Rapid In-Field Volatile Sampling for Detection of Botrytis cinerea Infection in Wine Grapes
Abstract
1. Introduction
2. Results and Discussion
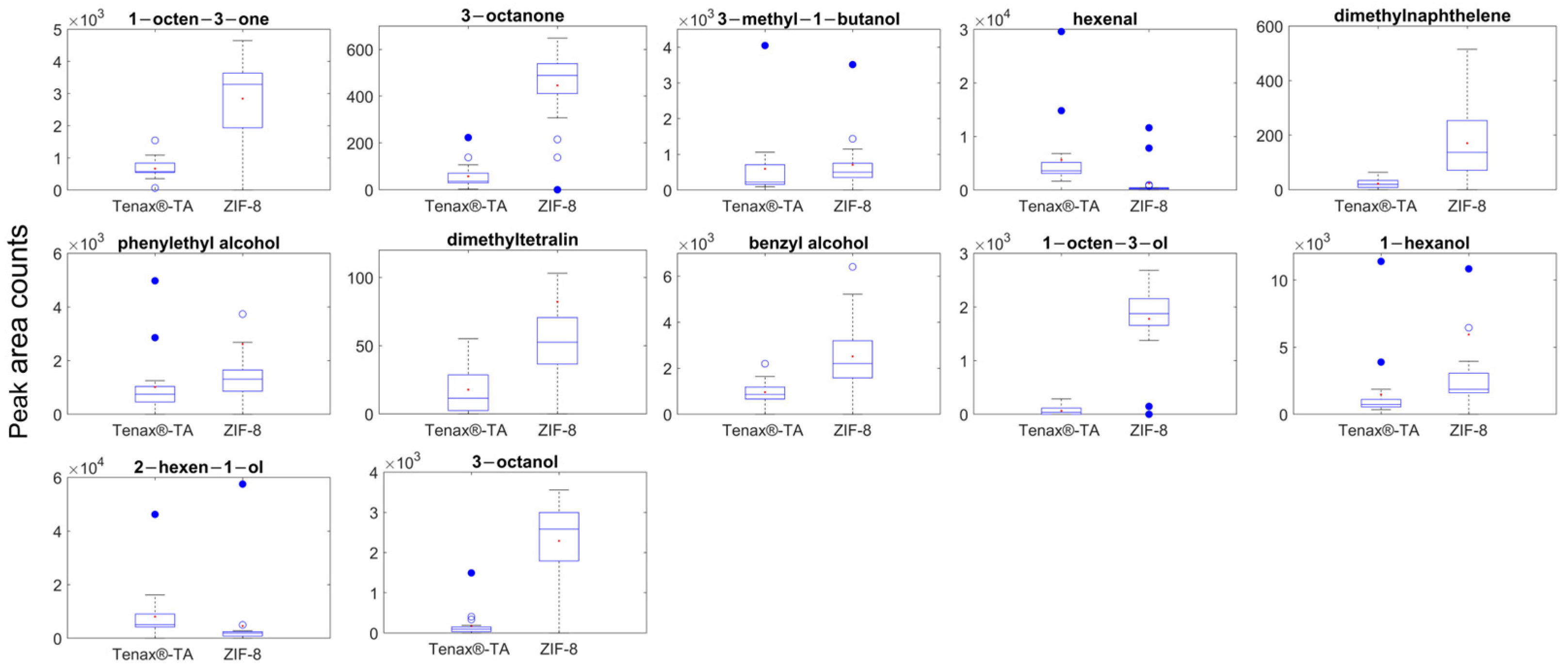
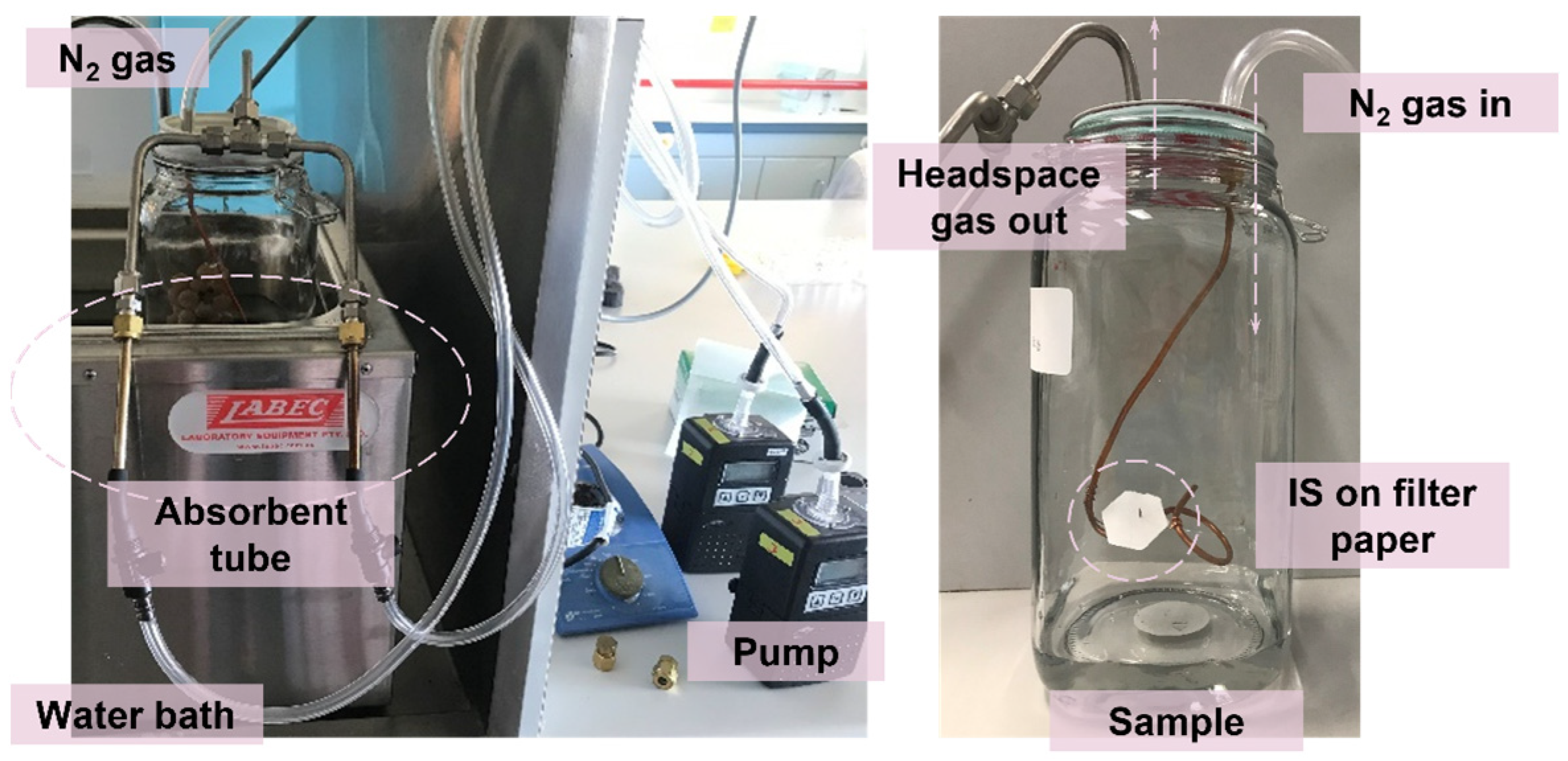
2.1. Controlled Environmental Condition for Gas Sampling
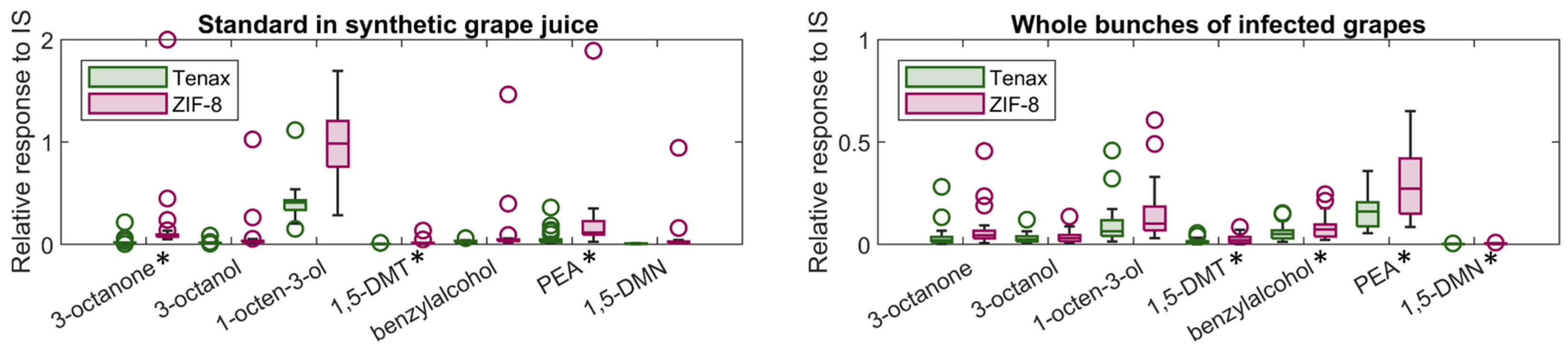
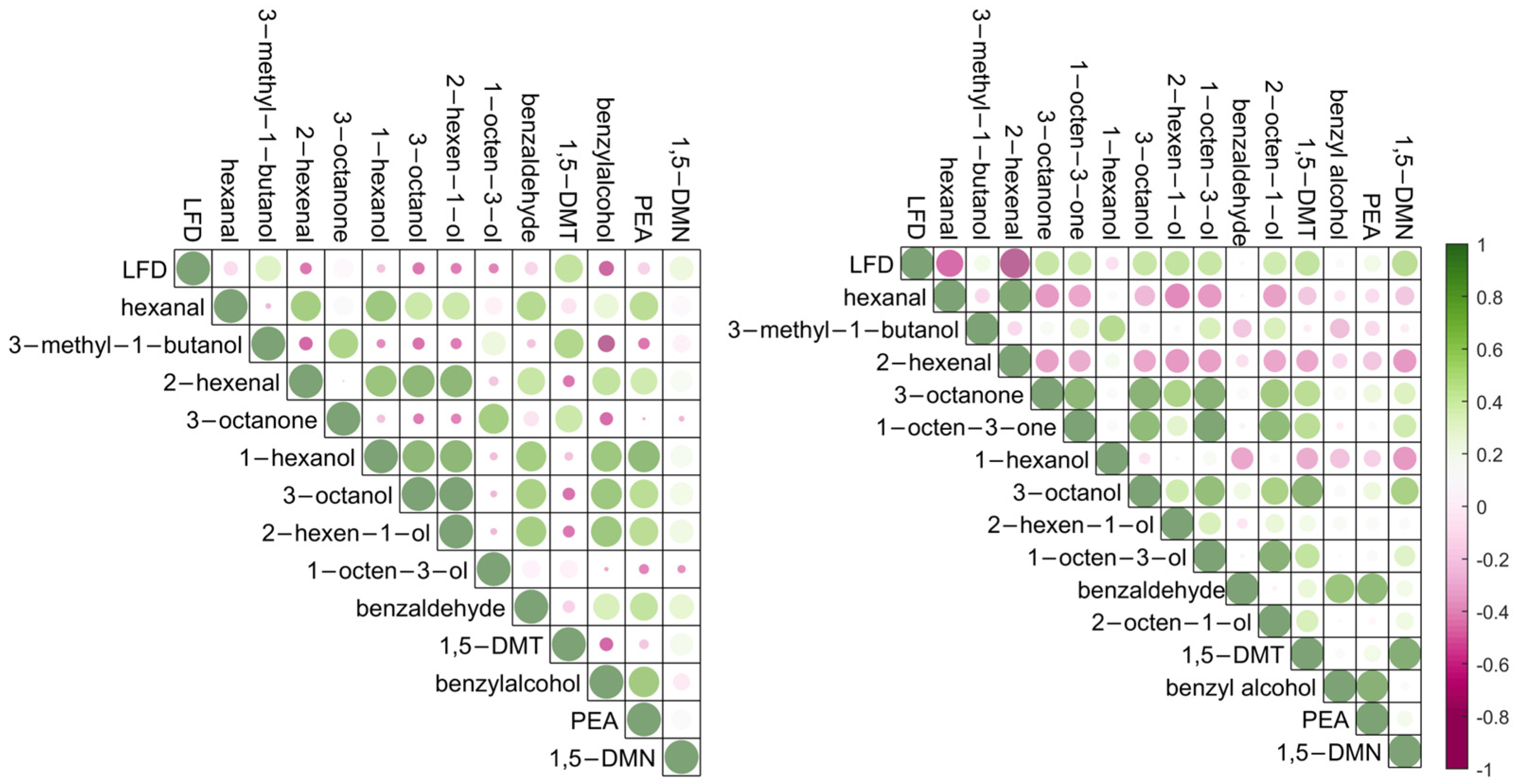
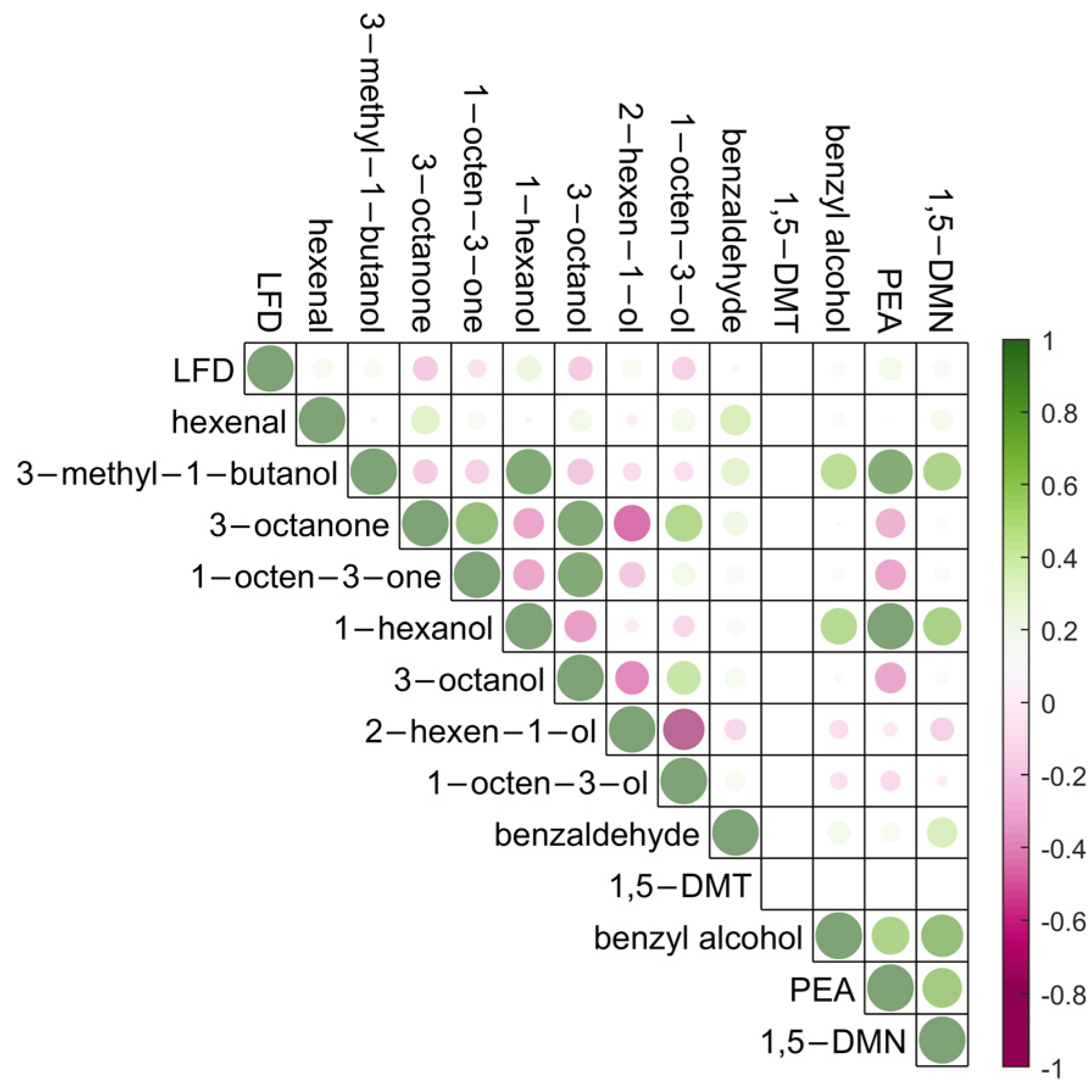
2.2. Correlation between Volatile Detection and B. cinerea Infection Severities
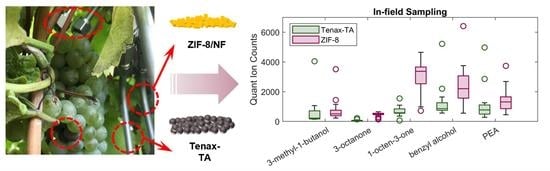
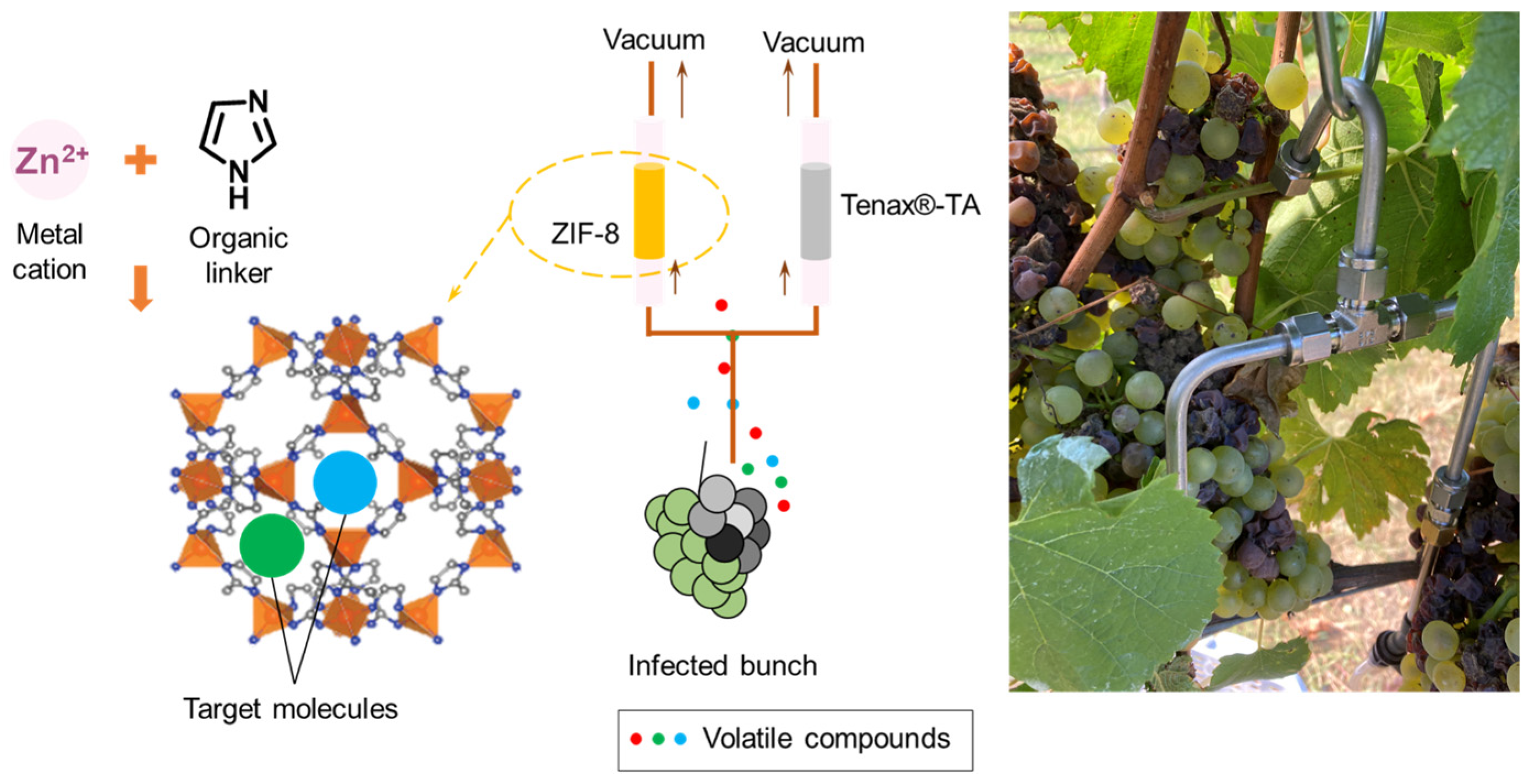
2.3. In-Field Volatile Sampling
3. Materials and Methods
3.1. Chemical and Materials
3.2. ZIF-8 Synthesis and Sampling Tube Preparation
3.3. VOCs Extraction Using ZIF-8
3.3.1. Volatile Extraction in a Closed Environment
3.3.2. In-Field Volatile Extraction
3.4. VOCs Detection Using GC-MS
3.4.1. Thermal Desorption Procedure
3.4.2. SPME Extraction Parameter
3.4.3. GC-MS Parameters and Statistics Processing
3.5. Botrytis cinerea Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine Bunch Rots: Impacts on Wine Composition, Quality, and Potential Procedures for the Removal of Wine Faults. J. Agric. Food Chem. 2013, 61, 5189–5206. [Google Scholar] [CrossRef]
