Gram-Scale Total Synthesis of TAB with Cardioprotective Activity and the Structure-Activity Relationship of Its Analogs
Abstract
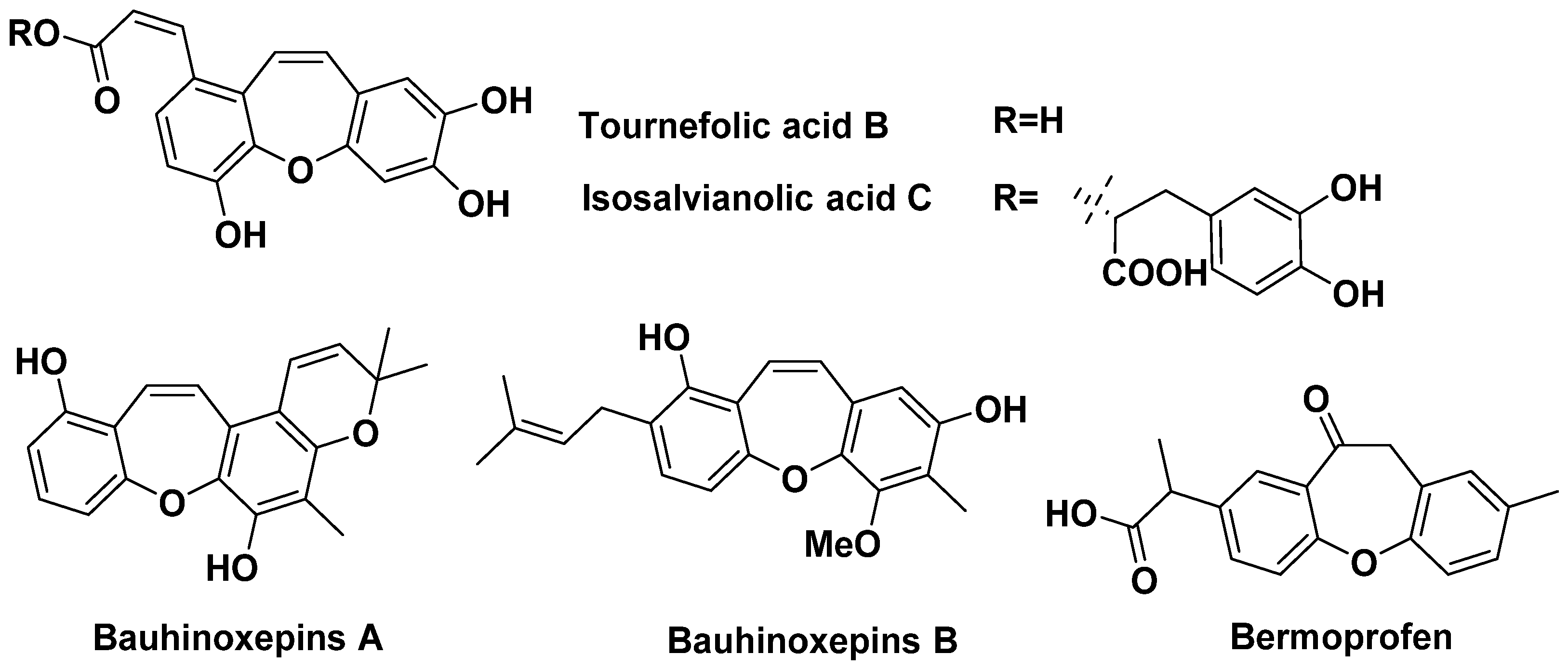
1. Introduction
2. Results
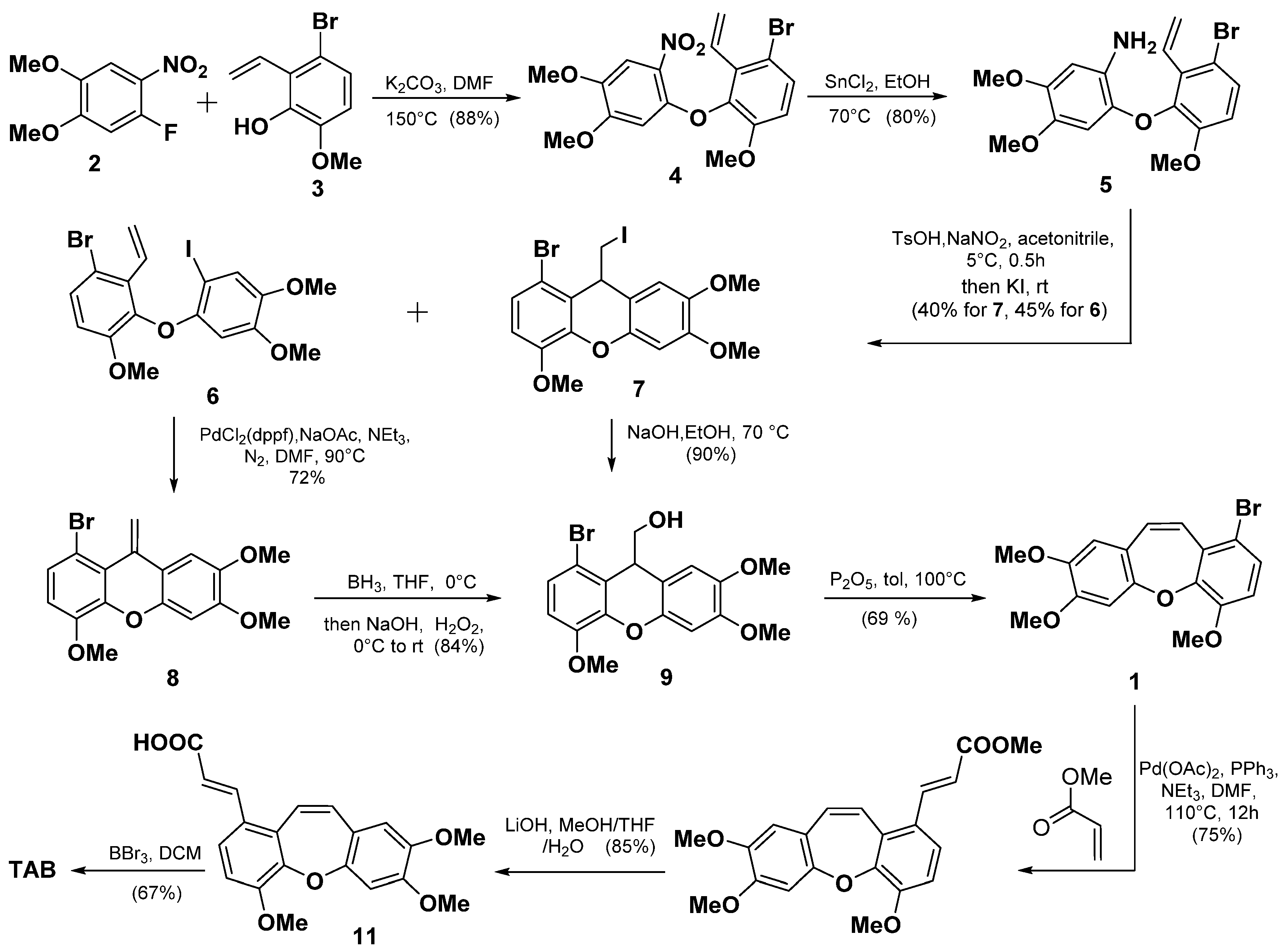
2.1. Total Synthesis of TAB
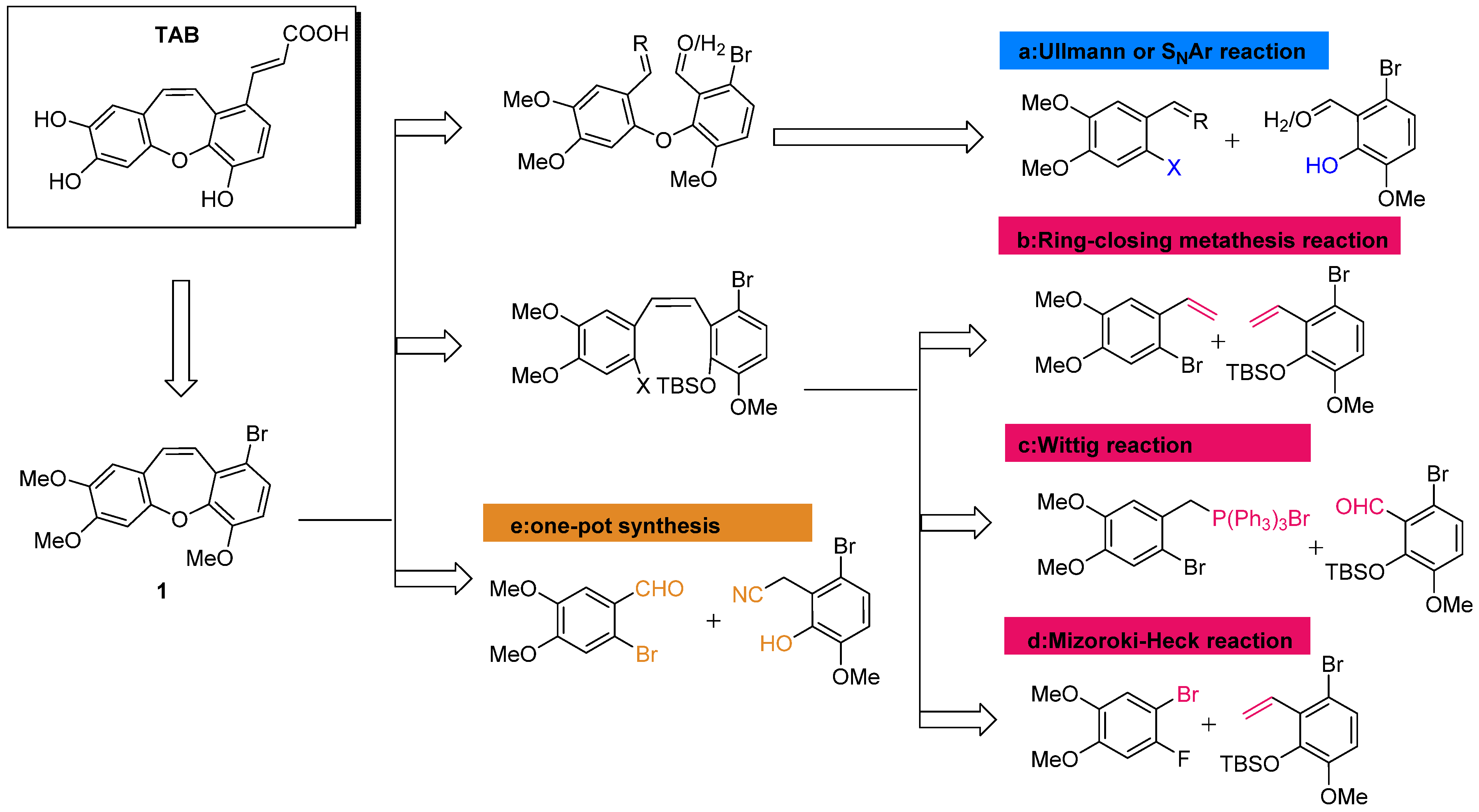
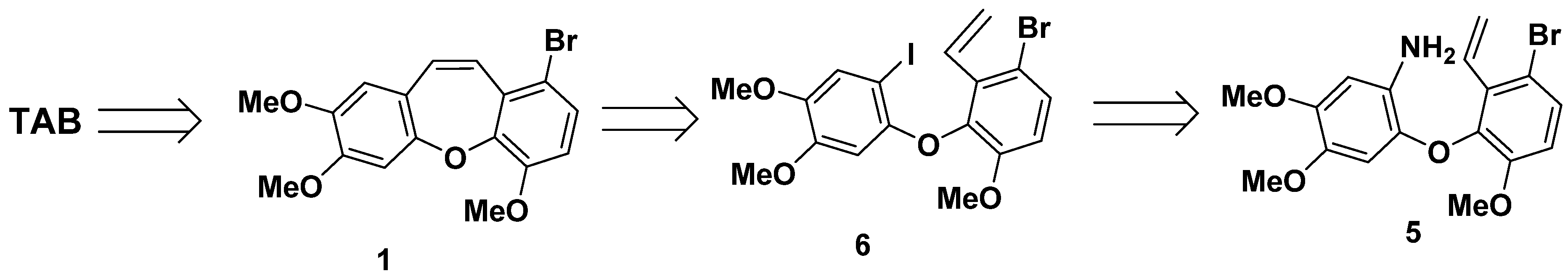
2.1.1. Retrosynthesis of TAB
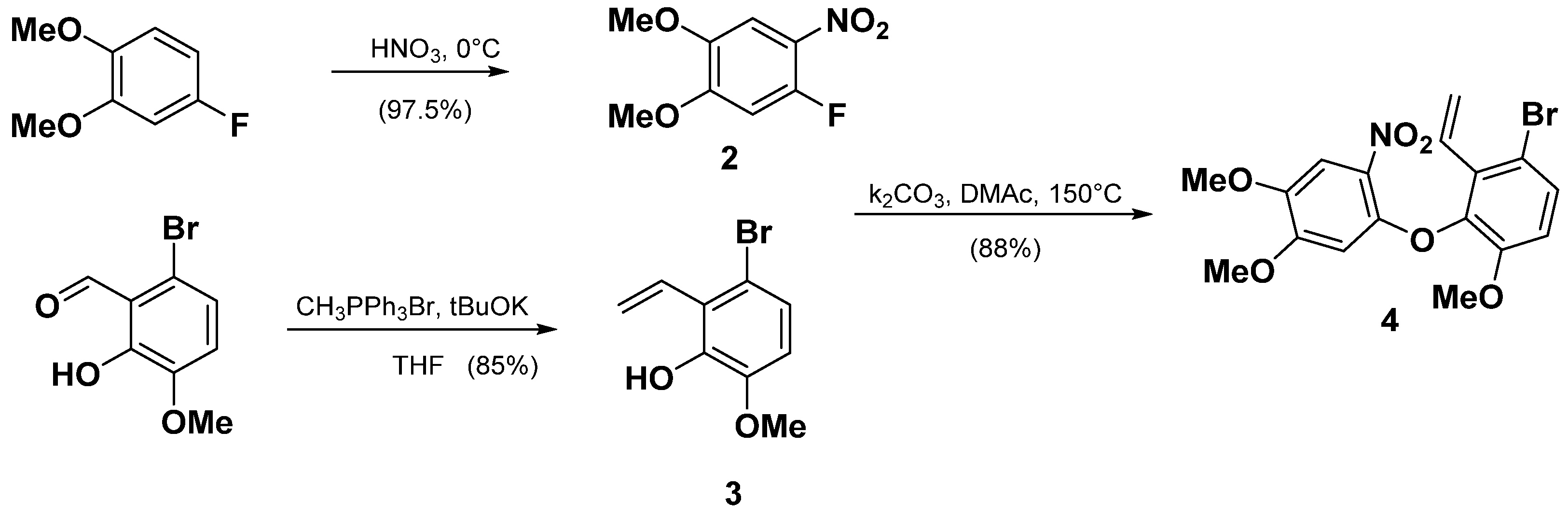
2.1.2. Synthesis of TAB
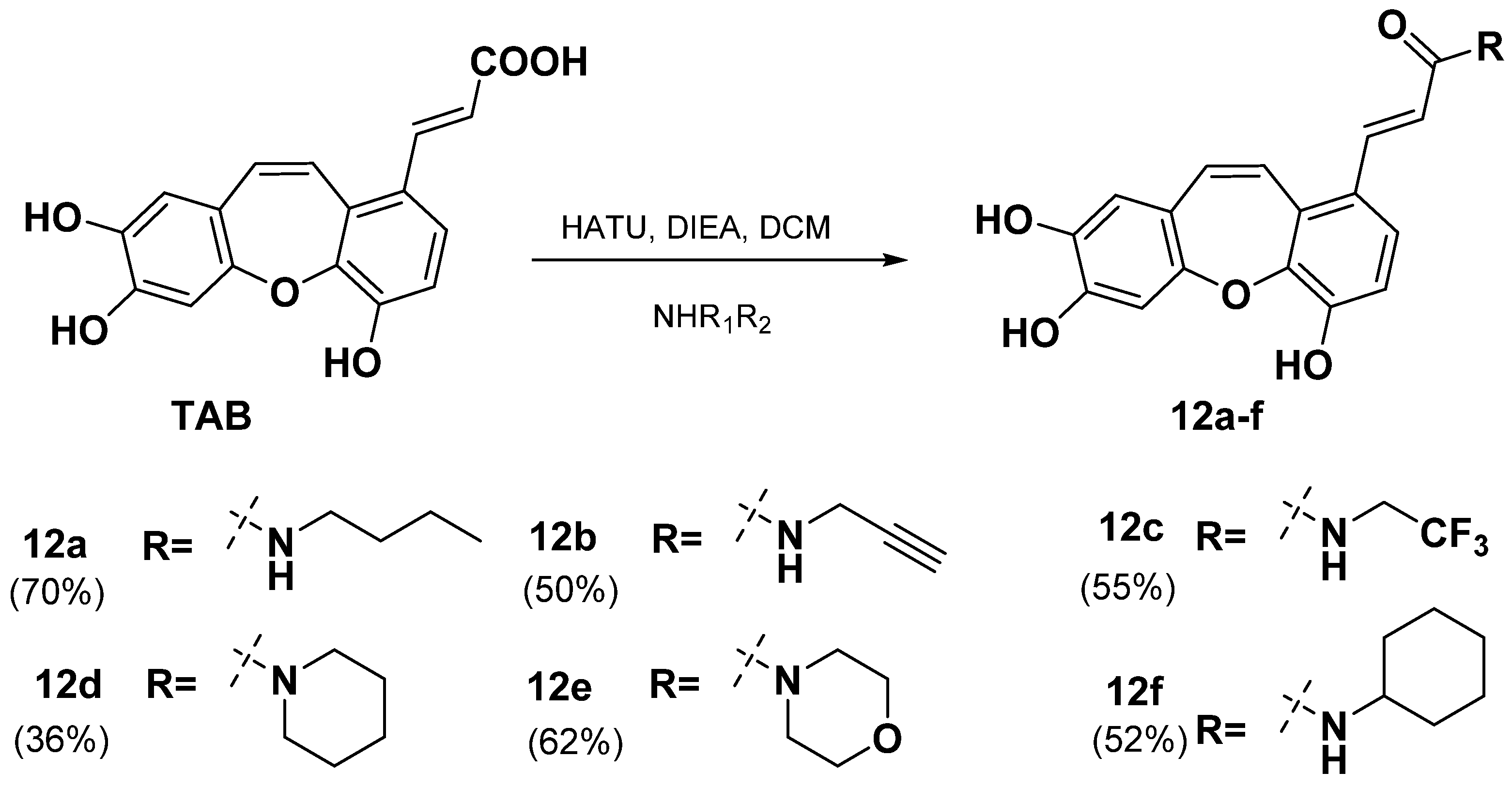
2.2. Synthesis of TAB Derivatives
2.3. Cardioprotective Activity
2.4. Structure-Activity Relationship
3. Discussion
4. Materials and Methods
4.1. General
4.2. Synthesis and Characterization Data
- 1-fluoro-4,5-dimethoxy-2-nitrobenzene (2)
- 3-bromo-6-methoxy-2-vinylphenol (3)
- 1-bromo-3-(4,5-dimethoxy-2-nitrophenoxy)-4-methoxy-2-vinylbenzene (4)
- 2-(3-bromo-6-methoxy-2-vinylphenoxy)-4,5-dimethoxyaniline (5)
- 1-bromo-3-(2-iodo-4,5-dimethoxyphenoxy)-4-methoxy-2-vinylbenzene (6) and 1-bromo-9-(iodomethyl)-4,6,7-trimethoxy-9H-xanthene (7)
- 1-bromo-4,6,7-trimethoxy-9-methylene-9H-xanthene (8)
- (1-bromo-4,6,7-trimethoxy-9H-xanthen-9-yl)methanol (9)
- 1-bromo-4,7,8-trimethoxydibenzo[b,f]oxepine (1)
- Methyl (E)-3-(4,7,8-trimethoxydibenzo[b,f]oxepin-1-yl)acrylate (10)
- (E)-3-(4,7,8-trimethoxydibenzo[b,f]oxepin-1-yl)acrylic acid (11)
- Tournefolic acid B (TAB)
- (E)-N-butyl-3-(4,7,8-trihydroxydibenzo[b,f]oxepin-1-yl)acrylamide (12a)
- (E)-N-(prop-2-yn-1-yl)-3-(4,7,8-trihydroxydibenzo[b,f]oxepin-1-yl)acrylamide (12b)
