Mechanistic Studies of Arene–Ruthenium(II) Complexes with Carbothioamidopyrazoles as Alternative Cancer Drugs
Abstract
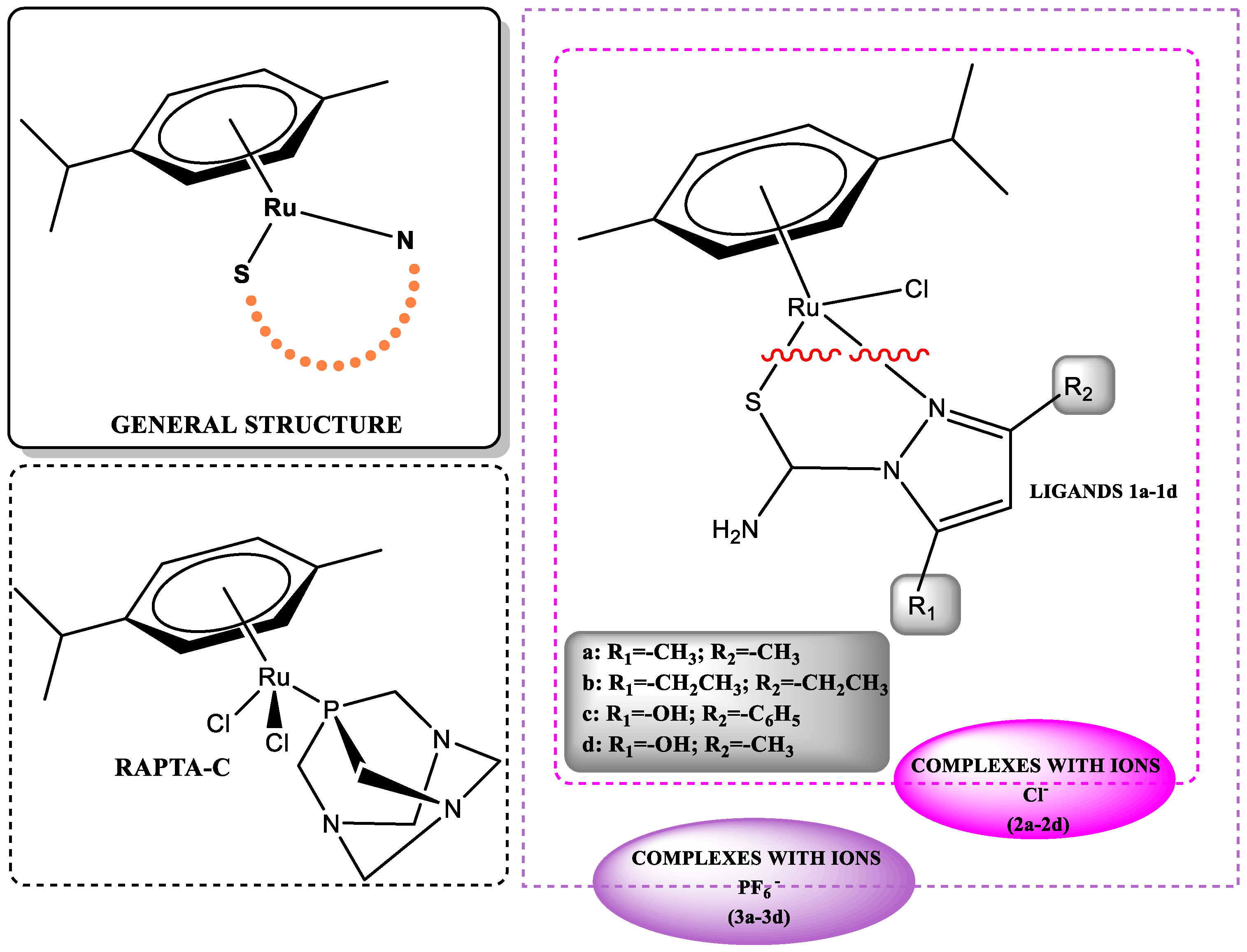
1. Introduction
2. Results
2.1. Cytotoxicity
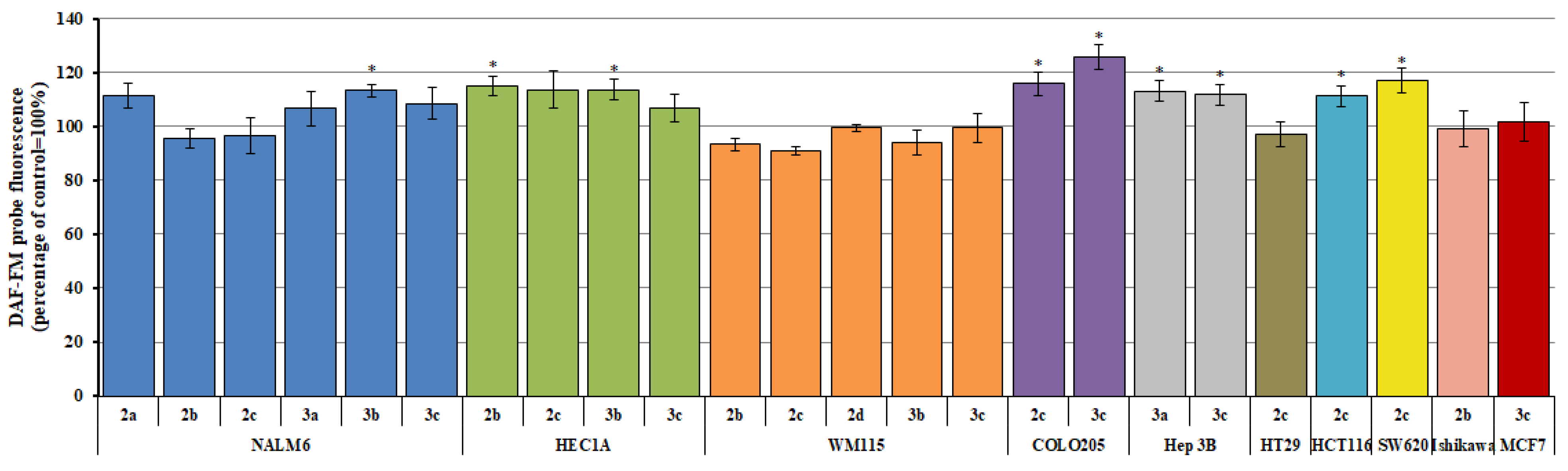
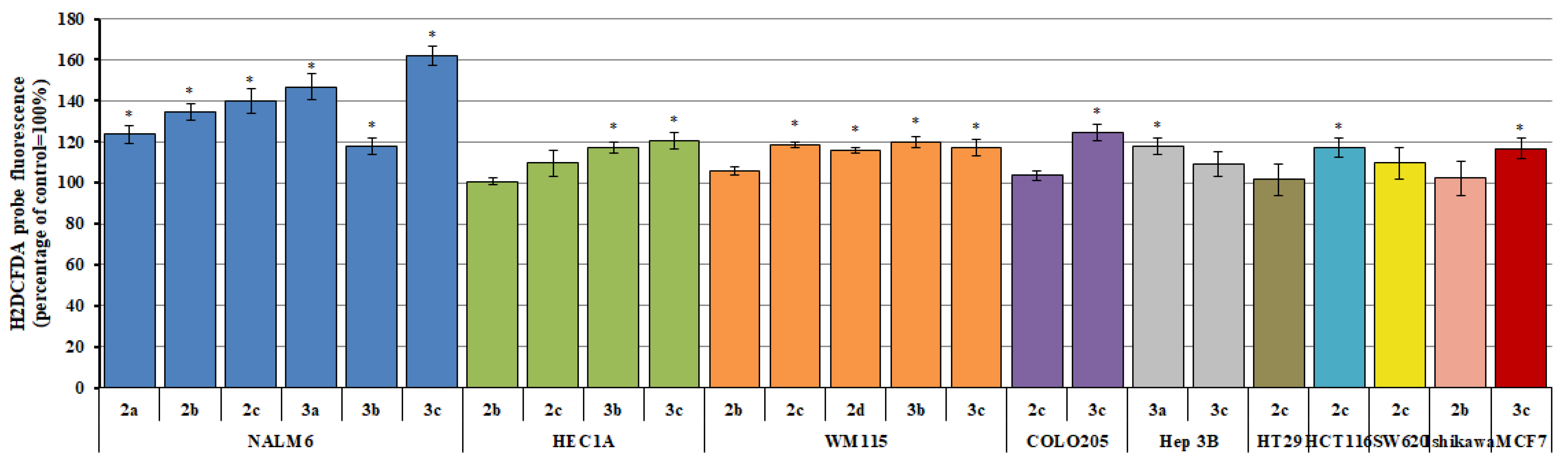
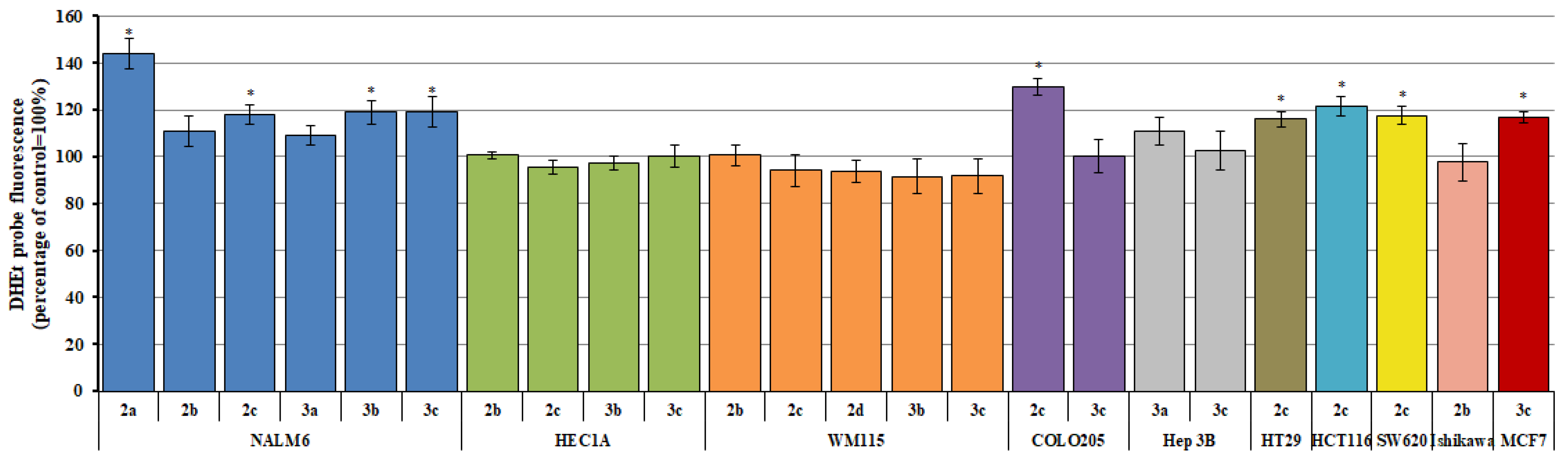
2.2. Reactive Oxygen/Nitrogen Species
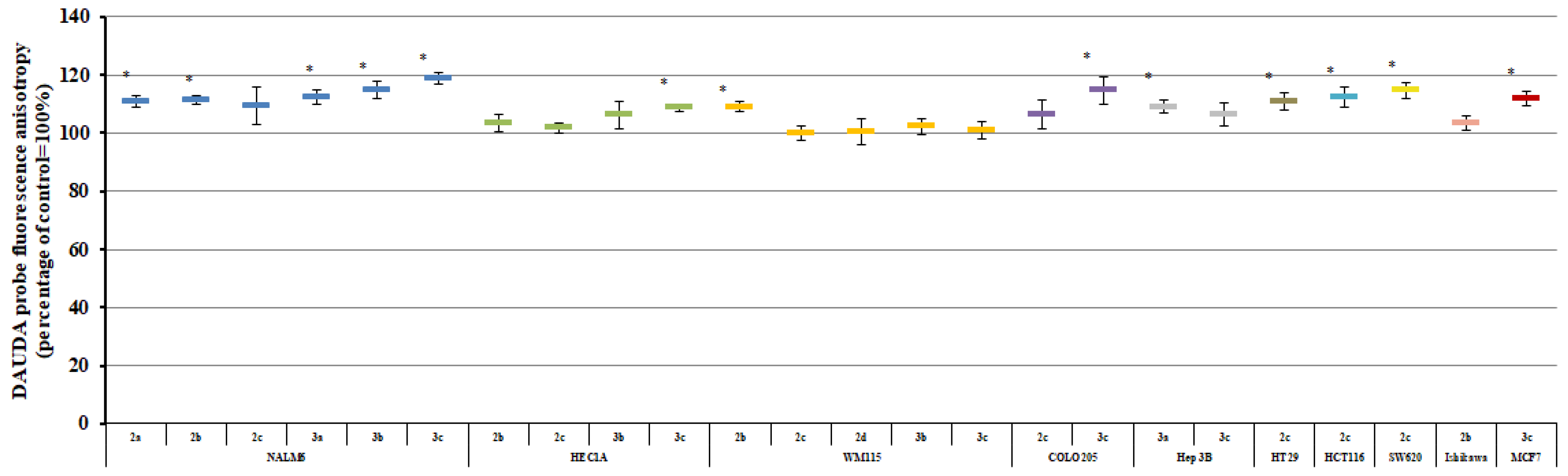
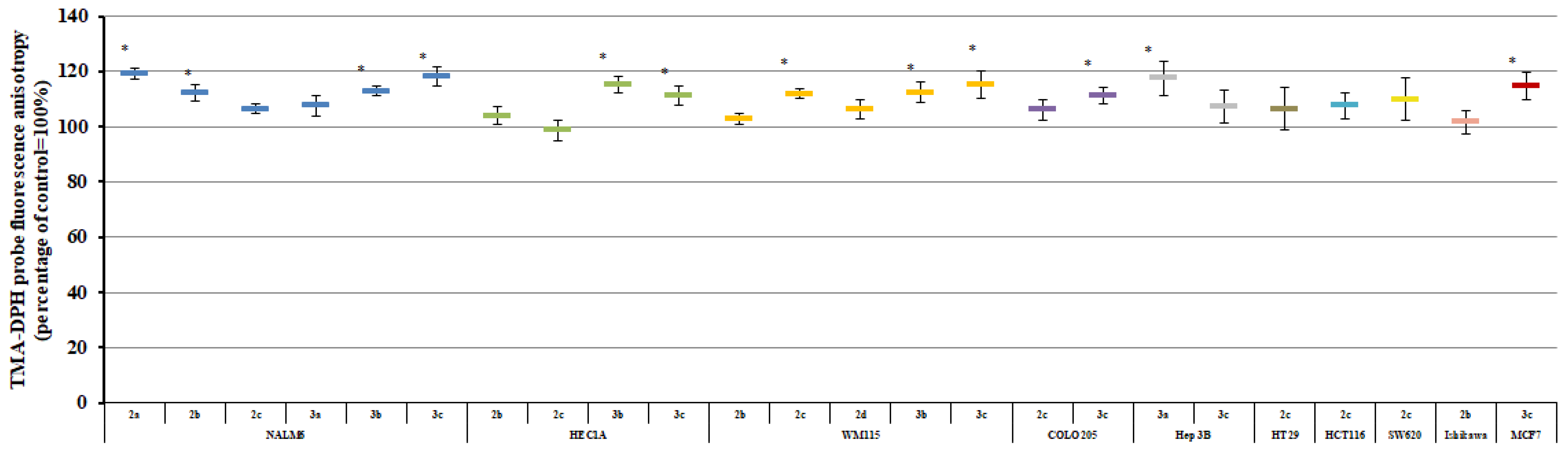
2.3. Plasma Membrane Fluidity
