New Hydrophilic Derivatives of Lasalocid and Their Complexes with Selected Metal Cations
Abstract
1. Introduction
2. Results and Discussion
2.1. ESI Mass Spectrometry
2.2. NMR Measurements
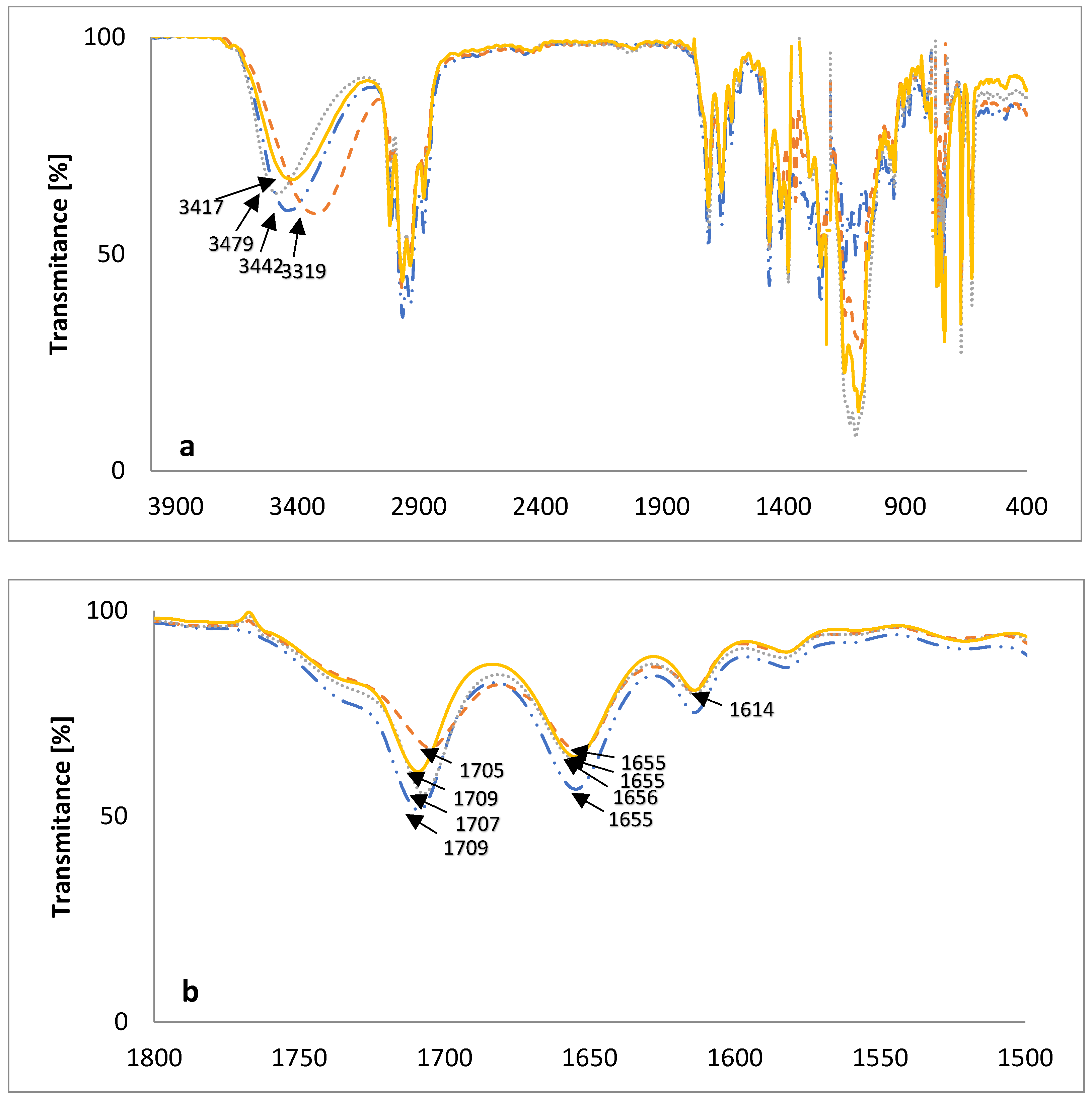
2.3. FT-IR Measurements
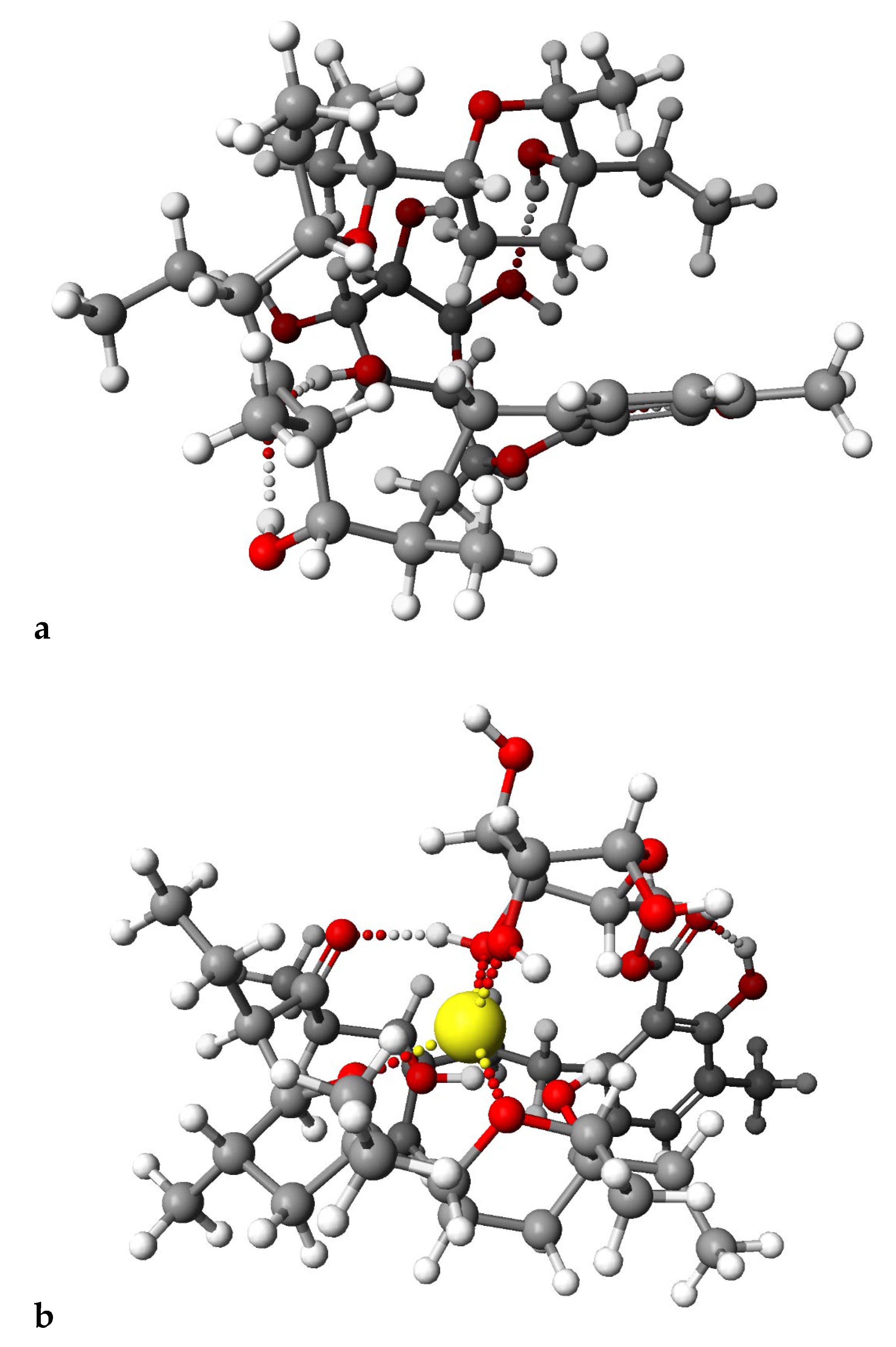
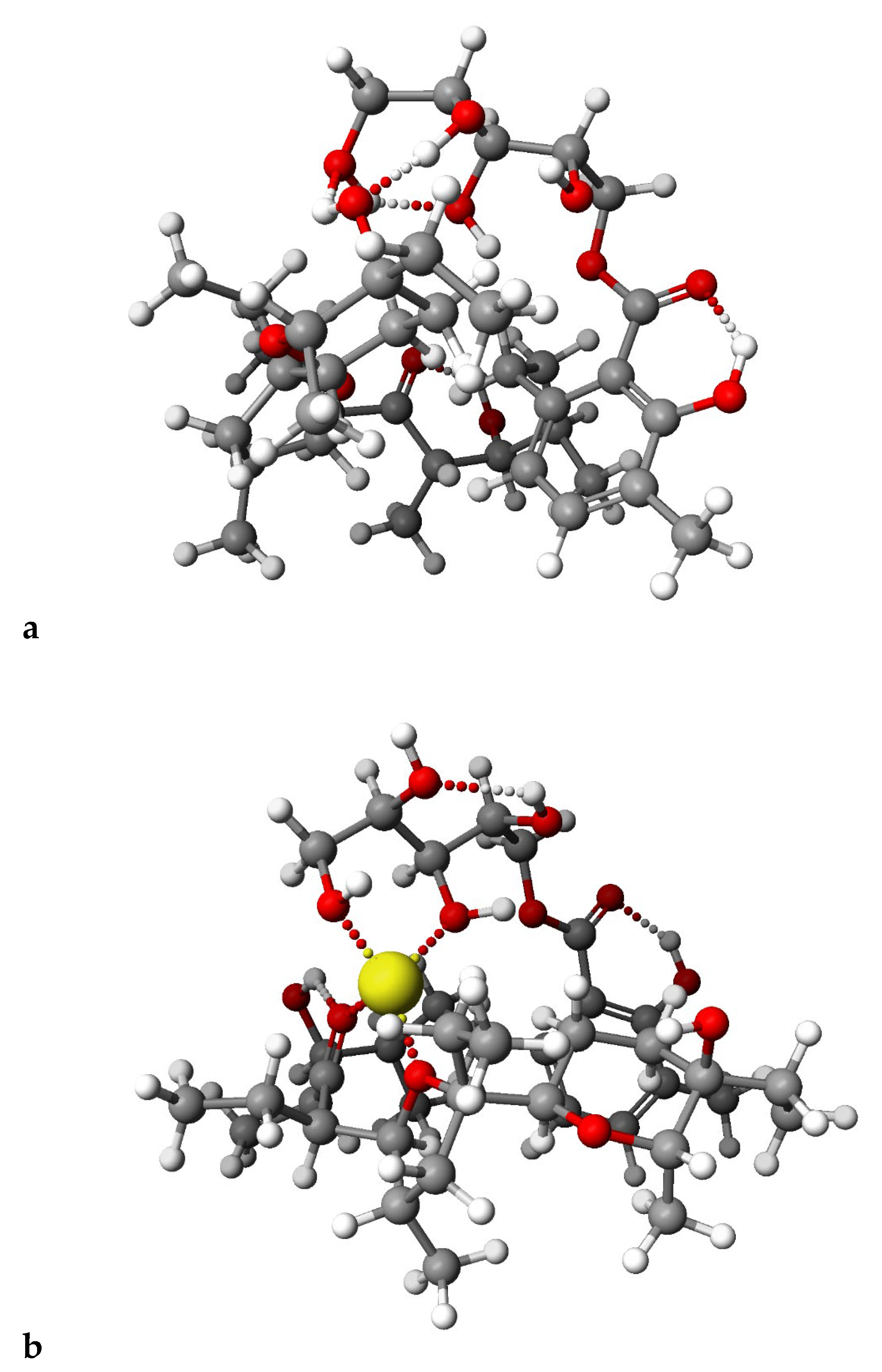
2.4. PM6 and DFT Study
3. Materials and Methods
3.1. Preparation of Lasalocid Acid
3.2. Preparation of the Glucose Ester (LasGlu) and Xylitol Ester (LasX)
3.3. Preparation of Complexes
3.4. ESI MS Measurements
3.5. NMR Measurements
3.6. FT-IR Measurement
3.7. Theoretical Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pressman, B.C.; Harris, E.J.; Jagger, W.S.; Johnson, J.H. Antibiotic-mediated transport of alkali ions across lipid barriers. Proc. Nat. Acad. Sci. USA 1967, 58, 1949–1956. [Google Scholar] [CrossRef]
- Pressman, B.C. Biological applications of ionophores. Annu. Rev. Biolchem. 1976, 45, 501–530. [Google Scholar] [CrossRef] [PubMed]
- Faul, M.M.; Huff, B.E. Strategy and Methodology Development for the Total Synthesis of Polyether Ionophore Antibiotics. Chem. Rev. 2000, 100, 2407–2473. [Google Scholar] [CrossRef] [PubMed]
- Butaye, P.; Deyriese, L.A.; Haesebrouck, F. Antimicrobial growth promoters used in animal feed: Effects of less well know antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C.; Fuchs, D.; Opelz, G. Salinomycin in cancer: A new mission for an old agent. Mol. Med. Rep. 2010, 3, 555–559. [Google Scholar] [CrossRef]