- (E)-N-(2,2,2-trifluoroethyl)-3-(4,7,8-trihydroxydibenzo[b,f]oxepin-1-yl)acrylamide (12c)
- (E)-1-(piperidin-1-yl)-3-(4,7,8-trihydroxydibenzo[b,f]oxepin-1-yl)prop-2-en-1-one (12d)
- (E)-1-morpholino-3-(4,7,8-trihydroxydibenzo[b,f]oxepin-1-yl)prop-2-en-1-one (12e)
- (E)-N-cyclohexyl-3-(4,7,8-trihydroxydibenzo[b,f]oxepin-1-yl)acrylamide (12f)
- 2-bromo-10,11-dihydro-5H-dibenzo[b,f]azepine (13)
- Methyl (E)-3-(10,11-dihydro-5H-dibenzo[b,f]azepin-2-yl)acrylate (14a) and phenethyl (E)-3-(10,11-dihydro-5H-dibenzo[b,f]azepin-2-yl)acrylate (14b)
- (E)-3-(10,11-dihydro-5H-dibenzo[b,f]azepin-2-yl)acrylic acid (15)
- (E)-N-butyl-3-(10,11-dihydro-5H-dibenzo[b,f]azepin-2-yl)acrylamide (16a)
- (E)-3-(10,11-dihydro-5H-dibenzo[b,f]azepin-2-yl)-N-(prop-2-yn-1-yl)acrylamide (16b)
- (E)-3-(10,11-dihydro-5H-dibenzo[b,f]azepin-2-yl)-N-(2,2,2-trifluoroethyl)acrylamide (16c)
- (E)-3-(10,11-dihydro-5H-dibenzo[b,f]azepin-2-yl)-1-(piperidin-1-yl)prop-2-en-1-one (16d)
4.3. Cardioprotective Activity Assays
4.3.1. Cell Culture and Treatment
4.3.2. Hypoxia/Reoxygenation Protocol
4.3.3. Cell Viability Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Golshiri, K.; Ataabadi, E.A.; Fernandez, P.; Danser, J.; Roks, A.J. The importance of the nitric oxide-cGMP pathway in age-related cardiovascular disease: Focus on phosphodiesterase-1 and soluble guanylate cyclase. Basic Clin. Pharmacol. Toxicol. 2020, 127, 67–80. [Google Scholar]
