Usnic Acid-Loaded Magnetite Nanoparticles—A Comparative Study between Synthesis Methods
Abstract
1. Introduction
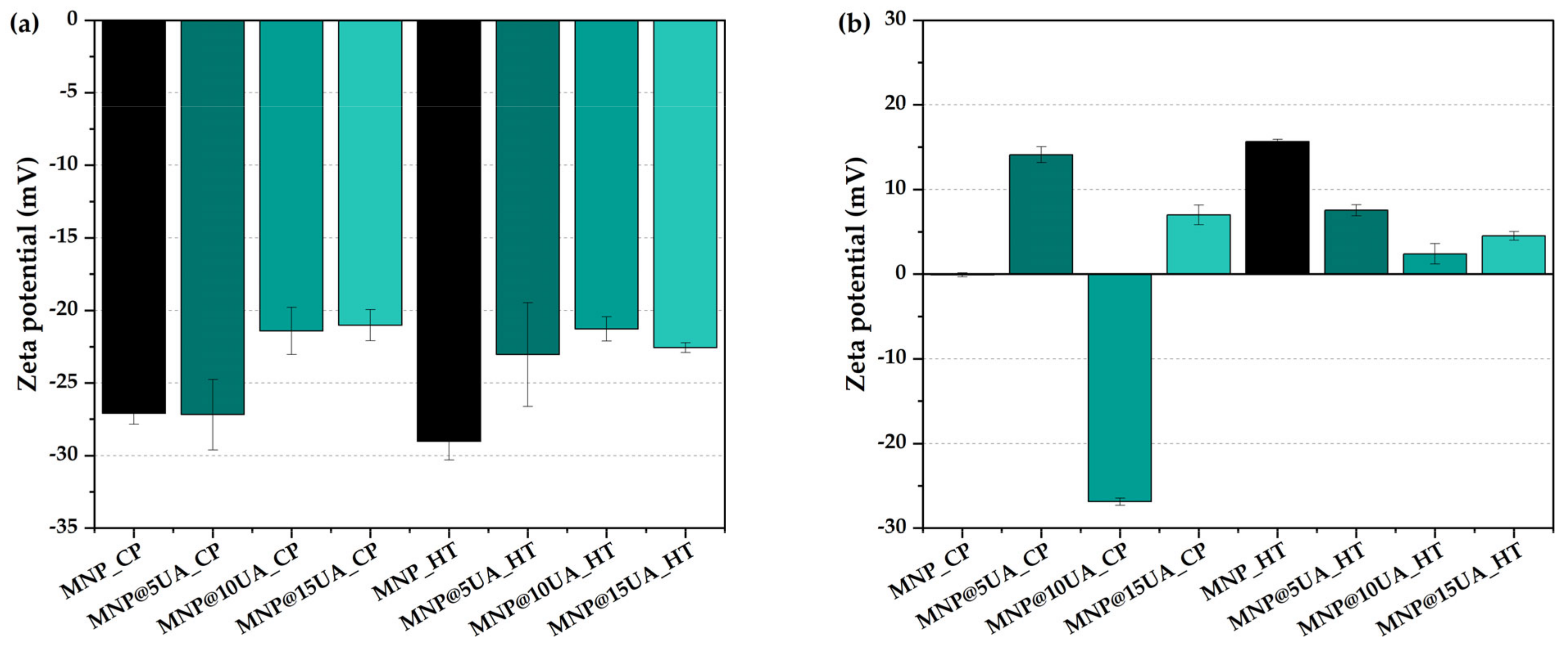
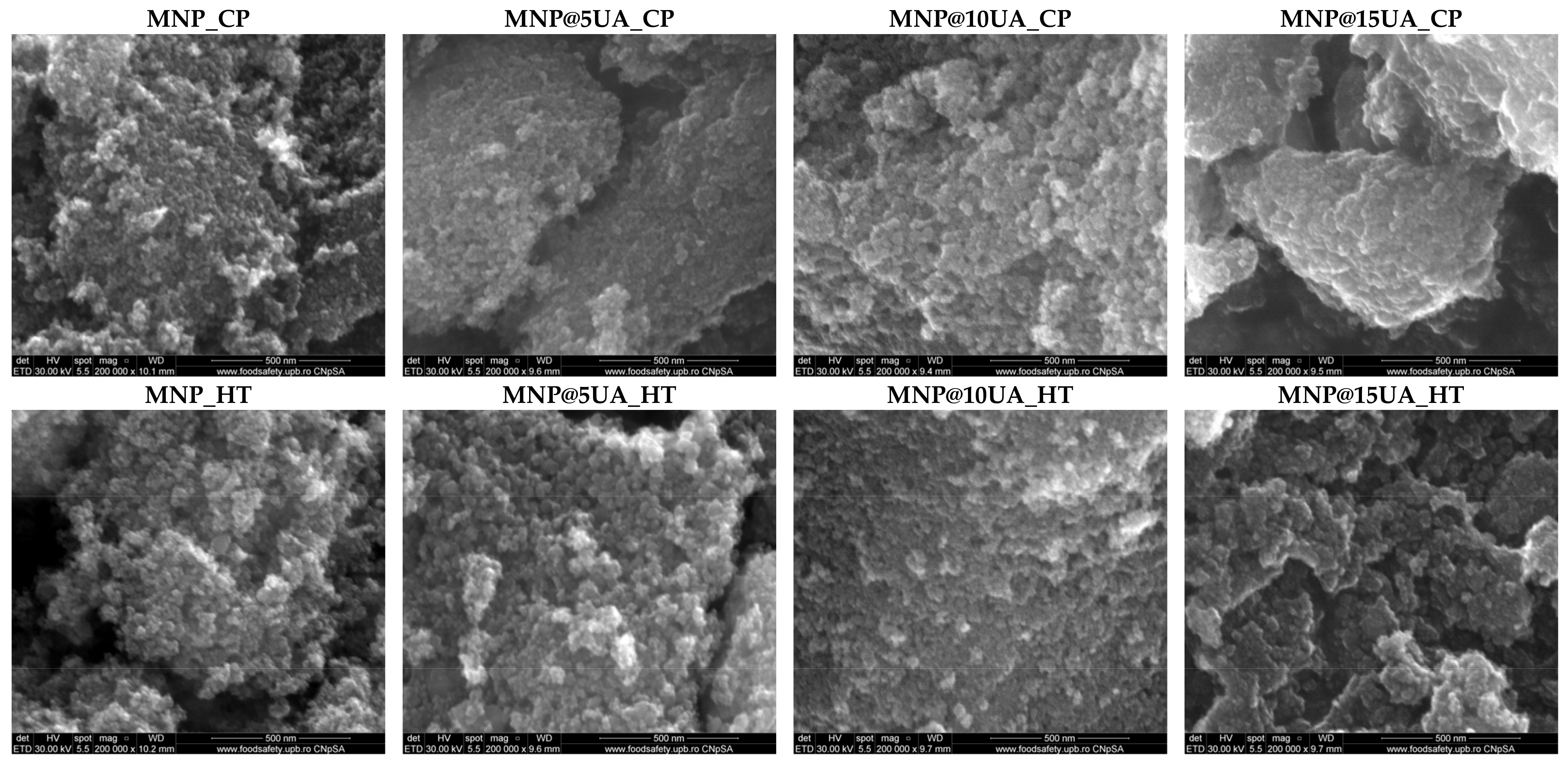
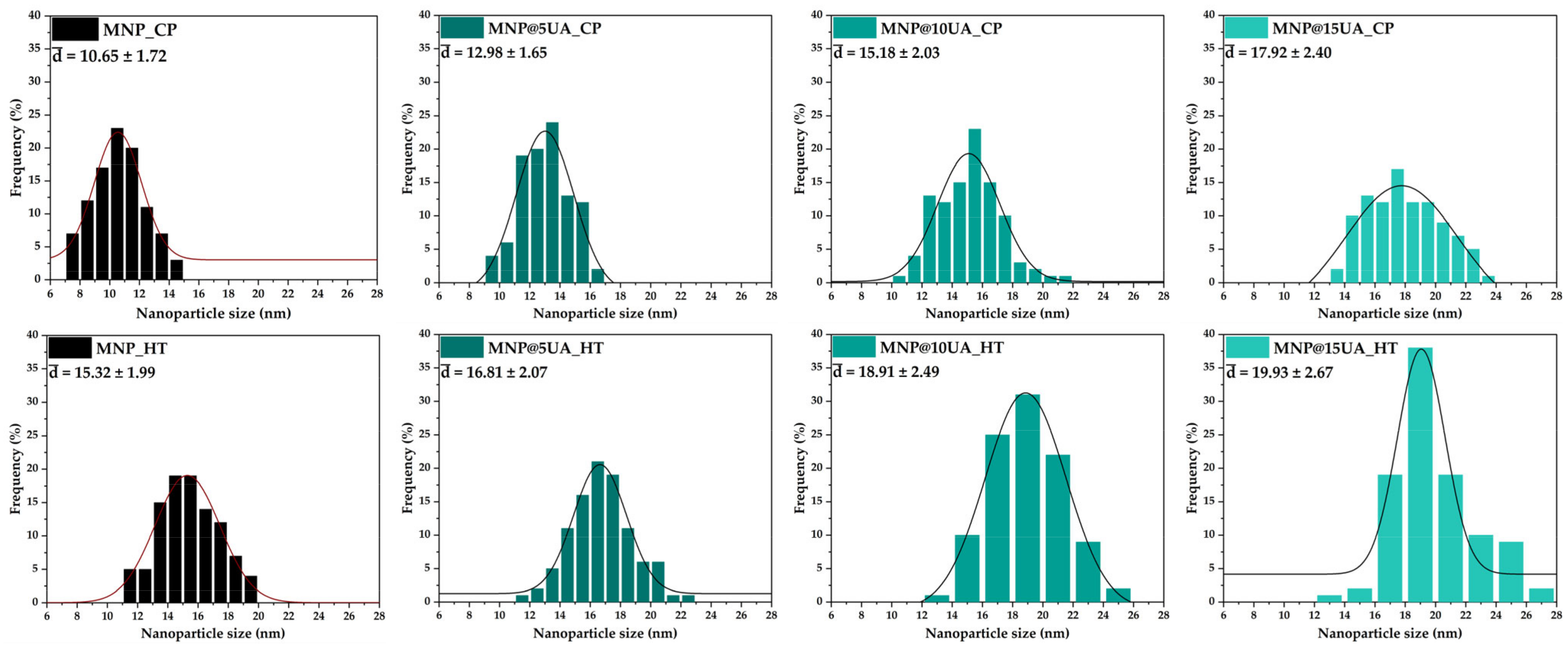
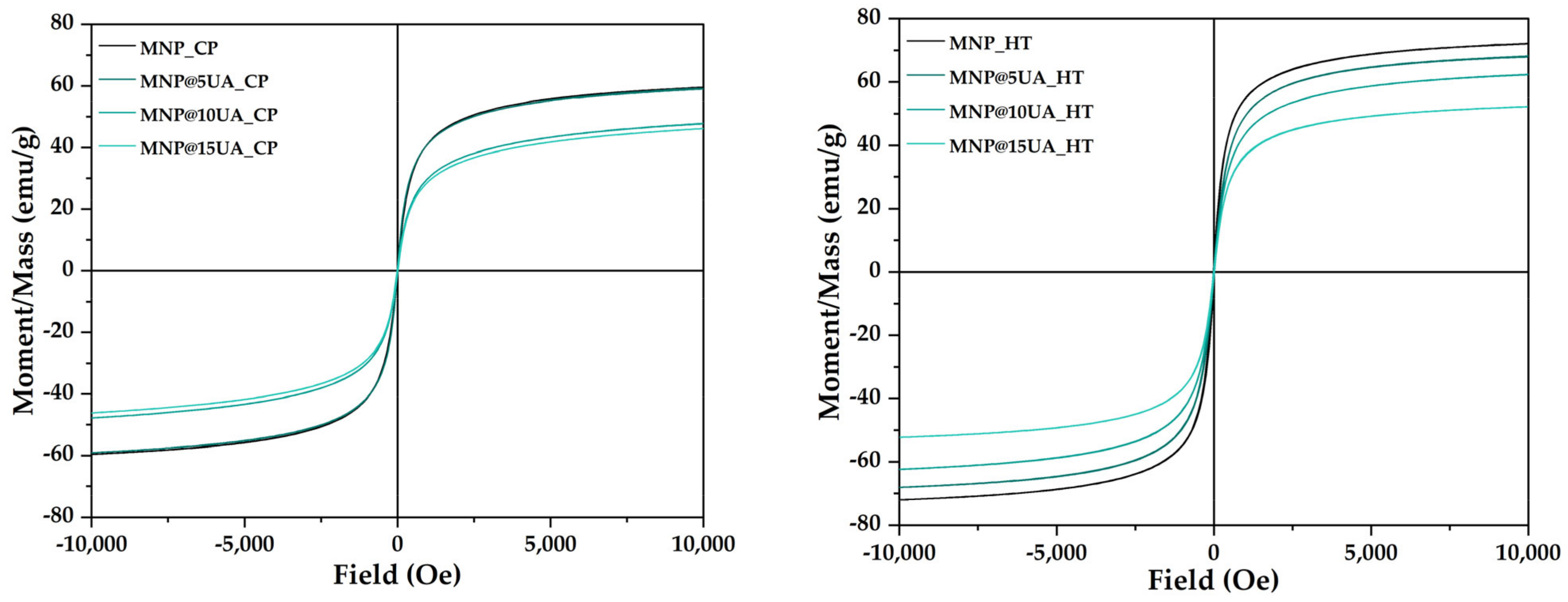
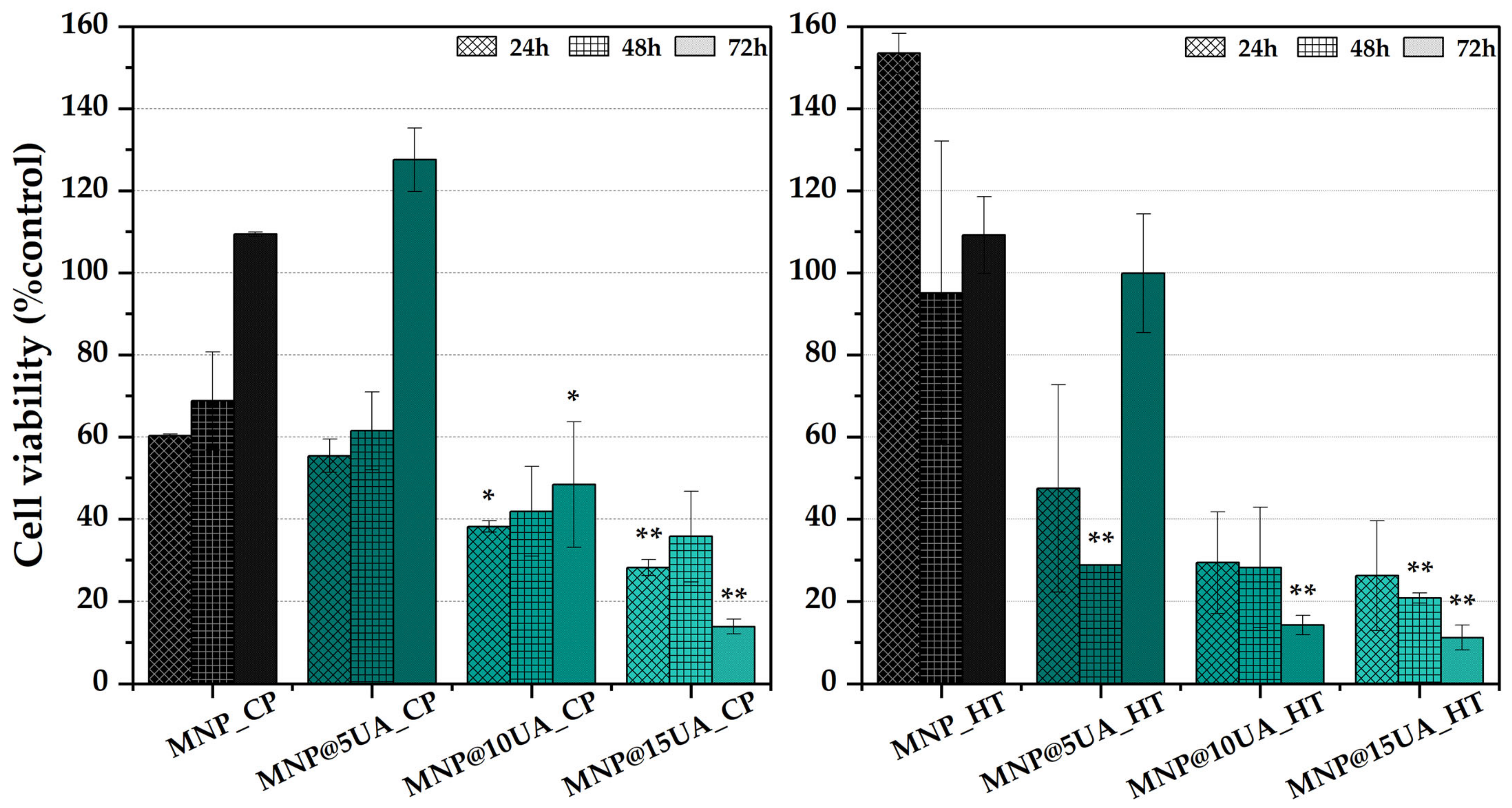
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Nanoparticle Synthesis
4.3. Morpho-Structural and Physicochemical Characterization
4.3.1. X-ray Diffraction (XRD)
4.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
4.3.3. Thermogravimetry and Differential Scanning Calorimetry (TG-DSC)
4.3.4. Dynamic Light Scattering (DLS) and Zeta Potential
4.3.5. Scanning Electron Microscopy (SEM)
4.3.6. Vibrating Sample Magnetometry (VSM)
4.4. Biological Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier drug delivery systems: Characterization, limitations, future perspectives and implementation of artificial intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Petrov, K.D.; Chubarov, A.S. Magnetite nanoparticles for biomedical applications. Encyclopedia 2022, 2, 1811–1828. [Google Scholar] [CrossRef]
- Dudchenko, N.; Pawar, S.; Perelshtein, I.; Fixler, D. Magnetite nanoparticles: Synthesis and applications in optics and nanophotonics. Materials 2022, 15, 2601. [Google Scholar] [CrossRef]
- Alromi, D.A.; Madani, S.Y.; Seifalian, A. Emerging application of magnetic nanoparticles for diagnosis and treatment of cancer. Polymers 2021, 13, 4146. [Google Scholar] [CrossRef]
- Crezee, J.; Franken, N.A.P.; Oei, A.L. Hyperthermia-based anti-cancer treatments. Cancers 2021, 13, 1240. [Google Scholar] [CrossRef]
- Jha, S.; Sharma, P.K.; Malviya, R. Hyperthermia: Role and risk factor for cancer treatment. Achieve Life Sci. 2016, 10, 161–167. [Google Scholar] [CrossRef]
- Fatima, H.; Charinpanitkul, T.; Kim, K.S. Fundamentals to apply magnetic nanoparticles for hyperthermia therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef]
- Gavilán, H.; Avugadda, S.K.; Fernández-Cabada, T.; Soni, N.; Cassani, M.; Mai, B.T.; Chantrell, R.; Pellegrino, T. Magnetic nanoparticles and clusters for magnetic hyperthermia: Optimizing their heat performance and developing combinatorial therapies to tackle cancer. Chem. Soc. Rev. 2021, 50, 11614–11667. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Medina-Cruz, D.; Saleh, B.; Vernet-Crua, A.; Ajo, A.; Roy, A.K.; Webster, T.J. Chapter 22—Drug-delivery nanocarriers for skin wound-healing applications. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 439–488. [Google Scholar] [CrossRef]
- Croce, N.; Pitaro, M.; Gallo, V.; Antonini, G. Toxicity of usnic acid: A narrative review. J. Toxicol. 2022, 2022, 8244340. [Google Scholar] [CrossRef]
- Frankos, V. Ntp Nomination for Usnic Acid and Usnea Barbata Herb; Food and Drug Administration, Division of Dietary Supplement Programs: Silver Spring, MD, USA, 2005.
