Study on Extraction, Physicochemical Properties, and Bacterio-Static Activity of Polysaccharides from Phellinus linteus
Abstract
1. Introduction
2. Results and Discussion
2.1. Glucose Labeling Curve Determination
2.2. Effect of Ultrasonic Time on the Yield of PLP
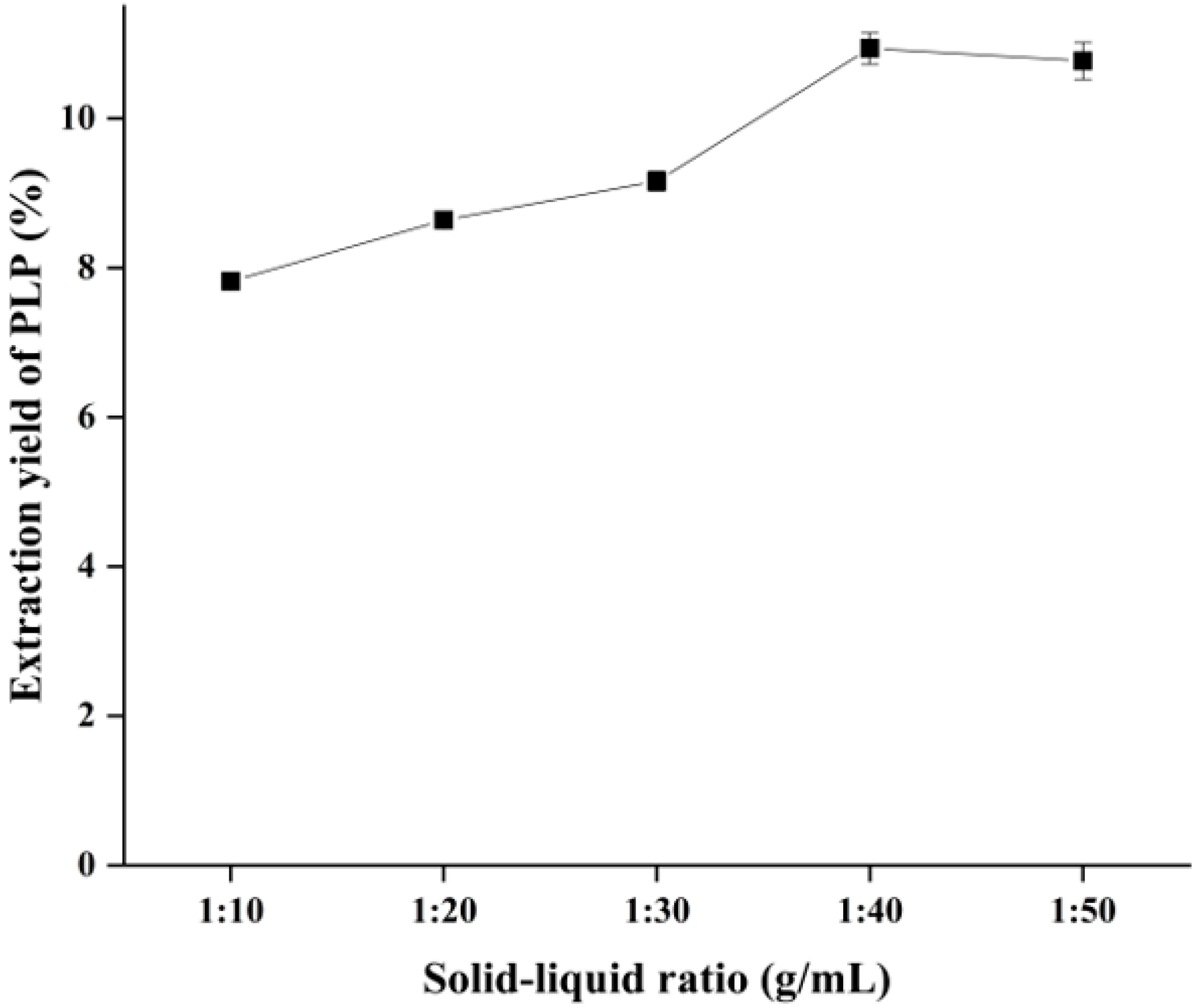
2.3. Effect of Solid–Liquid Ratio on the Yield of PLP
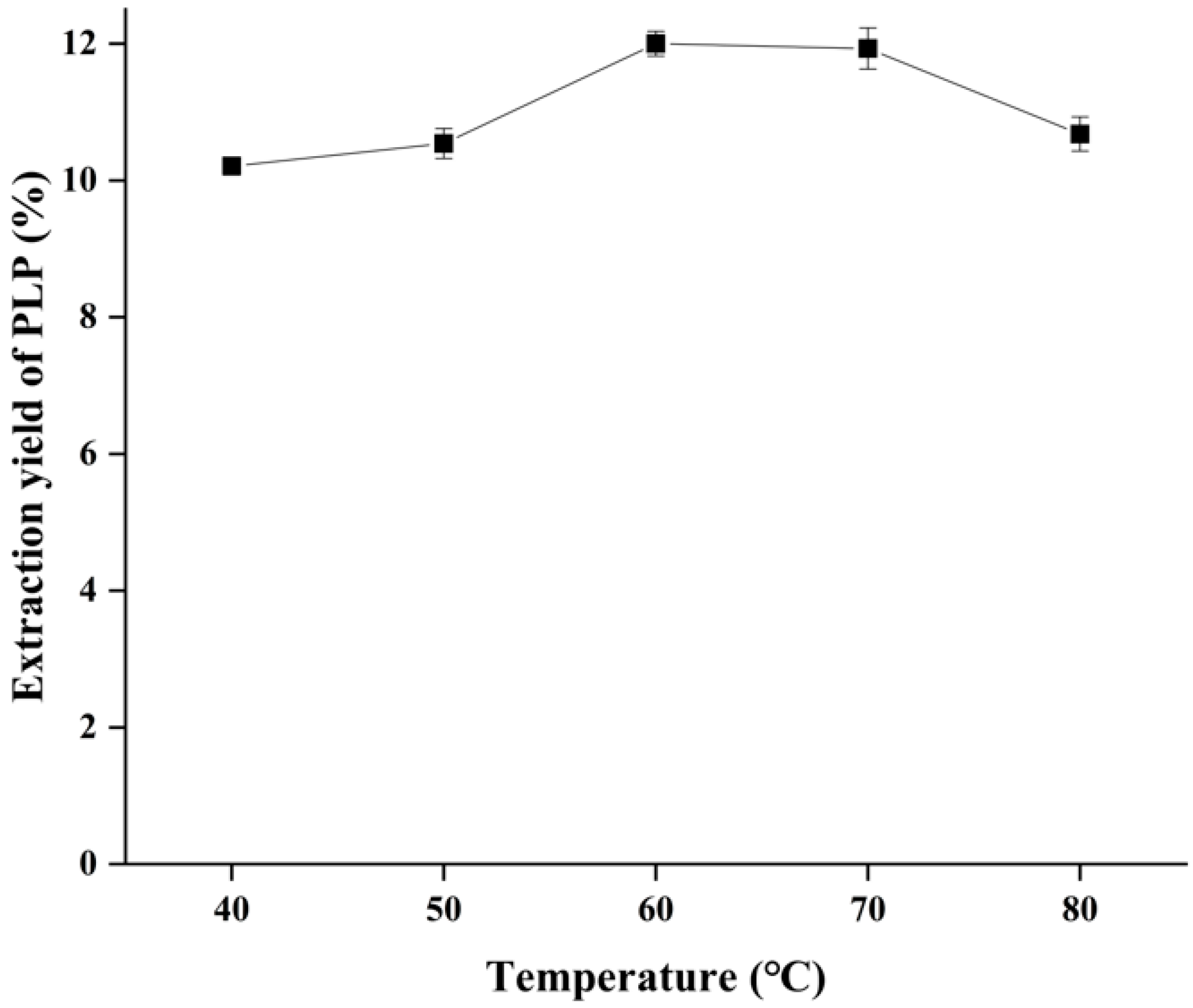
2.4. Effect of Ultrasonic Temperature on the Yield of PLP
2.5. Response Surface Test of Extraction PLP by Ultrasonic-Assisted Extraction
