Zinc Oxide Quantum Dots May Provide a Novel Potential Treatment for Antibiotic-Resistant Streptococcus agalactiae in Lama glama
Abstract
1. Introduction
2. Results
2.1. Results for the Isolation and Identification of S. agalactiae
2.2. Molecular Identification Results
2.3. Identification of Antibiotic Susceptibility
2.4. High-Throughput Sequencing Results
2.5. Virulence and Pathogenic Analysis
2.6. Analysis of Antibiotic Resistance Genes of S. agalactiae
2.7. Effect of ZnO QDs on S. agalactiae
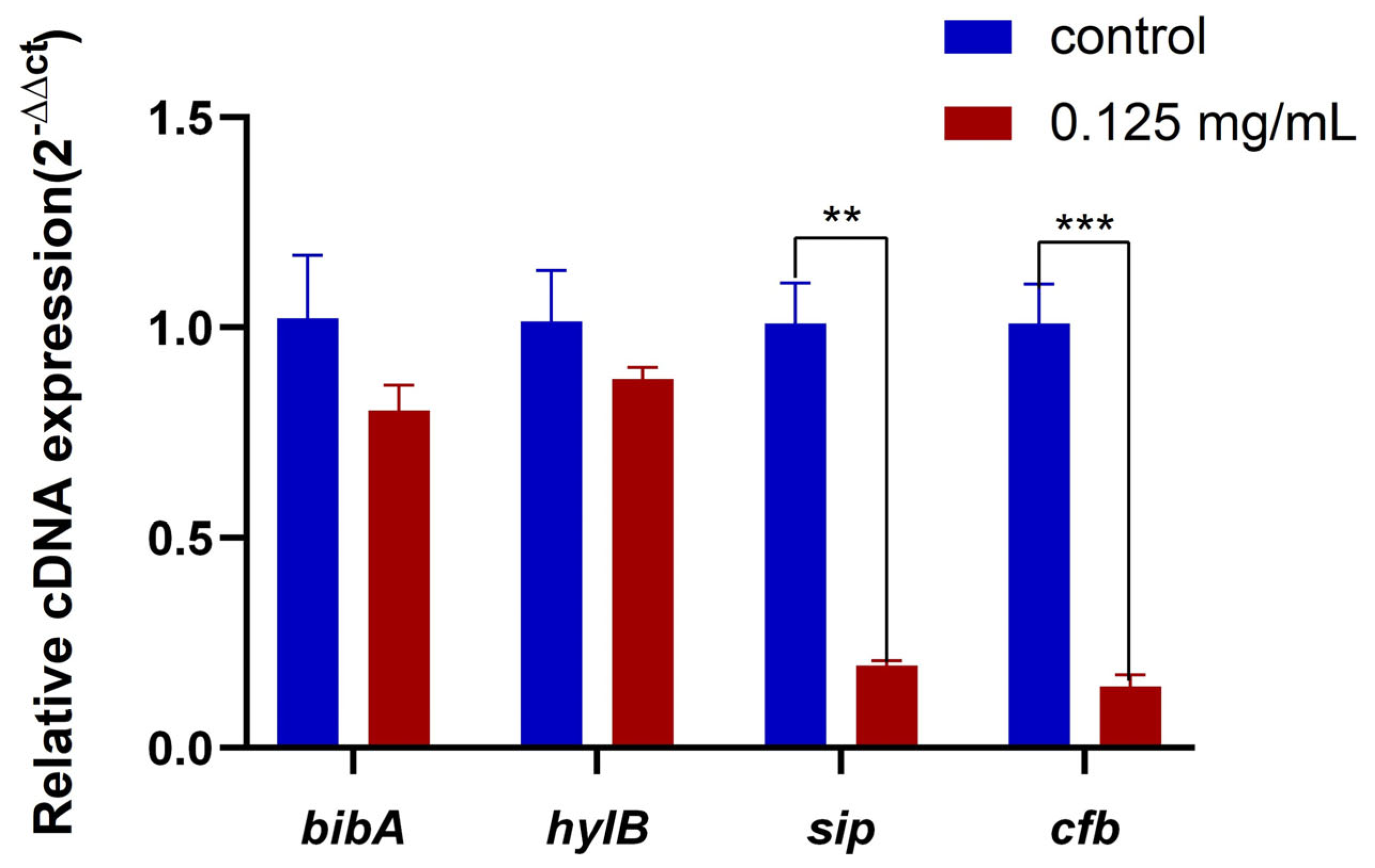
2.8. Effect of ZnO QDs on Virulence Genes of S. agalactiae
3. Discussion
4. Materials and Methods
4.1. Basic Information of Animals
4.2. Isolation and Identification of S. agalactiae
4.3. Molecular Identification
4.4. Antibiotic Susceptibility Testing
4.5. High-Throughput Sequencing
4.6. Antibacterial Effect of ZnO QDs
4.7. Detection of Virulence Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Raabe, V.N.; Shane, A.L. Group B Streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Liljander, A.; Kaspar, H.; Muriuki, C.; Fuxelius, H.H.; Bongcam-Rudloff, E.; de Villiers, E.P.; Huber, C.A.; Frey, J.; Daubenberger, C.; et al. Camel Streptococcus agalactiae populations are associated with specific disease complexes and acquired the tetracycline resistance gene tetM via a Tn916-like element. Vet. Res. 2013, 44, 86. [Google Scholar] [CrossRef] [PubMed]
- Tavella, A.; Bettini, A.; Cocchi, M.; Idrizi, I.; Colorio, S.; Viel, L.; Zanardello, C.; Zanolari, P. Isolation of Streptococcus agalactiae in a female llama (Lama glama) in South Tyrol (Italy). BMC Vet. Res. 2018, 14, 343. [Google Scholar] [CrossRef] [PubMed]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Paitan, Y. Current Trends in Antimicrobial Resistance of Escherichia coli. Curr. Top. Microbiol. Immunol. 2018, 416, 181–211. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Saravanakumar, K.; Malaikozhundan, B.; Divya, M.; Vaseeharan, B.; Durán-Lara, E.F.; Wang, M.-H. Biopolymer K-carrageenan wrapped ZnO nanoparticles as drug delivery vehicles for anti MRSA therapy. Int. J. Biol. Macromol. 2020, 144, 9–18. [Google Scholar] [CrossRef]
- Lei, C.; Sun, N.; Wu, H.; Zhao, Y.; Yu, C.; Janani, B.J.; Fakhri, A. Bio-photoelectrochemical degradation, and photocatalysis process by the fabrication of copper oxide/zinc cadmium sulfide heterojunction nanocomposites: Mechanism, microbial community and antifungal analysis. Chemosphere 2022, 308, 136375. [Google Scholar] [CrossRef]
- Yao, X.; BahrAluloom, Y.J.; Jawad, S.F.; Abdtawfeeq, T.H.; Al-janabi, D.R.; Ahmad, N.; Alshehri, A.; Hadrawi, S.K.; Al-Taee, M.M.; Riadi, Y. Multipurpose properties the Z-scheme dimanganese copper oxide/cadmium sulfide nanocomposites for photo-or photoelectro-catalytic, antibacterial applications, and thiamine detection process. J. Photochem. Photobiol. A: Chem. 2023, 436, 114374. [Google Scholar] [CrossRef]