- Nachliel, E.; Finkelstein, Y.; Gutman, M. The mechanism of monensin-mediated cation exchange based on real time measurements. Biochim. Biophys. Acta 1996, 1285, 131–145. [Google Scholar] [CrossRef]
- Rybachenko, V.I.; Pankiewicz, R.; Schroeder, G. Application of Molecular Receptors; Schidnyj wydawnyczyj dim: Donetsk, Ukraine, 2009; pp. 89–99. [Google Scholar]
- Pankiewicz, R.; Pawłowska, A.; Schroeder, G.; Przybylski, P.; Brzezinski, B.; Bartl, F. Multinuclear NMR, FT-IR, ESI MS studies and PM5 semiempirical calculations of new ethylene glycol ester of lasalocid acid and their complexes with K+ cation. J. Mol. Struct. 2004, 694, 55–61. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Schroeder, G.; Brzezinski, B. Spectroscopic, spectrometric and PM5 semiempirical investigation of new lasalocid 8-hydroxy-3,6-dioxaoctyl ester and its complexes with monovalent cations. J. Mol. Struct. 2005, 733, 217–229. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Kira, J.; Schroeder, G.; Ossowski, T.; Brzezinski, B. Potentiometric, ESI MS and AM1d studies of lasalocid esters–silver(I) complexes. J. Mol. Struct. 2006, 782, 73–80. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Schroeder, G.; Gierczyk, B.; Wojciechowski, G.; Brzezinski, B.; Bartl, F.; Zundel, G. 7Li-NMR and FTIR Studies of Lithium, Potassium, Rubidium and Cesium Complexes with Ionophore Lasalocid. Biopolymers 2001, 62, 173–182. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Schroeder, G.; Gierczyk, B.; Brzeziński, B.; Bartl, F. Multiclear NMR and FTIR Studies of New Polyoxaalkyl Esters of Lasalocid and Their Complexes with Lithium and Sodium Cations. Biopolymers 2002, 65, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Huczyński, A.; Pospieszny, T.; Wawrzyn, R.; Ratajczak-Sitarz, M.; Katrusiak, A.; Brzeziński, B.; Bartl, F. Structural and Spectroscopic Studies of New o-, m-, and p-Nitrobenzyl esters of Lasalocid Acid; Elsevier: Amsterdam, The Netherlands, 2007; pp. 105–114. [Google Scholar]
- Rutkowski, J.; Brzeziński, B. Structures and Properties of Naturally Occurring Polyether Antibiotics. BioMed Res. Int. 2013, 2013, 162513. [Google Scholar] [CrossRef] [PubMed]
- Kevin, D.A., II.; Meujo, D.A.F.; Hamann, M.T. Polyether ionophores: Broad-spectrum and promising for the control of drug-resistant bacteria and parasites. Expert Opin. Drug. Discov. 2009, 4, 109–146. [Google Scholar] [CrossRef]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of Selective Inhibitors of Cancer stem Cells by High-Throughput Screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Huczyński, A. Polyether ionophores-promising bioactive molecules for cancer therapy. Bioorg. Med. Chem. Lett. 2012, 22, 7002–7010. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, S.H.; Yu, S.N.; Park, S.G.; Kim, Y.W.; Nam, H.W.; An, H.H.; Yu, H.S.; Kim, Y.W.; Ji, J.H.; et al. Lasalocid Induces Cytotoxic Apoptosis and Cytoprotective Autophagy through Reactive Oxygen Species in Human Prostate Cancer PC-3 Cells; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1016–1024. [Google Scholar]