- Armijo, G.; Schlechter, R.; Agurto, M.; Muñoz, D.; Nuñez, C.; Arce-Johnson, P. Grapevine Pathogenic Microorganisms: Understanding Infection Strategies and Host Response Scenarios. Front. Plant Sci. 2016, 7, 382. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, J.; Wang, X.D.; Yang, L.; Jiang, D.H.; Li, G.Q.; Hsiang, T.; Zhuang, W.Y. Morphological and phylogenetic identification of Botrytis sinoviticola, a novel cryptic species causing gray mold disease of table grapes (Vitis vinifera) in China. Mycologia 2014, 106, 43–56. [Google Scholar] [CrossRef]
- Santos, H.; Augusto, C.; Reis, P.; Rego, C.; Figueiredo, A.C.; Fortes, A.M. Volatile Metabolism of Wine Grape Trincadeira: Impact of Infection with Botrytis cinerea. Plants 2022, 11, 141. [Google Scholar] [CrossRef]
- Jiang, L.; Qiu, Y.; Dumlao, M.C.; Donald, W.A.; Steel, C.C.; Schmidtke, L.M. Detection and prediction of Botrytis cinerea infection levels in wine grapes using volatile analysis. Food Chem. 2023, 421, 136120. [Google Scholar] [CrossRef] [PubMed]
- Piechulla, B.; Lemfack, M.C.; Kai, M. Effects of discrete bioactive microbial volatiles on plants and fungi. Plant Cell Environ. 2017, 40, 2042–2067. [Google Scholar] [CrossRef]
- Elhamouly, N.A.; Hewedy, O.A.; Zaitoon, A.; Miraples, A.; Elshorbagy, O.T.; Hussien, S.; El-Tahan, A.; Peng, D. The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation. Front. Plant Sci. 2022, 13, 1044896. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Lorrain, B.; Jourdes, M.; Pasquier, G.; Fermaud, M.; Geny, L.; Rey, P.; Doneche, B.; Teissedre, P.-L. Assessment of grey mould (Botrytis cinerea) impact on phenolic and sensory quality of Bordeaux grapes, musts and wines for two consecutive vintages. Aust. J. Grape Wine Res. 2012, 18, 215–226. [Google Scholar] [CrossRef]
- Sadoughi, N.; Schmidtke, L.M.; Antalick, G.; Blackman, J.W.; Steel, C.C. Gas Chromatography–Mass Spectrometry Method Optimized Using Response Surface Modeling for the Quantitation of Fungal Off-Flavors in Grapes and Wine. J. Agric. Food Chem. 2015, 63, 2877–2885. [Google Scholar] [CrossRef]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Kataoka, H.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Boschfuste, J.; Riuaumatell, M.; Guadayol, J.; Caixach, J.; Lopeztamames, E.; Buxaderas, S. Volatile profiles of sparkling wines obtained by three extraction methods and gas chromatography–mass spectrometry (GC–MS) analysis. Food Chem. 2007, 105, 428–435. [Google Scholar] [CrossRef]
- Vas, G.; Vékey, K. Solid-phase microextraction: A powerful sample preparation tool prior to mass spectrometric analysis. J. Mass Spectrom. 2004, 39, 233–254. [Google Scholar] [CrossRef] [PubMed]
- De Fátima Alpendurada, M. Solid-phase microextraction: A promising technique for sample preparation in environmental analysis. J. Chromatogr. A 2000, 889, 3–14. [Google Scholar] [CrossRef]