- Liu, Z.; Hadi, M.A.; Aljuboory, D.S.; Ali, F.A.; Jawad, M.A.; Ameen, A.-A.; Hadrawi, S.K.; Mundher, T.; Riadi, Y.; Amer, R.F. High efficiency of Ag0 decorated Cu2MoO4 nanoparticles for heterogeneous photocatalytic activation, bactericidal system, and detection of glucose from blood sample. J. Photochem. Photobiol. B Biol. 2022, 236, 112571. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Xiong, H.M. Photoluminescent ZnO nanoparticles and their biological applications. Materials 2015, 8, 3101–3127. [Google Scholar] [CrossRef]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V.R. Antibacterial effect of chronic exposure of low concentration ZnO nanoparticles on E. Coli. J. Environ. Sci. Health Part A 2013, 48, 871–878. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, J.; Lin, H.; Ren, X.; Tian, H.; Liang, Y.; Wang, W.; Wang, Y.; Yin, M.; Huang, Y. In situ fabrication of nano ZnO/BCM biocomposite based on MA modified bacterial cellulose membrane for antibacterial and wound healing. Int. J. Nanomed. 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Li, B.; Chen, D.; Dong, X.; Wang, Y.; Gu, Y. Versatile antimicrobial peptide-based ZnO quantum dots for in vivo bacteria diagnosis and treatment with high specificity. Biomaterials 2015, 53, 532–544. [Google Scholar] [CrossRef]
- Shu, G.; Xu, D.; Xie, S.; Chang, L.-J.; Liu, X.; Yang, J.; Li, Y.; Wang, X. The antioxidant, antibacterial, and infected wound healing effects of ZnO quantum dots-chitosan biocomposite. Appl. Surf. Sci. 2023, 611, 155727. [Google Scholar] [CrossRef]
- Li, Y.; Xie, S.; Xu, D.; Shu, G.; Wang, X. Antibacterial activity of ZnO quantum dots and its protective effects of chicks infected withSalmonella pullorum. Nanotechnology 2021, 32, 505104. [Google Scholar] [CrossRef]
- M100-S23; Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- Li, R.; Zhu, H.; Ruan, J.; Qian, W.; Fang, X.; Shi, Z.; Li, Y.; Li, S.; Shan, G.; Kristiansen, K. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010, 20, 265–272. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J.J.B. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.T.; Wong, K.; Jackman, S.D.; Schein, J.E.; Jones, S.J.; Birol, I. ABySS: A parallel assembler for short read sequence data. Genome Res. 2009, 19, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Liao, Y.C. CISA: Contig integrator for sequence assembly of bacterial genomes. PLoS ONE 2013, 8, e60843. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Liu, B.; Pop, M.J.N.A.R. ARDB—antibiotic resistance genes database. Nucleic Acids Res. 2009, 37, D443–D447. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- Zimmermann, P.; Gwee, A.; Curtis, N. The controversial role of breast milk in GBS late-onset disease. J. Infect. 2017, 74 (Suppl. S1), S34–S40. [Google Scholar] [CrossRef]
