Cyclopentenylcytosine (CPE-C): In Vitro and In Vivo Evaluation as an Antiviral against Adenoviral Ocular Infections
Abstract
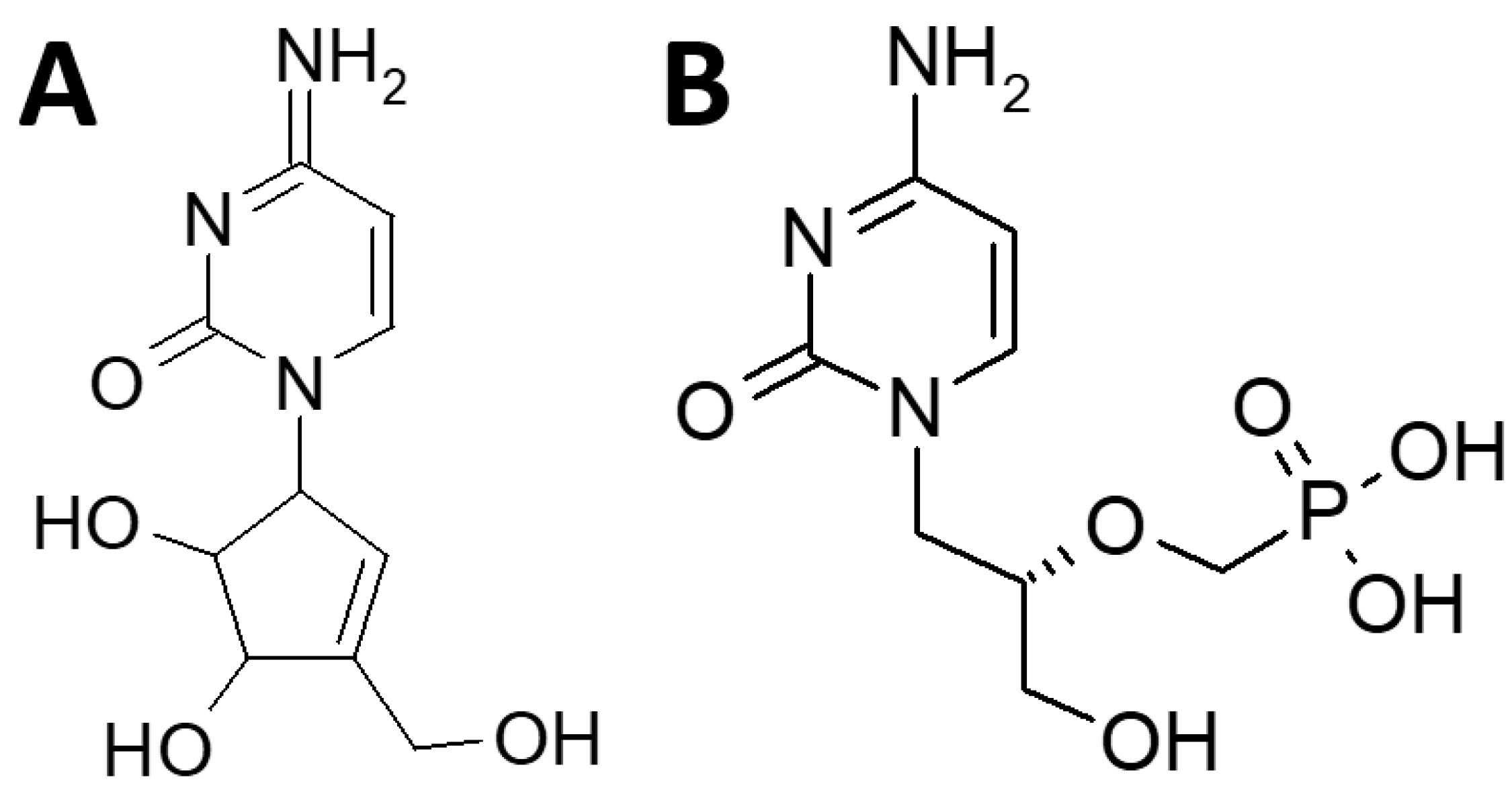
1. Introduction
2. Results
2.1. In Vitro Antiviral Testing (EC50 Determinations)
2.2. In Vivo Ocular Toxicity in a Draize Rabbit Model
2.3. In Vivo Antiviral Efficacy in Ad5/NZW Rabbit Ocular Replication Model
3. Discussion
4. Materials and Methods
4.1. Test Drugs
4.2. Adenovirus Isolates and Cells
4.3. Animals
4.4. In Vitro Antiviral Testing (EC50 Determinations)
4.5. Ocular Toxicity in Draize Rabbit Model
4.6. Ad5/NZW Rabbit Ocular Replication Model for In Vivo Antiviral Efficacy Testing
4.7. Determination of Viral Titers Using the Viral Plaque Assay
4.8. Statistical Analysis of In Vivo Efficacy Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gordon, Y.J.; Aoki, K.; Kinchington, P.R. Adenovirus keratoconjunctivitis. In Ocular Infection & Immunity; Pepose, J.S., Holland, G.N., Wilhelmus, K.R., Eds.; Mosby: St Louis, MO, USA, 1996; pp. 877–894. [Google Scholar]
- Kaneko, H.; Maruko, I.; Iida, T.; Ohguchi, T.; Aoki, K.; Ohno, S.; Suzutani, T. The possibility of human adenovirus detection from the conjunctiva in asymptomatic cases during nosocomial infection. Cornea 2008, 27, 527–530. [Google Scholar] [CrossRef]
- Lenaerts, L.; De Clercq, E.; Naesens, L. Clinical features and treatment of adenovirus infections. Rev. Med. Virol. 2008, 18, 357–374. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Gordon, Y.J. Update on antiviral treatment of adenoviral ocular infections. Am. J. Ophthalmol. 2008, 146, 635–637. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.; Araullo-Cruz, T.; De Clercq, E. Pretreatment with topical 0.1% (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine inhibits adenovirus type 5 replication in the New Zealand rabbit ocular model. Cornea 1992, 11, 529–533. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.G.; Araullo-Cruz, T. Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular replication model. Investig. Ophthalmol. Vis. Sci. 1994, 35, 4135–4143. [Google Scholar]
- Romanowski, E.G.; Gordon, Y.J. Efficacy of topical cidofovir on multiple adenoviral serotypes in the New Zealand rabbit ocular model. Investig. Ophthalmol. Vis. Sci. 2000, 41, 460–463. [Google Scholar]
- Romanowski, E.G.; Yates, K.A.; Gordon, Y.J. Antiviral prophylaxis with twice daily topical cidofovir protects against challenge in the adenovirus type 5/New Zealand rabbit ocular model. Antivir. Res. 2001, 52, 275–280. [Google Scholar] [CrossRef]
- Romanowski, E.G. Is there an anti-adenoviral drug on the horizon? Expert Rev. Ophthalmol. 2013, 8, 427–435. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Gordon, Y.J.; Araullo-Cruz, T.; Yates, K.A.; Kinchington, P.R. The antiviral resistance and replication of cidofovir-resistant adenovirus variants in the New Zealand White rabbit ocular model. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1812–1815. [Google Scholar]