- Llop, A.; Borrull, F.; Pocurull, E. Fully automated determination of N -nitrosamines in environmental waters by headspace solid-phase microextraction followed by GC-MS-MS. J. Sep. Sci. 2010, 33, 3692–3700. [Google Scholar] [CrossRef]
- Siebert, T.E.; Smyth, H.E.; Capone, D.L.; Neuw Hner, C.; Pardon, K.H.; Skouroumounis, G.K.; Herderich, M.J.; Sefton, M.A.; Pollnitz, A.P. Stable isotope dilution analysis of wine fermentation products by HS-SPME-GC-MS. Anal. Bioanal. Chem. 2005, 381, 937–947. [Google Scholar] [CrossRef]
- Ziółkowska, A.; Wąsowicz, E.; Jeleń, H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016, 213, 714–720. [Google Scholar] [CrossRef]
- Brown, S.D.; Rhodes, D.J.; Pritchard, B.J. A validated SPME-GC–MS method for simultaneous quantification of club drugs in human urine. Forensic Sci. Int. 2007, 171, 142–150. [Google Scholar] [CrossRef]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater. 2013, 244–245, 444–456. [Google Scholar] [CrossRef]
- Padial, N.M.; Quartapelle Procopio, E.; Montoro, C.; López, E.; Oltra, J.E.; Colombo, V.; Maspero, A.; Masciocchi, N.; Galli, S.; Senkovska, I.; et al. Highly Hydrophobic Isoreticular Porous Metal-Organic Frameworks for the Capture of Harmful Volatile Organic Compounds. Angew. Chem. Int. Ed. 2013, 52, 8290–8294. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D. Adsorption of volatile organic compounds by metal–organic frameworks MIL-101: Influence of molecular size and shape. J. Hazard. Mater. 2011, 195, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Suwannakot, P.; Lisi, F.; Ahmed, E.; Liang, K.; Babarao, R.; Gooding, J.J.; Donald, W.A. Metal–Organic Framework-Enhanced Solid-Phase Microextraction Mass Spectrometry for the Direct and Rapid Detection of Perfluorooctanoic Acid in Environmental Water Samples. Anal. Chem. 2020, 92, 6900–6908. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-Y.; Yang, C.-X.; Chang, N.; Yan, X.-P. Metal–Organic Frameworks for Analytical Chemistry: From Sample Collection to Chromatographic Separation. Acc. Chem. Res. 2012, 45, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Kumar, P.; Kwon, E.E.; Kim, K.-H. Metal-organic frameworks as superior media for thermal desorption-gas chromatography application: A critical assessment of MOF-5 for the quantitation of airborne formaldehyde. Microchem. J. 2017, 132, 219–226. [Google Scholar] [CrossRef]
- Gu, Z.-Y.; Wang, G.; Yan, X.-P. MOF-5 Metal−Organic Framework as Sorbent for In-Field Sampling and Preconcentration in Combination with Thermal Desorption GC/MS for Determination of Atmospheric Formaldehyde. Anal. Chem. 2010, 82, 1365–1370. [Google Scholar] [CrossRef]