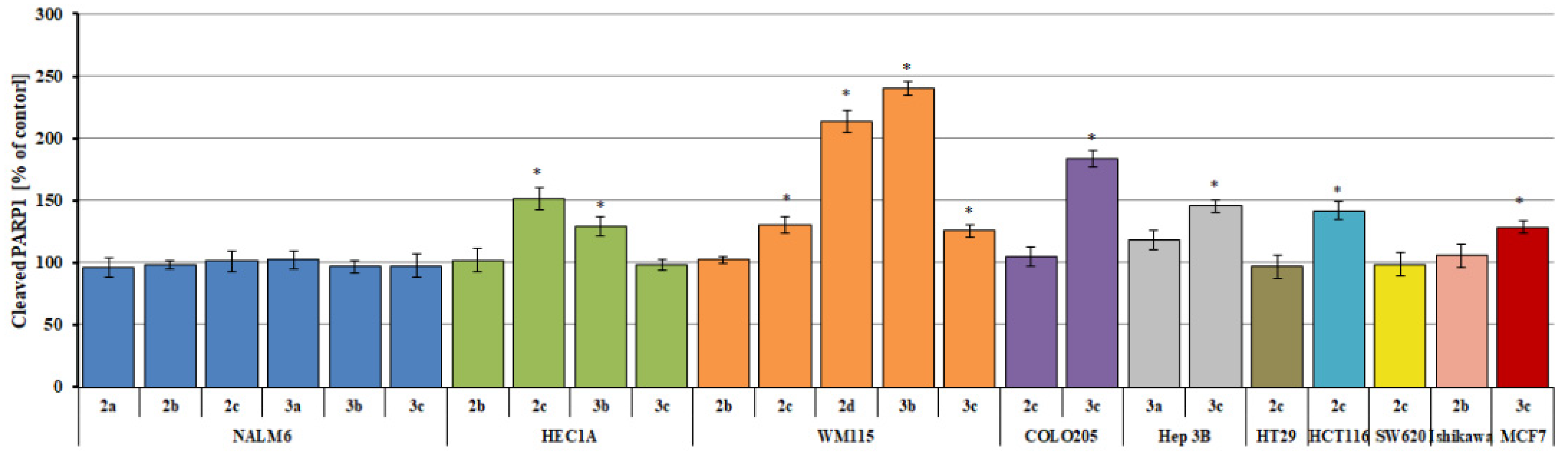
2.4. Measurement of Cleaved PARP1 Levels
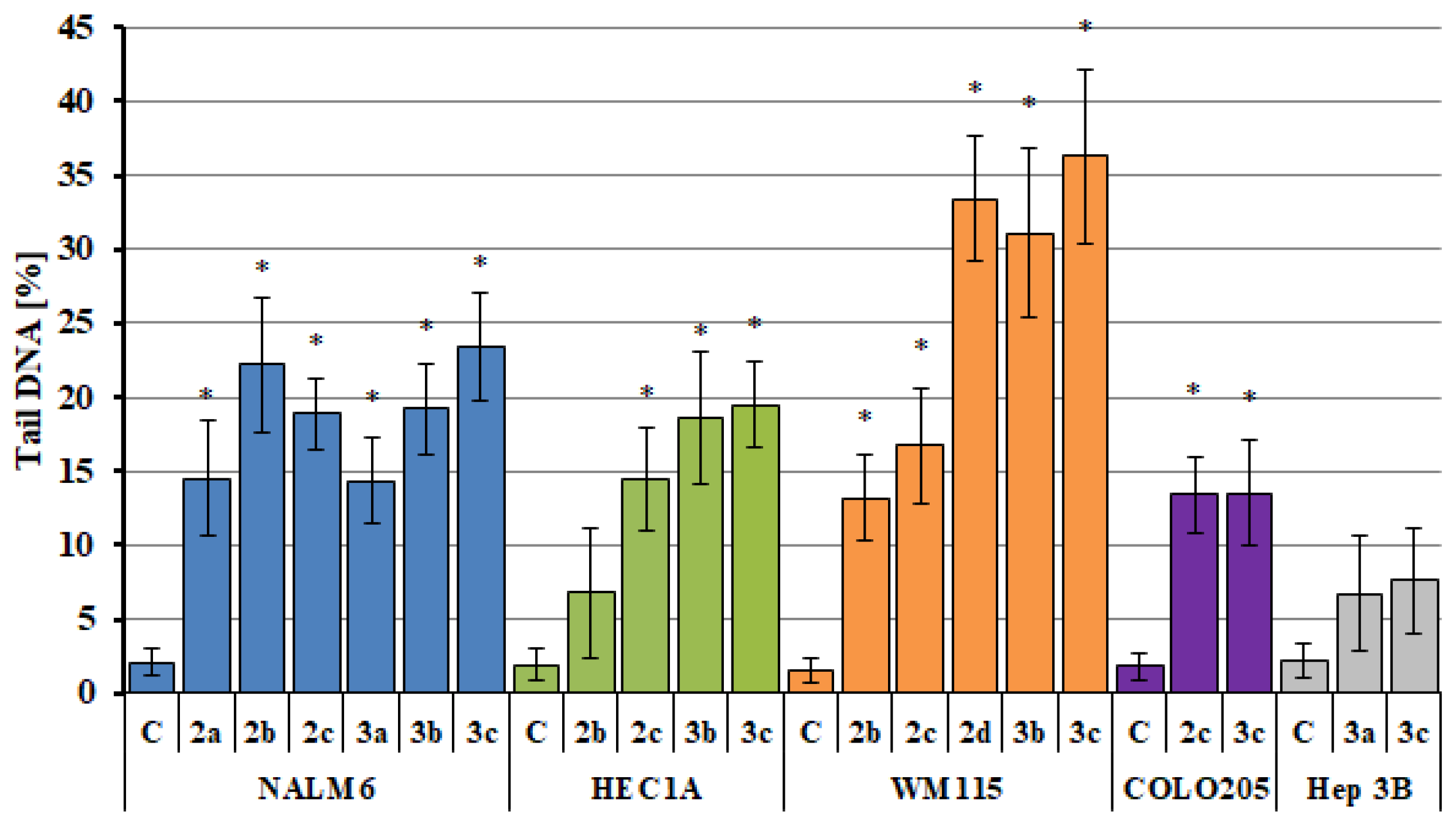
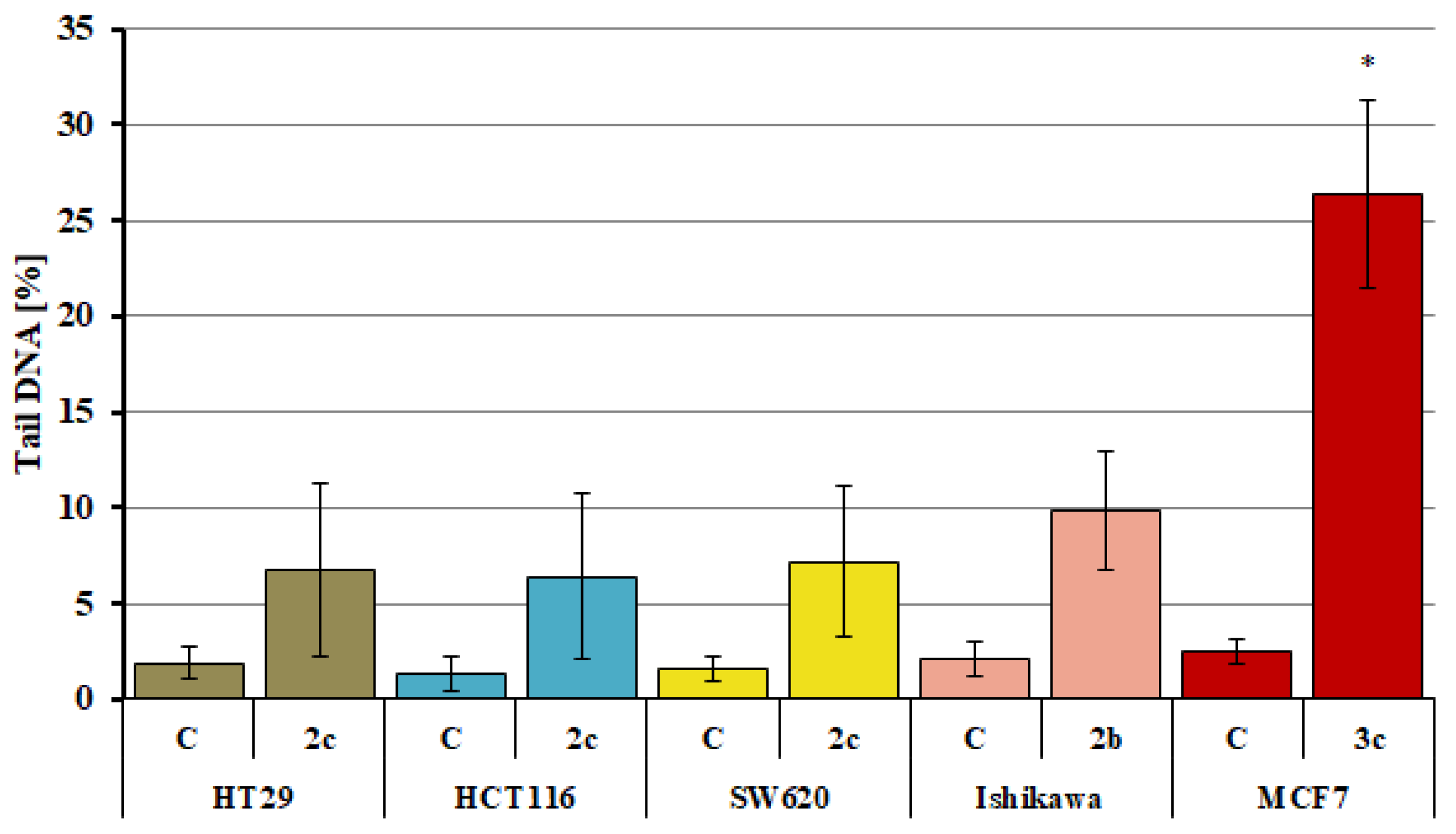
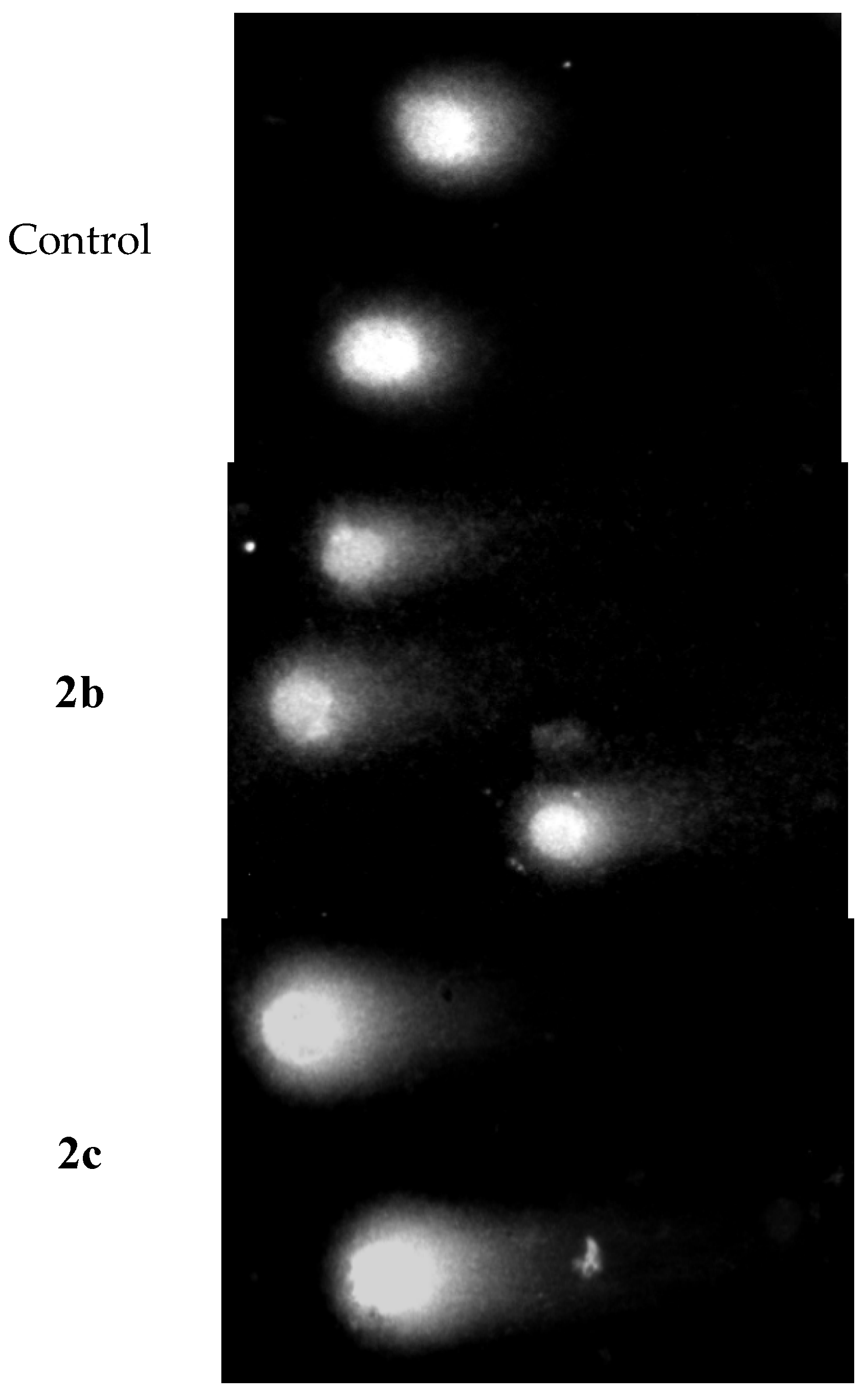
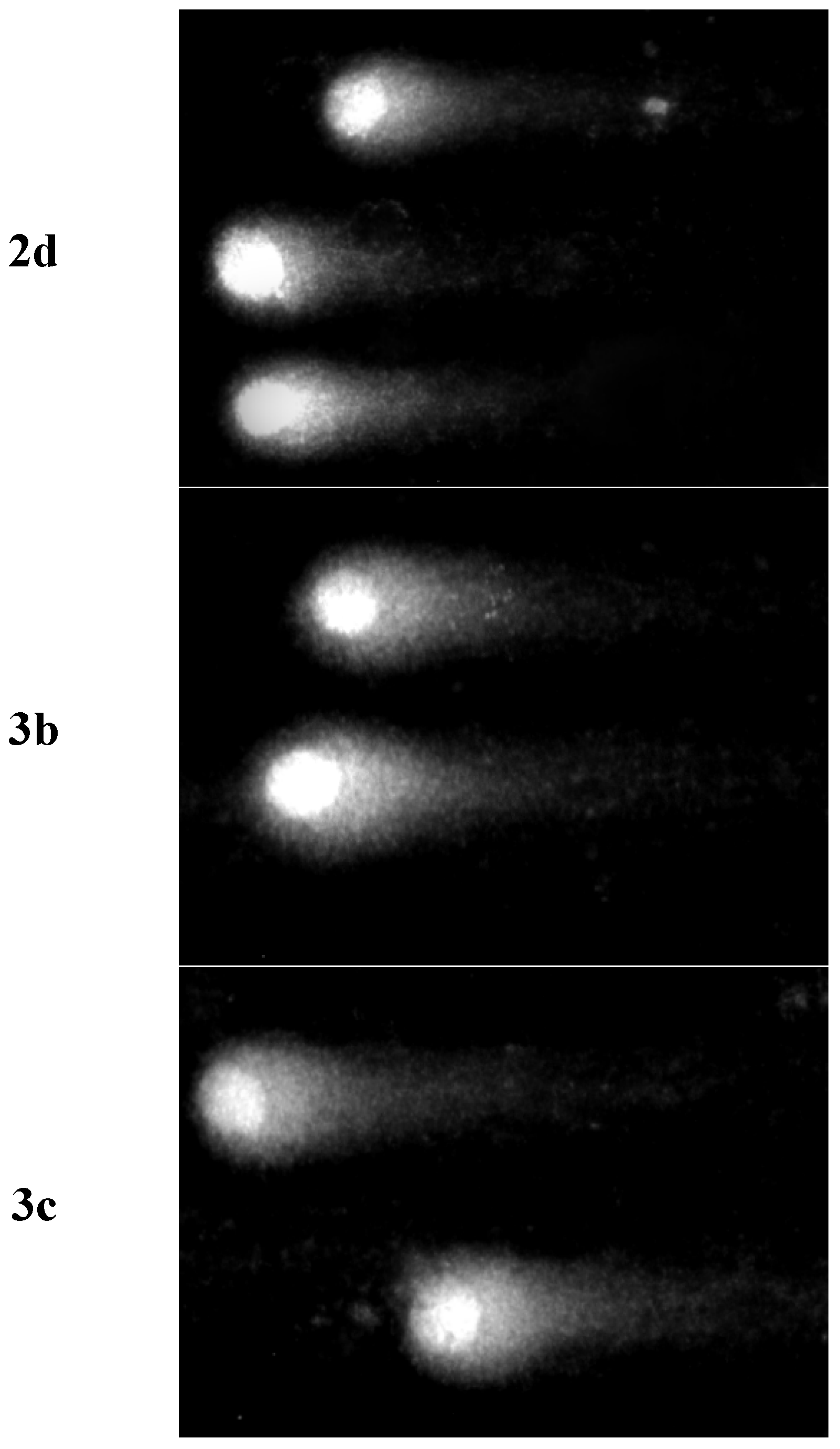
2.5. Analysis of DNA Damage Using the Alkaline Version of the Comet Assay (Single Cell Electrophoresis)—DNA Comet Assay to Assess DNA Damage of Cancer Cells
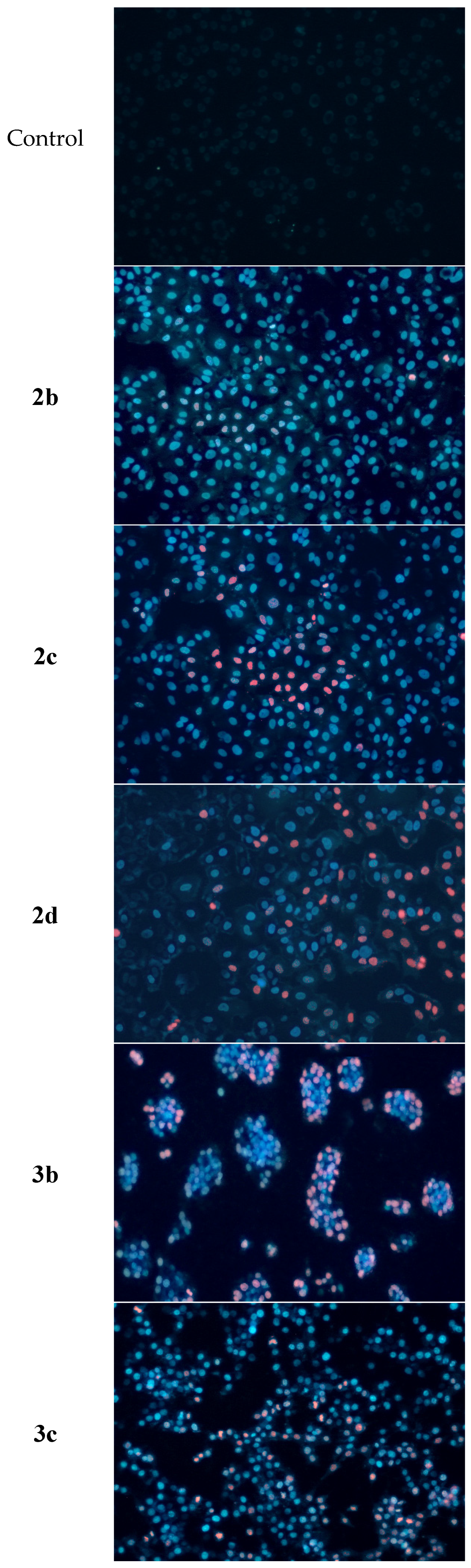