2.5.1. Response Surface Test Protocol and Results
2.5.2. Analysis of Variance
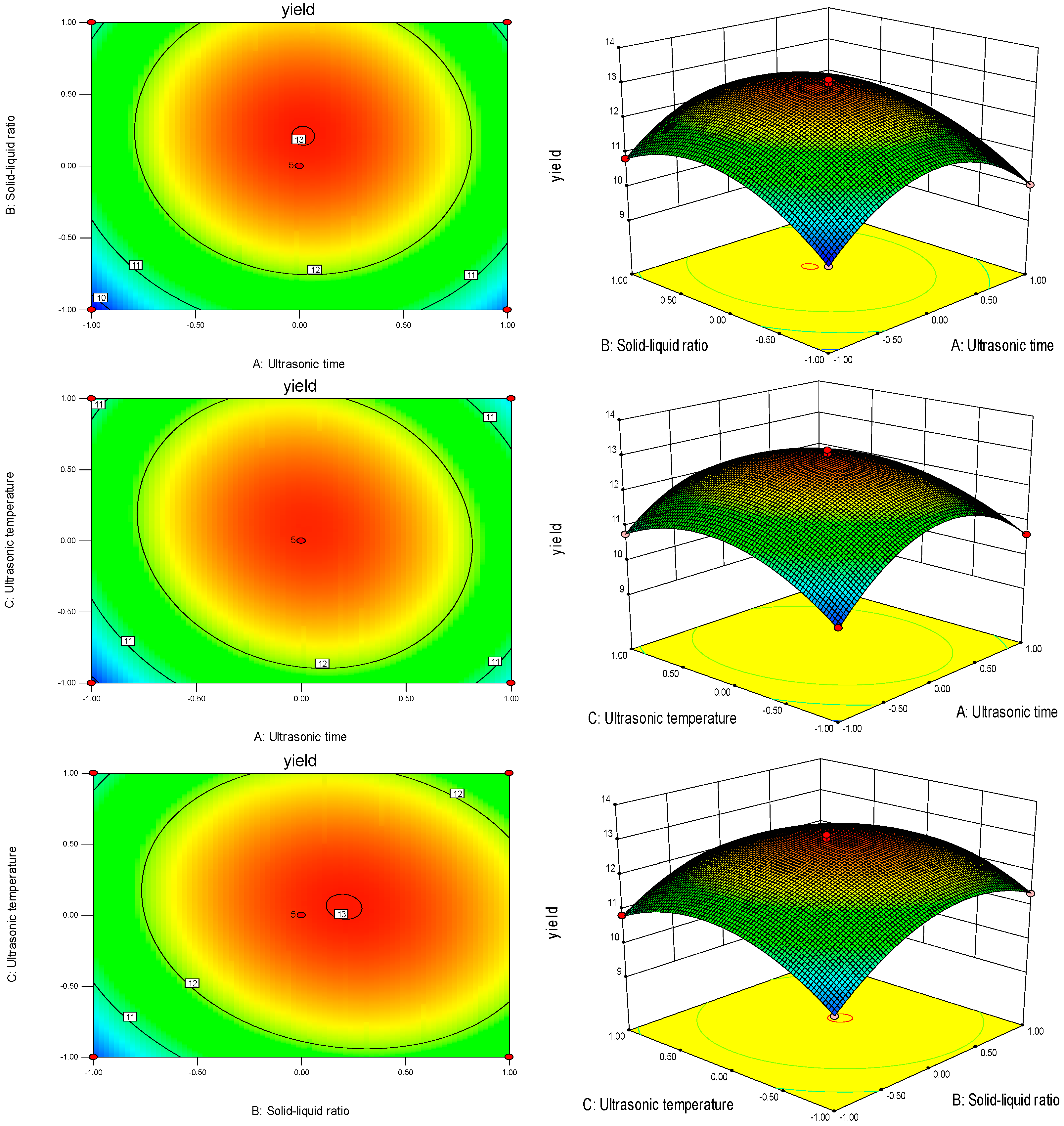
2.5.3. Results of Interactions between Factors
2.5.4. Validation Experiments
2.6. Physicochemical Properties of PLP
2.6.1. Results of Total Sugar Content Measurement