- Rice, S.; Maurer, D.; Fennell, A.; Dharmadhikari, M.; Koziel, J. Evaluation of Volatile Metabolites Emitted In-Vivo from Cold-Hardy Grapes during Ripening Using SPME and GC-MS: A Proof-of-Concept. Molecules 2019, 24, 536. [Google Scholar] [CrossRef]
- Fung, A.G.; Yamaguchi, M.S.; McCartney, M.M.; Aksenov, A.A.; Pasamontes, A.; Davis, C.E. SPME-based mobile field device for active sampling of volatiles. Microchem. J. 2019, 146, 407–413. [Google Scholar] [CrossRef]
- Stierlin, É.; Michel, T.; Fernandez, X. Field analyses of lavender volatile organic compounds: Performance evaluation of a portable gas chromatography–mass spectrometry device. Phytochem. Anal. 2020, 31, 778–785. [Google Scholar] [CrossRef]
- Schueuermann, C.; Steel, C.C.; Blackman, J.W.; Clark, A.C.; Schwarz, L.J.; Moraga, J.; Collado, I.G.; Schmidtke, L.M. A GC–MS untargeted metabolomics approach for the classification of chemical differences in grape juices based on fungal pathogen. Food Chem. 2019, 270, 375–384. [Google Scholar] [CrossRef]
- Dankó, T.; Szelényi, M.; Janda, T.; Molnár, B.P.; Pogány, M. Distinct volatile signatures of bunch rot and noble rot. Physiol. Mol. Plant Pathol. 2021, 114, 101626. [Google Scholar] [CrossRef]
- Callejón, R.M.; Ubeda, C.; Ríos-Reina, R.; Morales, M.L.; Troncoso, A.M. Recent developments in the analysis of musty odour compounds in water and wine: A review. J. Chromatogr. A 2016, 1428, 72–85. [Google Scholar] [CrossRef]