- Hillenkamp, J.; Reinhard, T.; Ross, R.S.; Böhringeret, D.; Cartsburg, O.; Roggendorf, M.; De Clercq, E.; Godehardt, E.; Sundmacher, R. Topical treatment of acute adenoviral keratoconjunctivitis with 0.2% cidofovir and 1% cyclosporine: A controlled clinical pilot study. Arch. Ophthalmol. 2001, 119, 1487–1491. [Google Scholar] [CrossRef]
- Hillenkamp, J.; Reinhard, T.; Ross, R.S.; Böhringeret, D.; Cartsburg, O.; Roggendorf, M.; De Clercq, E.; Godehardt, E.; Sundmacher, R. The effects of cidofovir 1% with and without cyclosporin a 1% as a topical treatment of acute adenoviral keratoconjunctivitis: A controlled clinical pilot study. Ophthalmology 2002, 109, 845–850. [Google Scholar] [CrossRef]
- Mentel, R.; Kinder, M.; Wegner, U.; von Janta-Lipinski, M.; Matthes, E. Inhibitory activity of 3’-fluoro-2’ deoxythymidine and related nucleoside analogues against adenoviruses in vitro. Antivir. Res. 1997, 34, 113–119. [Google Scholar] [CrossRef]
- Mentel, R.; Wegner, U. Evaluation of the efficacy of 2′,3′-dideoxycytidine against adenovirus infection in a mouse pneumonia model. Antivir. Res. 2000, 47, 79–87. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Yates, K.A.; Gordon, Y.J. The in vitro and in vivo evaluation of ddC as a topical antiviral for ocular adenovirus infections. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5295–5299. [Google Scholar] [CrossRef]
- Nwanegbo, E.C.; Romanowski, E.G.; Gordon, Y.J.; Gambotto, A. Efficacy of topical immunoglobulins against experimental adenoviral ocular infection. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4171–4176. [Google Scholar] [CrossRef]
- Epstein, S.P.; Pashinsky, Y.Y.; Gershon, D.; Winicov, I.; Srivilasa, C.; Kristic, K.J.; Asbell, P.A. Efficacy of topical cobalt chelate CTC-96 against adenovirus in a cell culture model and against adenovirus keratoconjunctivitis in a rabbit model. BMC Ophthalmol. 2006, 6, 22. [Google Scholar] [CrossRef]
- Teuchner, B.; Nagl, M.; Schidlbauer, A.; Ishiko, H.; Dragosits, E.; Ulmer, H.; Aoki, K.; Ohno, S.; Mizuki, N.; Gottardi, W.; et al. Tolerability and efficacy of N-chlorotaurine in epidemic keratoconjunctivitis—A double-blind, randomized, phase-2 clinical trial. J. Ocul. Pharmacol. Ther. 2005, 21, 157–165. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Yates, K.A.; Teuchner, B.; Nagl, M.; Irschick, E.U.; Gordon, Y.J. N-chlorotaurine is an effective antiviral agent against adenovirus in vitro and in the Ad5/NZW rabbit ocular model. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2021–2026. [Google Scholar] [CrossRef]
- Isenberg, S.J.; Apt, L.; Valenton, M.; Del Signore, M.; Cubillan, L.; Labrador, M.A.; Chan, P.; Berman, N.G. A controlled trial of povidone-iodine to treat infectious conjunctivitis in children. Am. J. Ophthalmol. 2002, 134, 681–688. [Google Scholar] [CrossRef]