- Macedo, D.C.S.; Almeida, F.J.F.; Wanderley, M.S.O.; Ferraz, M.S.; Santos, N.P.S.; López, A.M.Q.; Santos-Magalhães, N.S.; Lira-Nogueira, M.C.B. Usnic acid: From an ancient lichen derivative to promising biological and nanotechnology applications. Phytochem. Rev. 2021, 20, 609–630. [Google Scholar] [CrossRef]
- Ingólfsdóttir, K. Usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Šulc, R.; Šídlová, M.; Formáček, P.; Snop, R.; Škvára, F.; Polonská, A. A study of physicochemical properties of stockpile and ponded coal ash. Materials 2022, 15, 3653. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.; Kreyenschulte, C.; Agostini, G.; Lund, H.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. A robust iron catalyst for the selective hydrogenation of substituted (iso)quinolones. Chem. Sci. 2018, 9, 8134–8141. [Google Scholar] [CrossRef] [PubMed]
- Alpsoy, L.; Baykal, A.; Amir, M.; Ülker, Z.; Nawaz, M. SPION@APTES@FA-PEG@Usnic acid bionanodrug for cancer therapy. J. Supercond. Nov. Magn. 2018, 31, 1395–1401. [Google Scholar] [CrossRef]
- Ficai, D.; Ficai, A.; Vasile, B.S.; Ficai, M.; Oprea, O.; Guran, C.; Andronescu, E. Synthesis of rod-like magnetite by using low magnetic field. Dig. J. Nanomater. Biostruct. 2011, 6, 943–951. [Google Scholar]
- Chircov, C.; Stefan, R.-E.; Dolete, G.; Andrei, A.; Holban, A.M.; Oprea, O.-C.; Vasile, B.S.; Neacsu, I.A.; Tihauan, B. Dextran-coated iron oxide nanoparticles loaded with curcumin for antimicrobial therapies. Pharmaceutics 2022, 14, 1057. [Google Scholar] [CrossRef]
- Puiu, R.A.; Balaure, P.C.; Constantinescu, E.; Grumezescu, A.M.; Andronescu, E.; Oprea, O.C.; Vasile, B.S.; Grumezescu, V.; Negut, I.; Nica, I.C.; et al. Anti-cancer nanopowders and maple-fabricated thin films based on spions surface modified with paclitaxel loaded beta-cyclodextrin. Pharmaceutics 2021, 13, 1356. [Google Scholar] [CrossRef]
- Khan, F.; Yu, H.; Kim, Y.M. Bactericidal activity of usnic acid-chitosan nanoparticles against persister cells of biofilm-forming pathogenic bacteria. Mar. Drugs 2020, 18, 270. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.D.A.; Silva, H.; Silva Júnior, J.G.D.; Albuquerque, M.; Coelho, L.; Aires, A.L. The natural compound hydrophobic usnic acid and hydrophilic potassium usnate derivative: Applications and comparisons. Molecules 2021, 26, 5995. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, Q.; Lu, T.; Qi, W.; Zhang, H.; Wang, M.; Qi, Z.; Chen, W. Effect of phosphate on the adsorption of antibiotics onto iron oxide minerals: Comparison between tetracycline and ciprofloxacin. Ecotoxicol. Environ. Saf. 2020, 205, 111345. [Google Scholar] [CrossRef]