- Darriet, P.; Pons, M.; Henry, R.; Dumont, O.; Findeling, V.; Cartolaro, P.; Calonnec, A.; Dubourdieu, D. Impact Odorants Contributing to the Fungus Type Aroma from Grape Berries Contaminated by Powdery Mildew (Uncinula necator); Incidence of Enzymatic Activities of the Yeast Saccharomyces cerevisiae. J. Agric. Food Chem. 2002, 50, 3277–3282. [Google Scholar] [CrossRef] [PubMed]
- Hung, R.; Lee, S.; Rodriguez-Saona, C.; Bennett, J.W. Common gas phase molecules from fungi affect seed germination and plant health in Arabidopsis thaliana. AMB Express 2014, 4, 53. [Google Scholar] [CrossRef]
- Archbold, D.D.; Hamilton-Kemp, T.R.; Barth, M.M.; Langlois, B.E. Identifying Natural Volatile Compounds That Control Gray Mold (Botrytis cinerea) during Postharvest Storage of Strawberry, Blackberry, and Grape. J. Agric. Food Chem. 1997, 45, 4032–4037. [Google Scholar] [CrossRef]
- Lan, Y.-B.; Xiang, X.-F.; Yang, W.-X.; Zhu, B.-Q.; Pu, H.-T.; Duan, C.-Q. Characterization of free and glycosidically bound volatile compounds, fatty acids, and amino acids in Vitis davidii Foex grape species native to China. Food Sci. Biotechnol. 2020, 29, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Liu, X.-L.; Li, Y.-S.; Zhu, G.-Q.; Ban, Y.-J.; Xu, L.-Y.; Yang, W.-S. An Organophilic Pervaporation Membrane Derived from Metal-Organic Framework Nanoparticles for Efficient Recovery of Bio-Alcohols. Angew. Chem. Int. Ed. 2011, 50, 10636–10639. [Google Scholar] [CrossRef]