- Trinavarat, A.; Atchaneeyasakul, L.O. Treatment of epidemic keratoconjunctivitis with 2% povidone-iodine: A pilot study. J. Ocul. Pharmacol. Ther. 2012, 28, 53–58. [Google Scholar] [CrossRef]
- Pelletier, J.S.; Stewart, K.; Trattler, W.; Ritterband, D.C.; Braverman, S.; Samson, C.M.; Liang, B.; Capriotti, J.A. A combination povidone-iodine 0.4%/dexamethasone 0.1% ophthalmic suspension in the treatment of adenoviral conjunctivitis. Adv. Ther. 2009, 26, 776–783. [Google Scholar] [CrossRef]
- Clement, C.; Capriotti, J.A.; Kumar, M.; Hobden, J.A.; Foster, T.P.; Bhattacharjee, P.S.; Thompson, H.W.; Mahmud, R.; Liang, B.; Hill, J.M. Clinical and antiviral efficacy of an ophthalmic formulation of dexamethasone povidone-iodine in a rabbit model of adenoviral keratoconjunctivitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 339–344. [Google Scholar] [CrossRef]
- Yoon, J.; Jekle, A.; Najafi, R.; Ruado, F.; Zuck, M.; Khosrovi, B.; Memarzadeh, B.; Debabov, D.; Wang, L.; Anderson, M. Virucidal mechanism of action of NVC-422, a novel antimicrobial drug for the treatment of adenoviral conjunctivitis. Antivir. Res. 2011, 92, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, A.Y.; Akileswaran, L.; Stroman, D.; Najafi-Tagol, K.; Kleiboeker, S.; Chodosh, J.; Margaret, A.; Wald, A.; Van Gelder, R.N. Determinants of Outcomes of Adenoviral Keratoconjunctivitis. Ophthalmology 2018, 125, 1344–1353. [Google Scholar] [CrossRef]
- Yabiku, S.T.; Yabiku, M.M.; Bottós, K.M.; Araújoet, A.L.; de Freitas, D.; Belfort Jr., R. Ganciclovir 0.15% ophthalmic gel in the treatment of adenovirus keratoconjunctivitis. Arq. Bras. Oftalmol. 2011, 74, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Ying, B.; Tollefson, A.E.; Spencer, J.F.; Balakrishan, L.; Dewhurst, S.; Capella, C.; Buller, R.M.L.; Toth, K.; Wold, W.S.M. Ganciclovir inhibits human adenovirus replication and pathogenicity in permissive immunosuppressed Syrian hamsters. Antimicrob. Agents Chemother. 2014, 58, 7171–7181. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Hussein, I.T.M.; Cardinale, S.C.; Butler, M.M.; Morin, L.R.; Bowlin, T.L.; Yates, K.A.; Shanks, R.M.Q.; Kowalski, R.P. Filociclovir is an active antiviral agent against ocular adenovirus isolates in vitro and in the Ad5/NZW rabbit ocular model. Pharmaceuticals 2021, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, E.G.; Yates, K.A.; Paull, J.R.A.; Heery, G.P.; Shanks, R.M.Q. Topical astodrimer sodium, a non-toxic polyanionic dendrimer, demonstrates antiviral activity in an experimental ocular adenovirus infection model. Molecules 2021, 26, 3419. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Yates, K.A.; Daniels, E.J.; Strem, B.M.; Romanowski, J.E.; Kowalski, R.P. Ranpirnase (OKG-0301), a novel ribonuclease, demonstrates antiviral activity against adenovirus in the Ad5/NZW rabbit ocular replication model. Pathogens 2022, 11, 1485. [Google Scholar] [CrossRef]