- Chircov, C.; Matei, M.-F.; Neacșu, I.A.; Vasile, B.S.; Oprea, O.-C.; Croitoru, A.-M.; Trușcă, R.-D.; Andronescu, E.; Sorescu, I.; Bărbuceanu, F. Iron oxide–silica core–shell nanoparticles functionalized with essential oils for antimicrobial therapies. Antibiotics 2021, 10, 1138. [Google Scholar] [CrossRef]
- Sunaryono; Taufiq, A.; Munaji; Indarto, B.; Triwikantoro; Zainuri, M.; Darminto. Magneto-elasticity in hydrogels containing Fe3O4 nanoparticles and their potential applications. In AIP Conference Proceedings; INTERNATIONAL CONFERENCE ON THEORETICAL AND APPLIED PHYSICS (LCTAP 2012) 19–20 October 2012 Central of Kalimantan, Indonesia; American Institute of Physics: College Park, MD, USA, 2013. [Google Scholar]
- Morales, I.; Costo, R.; Mille, N.; Da Silva, G.B.; Carrey, J.; Hernando, A.; De la Presa, P. High frequency hysteresis losses on γ-Fe2O3 and Fe3O4: Susceptibility as a magnetic stamp for chain formation. Nanomaterials 2018, 8, 970. [Google Scholar] [CrossRef]
- Maldonado-Camargo, L.; Unni, M.; Rinaldi, C. Magnetic characterization of iron oxide nanoparticles for biomedical applications. In Biomedical Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 47–71. [Google Scholar]
- Chircov, C.; Bîrcă, A.C.; Vasile, B.S.; Oprea, O.-C.; Huang, K.-S.; Grumezescu, A.M. Microfluidic synthesis of -nh2- and -cooh-functionalized magnetite nanoparticles. Nanomaterials 2022, 12, 3160. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the nano-drug delivery system in treatment of cardiovascular diseases. Front. Bioeng. Biotechnol. 2020, 7, 489. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M.; Vasile, B.S.; Oprea, O.; Nicoară, A.I.; Yang, C.-H.; Huang, K.-S.; Andronescu, E. Synthesis of magnetite nanoparticles through a lab-on-chip device. Materials 2021, 14, 5906. [Google Scholar] [CrossRef]
- Karimi Pasandideh, E.; Kakavandi, B.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Esrafili, A.; Rezaei Kalantary, R. Silica-coated magnetite nanoparticles core-shell spheres (Fe3O4@SiO2) for natural organic matter removal. J. Environ. Health Sci. Eng. 2016, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, M.; Vijayalakshmi, U. Fabrication of Fe3O4-silica core-shell magnetic nano-particles and its characterization for biomedical applications. Mater. Today Proc. 2019, 9, 371–379. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Karaagac, O.; Köçkar, H. Improvement of the saturation magnetization of peg coated superparamagnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2022, 551, 169140. [Google Scholar] [CrossRef]
