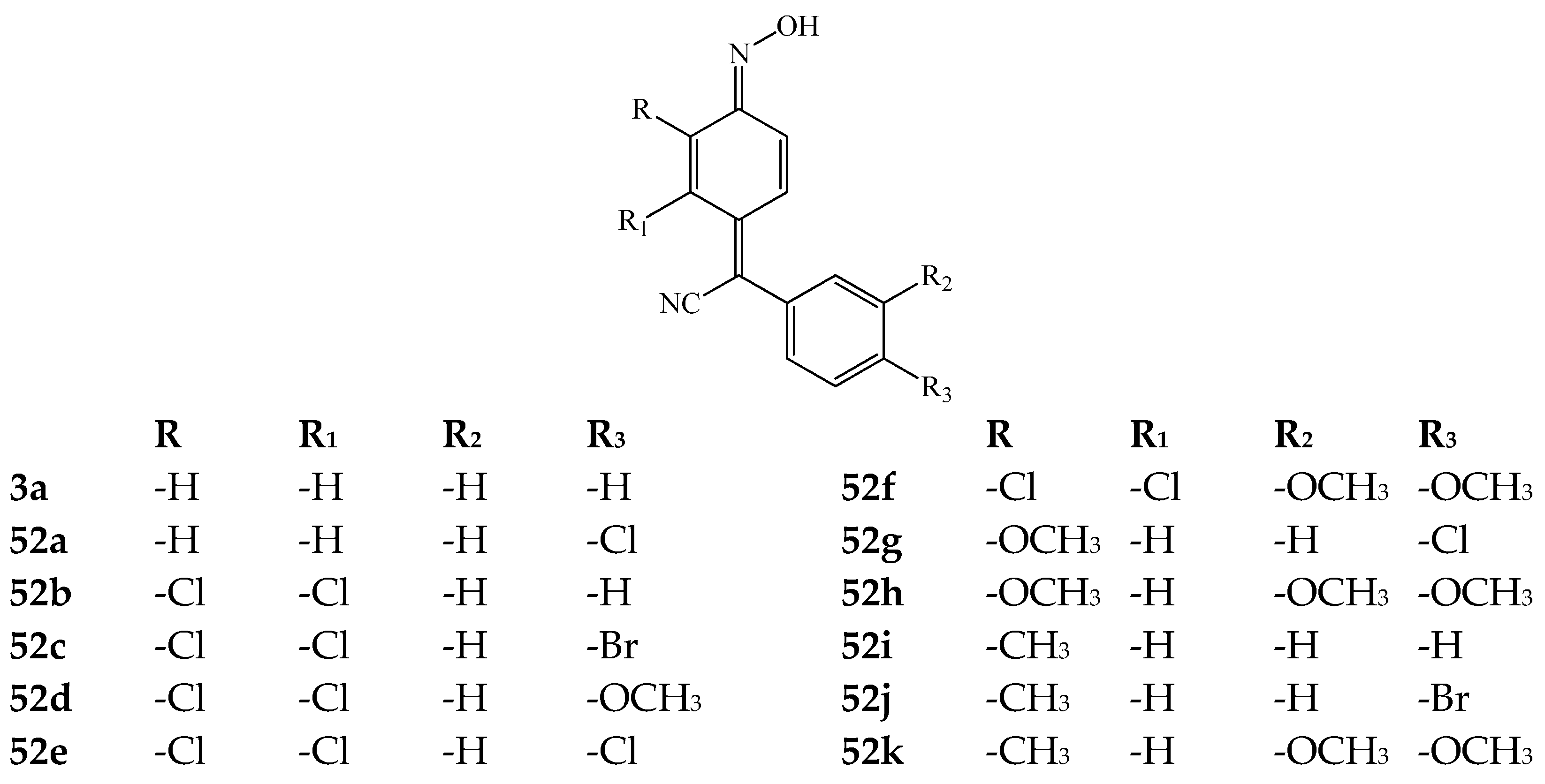

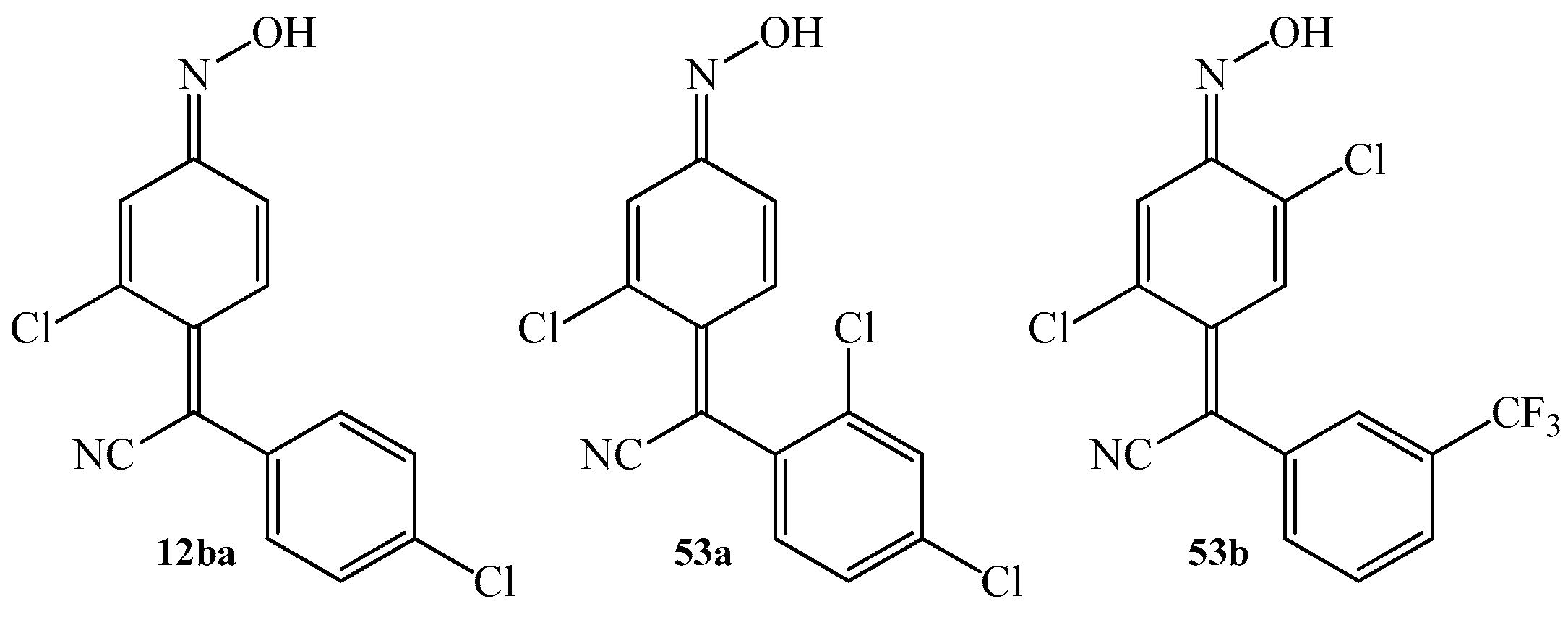


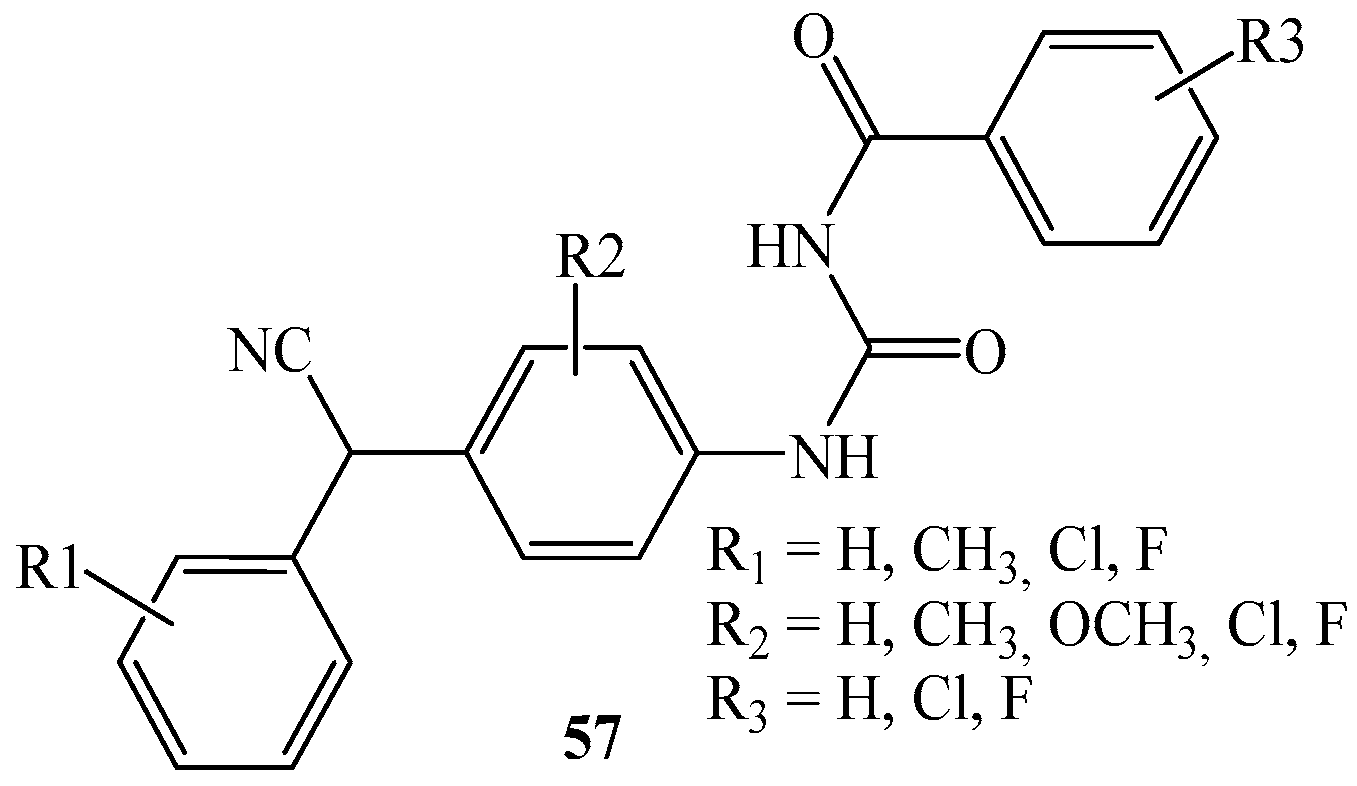
| Product (Yield [%]) | R | R1 | R2 | R3 | Product (Yield [%]) | R | R1 | R2 | R3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12aa | (53) | -H | -H | -H | -Cl | 12be | (94) | -Cl | -Cl | -H | -Cl |
| 12ab | (25) | -H | -H | -H | -OCH3 | 12bf | (92) | -Cl | -Cl | -Cl | -H |
| 12ac | (76) | -H | -H | -H | -CH3 | 12bg | (77) | -Cl | -Cl | -H | -CH3 |
| 12ad | (92) | -H | -Cl | -H | -H | 12bh | (80) | -Cl | -OCH3 | -H | -H |
| 12ae | (93) | -H | -Cl | -H | -Cl | 12bi | (81) | -Cl | -OCH3 | -H | -Cl |
| 12af | (53) | -H | -Cl | -Cl | -H | 12bj | (80) | -Cl | -OCH3 | -H | -OCH3 |
| 12ag | (77) | -H | -Cl | -H | -CH3 | 12bk | (60) | -Cl | -CH3 | -H | -H |
| 12ah | (87) | -H | -OCH3 | -H | -H | 12bm | (80) | -Cl | -CH3 | -Cl | -H |
| 12ai | (82) | -H | -OCH3 | -H | -Cl | 12bn | (43) | -Cl | -CH3 | -H | -CH3 |
| 12aj | (88) | -H | -OCH3 | -H | -OCH3 | 12ce | (91) | -OCH3 | -Cl | -H | -Cl |