- Haenni, M.; Lupo, A.; Madec, J.Y. Antimicrobial Resistance in Streptococcus spp. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Guo, H.; Fu, M.; Peng, Q.; Chen, Z.; Liu, J.; Qiu, Y.; Huang, Y. Antimicrobial resistance and molecular characterization of Streptococcus agalactiae from pregnant women in southern China. J. Infect. Dev. Ctries. 2019, 13, 802–809. [Google Scholar] [CrossRef]
- Rigvava, S.; Kharebava, S.; Giorgobiani, T.; Dvalidze, T.; Goderdzishvili, M. Identification And Antibiotic Susceptibility Patterns Of Streptococcus agalactiae. Georgian Med. News 2019, 297, 149–153. [Google Scholar]
- Gibson, P.S.; Bexkens, E.; Zuber, S.; Cowley, L.A.; Veening, J.W. The acquisition of clinically relevant amoxicillin resistance in Streptococcus pneumoniae requires ordered horizontal gene transfer of four loci. PLoS Pathog. 2022, 18, e1010727. [Google Scholar] [CrossRef]
- Suchland, R.J.; Sandoz, K.M.; Jeffrey, B.M.; Stamm, W.E.; Rockey, D.D. Horizontal transfer of tetracycline resistance among Chlamydia spp. in vitro. Antimicrob. Agents Chemother. 2009, 53, 4604–4611. [Google Scholar] [CrossRef]
- Remenant, B.; de Cambiaire, J.-C.; Cellier, G.; Jacobs, J.M.; Mangenot, S.; Barbe, V.; Lajus, A.; Vallenet, D.; Medigue, C.; Fegan, M. Ralstonia syzygii, the blood disease bacterium and some Asian R. solanacearum strains form a single genomic species despite divergent lifestyles. PLoS ONE 2011, 6, e24356. [Google Scholar] [CrossRef]
- Schweizer, I.; Peters, K.; Stahlmann, C.; Hakenbeck, R.; Denapaite, D. Penicillin-binding protein 2x of Streptococcus pneumoniae: The mutation Ala707Asp within the C-terminal PASTA2 domain leads to destabilization. Microb. Drug Resist. 2014, 20, 250–257. [Google Scholar] [CrossRef]
- Piccinelli, G.; Carlentini, G.; Gargiulo, F.; Caruso, A.; De Francesco, M.A. Analysis of Point Mutations in the pbp2x, pbp2b, and pbp1a Genes of Streptococcus agalactiae and Their Relation with a Reduced Susceptibility to Cephalosporins. Microb. Drug Resist. 2017, 23, 1019–1024. [Google Scholar] [CrossRef]
- André, E.; Goeminne, L.; Cabibbe, A.; Beckert, P.; Mukadi, B.K.; Mathys, V.; Gagneux, S.; Niemann, S.; Van Ingen, J.; Cambau, E.J.C.M.; et al. Consensus numbering system for the rifampicin resistance-associated rpoB gene mutations in pathogenic mycobacteria. Clin. Microbiol. Infect. 2017, 23, 167–172. [Google Scholar] [CrossRef]
- Terzi, H.A.; Kulah, C.; Ciftci, I.H. The effects of active efflux pumps on antibiotic resistance in Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2014, 30, 2681–2687. [Google Scholar] [CrossRef]