- Boesch, M.; Sopper, S.; Wolf, D. Ionophore Antibiotic as Cancer Cell-Selective Drugs: Open Question. Oncologist 2016, 21, 1291–1293. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Schroeder, G.; Przybylski, P.; Brzezinski, B.; Bartl, F. Lasalocid polyoxaalkyl esters complexes with Li+, Na+, K+, Rb+ and Cs+ cations studied by ESI MS and semiempirical methods. J. Mol. Struct. 2004, 688, 171–176. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Schroeder, G.; Brzezinski, B. Spectroscopic and PM5 semiempirical study of a new lasalocid ester with 2-allyloxyethanol and its complexes with monovalent cations. J. Mol. Struct. 2006, 789, 1–7. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Schroeder, G.; Brzezinski, B.; Bartl, F. Spectroscopic and PM5 semiempirical study of new lasalocid 5-hydroxypentyl ester and its complexes with monovalent cations. J. Mol. Struct. 2004, 699, 53–64. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Schroeder, G.; Brzezinski, B. NMR, FT-IR, ESI MS and PM5 semiempirical study of new lasalocid 5-hydroxy-3-oxapentyl ester and its complexes with monovalent cations. J. Mol. Struct. 2005, 733, 155–165. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Pawłowska, A.; Schroeder, G.; Przybylski, P.; Brzezinski, B.; Bartl, F. NMR, FT-IR, ESI MS studies and PM5 semiempirical calculations of lasalocid ethylene glycol ester complexes with Li+ and Na+ cations. J. Mol. Struct. 2004, 694, 155–163. [Google Scholar] [CrossRef]
- Pankiewicz, R.; Schroeder, G.; Brzezinski, B.; Bartl, F. HMR, FT-IR and ESI-MS study of new lasalocid ester with 2-(hydroxymethyl)-12-crown-4 and its complexes with monovalent cations. J. Mol. Struct. 2005, 749, 128–137. [Google Scholar] [CrossRef]