- Johoson, J.A.; Cavallari, L.H. Pharmacogenetics and cardiovascular diseasee implications for personalized medicine. Pharmacol. Rev. 2013, 65, 987–1009. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxidative Med. Cell. Longev. 2016, 2014, 1656450. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Engelman, R.M.; Wei, Z.; Bagchi, D.; Rousou, J.A.; Das, D.K. Attenuation of myocardial reperfusion injury by reducing intracellular calcium overloading with dihydropyridines. Biochem. Pharmacol. 1993, 45, 1333–1341. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, H.; Liu, L.X.; Yan, W.; Guo, H.J.; Li, W.J.; Tian, C.; Li, H.H.; Wang, H.X. NOD2 contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and inflammation. Life Sci. 2016, 149, 10–17. [Google Scholar] [CrossRef]
- McCully, J.D.; Wakiyama, H.; Hsieh, Y.J.; Jones, M.; Levitsky, S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. AJP Heart Circ. Physiol. 2004, 286, 1923–1935. [Google Scholar] [CrossRef]
- Kong, R.; Gao, Y.; Sun, B.; Chen, H.; Wang, G.; Wang, X.; Zhu, H.; Pan, S.; Xue, D.; Jiang, H. The strategy of combined ischemia preconditioning and salvianolic acid-B pretreatment to prevent hepatic ischemia-reperfusion injury in rats. Dig. Dis. Sci. 2009, 54, 2568–2576. [Google Scholar] [CrossRef]
- Chen, J.; Wong, H.S.; Leung, H.Y.; Leong, P.K.; Chan, W.M.; Chen, N.; Ko, K.M. An ursolic acid-enriched extract of Cynomorium songaricum protects against carbon tetrachloride hepatotoxicity and gentamicin nephrotoxicity in rats possibly through a mitochondrial pathway: A comparison with ursolic acid. J. Funct. Foods 2014, 7, 330–341. [Google Scholar] [CrossRef]