2.6. Determination of Apoptotic and Necrotic Cell Fractions by Fluorescence Microscopy (Double Staining of Cells with Fluorescent Dyes Hoechst 33258 and Propidium Iodide)
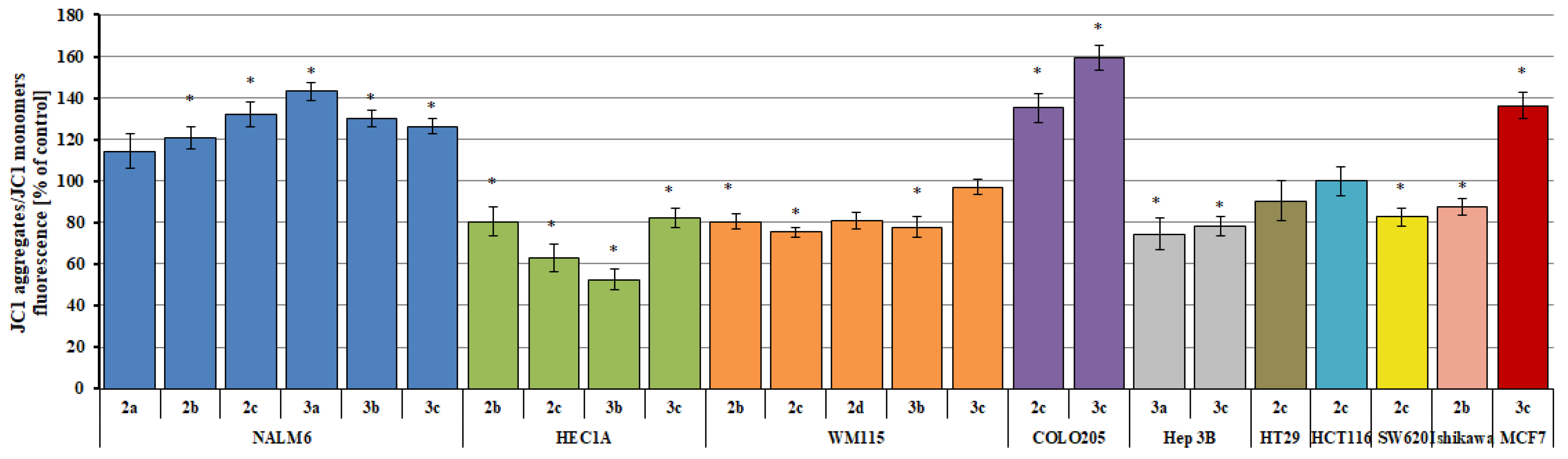
2.7. Changes in the Transmembrane Mitochondrial Potential (ΔΨm)
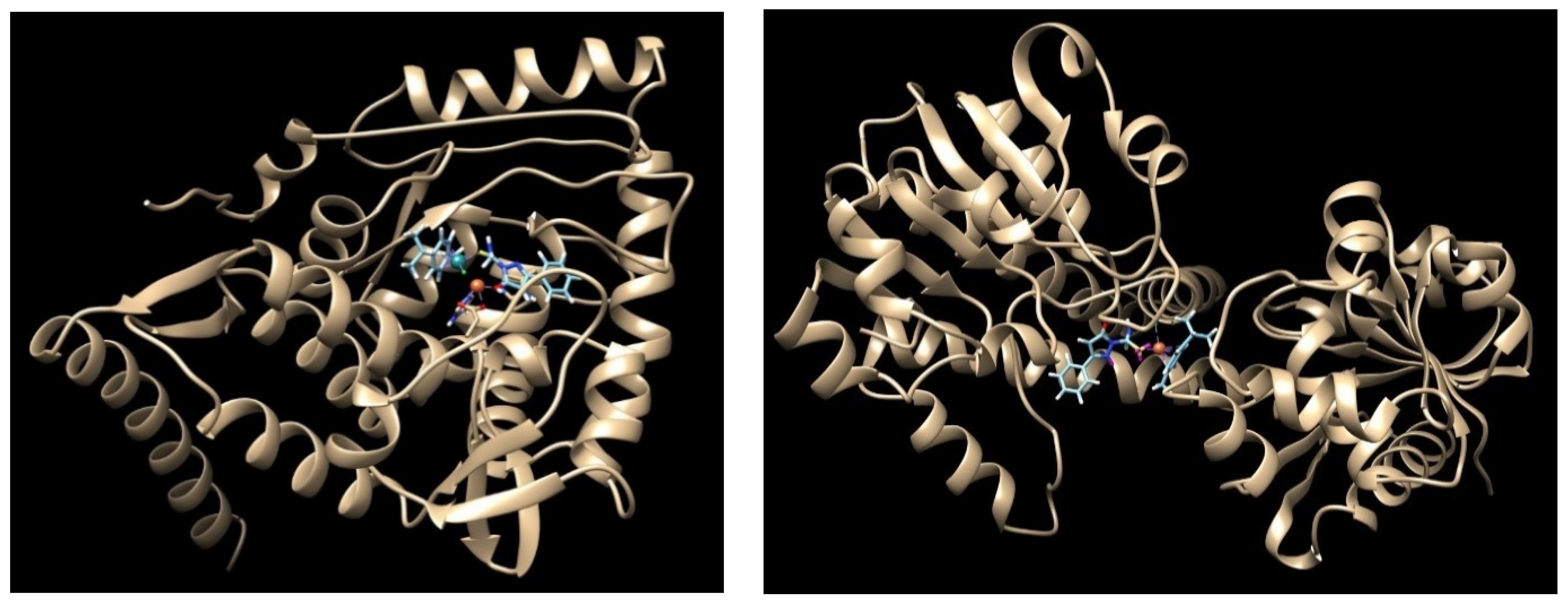
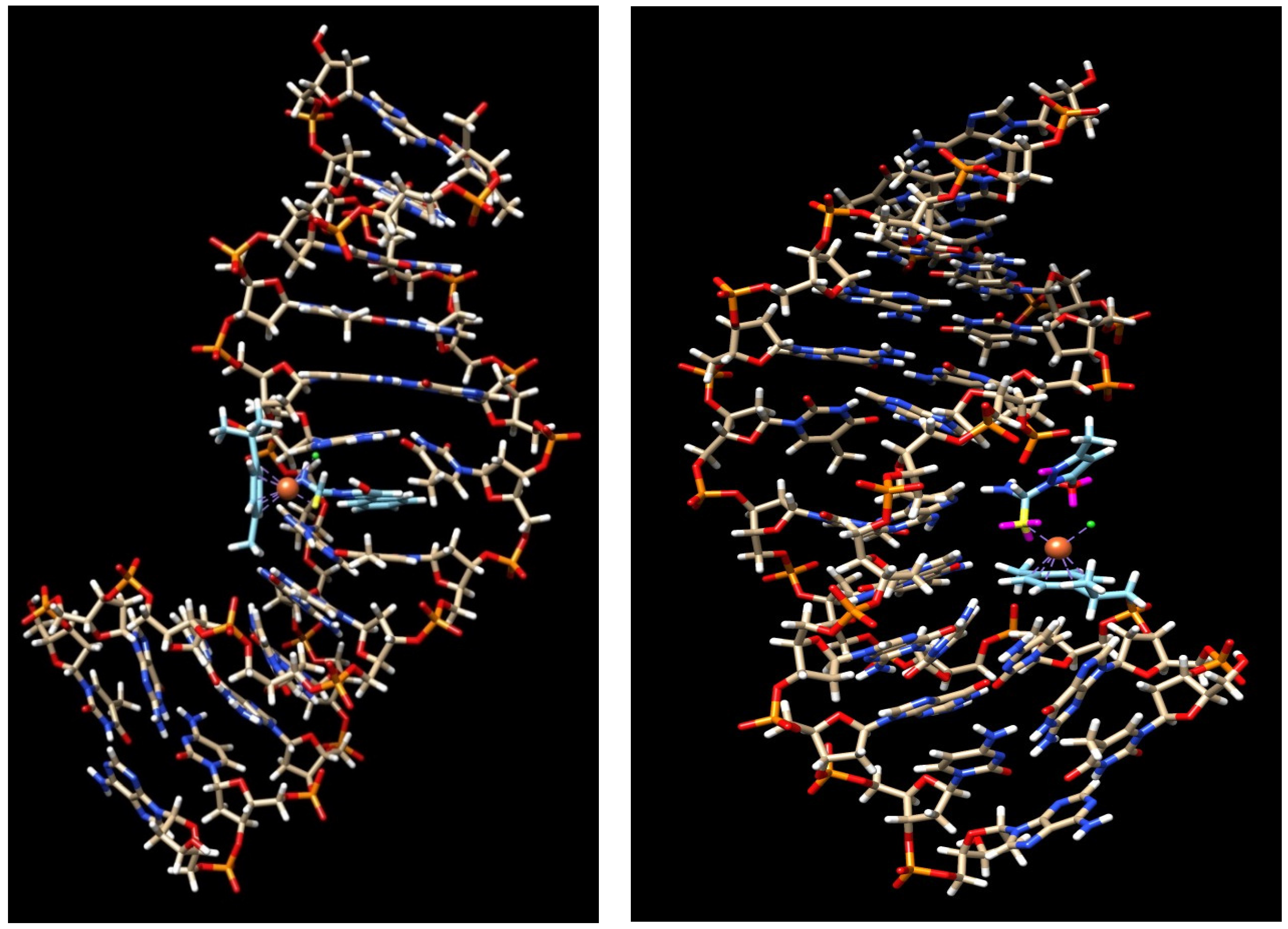
2.8. Computational Results
2.9. Lipophilicity Based on RP-TLC
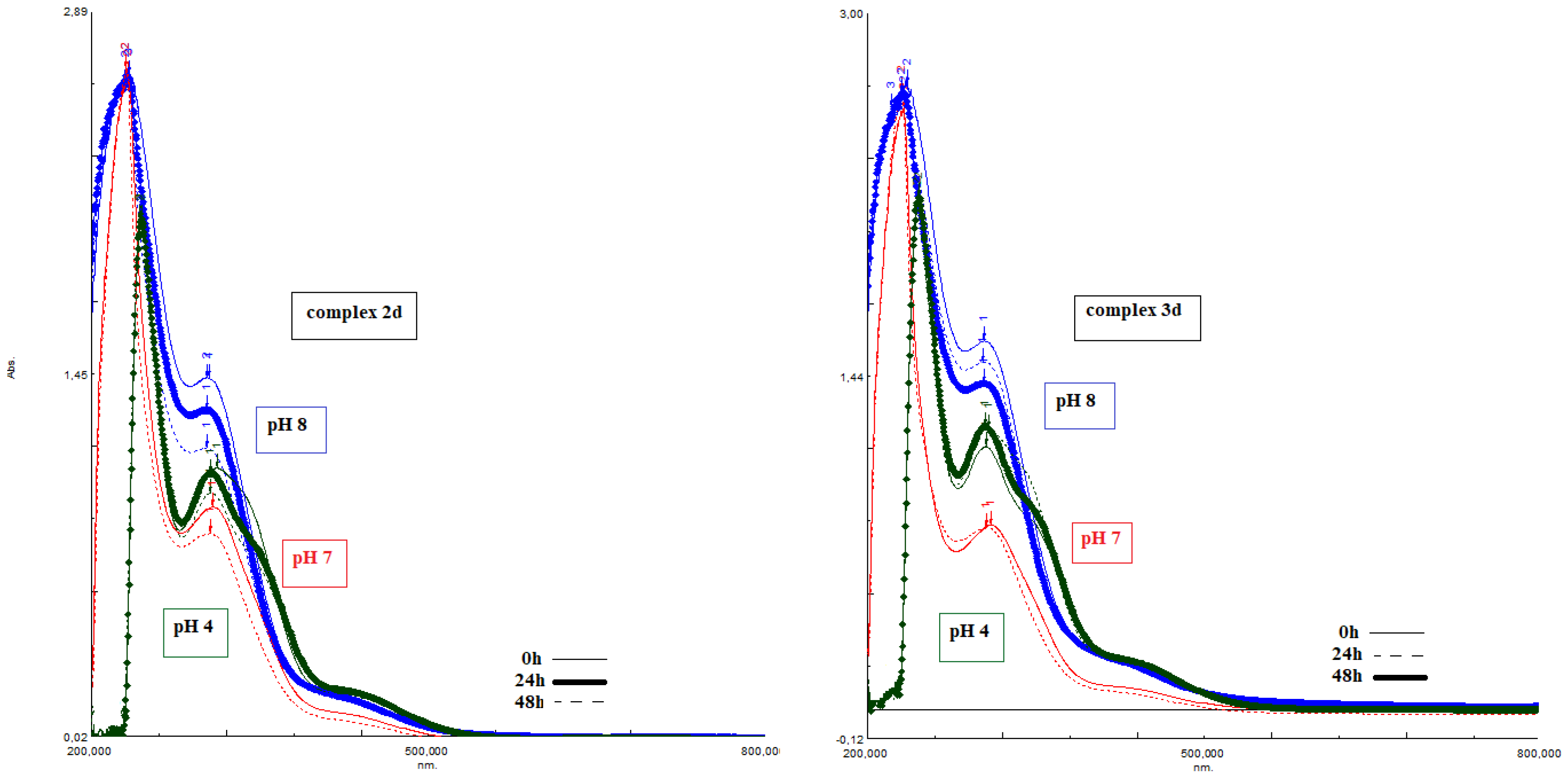
2.10. The pH Dependent of Stability of the Complexes
3. Discussion
4. Experimental Section, Material and Methods
4.1. Cell Lines and Cell Culture
4.2. Cytotoxicity Assay
4.3. Measurement of Membrane Fluidity
4.4. Measurement of Reactive Oxygen and Nitrogen Species
4.5. Measurement of Changes in Mitochondrial Potential (ΔΨm) Using the Microplate Spectrofluorimetric Method with the JC1 Fluorescent Probe
4.6. Analysis of DNA Damage Using the Comet Method in the Alkaline Version (Single Cell Electrophoresis)
4.7. Measurement of Cleaved PARP Levels
4.8. Determination of Apoptotic and Necrotic Cell Fractions by Fluorescence Microscopy (Double Staining of Cells with Fluorescent Dyes Hoechst 33258 and Propidium Iodide)
- Live cells (weak, dull light blue fluorescence);
- Cells in the early phase of PCD (bright, light blue fluorescence);
- Cells in the late phase of PCD (pink and purple fluorescence);
- Necrotic cells (intense red fluorescence).