- Marquez, V.E.; Lim, M.I.; Treanor, S.P.; Plowman, J.; Priest, M.A.; Markovac, A.; Khan, M.S.; Kaskar, B.; Driscoll, J.S. Cyclopentenylcytosine. A carbocyclic nucleoside with antitumor and antiviral properties. J. Med. Chem. 1988, 31, 1687–1694. [Google Scholar] [CrossRef]
- De Clercq, E.; Murase, J.; Marquez, V.E. Broad-spectrum antiviral and cytocidal activity of cyclopentenylcytosine, a carbocyclic nucleoside targeted at CTP synthetase. Biochem. Pharmacol. 1991, 41, 1821–1829. [Google Scholar] [CrossRef]
- Seley-Radtke, K.L.; Yates, M.K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 2018, 154, 66–86. [Google Scholar] [CrossRef]
- Naesens, L.; Snoeck, R.; Andrei, G.; Balzarini, J.; Neyts, J.; DeClercq, E. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: A review of their pharmacology and clinical potential in the treatment of viral infections. Antivir. Chem. Chemother. 1997, 8, 1–23. [Google Scholar] [CrossRef]
- Politi, P.M.; Xie, F.; Dahut, W.; Ford, H., Jr.; Kelley, J.A.; Bastian, A.; Setser, A.; Allegra, C.J.; Chen, A.P.; Hamilton, J.M. Phase I clinical trial of continuous infusion cyclopentenyl cytosine. Cancer Chemother. Pharmacol. 1995, 36, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Morrey, J.D.; Smee, D.F.; Sidwell, R.W.; Tseng, C. Identification of active antiviral compounds against a New York isolate of West Nile virus. Antivir. Res. 2002, 55, 107–116. [Google Scholar] [CrossRef]
- De Clercq, E. Another ten stories in antiviral drug discovery (part C): “Old” and “new” antivirals, strategies, and perspectives. Med. Res. Rev. 2009, 29, 611–645. [Google Scholar] [CrossRef]
- Romanowski, E.G.; Araullo-Cruz, T.; Gordon, Y.J. Multiple adenoviral serotypes demonstrate host range extension in the New Zealand rabbit ocular model. Investig. Ophthalmol. Vis. Sci. 1998, 39, 532–536. [Google Scholar]
- Draize, J.H.; Woodward, G.; Calvery, H.O. Methods for the study of irritation and toxicity of articles applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Kay, J.H.; Calandra, J.C. Interpretation of eye irritation tests. J. Soc. Cos. Chem. 1962, 13, 281–289. [Google Scholar]
- Zhou, X.; Robinson, C.M.; Rajaiya, J.; Dehghan, S.; Seto, D.; Jones, M.S.; Dyer, D.W.; Chodosh, J. Analysis of human adenovirus type 19 associated with epidemic keratoconjunctivitis and its reclassification as adenovirus type 64. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2804–2811. [Google Scholar] [CrossRef]

| Mean and Standard Deviation EC50 Concentrations of CPE-C and Cidofovir | |||||
|---|---|---|---|---|---|
| CPE-C | Cidofovir | ||||
| HAdV Type | EC50 [μg/mL] | EC50 [μM] | EC50 [μg/mL] | EC50 [μM] | p * |
| HAdV1 | 0.051 ± 0.007 | 0.214 ± 0.027 | 5.268 ± 0.588 | 18.87 ± 2.110 | 0.004 |