- Srivastava, A.; Raghuwanshi, R. 10—Landscape of natural product diversity in land-plants as source for anticancer molecules. In Evolutionary Diversity as a Source for Anticancer Molecules; Srivastava, A.K., Kannaujiya, V.K., Singh, R.K., Singh, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 233–254. [Google Scholar] [CrossRef]
- Lauterwein, M.; Oethinger, M.; Belsner, K.; Peters, T.; Marre, R. In vitro activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (−)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob. Agents Chemother. 1995, 39, 2541–2543. [Google Scholar] [CrossRef]
- Tozatti, M.G.; Ferreira, D.S.; Flauzino, L.G.B.; da Silva Moraes, T.; Martins, C.H.; Groppo, M.; Silva, M.L.A.e.; Januario, A.H.; Pauletti, P.M.; Cunha, W.R. Activity of the lichen usnea steineri and its major metabolites against gram–positive, multidrug–resistant bacteria. Nat. Prod. Commun. 2016, 11, 1934578X1601100419. [Google Scholar] [CrossRef]
- Francolini, I.; Piozzi, A.; Donelli, G. Usnic acid: Potential role in management of wound infections. Adv. Microbiol. Infect. Dis. Public Health 2019, 13, 31–41. [Google Scholar]
- Ingólfsdóttir, K.n.; Chung, G.A.; Skúlason, V.G.; Gissurarson, S.R.; Vilhelmsdóttir, M. Antimycobacterial activity of lichen metabolites in vitro. Eur. J. Pharm. Sci. 1998, 6, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Ramos, D.F.; Almeida da Silva, P.E. Antimycobacterial activity of usnic acid against resistant and susceptible strains of mycobacterium tuberculosis and non-tuberculous mycobacteria. Pharm. Biol. 2010, 48, 260–263. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, A.M.; Iordache, F. Chapter 4—Magnetite nanostructures: Trends in anti-infectious therapy. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Rai, M., Kon, K., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 51–67. [Google Scholar] [CrossRef]
- Holban, A.; Grumezescu, A.; Andronescu, E.; Grumezescu, V.; Chifiriuc, M.; Rǎdulescu, R. Magnetite—Usnic acid nanostructured bioactive material with antimicrobial activity. Rev. Romana Mater. Rom. J. Mater. 2013, 43, 402–407. [Google Scholar]
- Yamamoto, Y.; Miura, Y.; Kinoshita, Y.; Higuchi, M.; Yamada, Y.; Murakami, A.; Ohigashi, H.; Koshimizu, K. Screening of tissue cultures and thalli of lichens and some of their active constituents for inhibition of tumor promoter-induced Epstein-Barr virus activation. Chem. Pharm. Bull. 1995, 43, 1388–1390. [Google Scholar] [CrossRef] [PubMed]