- MO-G Version 1.2A; Fujitsu Limited: Tokyo, Japan, 2013.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
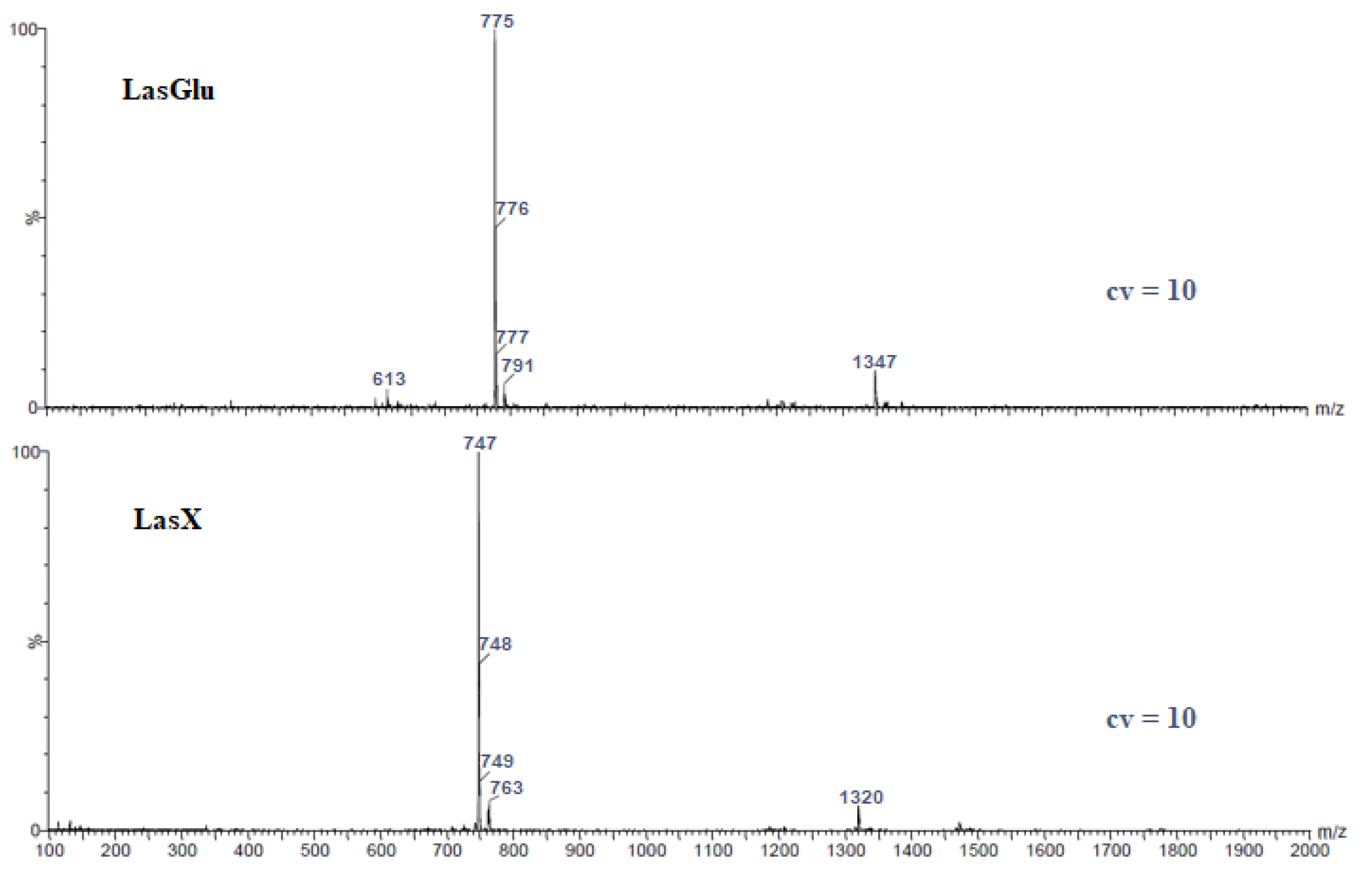
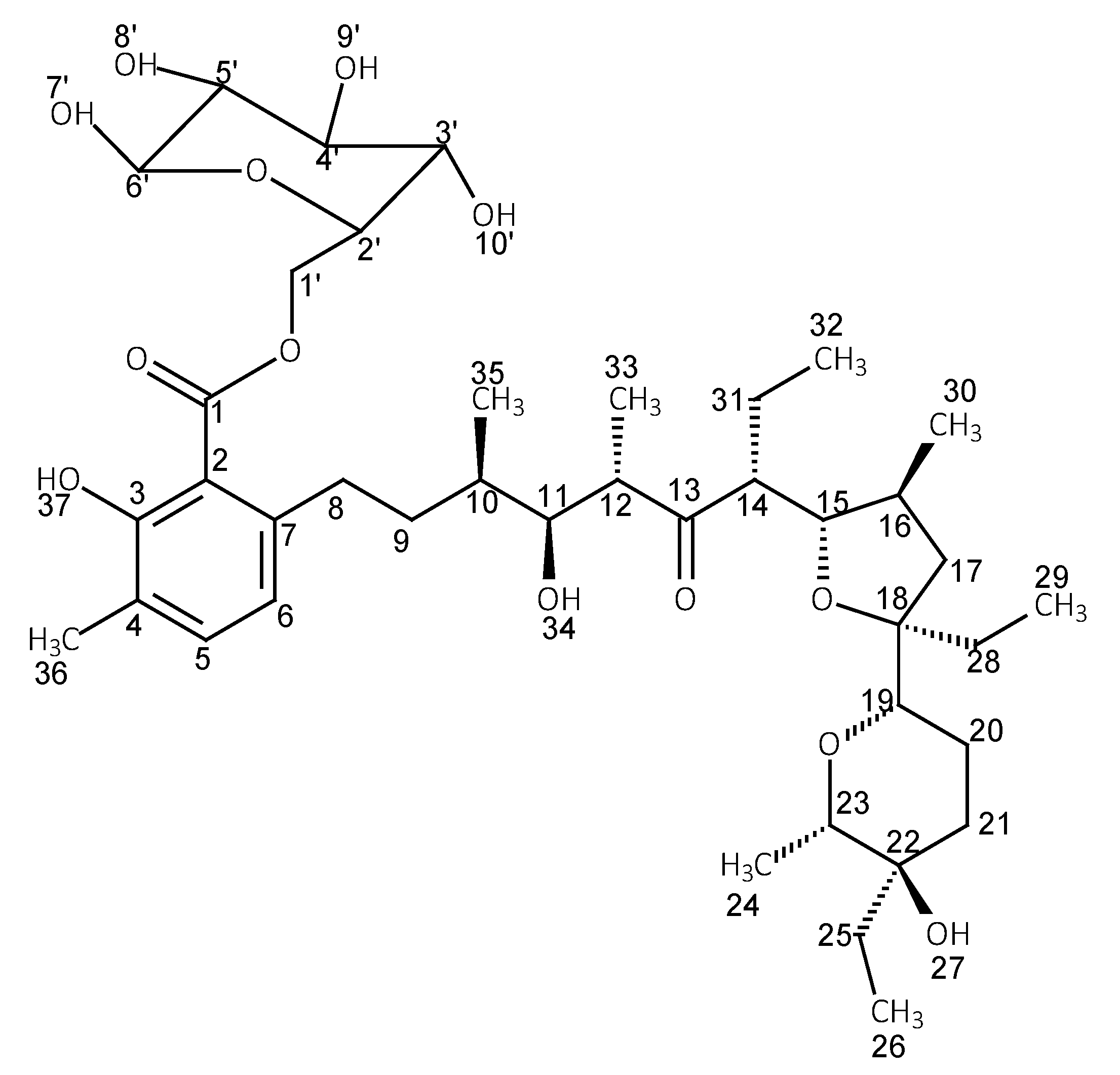
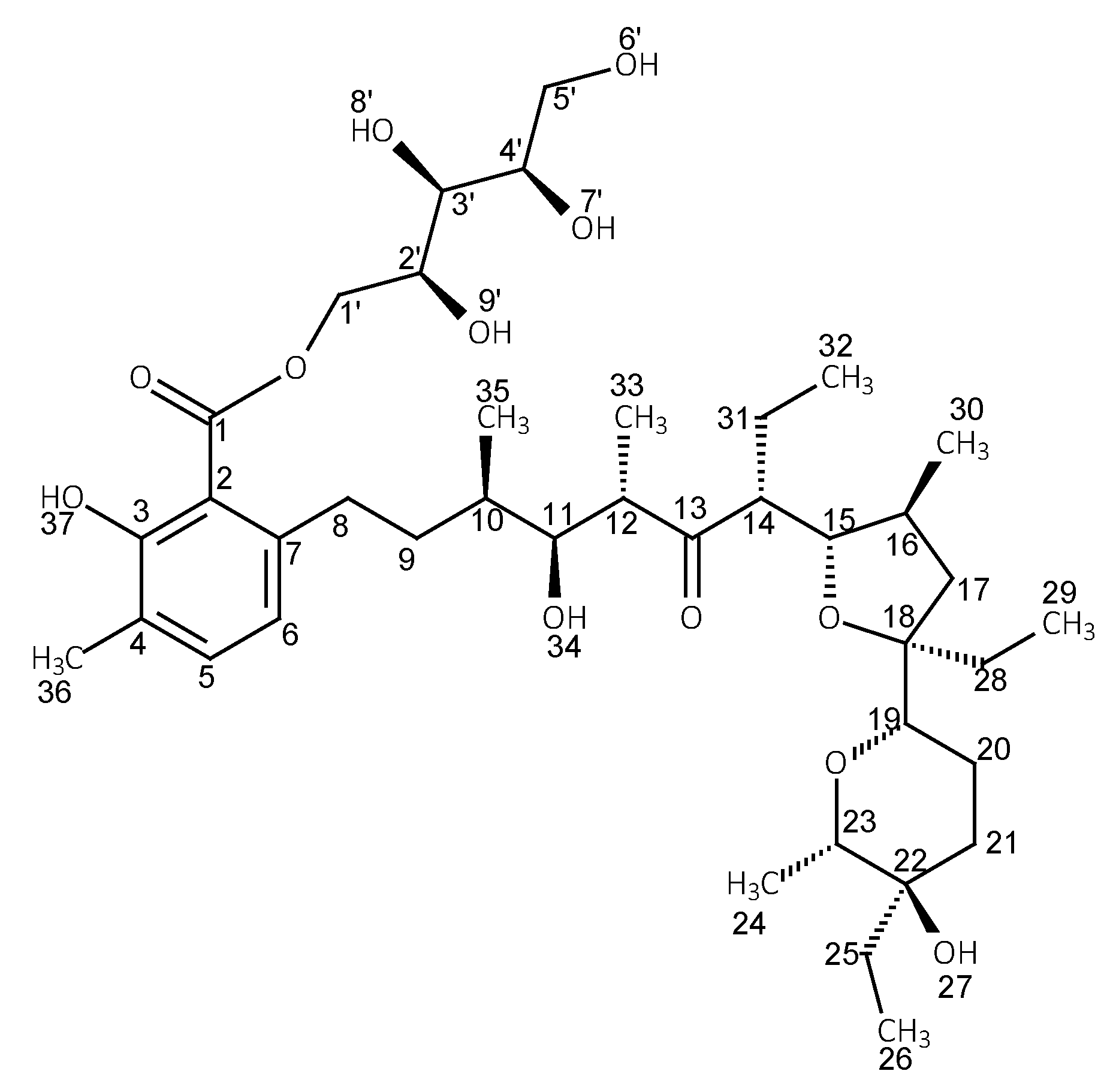

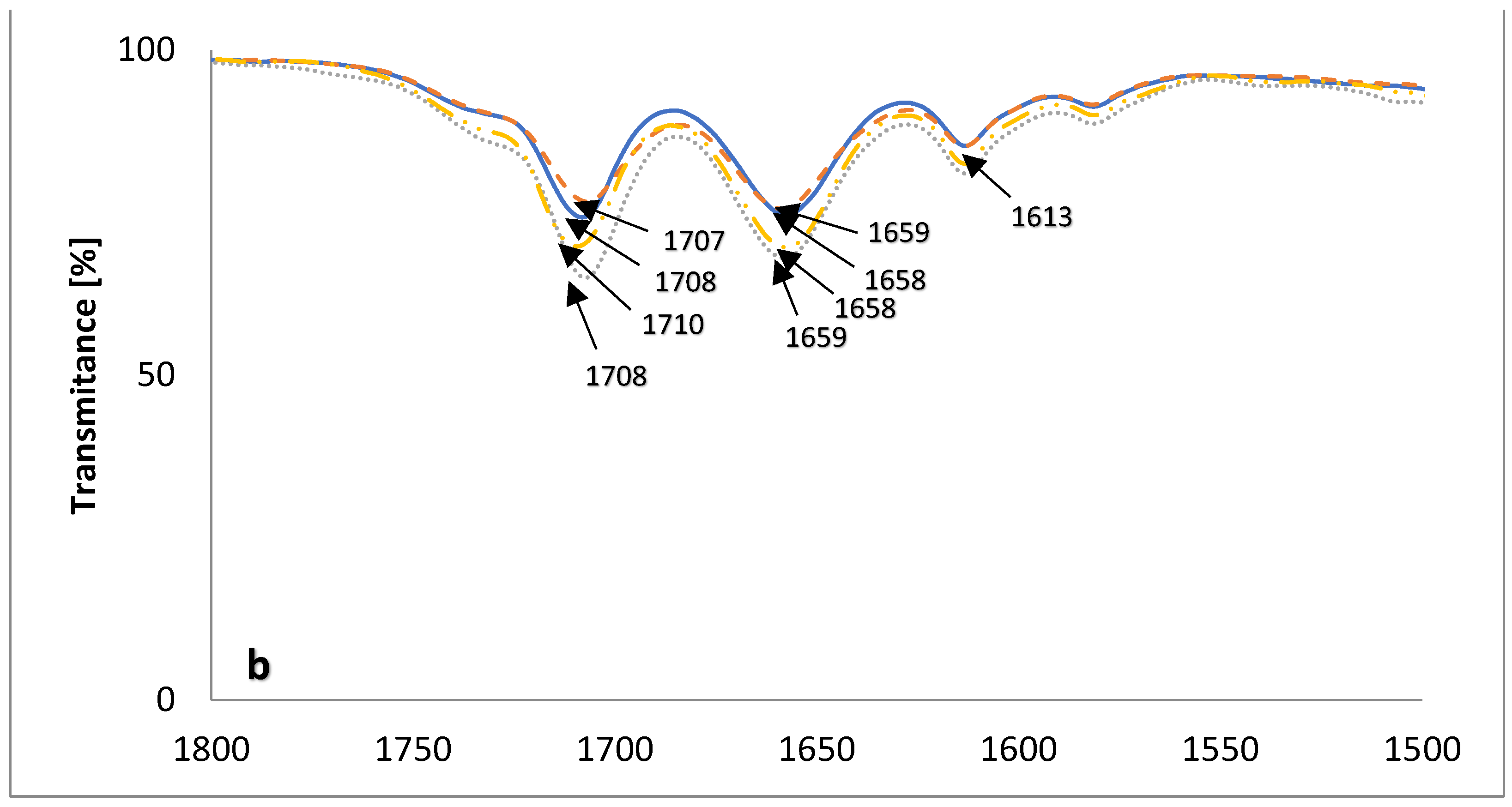
| Ester | Main Peaks (m/z) |
|---|---|
| LasGlu | 613 (w), 775, 776, 777 (w), 791 (w), 1347 (w) |
| LasX | 747, 748, 749 (w), 763 (w), 1320 (w) |
| (w) weak signal. |
| No. of Atom | LasH | LasGlu | LasX | Δ LasGlu | Δ LasX | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | Δ1H | Δ13C | Δ1H | Δ13C | |
| 1 | – | 171.37 | – | 175.9 | – | 172.23 | – | 4.5 | – | 0.86 |
| 2 | – | 111.54 | – | 117.5 | – | 111.61 | – | 6.0 | – | 0.07 |
| 3 | – | 160.29 | – | 163.51 | – | 160.93 | – | 3.2 | – | 0.64 |
| 4 | – | 124.08 | – | 123.42 | – | 124.28 | – | −0.7 | – | 0.2 |
| 5 | 7.13 | 134.99 | 7.16 | 132.33 | 7.17 | 135.32 | 0.03 | −2.7 | 0.04 | 0.33 |
| 6 | 6.63 | 121.8 | 6.87 | 119.74 | 6.66 | 121.77 | 0.24 | −2.1 | 0.03 | −0.03 |
| 7 | – | 143.43 | – | 144.4 | – | 143.72 | – | 1.0 | – | 0.29 |
| 8 | 2.81; 3.00 | 33.75 | 2.90; 3.87 | 34.05 | 2.66; 3.10 | 34.58 | 0.09; 0.87 | 0.3 | 0.15; 0.10 | 0.83 |
| 9 | 2.19 | 35.38 | 2.25 | 34.83 | 2.48 | 35.24 | 0.06 | −0.6 | 0.29 | −0.14 |
| 10 | 1.76 | 33.95 | 1.99 | 34.0 | 1.64 | 35 | 0.23 | 0.0 | −0.12 | 1.05 |
| 11 | 3.47 | 70.56 | 5.29 | 71.42 | 3.46 | 71.1 | 1.82 | 0.9 | −0.01 | 0.54 |