4.9. Statistical Analysis
4.10. Molecular Docking
4.11. Lipophilicity of Chromatography Methods of RP-TLC
4.12. The pH Dependent of Stability of the Complexes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bruijnincx, P.C.A.; Sadler, P.J. New trends of metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef]
- Miller, M.C.; Stineman, C.N.; Vance, J.R.; West, D.X.; Hall, I.H. The cytotoxicity of copper(II) complexes of 2-acetyl-pirydyl-4N-substituted thiosemicarbazones. Anticancer Res. 1998, 18, 4131–4139. [Google Scholar] [PubMed]
- Zeglis, B.M.; Divilov, V.; Lewis, J.S. Role of Metalation in the Topoisomerase IIα Inhibition and Antiproliferation Activity of a Series of α-Heterocyclic-N4-Substituted Thiosemicarbazones and Their Cu(II) Complexes. J. Med. Chem. 2011, 54, 2391–2398. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Chen, D.; Cui, C.; Dou, Q.P. Disulfiram promotes the conversion of carcinogenic cadmium to a proteasome inhibitor with pro-apoptotic activity in human cancer cells. Toxicol. Appl. Pharmacol. 2008, 229, 206–214. [Google Scholar] [CrossRef]
- Abdul Manan, M.A.F.; Tahir, M.I.M.; Crouse, K.A.; Rosli, R.; How, F.N.F.; Watkin, D.J. The crystal structure and cytotoxicity of Centro-symmetric Copper(II) complex derived from S-methyldithiocarbazate with isatin. J. Chem. Crystallogr. 2011, 41, 1866–1871. [Google Scholar] [CrossRef]
- Zhang, H.; Thomas, R.; Oupicky, D.; Peng, F. Synthesis and characterization of new copper thosemicarbazone complexes with an ONNS quadridentate system: Cell growth inhibition, S–phase cell cycle arrest and proapoptotic activities on cisplatin–resistant neuroblastoma cells. J. Biol. Inorg.Chem. 2008, 13, 47–55. [Google Scholar] [CrossRef]
- Pellei, M.; Papini, G.; Trasatti, A.; Giorgetti, M.; Tonelli, D.; Minicucci, M.; Marzano, C.; Gandin, V.; Aquilanti, G.; Dolmella, A.; et al. Nitroimidazole and glucosamine conjugated heteroscorpionate ligands and related copper(II) complexes. Syntheses, biological activity and XAS studies. Dalton Trans. 2011, 40, 9877–9888. [Google Scholar] [CrossRef]
- Roy, S.; Maheswari, P.U.; Lutz, M.; Spek, A.L.; den Dulk, H.; Barends, S.; van Wezel, G.P.; Hartl, F.; Reedijk, J. DNA cleavage and antitumour activity of platinum(II) and copper(II) compounds derived from 4-methyl-2-N-(2-pyridylmethyl)aminophenol: Spectroscopic, electrochemical and biological investigation. Dalton Trans. 2009, 48, 10846–10860. [Google Scholar] [CrossRef]
- Gama, S.; Mendes, F.; Marques, F.; Santos, I.C.; Carvalho, M.F.; Correia, I.; Pessoa, J.C.; Santos, I.; Paulo, A. Copper(II) complexes with tridentate pyrazole-based ligands: Synthesis, characterization, DNA cleavage activity and cytotoxicity. J. Inorg. Biochem. 2011, 105, 637–644. [Google Scholar] [CrossRef]
- Flocke, L.S.; Trondl, R.; Jakupec, M.A.; Keppler, B.K. Molecularmode of action of NKP-1339—A clinically investigated rutheniumbaseddrug–Involves ER- and ROS-related effects in colon carcinomacell lines. Investig. New Drugs 2016, 34, 261–268. [Google Scholar] [CrossRef]
- Alessio, E.; Messori, L. NAMI-A and KP1019/1339, Two Iconic Ruthenium Anticancer Drug Candidates Face-to-Face: A Case Story in Medicinal Inorganic Chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef]
- Riccardi, C.; Musumeci, D.; Trifuoggi, M.; Irace, C.; Paduano, L.; Montesarchio, D. Anticancer Ruthenium(III) Complexes and Ru(III)-Containing Nanoformulations: An Update on the Mechanism of Action and Biological Activity. Pharmaceuticals 2019, 12, 146. [Google Scholar] [CrossRef]
- Hairat, S.; Zaki, M. Half sandwiched RutheniumII complexes: En Route towards the targeted delivery by Human Serum Albumin (HSA). J. Organomet. Chem. 2021, 937, 121732–121834. [Google Scholar] [CrossRef]
- Nazarov, A.A.; Hartinger, C.G.; Dyson, P.J. Opening the lipid on piano–stool complexes: An account of ruthenium(II)–arene complexes with medicinal applications. J. Organomet. Chem. 2014, 751, 251–260. [Google Scholar] [CrossRef]
- Allardyce, C.S.; Dyson, P.J. Theoretical Determination of Influence of the Metallic State of Oxidation toward Cytotoxic Activity: Case of Ruthenium Complexes. Platin. Met. Rev. 2001, 45, 62–69. [Google Scholar]
- Namiecińska, E.; Sadowska, B.; Więckowska-Szakiel, M.; Dołęga, A.; Pasternak, B.; Grażul, M.; Budzisz, E. Anticancer and antimicrobial properties of novel 6-p-cymene ruthenium(II) complexes containing a N,S-type ligand, their structural and theoretical characterization. RSC Adv. 2019, 9, 38629–38645. [Google Scholar] [CrossRef]
- Namiecińska, E.; Grażul, M.; Sadowska, B.; Więckowska-Szakiel, M.; Hikisz, P.; Pasternak, B.; Budzisz, E. Arene-Ruthenium(II) Complexes with Carbothiamidopyrazoles as a Potential Alternative for Antibiotic Resistance in Human. Molecules 2022, 27, 468. [Google Scholar] [CrossRef]
- Kovacic, P. Unifying mechanism for anticancer agents involving electron transfer and oxidative stress: Clinical implications. Med. Hypotheses 2007, 69, 510–516. [Google Scholar] [CrossRef]
- Engbrecht, M.; Mangerich, A. The Nucleolus and PARP1 in Cancer Biology. Cancers 2020, 12, 1813. [Google Scholar] [CrossRef]
- Shweshein, K.S.A.M.; Andrić, F.; Radoičići, A.; Zlatar, M.; Gruden-Pavlović, M.; Tešić, Ž.; Milojkovi-Opsenica, D. Lipohilicity Assessment of Ruthenium(II)–Arene Complexes by the Means of Reversed–Phase Thin–Layer Chromatography and DFT Calculations. Sci. World J. 2014, 14, 862796. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K.J. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Ronconi, L.; Sadler, P.J. Using coordination chemistry to design new Medicines. Coord. Chem. Rev. 2007, 251, 1633–1648. [Google Scholar] [CrossRef]
- Pettinari, R.; Marchetti, F.; Tombesi, A.; Di Nicola, C.; Pettinari, C.; Guo, C.; Zhang, Z.; Galindo, A.; Fadaei-Tirani, F.; Hadiji, M.; et al. Arene-ruthenium(II) complexes with pyrazole-based ligands bearing a pyridine moiety: Synthesis, structure, DFT calculations, and cytotoxicity. Inorg. Chim. Act. 2021, 528, 120610. [Google Scholar] [CrossRef]
- Huxham, L.A.; Cheu, E.L.S.; Patrick, B.O.; James, B.R. The synthesis, structural characterization, and in vitro anti-cancer activity of chloro(p-cymene) complexes of ruthenium(II) containing a disulfoxide ligand. Inorg. Chim. Act. 2003, 352, 238–246. [Google Scholar] [CrossRef]
- Vajs, J.; Steiner, I.; Brozovic, A.; Pevec, A.; Ambriović-Ristov, A.; Matković, M.; Piantanida, I.; Urankar, D.; Osmak, M.; Košmrlj, J. The 1,3-diaryltriazenido(p-cymene)ruthenium(II) complexes with a high in vitro anticancer activity. J. Inorg. Biochem. 2015, 153, 42–48. [Google Scholar] [CrossRef]
- Tabrizi, L.; Chiniforoshan, H.J. Ruthenium(II) p-cymene complexes of naphthoquinone derivatives as antitumor agents: A structure–activity relationship study. Organomet. Chem. 2016, 822, 211–220. [Google Scholar] [CrossRef]
- López-Lazaro, M. Two preclinical tests to evaluate anticancer activity and to help validate drug candidates for clinical trials. Oncoscience 2015, 2, 91–98. [Google Scholar] [CrossRef]
- López-Lazaro, M. How many times should we screen a chemical library to discover an anticancer drug? Drug Discov. Today 2015, 20, 167–169. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar]