- Yalage Don, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 2020, 10, 4498. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Jang, M.-S.; Cho, H.-Y.; Kwon, H.-J.; Kim, S.; Ahn, W.-S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Dumlao, M.C.; Donald, W.A.; Steel, C.C.; Schmidtke, L.M. Rapid In-Field Volatile Sampling for Detection of Botrytis cinerea Infection in Wine Grapes. Molecules 2023, 28, 5227. https://doi.org/10.3390/molecules28135227
Jiang L, Dumlao MC, Donald WA, Steel CC, Schmidtke LM. Rapid In-Field Volatile Sampling for Detection of Botrytis cinerea Infection in Wine Grapes. Molecules. 2023; 28(13):5227. https://doi.org/10.3390/molecules28135227
Chicago/Turabian StyleJiang, Liang, Morphy C. Dumlao, William A. Donald, Christopher C. Steel, and Leigh M. Schmidtke. 2023. "Rapid In-Field Volatile Sampling for Detection of Botrytis cinerea Infection in Wine Grapes" Molecules 28, no. 13: 5227. https://doi.org/10.3390/molecules28135227
APA StyleJiang, L., Dumlao, M. C., Donald, W. A., Steel, C. C., & Schmidtke, L. M. (2023). Rapid In-Field Volatile Sampling for Detection of Botrytis cinerea Infection in Wine Grapes. Molecules, 28(13), 5227. https://doi.org/10.3390/molecules28135227