- Coleman, M.; Armistead, B.; Orvis, A.; Quach, P.; Brokaw, A.; Gendrin, C.; Sharma, K.; Ogle, J.; Merillat, S.; Dacanay, M.; et al. Hyaluronidase Impairs Neutrophil Function and Promotes Group B Streptococcus Invasion and Preterm Labor in Nonhuman Primates. mBio 2021, 12, eaah4576. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Bi, Y.; Wang, Y.; Liu, H. Genetic and functional characterization of the hyaluronate lyase HylB and the beta-N-acetylglucosaminidase HylZ in Streptococcus zooepidemicus. Curr. Microbiol. 2015, 70, 35–42. [Google Scholar] [CrossRef]
- Jin, T.; Brefo-Mensah, E.; Fan, W.; Zeng, W.; Li, Y.; Zhang, Y.; Palmer, M. Crystal structure of the Streptococcus agalactiae CAMP factor provides insights into its membrane-permeabilizing activity. J. Biol. Chem. 2018, 293, 11867–11877. [Google Scholar] [CrossRef]
- Hensler, M.E.; Quach, D.; Hsieh, C.J.; Doran, K.S.; Nizet, V. CAMP factor is not essential for systemic virulence of Group B Streptococcus. Microb. Pathog. 2008, 44, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Palmer, M. Characterization of Streptococcus agalactiae CAMP factor as a pore-forming toxin. J. Biol. Chem. 2003, 278, 38167–38173. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Wang, K.Y.; Xiao, D.; Chen, D.F.; Geng, Y.; Wang, J.; He, Y.; Wang, E.L.; Huang, J.L.; Xiao, G.Y. Safety and immunogenicity of an oral DNA vaccine encoding Sip of Streptococcus agalactiae from Nile tilapia Oreochromis niloticus delivered by live attenuated Salmonella typhimurium. Fish Shellfish Immunol. 2014, 38, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Tsay, L.Y. ZnO quantum dots-decorated ZnO nanowires for the enhancement of antibacterial and photocatalytic performances. Nanotechnology 2015, 26, 395704. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloy. Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. ZnO nanofluids: Green synthesis, characterization, and antibacterial activity. Mater. Chem. Phys. 2010, 121, 198–201. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Liu, H.Z.; Liu, J.F.; Sun, Y.; Song, Y. Pathogenic mechanism, detection methods and clinical significance of group B Streptococcus. Future Microbiol. 2021, 16, 671–685. [Google Scholar] [CrossRef]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767. [Google Scholar]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater 2008, 9, 035004. [Google Scholar] [CrossRef]
- Carvalho-Castro, G.A.; Silva, J.R.; Paiva, L.V.; Custódio, D.A.C.; Moreira, R.O.; Mian, G.F.; Prado, I.A.; Chalfun-Junior, A.; Costa, G.M. Molecular epidemiology of Streptococcus agalactiae isolated from mastitis in Brazilian dairy herds. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2017, 48, 551–559. [Google Scholar] [CrossRef]