| 12 | 2.9 | 48.99 | 2.94 | 49.51 | 2.83 | 49.63 | 0.04 | 0.5 | −0.07 | 0.64 |
| 13 | – | 215.28 | – | 219.90 | – | 216.04 | – | 4.6 | – | 0.76 |
| 14 | 2.71 | 55.16 | 2.87 | 55.93 | 2.6 | 54.97 | 0.16 | 0.8 | -0.11 | −0.19 |
| 15 | 3.79 | 84.51 | 3.83 | 83.45 | 3.77 | 84.52 | 0.04 | −1.1 | -0.02 | 0.01 |
| 16 | ~1.6 | 36.1 | ~1.6 | 34.47 | ~1.6 | 36.71 | 0 | −1.6 | 0 | 0.61 |
| 17 | 1.50; 1.82 | 39.22 | 1.45; 1.88 | 38.22 | 1.50; 1.85 | 38.56 | 0.05; 0.06 | −1.0 | 0; −0.03 | −0.66 |
| 18 | – | 71.43 | – | 76.42 | – | 71.58 | – | 5.0 | – | 0.15 |
| 19 | 3.55 | 72.56 | 3.63 | 69.11 | 3.69 | 73.42 | 0.08 | −3.5 | 0.14 | 0.86 |
| 20 | 1.45; 1.66 | 20.64 | 1.43; 1.6 | 20.18 | 1.45; 1.7 | 19.79 | −0.02; −0.06 | −0.5 | –; 0.04 | −0.85 |
| 21 | 1.26 | 29.86 | 1.93 | 31.57 | 1.62 | 29.85 | 0.67 | 1.7 | 0.36 | −0.01 |
| 22 | – | 85.8 | – | 87.83 | – | 87.49 | – | 2.0 | – | 1.69 |
| 23 | 3.74 | ~77 | 3.76 | 76.42 | 3.81 | 77.71 | 0.02 | – | 0.07 | – |
| 24 | 1.16 | 14.02 | 0.76 | 13.83 | 1.15 | 14.03 | −0.40 | −0.2 | −0.01 | 0.01 |
| 25 | 1.55 | 30.61 | 1.26 | 31.51 | 2.17 | 30.71 | −0.29 | 0.9 | 0.62 | 0.1 |
| 26 | 0.84 | 6.39 | 0.82 | 6.82 | 0.94 | 6.55 | −0.02 | 0.4 | 0.10 | 0.16 |
| 27 | ~2.4 | – | 4.08 | – | 2.23 | – | 1.68 | – | −0.17 | – |
| 28 | ~1.4 | 29.3 | 1.75 | 30.35 | 1.15 | 30.02 | −0.35 | 1.1 | 0.25 | 0.72 |
| 29 | 0.79 | 8.54 | 0.74 | 9.74 | 0.98 | 9.34 | −0.05 | 1.2 | 0.19 | 0.8 |
| 30 | 1 | 15.99 | 1.01 | 15.47 | 1.03 | 16.06 | 0.01 | −0.5 | 0.03 | 0.07 |
| 31 | 1.40; 1.87 | 17.91 | 1.88 | 16.33 | 1.46 | 16.83 | 1 signal | −1.6 | 1 signal | −1.08 |