- Gupta, S.; Vandevord, J.M.; Loftus, L.M.; Toupin, N.; Al-Afyouni, M.H.; Rohrabaugh, T.N.; Turro, C.; Kodanko, J.J. Ru(II)–Based Acetylacetonate Complexes Induce Apoptosis Selectively in Cancer Cells. Inorg. Chem. 2021, 60, 18964–18974. [Google Scholar] [CrossRef]
- Pavlović, M.; Tadić, A.; Gligorijević, N.; Poljarević, J.; Petrović, T.; Dojčinović, B.; Savić, A.; Radulović, S.; Grgurić-Šipka, S.; Aranđelović, S. Synthesis, chemical characterization, PARP inhibition, DNA binding and cellular uptake of novel ruthenium(II)-arene complexes bearing benzamide derivatives in human breast cancer cells. J. Inorg. Biochem. 2020, 210, 111155. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Robalo, M.P.; Tomaz, A.I.; Fernandes, A.R.; Roma-Rodrigues, C.; Teixeira, R.G.; Marques, F.; Folgueira, M.; Yáñez, J.; Gonzalez, A.A.; et al. Half–sandwich Ru(p–cymene) Compounds with Diphosphanes: In Vitro and In Vivo Evaluation As Potential Anticancer metallodrugs. Inorg. Chem. 2021, 60, 2914–2930. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Tian, Z.; Liu, X.; Gong, Y.; Zheng, H.; Ge, X.; Liu, Z. Imine–N–heterocyclic carbenes as versatile Ligands in Ruthenium(II) p–Cymene Anticancer Complexes: A Structure–Activity Relationship Study. Chem. Asian J. 2018, 13, 2923–2933. [Google Scholar] [CrossRef]
- Xu, Z.; Kong, D.; He, X.; Guo, L.; Ge, X.; Liu, X.; Zhang, H.; Li, J.; Yang, Y.; Liu, Z. Mitochondria–targeted half–sandwich rutheniumII diimine complexes anticancer and antimetastasis via ROS–mediated signalling. Inorg. Chem. Front. 2018, 5, 2100–2105. [Google Scholar] [CrossRef]
- Pitchaimani, J.; Raja, C.M.R.; Sujatha, S.; Mahapatra, K.S.; Moon, D.; Anthony, S.P.; Madhu, V. Arene ruthenium(II) complexes with chalcone, aminoantipyrine and aminopyrimidine based ligands: Synthesis, structure and preliminary evaluation of anti–leucemia activity. RSC Adv. 2016, 6, 90982–90992. [Google Scholar] [CrossRef]
- Spoerlein-Guettler, C.; Mahal, K.; Schobert, R.; Biersack, B. Ferrocene and (arene)ruthenium(II) complexes of the natural anticancer naphthoquinone plumbagin with enhanced efficacy against resistant cancer cells and a genuine mode of action. J. Inorg. Biochem. 2014, 138, 64–72. [Google Scholar] [CrossRef]
- Paitandi, R.P.; Sharma, V.; Singh, V.D.; Dwivedi, B.K.; Mobin, S.M.; Pandey, D.S. Pyrazole appended quinoline-BODIPY based arene ruthenium complexes: Their anticancer activity and potential applications in cellular imaging. Dalton Trans. 2018, 47, 17500–17514. [Google Scholar] [CrossRef]
- Chen, C.; Xu, C.; Li, T.; Lu, S.; Luo, F.; Wang, H. Novel NHC-coordinated ruthenium(II) arene complexes achieve synergistic efficacy as safe and effective anticancer therapeutics. Eur. J. Med. Chem. 2020, 203, 112605. [Google Scholar] [CrossRef]
- Vyas, K.M.; Sharma, D.; Magani, S.K.J.; Mobin, S.M.; Mukhopadhyay, S. In vitro evaluation of cytotoxicity and antimetastatic properties of novel arene ruthenium(II)-tetrazolato compounds on human cancer cell lines. Appl. Organomet. Chem. 2021, 35, e6187. [Google Scholar] [CrossRef]
- Kumar, R.R.; Ramesh, R.; Małecki, J.G. Synthesis and structure of arene ruthenium(II) benzhydrazone complexes: Antiproliferative activity, apoptosis induction and cell cycle analysis. J. Organomet. Chem. 2018, 862, 95–104. [Google Scholar] [CrossRef]
- Mohan, N.; Subarkhan, M.M.K.; Ramesh, R. Synthesis, antiproliferative activity and apoptosis–promoting effects of arene ruthenium(II) complexes with N, O chelating ligands. J. Organomet. Chem. 2018, 859, 124–131. [Google Scholar] [CrossRef]
- Colina-Vegas, L.; Oliveira, K.; Cunha, B.; Cominetti, M.; Navarro, M.; Azevedo Batista, A. Anti-Proliferative and Anti-Migration Activity of Arene-Ruthenium(II) Complexes with Azole Therapeutic Agents. Inorganics 2018, 6, 132. [Google Scholar] [CrossRef]
- Kasim, M.M.S.; Sundar, S.; Rengan, R. Synthesis and structure of new binuclear ruthenium(II) arene benzil bis(benzoylhydrazone) complexes: Investigation on antiproliferative activity and apoptosis induction. Inorg. Chem. Front. 2018, 5, 585–596. [Google Scholar] [CrossRef]
- Pettinari, R.; Pettinari, C.; Marchetti, F.; Skelton, B.W.; White, A.H.; Bonfili, L.; Cuccioloni, M.; Mozzicafreddo, M.; Cecarini, V.; Angeletti, M.J. Arene-ruthenium(II) acylpyrazolonato complexes: Apoptosis-promoting effects on human cancer cells. Med. Chem. 2014, 57, 4532–4542. [Google Scholar] [CrossRef]
- Subarkhan, M.M.K.; Ramesh, R.; Liu, Y. Synthesis and molecular structure of arene ruthenium(ii) benzhydrazone complexes: Impact of substitution at the chelating ligand and arene moiety on antiproliferative activity. New J. Chem. 2016, 40, 9813–9823. [Google Scholar] [CrossRef]
- Leo, A.J. Calculating log Poct from structures. Chem. Rev. 1993, 93, 1281–1306. [Google Scholar] [CrossRef]
- Muller, M.T.; Zehender, J.B.; Escher, B.I. Liposome–water and octanol–water partitioning of alkohol ethoxylates. Environ. Toxicol. Chem. 1999, 18, 2191–2198. [Google Scholar]
- Morais, T.S.; Santos, F.C.; Jorge, T.F.; Côrte-Real, L.; Madeira, P.J.A.; Marques, F.; Robalo, M.P.; Matos, A.; Santos, I.; Garcia, M.H. New water–soluble ruthenium(II) cytotoxic complex: Biological activity and cellular distribution. J. Inorg. Biochem. 2014, 130, 1–14. [Google Scholar] [CrossRef]
- Wolters, D.A.; Stefanopoulou, M.; Dyson, P.J.; Groessl, M. Combination of metallomics and proteomics to study the effects of the metallodrug RAPTA–T on human cancer cells. Metallomics 2012, 4, 1185–1196. [Google Scholar] [CrossRef]
- Groessl, M.; Zava, O.; Dyson, P.J. Cellular uptake and subcellular distribution of ruthenium–based metallodrugs under clinical investigation versus cisplatin. Metallomics 2011, 3, 591–599. [Google Scholar] [CrossRef]
- Aitken, J.B.; Antony, S.; Weekley, C.M.; Lai, B.; Spiccia, L.; Harris, H.H. Distinct cellular fates for KP1019 and NAMI–A determined by X-ray fluorescence imaging of single cells. Metallomics 2012, 4, 1051–1056. [Google Scholar] [CrossRef]
- Pasternak, K.; Wróbel, D.; Nowacka, O.; Pieszyński, I.; Bryszewska, M.; Kujawa, J. The effect of MLS laser radiation on cel lipid membranę. Ann. Agric. Environ. Med. 2018, 25, 108–113. [Google Scholar] [CrossRef]
- Owusu-Ansah, E.; Yavari, A.; Banerjee, U. A protocol for in vivo detection of reactive oxygen species. Protoc. Exch. 2008. [Google Scholar] [CrossRef]
- Zhao, H.; Joseph, J.; Fales, H.M.; Sokoloski, E.A.; Levine, R.L.; Vasquez Vivar, J.; Kalyanaraman, B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. USA 2005, 102, 5727–5732. [Google Scholar] [CrossRef]
- Balcerczyk, A.; Soszynski, M.; Bartosz, G. On the specificity of 4-amino-5-methylamino-2′,7′-difluorofluorescein as a probe for nitric oxide. Free Radic. Biol. Med. 2005, 39, 327–335. [Google Scholar] [CrossRef]
- Reers, M.; Smith, T.W.; Bo Chen, L. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry 1991, 30, 4480–4486. [Google Scholar] [CrossRef]
- Cossarizza, A.; Baccarani-Contri, M.; Kalashnikova, G.; Franceschi, C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun. 1993, 30, 40–45. [Google Scholar] [CrossRef]