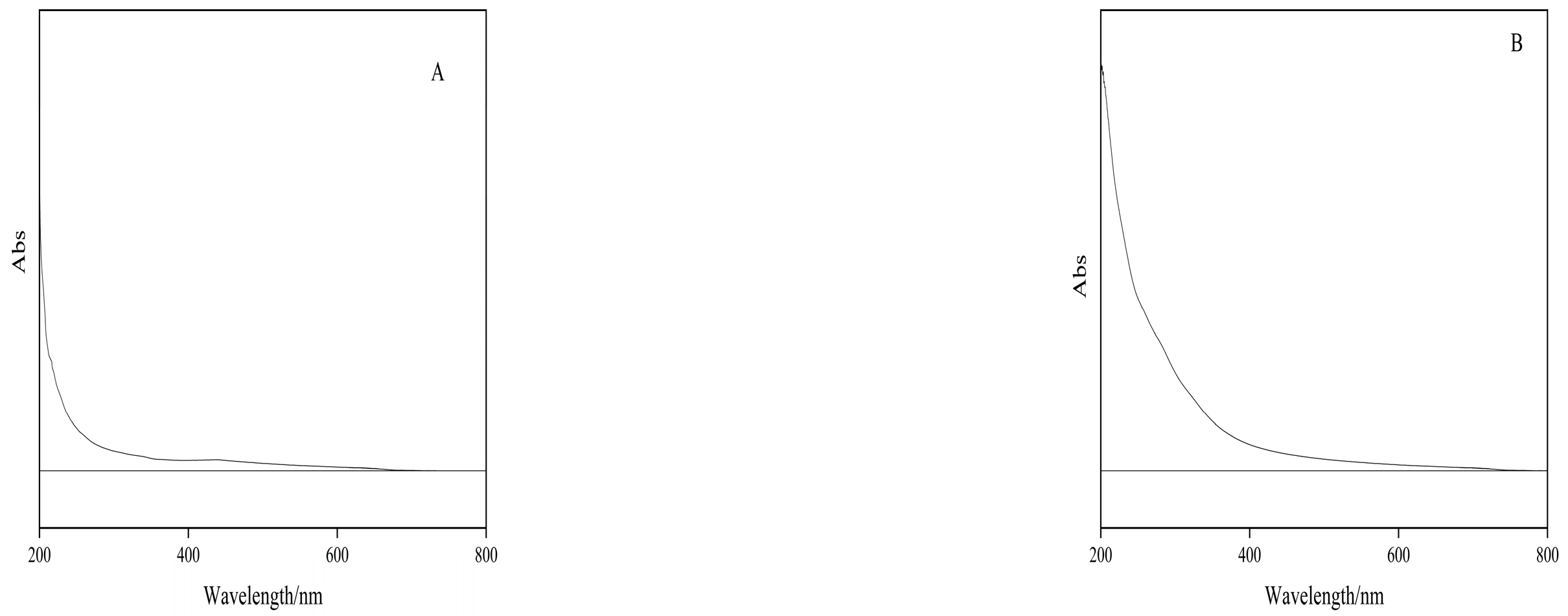
2.6.2. Results of Polysaccharide UV Full Wavelength Scan Analysis
2.6.3. Protein Content Measurement Results
2.6.4. Ash Content Measurement Results
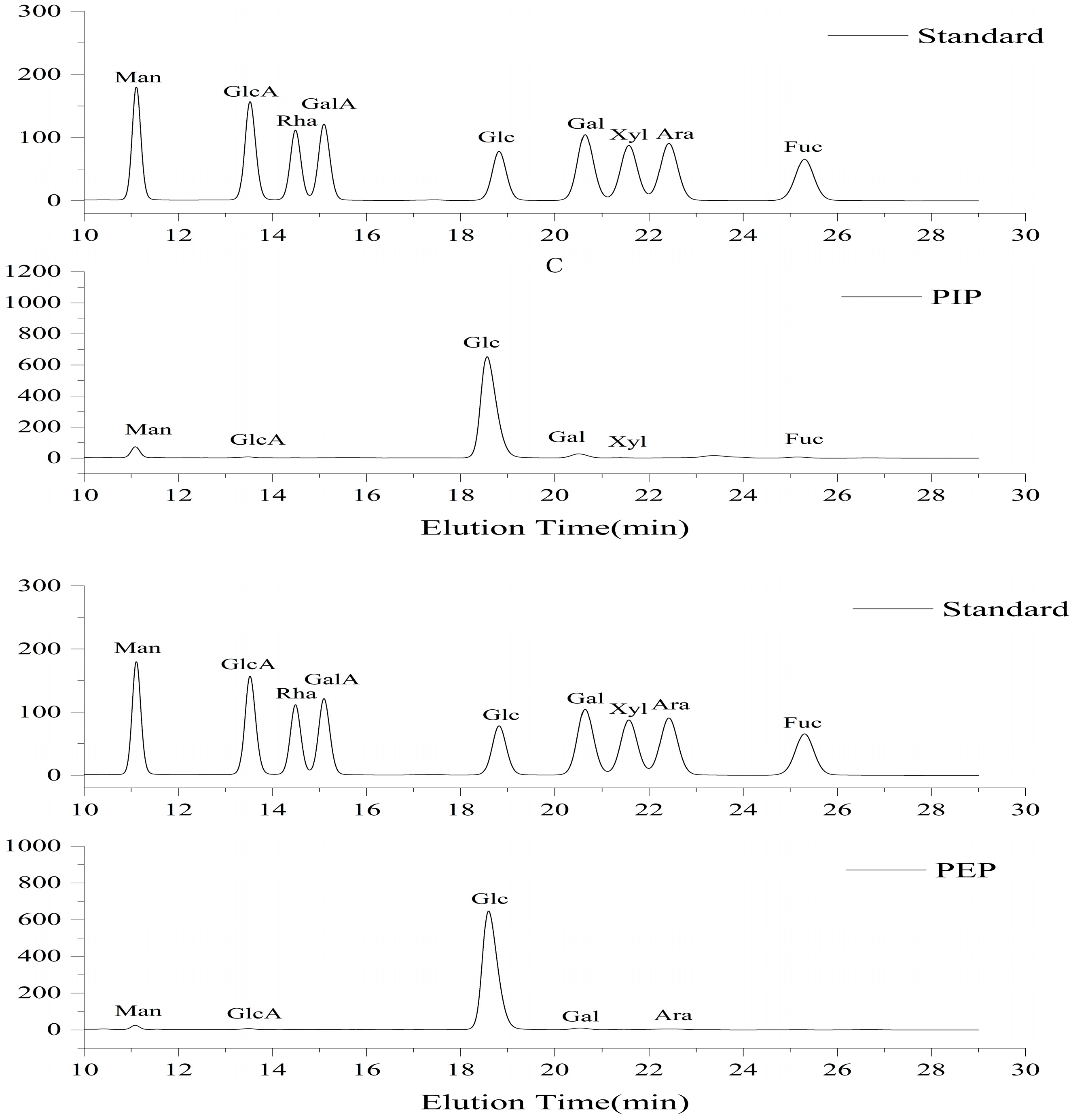
2.6.5. Results of Monosaccharide Composition Determination