- Lu, Y.; Kan, H.; Wang, Y.; Wang, D.; Wang, X.; Gao, J.; Zhu, L. Asiatic acid ameliorates hepatic ischemia/reperfusion injury in rats via mitochondria-targeted protective mechanism. Toxicol. Appl. Pharm. 2018, 338, 214–223. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Zhang, J.; Sun, K.; Li, Q.; Kuang, B.; Wang, M.M.Z.; Hou, S.; Gong, H. Methyl eugenol attenuates liver ischemia reperfusion injury via activating PI3K/Akt signaling. Int. Immunopharmacol. 2021, 99, 108023. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wu, S.; Jiang, Y.; Wang, L.; Li, G.; Qing, Y.; Liu, J.; Zhang, D. Inhibition of autophagy by geniposide protects against myocardial ischemia/reperfusion injury. Int. Immunopharmacol. 2020, 85, 106609. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, Y.; Liu, M.; Han, X.; Zhang, J.; Zhang, X.; Chu, L. Protective effect of quercetin against myocardial ischemia as a Ca2+ channel inhibitor: Involvement of inhibiting contractility and Ca2+ influx via L-type Ca2+ channels. Arch. Pharmacal Res. 2020, 43, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bian, L.; Fu, X.; Ai, Q.; Sui, Y.; Zhang, A.; Gao, H.; Zhong, L.L.; Lu, D. Gastrodin pretreatment alleviates rat brain injury caused by cerebral ischemic-reperfusion. Brain Res. 2019, 1712, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sun, X.; Xu, X.; Yu, Y.L.; Zhu, Y.D. Application of Tournefolic acid B in the Preparation for the Treatment and Prevention of Ischemic Heart Disease. CN Patent 106562950A, 19 April 2017. [Google Scholar]