| 12ak | (72) | -H | -CH3 | -H | -H | 12cg | (80) | -OCH3 | -Cl | -H | -CH3 |
| 12al | (92) | -H | -CH3 | -H | -Cl | 12cf | (65) | -OCH3 | -Cl | -Cl | -H |
| 12am | (82) | -H | -CH3 | -Cl | -H | 12ci | (84) | -OCH3 | -OCH3 | -H | -Cl |
| 12an | (53) | -H | -CH3 | -H | -CH3 | 12cj | (65) | -OCH3 | -OCH3 | -H | -OCH3 |
| 12ba | (100) | -Cl | -H | -H | -Cl | 12cl | (88) | -OCH3 | -CH3 | -H | -Cl |
| 12bb | (89) | -Cl | -H | -H | -OCH3 | 12cm | (80) | -OCH3 | -CH3 | -Cl | -H |
| 12bd | (100) | -Cl | -Cl | -H | -H | 12cn | (69) | -OCH3 | -CH3 | -H | -CH3 |
| Product (Yield (%)) | R | R1 | Product (Yield (%)) | R | R1 | ||
|---|---|---|---|---|---|---|---|
| 33aa | (67) | -H | -CH3 | 33ca | (65) | -OCH3 | -CH3 |
| 33ab | (69) | -H | -C2H5 | 33cb | (70) | -OCH3 | -C2H5 |
| 33ba | (86) | -Cl | -CH3 | 33da | (90) | -CH3 | -CH3 |
| 33bb | (76) | -Cl | -C2H5 | 33db | (92) | -CH3 | -C2H5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kula, K.; Nagatsky, R.; Sadowski, M.; Siumka, Y.; Demchuk, O.M. Arylcyanomethylenequinone Oximes: An Overview of Synthesis, Chemical Transformations, and Biological Activity. Molecules 2023, 28, 5229. https://doi.org/10.3390/molecules28135229
Kula K, Nagatsky R, Sadowski M, Siumka Y, Demchuk OM. Arylcyanomethylenequinone Oximes: An Overview of Synthesis, Chemical Transformations, and Biological Activity. Molecules. 2023; 28(13):5229. https://doi.org/10.3390/molecules28135229
Chicago/Turabian StyleKula, Karolina, Roman Nagatsky, Mikołaj Sadowski, Yevheniia Siumka, and Oleg M. Demchuk. 2023. "Arylcyanomethylenequinone Oximes: An Overview of Synthesis, Chemical Transformations, and Biological Activity" Molecules 28, no. 13: 5229. https://doi.org/10.3390/molecules28135229
APA StyleKula, K., Nagatsky, R., Sadowski, M., Siumka, Y., & Demchuk, O. M. (2023). Arylcyanomethylenequinone Oximes: An Overview of Synthesis, Chemical Transformations, and Biological Activity. Molecules, 28(13), 5229. https://doi.org/10.3390/molecules28135229