| Antibiotic Class | Antimicrobial | SIR |
|---|---|---|
| Aminoglycosides | Amikacin | R |
| Gentamicin | R | |
| β-lactams | Meropenem | R |
| Cefepime | R | |
| Ampicillin/Sulbactam | R | |
| Amoxicillin/Clavulanate | R | |
| Ceftriaxone | R | |
| Cefoperazone | R | |
| Chloramphenicols | Chloramphenicol | R |
| Quinolones | Ciprofloxacin | R |
| Glycylcyclines | Tigecycline | R |
| Tetracyclines | Doxycycline | R |
| Glycopeptides | Vancomycin | R |
| Lincomycins | Clindamycin | R |
| VF Name | Related Genes |
|---|---|
| Capsule | neuA, neuD, neuC, neuB, cpsL, cpsK, cpsJ, cpsI, cpsH, cpsG, cpsF, cpsE, cpsD, cpsC, cpsB, cpsY, uppS, cpsB, rgpB, rmlA, rmlC, rgpG, oppF |
| Beta-hemolysin | cylX, cylD, cylG, acpC, cylZ, cylA, cylI, cylJ, cylK |
| ABC transporter | fbpC, fagC, aatC |
| Periplasmic binding protein-dependent ABC transport systems | vctC, vctG, vctD |
| Type VII secretion system | essC, esxA |
| LPS | acpXL, fabZ |
| ClpE | clpE |
| Polysaccharide capsule | galE |
| Streptococcal enolase | eno |
| Mitogenic factor 2 | mf2 |
| PrrA/B | prrA |
| PEB1/CBF1 | pebA |
| RegX3 | regX3 |
| Lipoprotein diacylyceryl transferase | lgt |
| SodB | sodB |
| Cytolysin | cylR2 |
| PdgA | pdgA |
| PDH-B | pdhB |
| Lipoate protein ligase A1 | lplA1 |
| Exopolysaccharide | mrsA |
| Nucleoside diphosphate kinase | ndk |
| Sortase A | srtA |
| PhoP | phoP |
| Pyrimidine biosynthesis | carB |
| Lipoate protein ligase A1 | lplA1 |
| RTX toxin | rtxB |
| CAMP factor | cfb |
| Cytolysin | cylR2 |
| (p)ppGpp synthesis and hydrolysis | relA |
| Laminin-binding protein | lmb |
| Neuraminidase | nanA |
| Trehalose-recycling ABC transporter | sugC |
| ClpC | clpC |
| D-alanine-polyphosphoribitol ligase | dltA |
| Streptococcal plasmin receptor/GAPDH | gapA |
| Glutamine synthesis | glnA1 |
| C3-degrading protease | cppA |
| Polysaccharide capsule | manA |
| LisR/LisK | lisR |
| LOS | orfM |
| Fibronectin-binding protein | scpB |
| ClpP | clpP |
| Pneumococcal surface antigen A | psaA |
| Streptococcal lipoprotein rotamase A | slrA |
| SigA | sigA |
| PI-2b pili | lep |
| Lipoprotein-specific signal peptidase II | lspA |
| Hemolysin III | hlyIII |
| Hyaluronidase | hylB |
| Fibronectin-binding proteins | pavA |
| Serine protease | htrA |
| Phytotoxin phaseolotoxin | argK |
| MprA/B | mprA |
| GroEL | groEL |
| Cytolysin | cylR2 |
| Serine-threonine phosphatase | stp |
| Protein kinase G | pknG |
| Trigger factor | ropA |
| Listeria adhesion protein | lap |
| Surface immunogenic protein | sip |
| GbpC | gbpB |
| Polar flagella | flmH |
| Copper exporter | ctpV |
| Mitogenic factor 3 | mf3 |
| Cytolysin | cylR2 |