| HAdV2 | 0.047 ± 0.024 | 0.198 ± 0.101 | 5.106 ± 0.410 | 18.29 ± 1.469 | 0.002 |
| HAdV3 | 0.040 ± 0.004 | 0.165 ± 0.016 | 4.119 ± 0.910 | 14.75 ± 3.260 | 0.016 |
| HAdV4 | 0.032 ± 0.026 | 0.134 ± 0.110 | 4.605 ± 0.925 | 16.49 ± 3.310 | 0.013 |
| HAdV5 | 0.030 ± 0.033 | 0.126 ± 0.138 | 4.921 ± 0.486 | 17.63 ± 1.740 | 0.045 |
| HAdV7a | 0.055 ± 0.026 | 0.194 ± 0.057 | 5.367 ± 0.137 | 19.22 ± 0.491 | 0.012 |
| HAdV8 | 0.059 ± 0.018 | 0.246 ± 0.076 | 0.427 ± 0.173 | 1.556 ± 0.592 | 0.060 |
| HAdV19/64 | 0.047 ± 0.028 | 0.196 ± 0.118 | 4.898 ± 0.517 | 17.54 ± 1.850 | 0.004 |
| HAdV37 | 0.048 ± 0.008 | 0.202 ± 0.037 | 4.790 ± 0.678 | 17.16 ± 2.430 | 0.007 |
| Maximum Mean Total Score [40] | |||
|---|---|---|---|
| Group | Day 2 | Day 3 | Day 4 |
| 3% CPE-C | 0.0—N | 2.0—PN | 2.0—PN |
| 2% CPE-C | 0.0—N | 0.0—N | 0.0—N |
| 1% CPE-C | 0.0—N | 0.0—N | 0.0—N |
| 0.5% CPE-C | 1.0—PN | 1.0—PN | 1.0—PN |
| 3% CPE-C * | 1.0—PN | 1.0—PN | 1.0—PN |
| Saline Control | 3% CPE-C | 3% CPE-C | 0.5% Cidofovir | |
|---|---|---|---|---|
| 4X/Day | 4X/Day | 2X/Day | 2X/Day | |
| HAdV5-Positive Eye Cultures/Total | ||||
| Overall (Days 1–14) | 97/160 (61%) | 24/160 (15%) a | 31/160 (19%) a | 27/160 (17%) a |
| Early Phase (Days 1–5) | 70/80 (88%) | 18/23 (21%) a | 29/80 (36%) a | 26/80 (33%) a |
| Late Phase (Days 7–14) | 27/80 (34%) | 6/80 (8%) a | 2/80 (3%) a | 1/80 (1%) a |
| Mean ± Sd Combined HAdV5 Ocular Titers (Log10 PFU/mL) | ||||
| Early Phase (Days 1–5) (n = 80) | 1.0 ± 3.0 × 102 | 1.6 ± 4.8 × 101 b | 3.5 ± 18.1 × 101 b | 6.7 ± 12.7 × 100 b |
| Late Phase (Days 7–14) (n = 80) | 2.2 ± 9.2 × 101 | 0.9 ± 4.4 × 100 c | 3.6 ± 25.2 × 100 c | 0.3 ± 2.2 × 100 c |
| Duration of HAdV5 Shedding (Days) | ||||
| Mean ± Sd (n = 20) | 8.1 ± 3.4 | 1.3 ± 1.6 d | 2.5 ± 2.3 d | 2.0 ± 1.8 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanowski, E.G.; Yates, K.A.; Gordon, Y.J. Cyclopentenylcytosine (CPE-C): In Vitro and In Vivo Evaluation as an Antiviral against Adenoviral Ocular Infections. Molecules 2023, 28, 5078. https://doi.org/10.3390/molecules28135078
Romanowski EG, Yates KA, Gordon YJ. Cyclopentenylcytosine (CPE-C): In Vitro and In Vivo Evaluation as an Antiviral against Adenoviral Ocular Infections. Molecules. 2023; 28(13):5078. https://doi.org/10.3390/molecules28135078
Chicago/Turabian StyleRomanowski, Eric G., Kathleen A. Yates, and Y. Jerold Gordon. 2023. "Cyclopentenylcytosine (CPE-C): In Vitro and In Vivo Evaluation as an Antiviral against Adenoviral Ocular Infections" Molecules 28, no. 13: 5078. https://doi.org/10.3390/molecules28135078
APA StyleRomanowski, E. G., Yates, K. A., & Gordon, Y. J. (2023). Cyclopentenylcytosine (CPE-C): In Vitro and In Vivo Evaluation as an Antiviral against Adenoviral Ocular Infections. Molecules, 28(13), 5078. https://doi.org/10.3390/molecules28135078