- Scirpa, P.; Scambia, G.; Masciullo, V.; Battaglia, F.; Foti, E.; Lopez, R.; Villa, P.; Malecore, M.; Mancuso, S. A zinc sulfate and usnic acid preparation used as post-surgical adjuvant therapy in genital lesions by human papillomavirus. Minerva Ginecol. 1999, 51, 255–260. [Google Scholar] [PubMed]
- Perry, N.B.; Benn, M.H.; Brennan, N.J.; Burgess, E.J.; Ellis, G.; Galloway, D.J.; Lorimer, S.D.; Tangney, R.S. Antimicrobial, antiviral and cytotoxic activity of New Zealand lichens. Lichenologist 1999, 31, 627–636. [Google Scholar] [CrossRef]
- Sokolov, D.N.; Zarubaev, V.V.; Shtro, A.A.; Polovinka, M.P.; Luzina, O.A.; Komarova, N.I.; Salakhutdinov, N.F.; Kiselev, O.I. Anti-viral activity of (−)-and (+)-usnic acids and their derivatives against influenza virus a (H1N1) 2009. Bioorg. Med. Chem. Lett. 2012, 22, 7060–7064. [Google Scholar] [CrossRef]
- De Battisti, F.; Codolo, R.; Nicolato, A. Attività di una associazione antibatterico-antimicotico sulla sintomatologia della tinea pedis in un gruppo di sportivi. Chronic Dermat. 1991, 3, 375–380. [Google Scholar]
- Pires, R.H.; Lucarini, R.; Mendes-Giannini, M.J.S. Effect of usnic acid on Candida orthopsilosis and C. parapsilosis. Antimicrob. Agents Chemother. 2012, 56, 595–597. [Google Scholar] [CrossRef]
- Yu, X.; Guo, Q.; Su, G.; Yang, A.; Hu, Z.; Qu, C.; Wan, Z.; Li, R.; Tu, P.; Chai, X. Usnic acid derivatives with cytotoxic and antifungal activities from the lichen usnea longissima. J. Nat. Prod. 2016, 79, 1373–1380. [Google Scholar] [CrossRef]
- Fournet, A.; Ferreira, M.-E.; de Arias, A.R.; de Ortiz, S.T.; Inchausti, A.; Yalaff, G.; Quilhot, W.; Fernandez, E.; Hidalgo, M.E. Activity of compounds isolated from chilean lichens against experimental cutaneous leishmaniasis. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1997, 116, 51–54. [Google Scholar] [CrossRef]
- Bačkorová, M.; Bačkor, M.; Mikeš, J.; Jendželovský, R.; Fedoročko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. In Vitro 2011, 25, 37–44. [Google Scholar] [CrossRef]
- Zugic, A.; Tadic, V.; Savic, S. Nano- and microcarriers as drug delivery systems for usnic acid: Review of literature. Pharmaceutics 2020, 12, 156. [Google Scholar] [CrossRef]
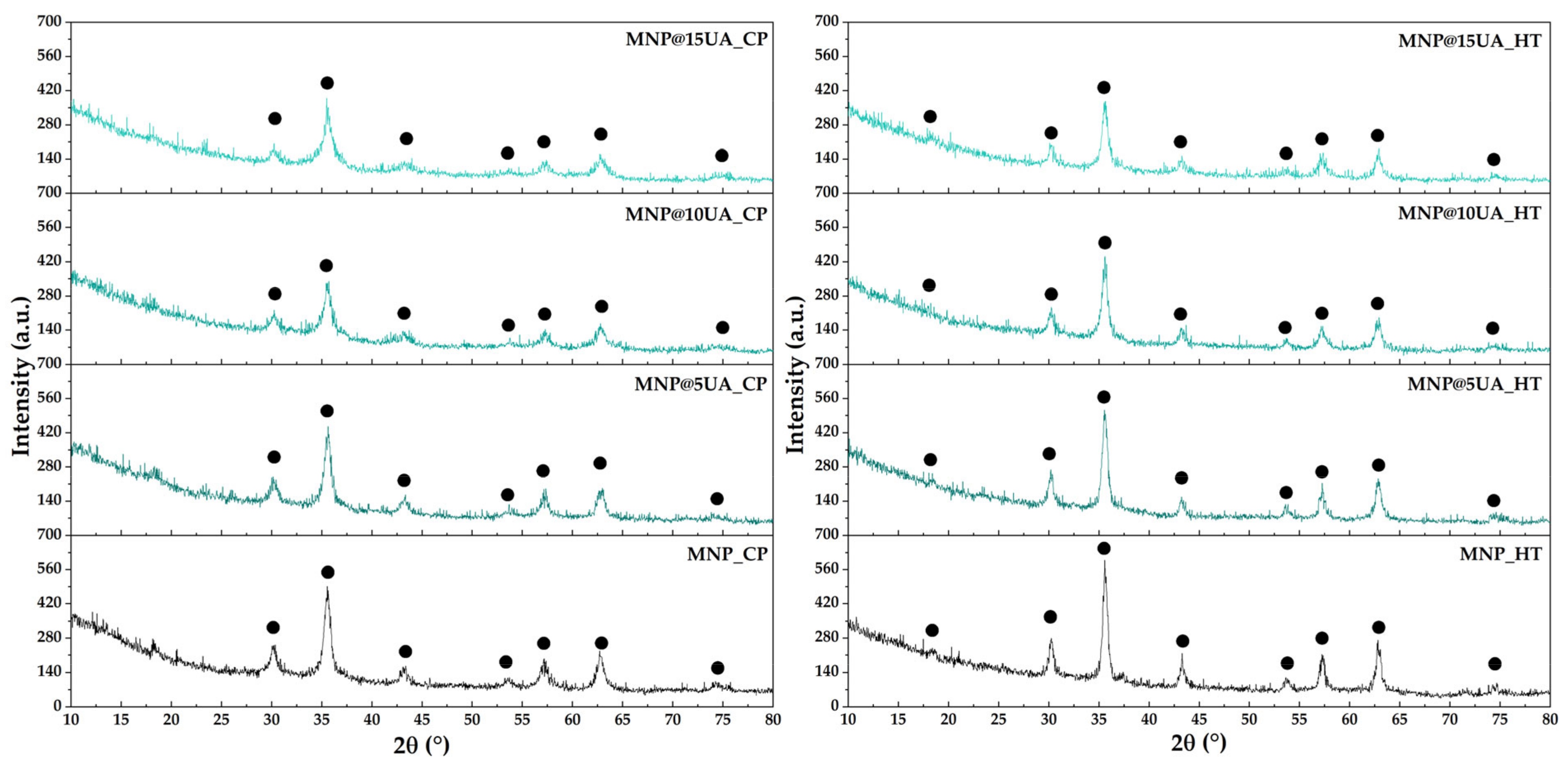
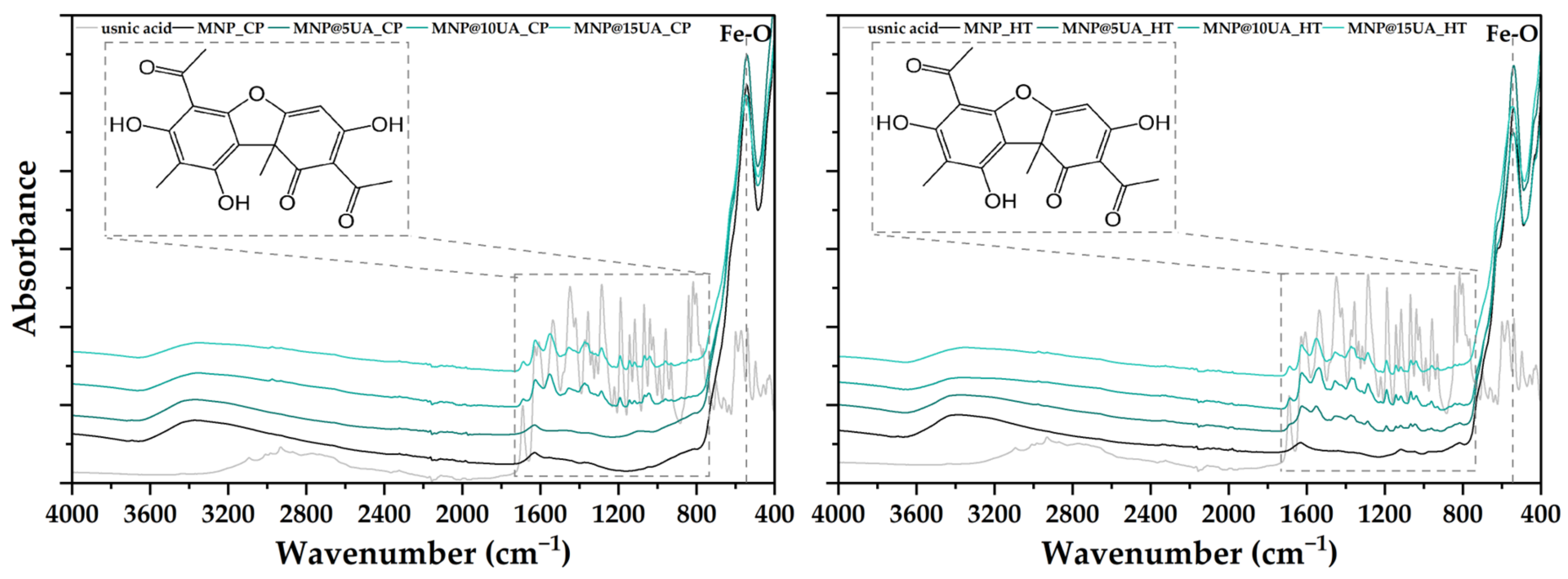
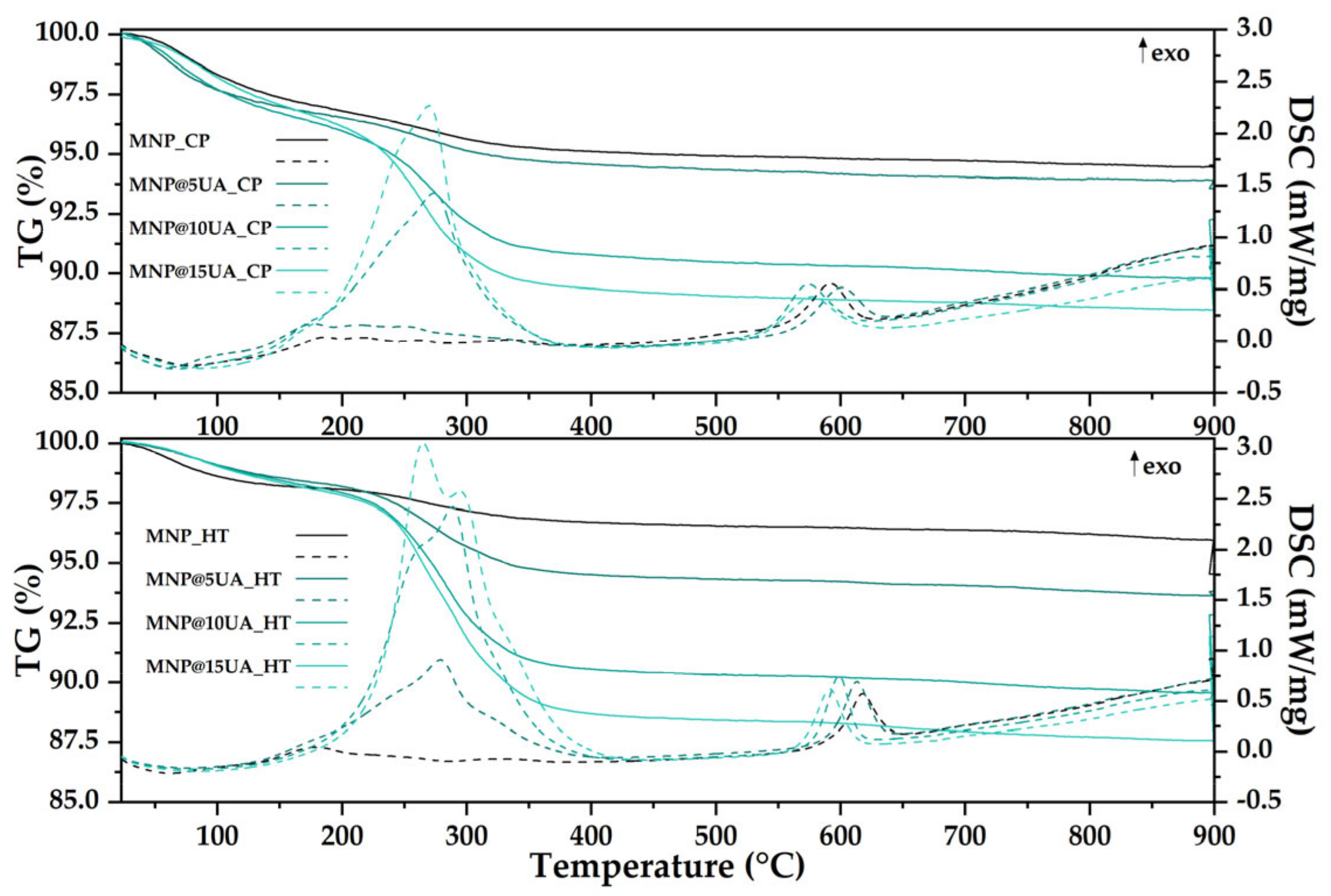
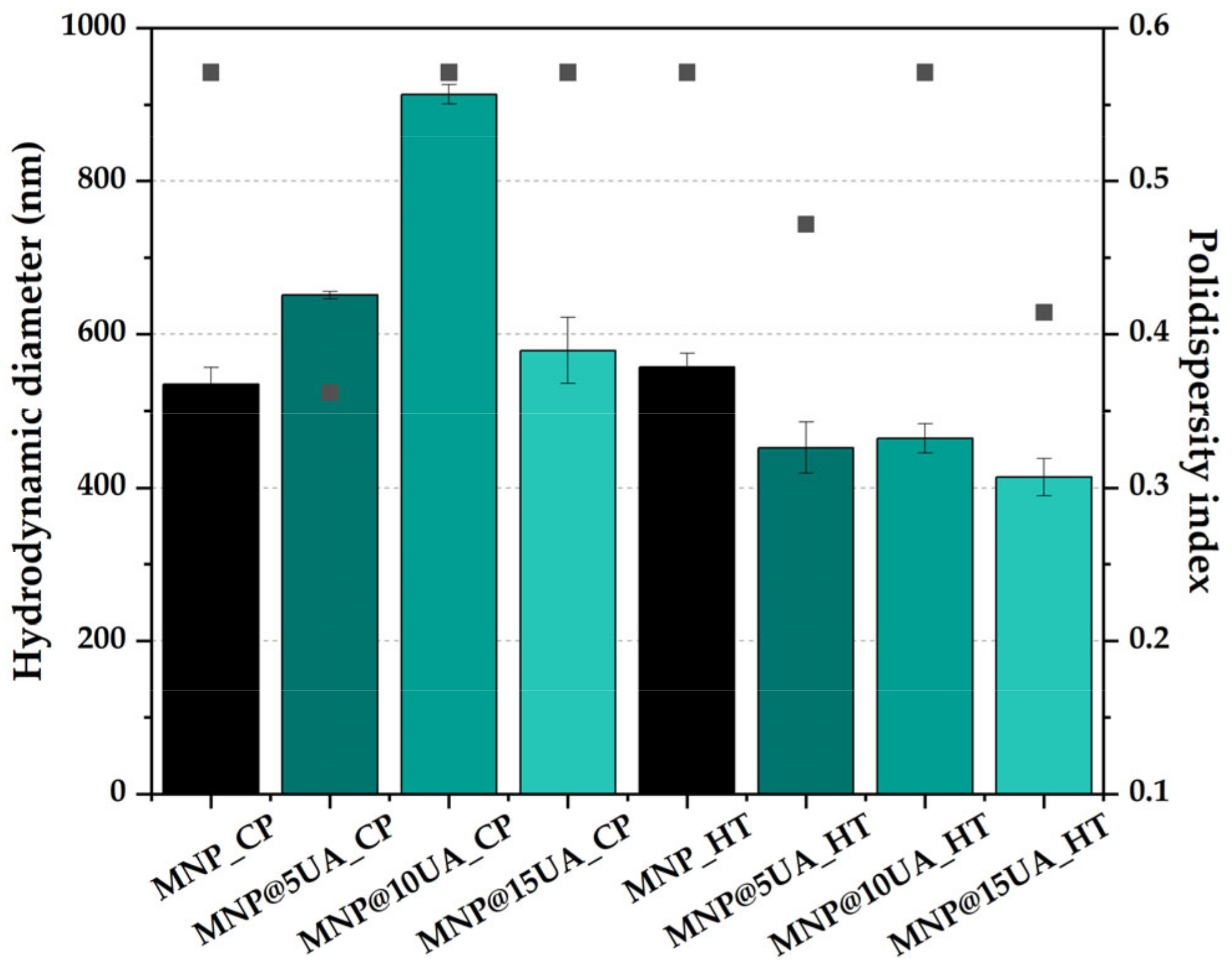
| Sample | Unit Cell Parameters | Average Crystallite Size ± Standard Deviation (SD) [nm] | |
|---|---|---|---|
| a = b = c [Å] | α = β = γ [°] | ||
| MNP_CP | 8.36 | 90 | 8.13 ± 0.17 |
| MNP@5UA_CP | 8.36 | 90 | 7.66 ± 0.19 |
| MNP@10UA_CP | 8.35 | 90 | 5.80 ± 0.23 |
| MNP@15UA_CP | 8.34 | 90 | 5.33 ± 0.26 |
| MNP_HT | 8.35 | 90 | 12.42 ± 0.80 |