| Group B Streptococcus immunogenic bacterial adhesin | bibA |
| Resistance Mechanism | Antibiotic Class | Antibiotic Resistance Ontology |
|---|---|---|
| antibiotic efflux | macrolide | mtrA |
| nitroimidazole | msbA | |
| diaminopyrimidine | oqxB | |
| fluoroquinolone | ||
| glycylcycline | ||
| nitrofuran | ||
| tetracycline | ||
| fluoroquinolone | efrB | |
| macrolide | ||
| rifamycin | ||
| fluoroquinolone | efrA | |
| macrolide | ||
| rifamycin | ||
| fluoroquinolone | adeH | |
| tetracycline | ||
| aminoglycoside | Pseudomonas | |
| aminoglycoside | MexD | |
| aaminocoumarin | ||
| cephalosporin | ||
| diaminopyrimidine | ||
| fluoroquinolone | ||
| macrolide | ||
| phenicol | ||
| tetracycline | ||
| acridine dye | arlR | |
| isinfecting agents and intercalating dyes fluoroquinolone | ||
| fluoroquinolone | norB | |
| pmrA | ||
| lincosamide | lmrP | |
| macrolide | ||
| streptogramin | ||
| tetracycline | ||
| acridine dye | mdtN | |
| disinfecting agents and intercalating dyes nucleoside | ||
| antibiotic target alteration | lincosamide | RlmA(II) |
| macrolide | ||
| peptide | mprF | |
| glycopeptide | vanRF | |
| vanRM | ||
| antibiotic inactivation | aminoglycoside | AA′(6′)-Ip |
| AAC(3)-Iib AN′(4′)-Ib | ||
| antibiotic target alteration;antibiotic target replacement | peptide | rpoB |
| rifamycin | ||
| antibiotic target replacement | diaminopyrimidine | DfrA42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Zhang, T.; Chen, Y.; Zhou, X.; Zhong, Y.; Liu, H.; Zhong, Z.; Hu, Y.; Liao, F.; Wang, X.; et al. Zinc Oxide Quantum Dots May Provide a Novel Potential Treatment for Antibiotic-Resistant Streptococcus agalactiae in Lama glama. Molecules 2023, 28, 5115. https://doi.org/10.3390/molecules28135115
Zhou Z, Zhang T, Chen Y, Zhou X, Zhong Y, Liu H, Zhong Z, Hu Y, Liao F, Wang X, et al. Zinc Oxide Quantum Dots May Provide a Novel Potential Treatment for Antibiotic-Resistant Streptococcus agalactiae in Lama glama. Molecules. 2023; 28(13):5115. https://doi.org/10.3390/molecules28135115
Chicago/Turabian StyleZhou, Ziyao, Ting Zhang, Yixin Chen, Xiaoxiao Zhou, Yalin Zhong, Haifeng Liu, Zhijun Zhong, Yanchun Hu, Fei Liao, Xianxiang Wang, and et al. 2023. "Zinc Oxide Quantum Dots May Provide a Novel Potential Treatment for Antibiotic-Resistant Streptococcus agalactiae in Lama glama" Molecules 28, no. 13: 5115. https://doi.org/10.3390/molecules28135115
APA StyleZhou, Z., Zhang, T., Chen, Y., Zhou, X., Zhong, Y., Liu, H., Zhong, Z., Hu, Y., Liao, F., Wang, X., & Peng, G. (2023). Zinc Oxide Quantum Dots May Provide a Novel Potential Treatment for Antibiotic-Resistant Streptococcus agalactiae in Lama glama. Molecules, 28(13), 5115. https://doi.org/10.3390/molecules28135115