| 32 | 0.84 | 12.53 | 0.78 | 12.61 | 0.94 | 13.05 | −0.06 | 0.1 | 0.10 | 0.52 |
| 33 | 0.9 | 13.49 | 1.13 | 13.62 | 0.92 | 13.41 | 0.23 | 0.1 | 0.02 | −0.08 |
| 34 | ~3.4 | – | ~3.4 | – | ~3.4 | – | 0 | – | 0 | – |
| 35 | 0.9 | 12.37 | 0.91 | 12.64 | 1.07 | 12.88 | 0.01 | 0.3 | 0.17 | 0.51 |
| 36 | 2.19 | 15.87 | 2.03 | 16.31 | 2.21 | 15.91 | −0.16 | 0.4 | 0.02 | 0.04 |
| 37 | 11.2 | – | 10.03 | – | 11.5 | – | −1.17 | – | 0.30 | – |
| No. of Atom | Glu | LasGlu | ||||
|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | Δ1H | Δ13C | |
| 1′ | 3.57 | 61.09 | 2.76 | 56.03 | −0.81 | −5.06 |
| 2′ | 3.4 | 72.98 | 3.83 | 71.5 | 0.43 | −1.48 |
| 3′ | 3.1 | 72.25 | 3.66 | 71.5 | 0.56 | −0.75 |
| 4′ | 3.54 | 71.88 | 3.48 | 71.4 | −0.06 | −0.48 |
| 5′ | 3.03 | 70.42 | 3.45 | 71 | 0.42 | 0.58 |
| 6′ | 4.89 | 92.13 | 4.28 | 87.9 | −0.61 | −4.23 |
| 7′ | 4.82 | – | 4.75 | – | −0.07 | – |
| 8′ | 4.7 | – | 4.73 | – | 0.03 | – |
| 9′ | 4.52 | – | 4.61 | – | 0.09 | – |
| 10′ | 6.23 | – | 6.32 | – | 0.09 | – |
| 11′ | 4.41 | – | – | – | – | – |
| No. of Atom | X | LasX | ||||
|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | Δ1H | Δ13C | |
| 1′ | 3.44 | 62.5 | 4.28 | 67.9 | 0.84 | 5.4 |
| 2′ | 4.53 | 72.2 | 3.83 | 71.5 | −0.7 | −0.7 |
| 3′ | 3.45 | 70 | 3.66 | 71.5 | 0.21 | 1.5 |
| 4′ | 3.53 | 72.2 | 3.48 | 71.4 | −0.05 | −0.8 |
| 5′ | 3.44 | 62.5 | 3.45 | 71 | 0.01 | 8.5 |
| 6′ | 1.32 | – | 1.27 | – | −0.05 | – |
| 7′ | 2.09 | – | 1.94 | – | −0.145 | – |
| 8′ | 1.48 | – | 1.41 | – | −0.069 | – |