- Nuydens, R.; Novalbos, J.; Dispersyn, G.; Weber, C.; Borgers, M.; Geerts, H. A rapid method for the evaluation of compounds with mitochondria–protective properties. J Neurosci Methods. 1999, 15, 153–159. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- González, P.; Marín, C.; Rodríguez-González, I.; Hitos, A.B.; Rosales, M.J.; Reina, M.; Díaz, J.G.; Gonzáles-Coloma, A.; Sánchez-Moreno, M. In vitro activity of C20–diterpenoid alkaloid derivatives in promastigotes and intracellular amastigotes of Leishmania infantum. Int. J. Antimicrob. Agents 2005, 25, 136–141. [Google Scholar] [CrossRef]
- Fandzloch, M.; Jędrzejewski, T.; Dobrzańska, L.; Esteban-Parra, G.M.; Wiśniewska, J.; Paneth, A.; Paneth, P.; Sitkowski, J. New organometallic ruthenium(II) complexes with purine analogs—A wide perspective on their biological application. Dalton Trans. 2021, 50, 5557–5573. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B. Gaussian, Version 16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.D.; Head-Gordon, M.J. Systematic optimization of long-range corrected hybrid density functionals. Chem. Phys. 2008, 128, 084106. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Goodwill, K.E.; Sabatier, C.; Marks, C.; Raag, R.; Fitzpatrick, P.F.; Stevens, R.C. Crystal structure of tyrosine hydroxylase at 2.3Å and its implications for inherited neurodegenerative diseases. Nat. Struct. Biol. 1997, 4, 578–585. [Google Scholar] [CrossRef]
- Sennett, N.C.; Kadirvelraj, R.; Wood, Z.A. Cofactor binding triggers a molecular switch to allosterically activate human UDP–α–D–glucose 6–dehydrogenase. Biochemistry 2012, 51, 9364–9374. [Google Scholar] [CrossRef]
- Volk, D.E.; Rice, J.S.; Luxon, B.A.; Yeh, H.J.; Liang, C.; Xie, G.; Sayer, J.M.; Jerina, D.M.; Gorenstein, D.G. NMR evidence for syn–anti interconversion of a trans opened (10R)–dA adduct of benza[a]pyrene (7S,8R)–diol (9R,10S)–epoxide in a DNA duplex. Biochemistry 2000, 39, 14040–14053. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, I.N.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Protein Data Bank Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; Mccabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Milliam, J.M. GaussView, Version 6.1; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
| Cell Line | Cytotoxic Effect of Investigated Compounds. IC50 (µM) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | 1d | 2a | 2b | 2c | 2d | 3a | 3b | 3c | 3d | 4 | |
| HT29 | >200 | >200 | >200 | >200 | 111.3 ± 4.9 | 106.6 ± 5.9 | 29.5 ± 2.1 | 168.1 ± 7.5 | 123.3 ± 3.9 | 122.8 ± 3.7 | 128.1 ± 4.2 | 185.2 ± 1.8 | >200 |
| Colo205 | >200 | >200 | >200 | >200 | 109.8 ± 6.7 | 114.6 ± 4.1 | 32.6 ± 3.3 | >200 | 59.6 ± 3.4 | 91.6 ± 1.6 | 26.7 ± 2.1 | >200 | >200 |
| SW620 | >200 | >200 | >200 | >200 | 117.6 ± 6.1 | 107.3 ± 3.4 | 49.4 ± 1.2 | >200 | 63.9 ± 2.7 | 78.8 ± 7.4 | 90.1 ± 1.7 | >200 | >200 |
| LoVo | >200 | >200 | >200 | >200 | >200 | 59.7 ± 3.2 | 42.6 ± 3.2 | >200 | 126.7 ± 3.7 | 99.1 ± 9.4 | 32.4 ± 1.8 | 110.3 ± 6.9 | 167.6 ± 7 |
| Caco2 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 107.2 ± 8.4 | 82.4 ± 8.4 | 172.2 ± 8.2 | 161.9 ± 6.1 | >200 |
| Hep 3b | >200 | >200 | >200 | >200 | >200 | >200 | 104.2 ± 4.4 | >200 | 55.3 ± 2.6 | 62.4 ± 2.7 | 52.1 ± 3.4 | >200 | 166.6 ± 6.8 |
| HEC1A | 110.8 ± 9.6 | 114.4 ± 11.3 | 109.5 ± 8.9 | 99.1 ± 5.7 | 62.3 ± 0.9 | 36.4 ± 1.7 | 24.5 ± 0.9 | 67.8 ± 6.1 | 77.2 ± 4.4 | 40.1 ± 2.1 | 39.9 ± 4.1 | 127.6 ± 2.1 | 107.8 ± 3.4 |
| Ishkiawa | 124.3 ± 4.8 | 100.1 ± 4.6 | 94 ± 2.4 | >200 | 90.7 ± 3.7 | 51.4 ± 1.9 | 142.3 ± 7.4 | 100 ± 6.7 | >200 | 121.4 ± 3.7 | 124.3 ± 0.9 | >200 | 120.1 ± 7.2 |
| Hela | >200 | >200 | >200 | >200 | >200 | 164.5 ± 8.5 | 124.3 ± 7.2 | 111.3 ± 1.7 | 98.7 ± 7.4 | 101.2 ± 6.7 | 78.8 ± 1.1 | >200 | >200 |
| A549 | >200 | >200 | >200 | >200 | 120.7 ± 3.6 | >200 | >200 | >200 | >200 | 162.4 ± 7.9 | 191.1 ± 4.3 | 152.2 ± 2.8 | >200 |
| HCC38 | >200 | >200 | >200 | >200 | 84.4 ± 1.1 | 63.7 ± 4.4 | >200 | >200 | >200 | >200 | 131.4 ± 2.2 | 187.6 ± 6.4 | >200 |
| MCF7 | >200 | >200 | >200 | >200 | >200 | 82.7 ± 3 | 83.4 ± 7.3 | >200 | >200 | 132.4 ± 5.3 | 30.4 ± 2.1 | >200 | >200 |
| WM115 | >200 | >200 | >200 | 90.9 ± 10.2 | 60.2 ± 6.3 | 54.9 ± 5.9 | 26.7 ± 3.3 | 8 ± 0.9 | 64.1 ± 5.1 | 51.7 ± 5.3 | 18.68 ± 1.7 | >200 | >200 |
| NALM6 | >200 | >200 | 152.3 ± 26.7 | >200 | 51.6 ± 5.7 | 40 ± 5.6 | 11.7 ± 1.6 | >200 | 46.1 ± 5.1 | 18.5 ± 2.3 | 19.8 ± 1.8 | >200 | >200 |
| HL60 | >200 | >200 | >200 | >200 | 88.9 ± 6.1 | 80.8 ± 3.9 | 86.5 ± 8 | >200 | 79.7 ± 7.2 | 52.9 ± 2.1 | 70.4 ± 6 | >200 | >200 |
| Compounds | Cytotoxic Effect of Reference Compounds, IC50 (µM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NALM6 | HEC1A | WM115 | COLO205 | Hep 3b | HT29 | HCT116 | SW620 | Ishikawa | MCF7 | |
| Cisplatin | 0.7 ± 0.3 | 89.4 ± 4.7 | 16.9 ± 4.3 | 26.7 ± 4.2 | 24.3 ± 2.9 | 141.3 ± 7.4 | 37.1 ± 2.8 | 26.4 ± 6.3 | 16.1 ± 2.5 | 11.4 ± 1.3 |
| NAMI-A | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 14.1 ± 1.5 |
| Cell Line | Cytotoxic Effect of Investigated Compounds, IC50 (µM) | ||||||
|---|---|---|---|---|---|---|---|
| 2a | 2b | 2c | 2d | 3a | 3b | 3c | |
| HMEC1 | 156.1 ± 4.9 | 124.6 ± 2.1 | 191.7 ± 6.5 | 163.0 ± 5.9 | >200 | 134.8 ± 6.7 | 164.5 ± 3.1 |
| Compounds | Conformers | 1TOH | 4EDF | 1FYY Intercallation | 1FYY Minor Groove |
|---|---|---|---|---|---|
| 2a | N17 | 50.4 | 47.0 | 49.9 | 35.2 |
| S17 | 57.8 | 62.8 | 55.2 | 46.0 | |
| 2b | N18 | 57.0 | 50.7 | 52.1 | 33.8 |
| S18 | 69.0 | 73.6 | 59.5 | 45.2 | |
| 2c | N1 | 57.2 | 60.3 | 61.5 | 42.3 |
| S1 | 73.2 | 77.9 | 66.7 | 46.8 | |
| 2d | N2 | 52.3 | 46.4 | 42.6 | 37.9 |
| S2 | 62.3 | 64.1 | 52.1 | 47.1 |
| Compounds | 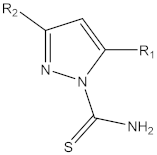 L | RM |
|---|---|---|
| 1a | L1: R1 = –CH3; R2 = –CH3 | 2.32 |
| 1b | L2: R1 = –CH2CH3; R2 = –CH2CH3 | 2.51 |
| 1c | L3: R1 = –OH; R2 = —C6H5 | 2.04 |
| 1d | L4: R1 = –OH; R2 = –CH3 | 1.29 |
| 2a | p-cymRuL1Cl | 2.40 |
| 2b | p-cymRuL2Cl | 2.59 |
| 2c | p-cymRuL3Cl | 2.27 |
| 2d | p-cymRuL4Cl | 1.64 |
| 3a | p-cymRuL1PF6 | 2.55 |
| 3b | p-cymRuL2PF6 | 2.73 |
| 3c | p-cymRuL3PF6 | 2.61 |
| 3d | p-cymRuL4PF6 | 2.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hikisz, P.; Namiecińska, E.; Paneth, P.; Budzisz, E. Mechanistic Studies of Arene–Ruthenium(II) Complexes with Carbothioamidopyrazoles as Alternative Cancer Drugs. Molecules 2023, 28, 3969. https://doi.org/10.3390/molecules28093969
Hikisz P, Namiecińska E, Paneth P, Budzisz E. Mechanistic Studies of Arene–Ruthenium(II) Complexes with Carbothioamidopyrazoles as Alternative Cancer Drugs. Molecules. 2023; 28(9):3969. https://doi.org/10.3390/molecules28093969
Chicago/Turabian StyleHikisz, Paweł, Ewelina Namiecińska, Piotr Paneth, and Elzbieta Budzisz. 2023. "Mechanistic Studies of Arene–Ruthenium(II) Complexes with Carbothioamidopyrazoles as Alternative Cancer Drugs" Molecules 28, no. 9: 3969. https://doi.org/10.3390/molecules28093969
APA StyleHikisz, P., Namiecińska, E., Paneth, P., & Budzisz, E. (2023). Mechanistic Studies of Arene–Ruthenium(II) Complexes with Carbothioamidopyrazoles as Alternative Cancer Drugs. Molecules, 28(9), 3969. https://doi.org/10.3390/molecules28093969