| MNP@5UA_HT | 8.35 | 90 | 10.88 ± 0.46 |
| MNP@10UA_HT | 8.35 | 90 | 9.20 ± 0.59 |
| MNP@15UA_HT | 8.35 | 90 | 9.45 ± 0.31 |
| Sample | Mass Loss RT-200 °C (%) | Endothermic Effect (°C) | Mass Loss 200–400 °C (%) | Exothermic Effect (°C) | Residual Mass (%) | Estimated Load (%) |
|---|---|---|---|---|---|---|
| MNP_CP | 3.20 | 77.1 | 1.70 | 592.2 | 94.44 | - |
| MNP_5UA_CP | 3.54 | 61.9 | 1.95 | 601.1 | 93.89 | 0.58 |
| MNP_10UA_CP | 4.10 | 67.0 | 5.18 | 574.4 | 89.79 | 4.92 |
| MNP_15UA_CP | 3.84 | 86.4 | 6.79 | 577.1 | 88.47 | 6.32 |
| MNP_HT | 1.92 | 63.1 | 1.37 | 617.8 | 95.94 | - |
| MNP_5UA_HT | 1.82 | 75.4 | 3.69 | 613.2 | 93.61 | 2.43 |
| MNP_10UA_HT | 2.07 | 82.5 | 7.37 | 598.8 | 89.54 | 6.67 |
| MNP_15UA_HT | 2.18 | 84.4 | 9.13 | 591.6 | 87.56 | 8.73 |
| Sample | Ms (emu/g) | Mr (emu/g) | Hc (Oe) |
|---|---|---|---|
| MNP_CP | 59.55 | 0.54 | 4.97 |
| MNP@5UA_CP | 59.14 | 0.53 | 4.26 |
| MNP@10UA_CP | 47.79 | 0.86 | 11.02 |
| MNP@15UA_CP | 46.12 | 0.71 | 9.22 |
| MNP_HT | 72.05 | 2.97 | 19.09 |
| MNP@5UA_HT | 68.08 | 1.60 | 13.80 |
| MNP@10UA_HT | 62.37 | 1.23 | 11.76 |
| MNP@15UA_HT | 52.20 | 0.65 | 7.04 |
| Sample | Synthesis Method | UA Concentration (wt. %) |
|---|---|---|
| MNP_CP | co-precipitation | 0 |
| MNP@5UA_CP | 5 | |
| MNP@10UA_CP | 10 | |
| MNP@15UA_CP | 15 | |
| MNP_HT | microwave-assisted hydrothermal method | 0 |
| MNP@5UA_HT | 5 | |
| MNP@10UA_HT | 10 | |
| MNP@15UA_HT | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chircov, C.; Bîrcă, A.C.; Dănciulescu, L.A.; Neacșu, I.A.; Oprea, O.-C.; Trușcă, R.-D.; Andronescu, E. Usnic Acid-Loaded Magnetite Nanoparticles—A Comparative Study between Synthesis Methods. Molecules 2023, 28, 5198. https://doi.org/10.3390/molecules28135198
Chircov C, Bîrcă AC, Dănciulescu LA, Neacșu IA, Oprea O-C, Trușcă R-D, Andronescu E. Usnic Acid-Loaded Magnetite Nanoparticles—A Comparative Study between Synthesis Methods. Molecules. 2023; 28(13):5198. https://doi.org/10.3390/molecules28135198
Chicago/Turabian StyleChircov, Cristina, Alexandra Cătălina Bîrcă, Lorena Alexandra Dănciulescu, Ionela Andreea Neacșu, Ovidiu-Cristian Oprea, Roxana-Doina Trușcă, and Ecaterina Andronescu. 2023. "Usnic Acid-Loaded Magnetite Nanoparticles—A Comparative Study between Synthesis Methods" Molecules 28, no. 13: 5198. https://doi.org/10.3390/molecules28135198
APA StyleChircov, C., Bîrcă, A. C., Dănciulescu, L. A., Neacșu, I. A., Oprea, O.-C., Trușcă, R.-D., & Andronescu, E. (2023). Usnic Acid-Loaded Magnetite Nanoparticles—A Comparative Study between Synthesis Methods. Molecules, 28(13), 5198. https://doi.org/10.3390/molecules28135198