| 9′ | 3.41 | – | 3.84 | – | 0.431 | – |
| 10′ | 1.47 | – | – | – | – | – |
| Complex | HOF (kJ/mol) | ΔHOF |
|---|---|---|
| LasGlu | −2742.24 | – |
| LasX | −2590.95 | |
| LasGlu Li+ (complexed) | −2495.57 | −368.47 |
| LasGlu Li+ (uncomplexed) | −2127.10 | |
| LasGlu Na+ (complexed) | −2611.59 | −422.41 |
| LasGlu Na+ (uncomplexed) | −2197.16 | |
| LasGlu K+ (complexed) | −2394.05 | −150.36 |
| LasGlu K+ (uncomplexed) | −2285.87 | |
| LasX Li+ (complexed) | −2256.91 | −281.10 |
| LasX Li+ (uncomplexed) | −1975.81 | |
| LasX Na+ (complexed) | −2369.08 | −323.21 |
| LasX Na+ (uncomplexed) | −2045.87 | |
| LasX K+ (complexed) | −2278.80 | −144.22 |
| LasX K+ (uncomplexed) | −2134.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papsdorf, M.; Pankiewicz, R. New Hydrophilic Derivatives of Lasalocid and Their Complexes with Selected Metal Cations. Molecules 2023, 28, 5114. https://doi.org/10.3390/molecules28135114
Papsdorf M, Pankiewicz R. New Hydrophilic Derivatives of Lasalocid and Their Complexes with Selected Metal Cations. Molecules. 2023; 28(13):5114. https://doi.org/10.3390/molecules28135114
Chicago/Turabian StylePapsdorf, Monika, and Radosław Pankiewicz. 2023. "New Hydrophilic Derivatives of Lasalocid and Their Complexes with Selected Metal Cations" Molecules 28, no. 13: 5114. https://doi.org/10.3390/molecules28135114
APA StylePapsdorf, M., & Pankiewicz, R. (2023). New Hydrophilic Derivatives of Lasalocid and Their Complexes with Selected Metal Cations. Molecules, 28(13), 5114. https://doi.org/10.3390/molecules28135114