- Yu, Y.; Xing, N.; Xu, X.; Zhu, Y.; Wang, S.; Sun, G.; Sun, X. Tournefolic acid B, derived from Clinopodium chinense (Benth.) Kuntze, protects against myocardial ischemia/reperfusion injury by inhibiting endoplasmic reticulum stress-regulated apoptosis via PI3K/AKT pathways. Phytomedicine 2018, 52, 178–186. [Google Scholar] [CrossRef]
- Lin, Y.; Chang, Y.Y.; Kuo, Y.; Shiao, M. Anti-Lipid-Peroxidative Principles from Tournefortia sarmentosa. J. Nat. Prod. 2002, 65, 745–747. [Google Scholar] [CrossRef]
- Chi, C.W.; Lin, Y.L.; Wang, Y.H.; Chen, C.F.; Wang, C.N.; Shiao, Y.J. Tournefolic acid B attenuates amyloid β protein-mediated toxicity by abrogating the calcium overload in mitochondria and retarding the caspase 8-truncated Bid-cytochrome c pathway in rat cortical neurons. Eur. J. Pharmacol. 2008, 586, 35–43. [Google Scholar] [CrossRef]
- Wang, C.N.; Pan, H.C.; Lin, Y.L.; Chi, C.W.; Shiao, Y. Ester Derivatives of Tournefolic Acid B Attenuate N-Methyl-Daspartate-Mediated Excitotoxicity in Rat Cortical Neurons. Mol Pharmacol. 2006, 69, 950–959. [Google Scholar] [CrossRef]
- Kittakoop, P.; Nopichai, S.; Thongon, N.; Charoenchai, P.; Thebtaranonth, Y. Bauhinoxepins A and B: New Antimycobacterial Dibenzo[b,f]oxepins from Bauhinia saccocalyx. Helv. Chim. Acta 2004, 87, 175–179. [Google Scholar] [CrossRef]
- Qian, T.X.; Li, L.N. Isosalvianolic acid C, a depside possessing a dibenzooxepin skeleton. Phytochemistry 1992, 31, 1068–1070. [Google Scholar]
- Lin, Y.; Wang, J.; He, L.; Hou, Y.; Fu, J.; Wei, D.; Jia, Q.; Lv, Y.; Wang, C.; Han, S. Isosalvianolic acid C-induced pseudo-allergic reactions via the mast cell specific receptor MRGPRX2. Int. Immunopharmacol. 2019, 71, 22–31. [Google Scholar] [CrossRef]
- Mori, M.; Nakamura, Y.; Shirai, Y.; Seto, Y.; Nakamura, H.; Makita, H.; Imasato, Y. Prolongation of antipyretic action and reduction of gastric ulcerogenicity in the rat by controlled-release granules of bermoprofen, a new nonsteroidal anti-inflammatory drug. J. Pharm. Sci. 1991, 80, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Fagan, P.J.; Hauptman, E.; Shapiro, R.; Casalnuovo, A. Using Intelligent/Random Library Screening to Design Focused Libraries for the Optimization of Homogeneous Catalysts: Ullmann Ether Formation. J. Am. Chem. Soc. 2000, 122, 5043–5051. [Google Scholar] [CrossRef]
- Yeager, G.; Schissel, D. An Umpoled synthon Approach to the synthesis of 2-Aryloxyphenols. Synthesis 1995, 1995, 28–30. [Google Scholar] [CrossRef]
- Krawczyk, H.; Mielecki, D.; Szczecinski, P.; Grzesiuk, E. Synthesis of derivatives of methoxydibenzo[b, f]oxepine in the presence of sodium azide. Tetrahedron 2016, 72, 3877–3884. [Google Scholar] [CrossRef]
- Jepsen, T.H.; Larsen, M.; Jørgensen, M.; Nielsen, M.B. Three-Step synthesis of (Thio)xanthene and Dibenzothiepine/Diebenzoxepine by an Intramolecular Mizoroki-Heck Reaction of Diaryl (Thio)Ethers. Synlett 2012, 23, 418–422. [Google Scholar]
- Arnold, L.A.; Luo, W.; Guy, R.K. Synthesis of Medium Ring Heterocycles Using an Intramolecular Heck Reaction. Org. Lett. 2004, 6, 3005–3007. [Google Scholar] [CrossRef]
- Comber, M.; Sargent, M. The Synthesis of Pacharin: A Dibenzoxepine from the Heartwood of Bauhinia racemose Lamk. J. Chem. Soc. Perkin Trans. 1 1990, 5, 1371–1373. [Google Scholar] [CrossRef]
- Matsuda, T.; Sato, S. Synthesis of Dibenzoheteropines of Group 13-16 Elements via Ring-Closing Metathesis. J. Org. Chem. 2013, 78, 3329–3335. [Google Scholar] [CrossRef]
- Choi, Y.L.; Lim, H.S.; Lim, H.J.; Heo, J.N. One-Pot Transition-Metal-Free Synthesis of Dibenzo[b,f]oxepins from 2-Halobenzaldehydes. Org. Lett. 2012, 14, 5102–5105. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; He, Q.; Xie, Y.; Yang, C. Copper-Assisted/Copper-Free Synthesis of Functionalized Dibenzo[b, f]oxepins and Their Analogs via a One-Pot Tandem Reaction. Helv. Chim. Acta 2013, 96, 296–308. [Google Scholar] [CrossRef]
- Carlos, L.; Castedo, L. A new synthesis of 10, 11- dihydrodlbenzo[b, f]oxepin-10-ones: Key intermediates to cularine alkaoids. Tetrahedron Lett. 1989, 30, 6927–6928. [Google Scholar]
- Stopks, T.; Marzo, L.; Daniliuc, C.G. Oxidative C-H Bond Functionalization and Ring Expansion with TMSCHN2: A Copper(I)-Catalyzed Approach to Dibenzoxepines and Dibenzoazepines. Angew. Chem. Int. Ed. 2015, 54, 5049–5053. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration v2018.10. Available online: https://www.molinspiration.com/ (accessed on 20 June 2023).
- Snow, A.; Weigele, M.; Larsen, L.; Nguyen, B.; Lake, T.; Castillo, G.; Sanders, V.; Lorimer, S.; Larsen, D.; Coffen, D.L.; et al. Preparation of Substituted N-aryl Benzamides and Related Compounds for Treatment of Amyloid Diseases and Synuclein Opathies and as Imaging Agents. U.S. Patent 20110104055, 5 May 2011. [Google Scholar]


| Compd. | Cell Viability (% of Control) | clogP a | |||||
|---|---|---|---|---|---|---|---|
| 0 μM | 0.5 μM | 1 μM | 2 μM | 4 μM | 8 μM | ||
| TAB | 56.0 ± 0.7 | 60.2 ± 0.4 | 77.2 ± 1.1 | 68.6 ± 0.9 | 65.7 ± 0.6 | 65.5 ± 1.2 | 2.69 |
| 10 | 56.5 ± 1.3 | 58.2 ± 1.3 | 56.8 ± 0.9 | 62.5 ± 1.6 | 62.0 ± 0.8 | 59.5 ± 1.4 | 4.20 |
| 11 | 56.3 ± 0.8 | 60.5 ± 0.7 | 60.4 ± 0.6 | 61.7 ± 1.2 | 67.4 ± 0.5 | 64.3 ± 0.6 | 3.58 |
| 12a | 56.6 ± 1.5 | 72.5 ± 0.5 | 75.7 ± 1.2 | 77.3 ± 1.5 | 74.6 ± 0.9 | 75.3 ± 1.1 | 3.99 |
| 12b | 56.4 ± 0.5 | 68.6 ± 1.5 | 70.7 ± 1.4 | 73.8 ± 0.9 | 77.3 ± 1.1 | 70.6 ± 0.7 | 2.71 |
| 12c | 56.7 ± 0.9 | 73.6 ± 0.5 | 78.5 ± 0.8 | 82.6 ± 1.1 | 80.4 ± 1.3 | 79.5 ± 0.8 | 3.48 |
| 12d | 56.5 ± 0.5 | 71.0 ± 0.6 | 75.6 ± 1.1 | 74.6 ± 0.8 | 72.5 ± 1.6 | 59.6 ± 1.7 | 3.70 |
| 12e | 56.1 ± 0.7 | 69.4 ± 1.2 | 74.1 ± 1.5 | 75.9 ± 1.2 | 73.7 ± 0.7 | 65.2 ± 0.3 | 2.64 |
| 12f | 56.7 ± 0.3 | 63.3 ± 1.3 | 68.5 ± 0.6 | 68.3 ± 1.0 | 66.2 ± 1.6 | 60.9 ± 1.2 | 4.46 |
| 14a | 56.9 ± 1.1 | 55.6 ± 1.0 | 57.4 ± 1.1 | 57.1 ± 1.4 | 59.7 ± 0.4 | 61.5 ± 1.1 | 4.62 |
| 14b | 56.4 ± 1.4 | 58.4 ± 1.6 | 56.1 ± 1.2 | 57.4 ± 1.7 | 58.5 ± 1.8 | 62.5 ± 0.8 | 6.41 |
| 15 | 56.0 ± 1.2 | 60.3 ± 1.0 | 64.8 ± 0.5 | 71.8 ± 1.4 | 69.3 ± 1.1 | 63.9 ± 0.9 | 4.00 |
| 16a | 55.8 ± 0.5 | 65.1 ± 0.6 | 65.7 ± 1.3 | 72.6 ± 0.3 | 70.8 ± 1.2 | 69.4 ± 0.5 | 5.30 |
| 16b | 56.4 ± 0.8 | 67.3 ± 0.6 | 75.0 ± 1.2 | 72.5 ± 1.7 | 68.2 ± 0.9 | 66.3 ± 0.6 | 4.02 |
| 16c | 56.2 ± 1.6 | 64.6 ± 1.5 | 72.4 ± 1.0 | 75.3 ± 1.2 | 69.3 ± 0.3 | 63.3 ± 0.5 | 4.79 |
| 16d | 56.7 ± 0.5 | 69.5 ± 1.5 | 71.4 ± 0.6 | 72.9 ± 1.1 | 65.4 ± 0.8 | 67.2 ± 1.0 | 5.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Sun, Z.; Wu, D.; Yi, F.; Wu, H.; Ma, G.; Xu, X. Gram-Scale Total Synthesis of TAB with Cardioprotective Activity and the Structure-Activity Relationship of Its Analogs. Molecules 2023, 28, 5197. https://doi.org/10.3390/molecules28135197
Sun Z, Sun Z, Wu D, Yi F, Wu H, Ma G, Xu X. Gram-Scale Total Synthesis of TAB with Cardioprotective Activity and the Structure-Activity Relationship of Its Analogs. Molecules. 2023; 28(13):5197. https://doi.org/10.3390/molecules28135197
Chicago/Turabian StyleSun, Zhonghao, Zhaocui Sun, Daoshun Wu, Fan Yi, Haifeng Wu, Guoxu Ma, and Xudong Xu. 2023. "Gram-Scale Total Synthesis of TAB with Cardioprotective Activity and the Structure-Activity Relationship of Its Analogs" Molecules 28, no. 13: 5197. https://doi.org/10.3390/molecules28135197
APA StyleSun, Z., Sun, Z., Wu, D., Yi, F., Wu, H., Ma, G., & Xu, X. (2023). Gram-Scale Total Synthesis of TAB with Cardioprotective Activity and the Structure-Activity Relationship of Its Analogs. Molecules, 28(13), 5197. https://doi.org/10.3390/molecules28135197