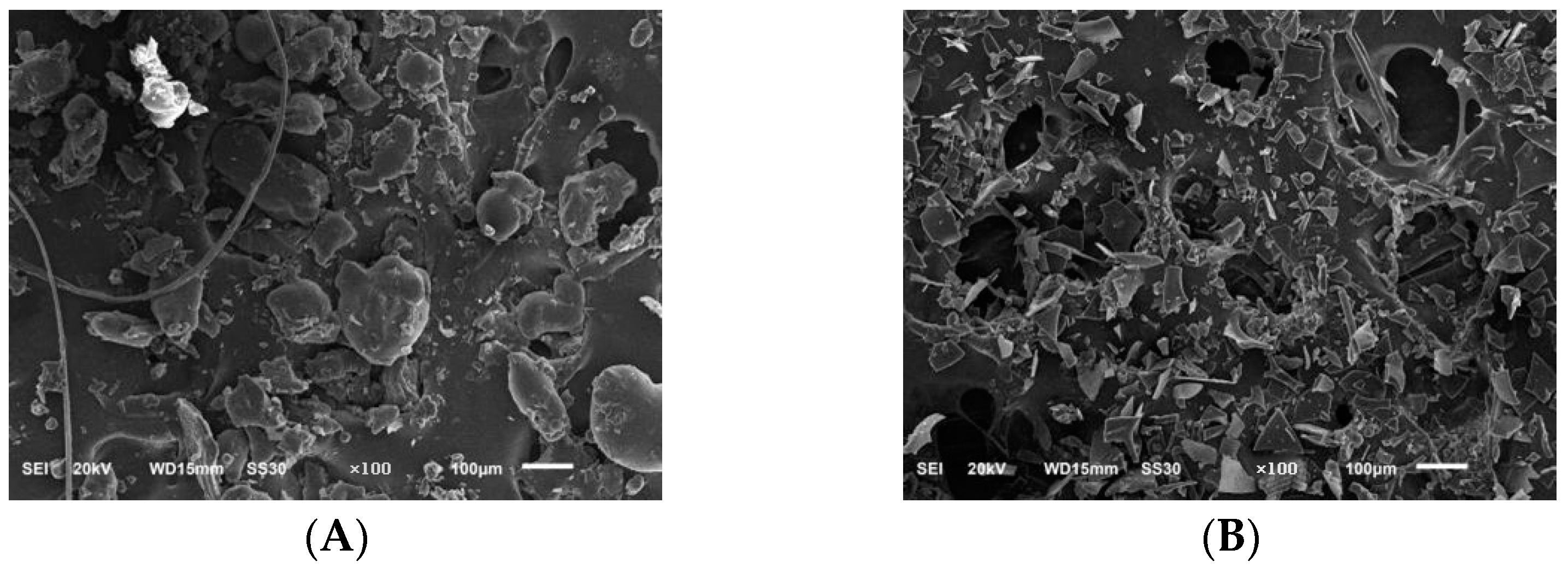
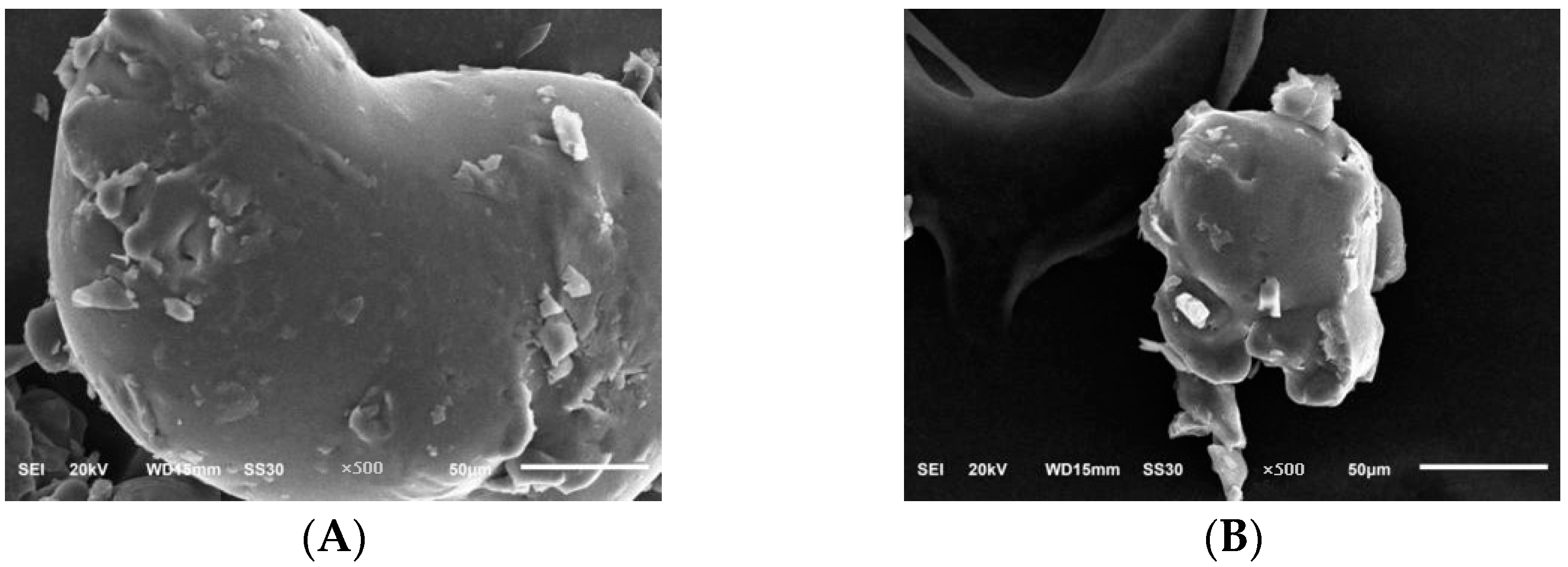
2.6.6. Scanning Electron Microscope Results Analysis
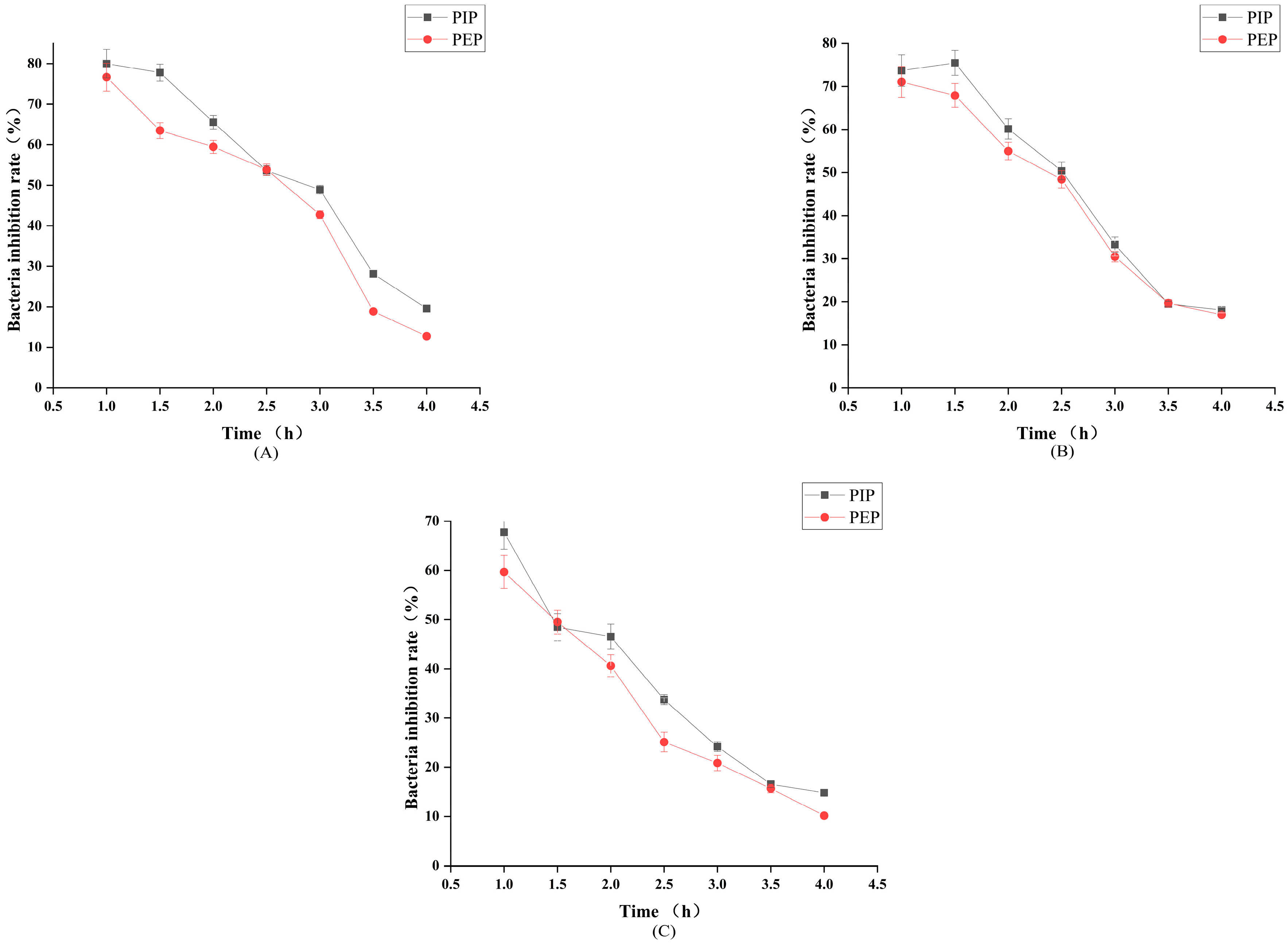
2.7. Results of the Analysis of the Antibacterial Properties of PLP
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Determination of the Glucose Standard Curves
3.3. The Extraction Process of PLP
3.4. Single-Factor Test on the Extraction of PLP
3.5. Response Surface Test on the Extraction of PLP
3.6. Physical and Chemical Property Analysis of PLP
3.6.1. Determination of Total Sugar Content
3.6.2. PLP UV Full Wavelength Scan Analysis
3.6.3. Determination of Protein Content
3.6.4. Determination of Ash Content
3.6.5. Determination of Monosaccharide Composition
3.6.6. Scanning Electron Microscopy Analysis of PLP
3.7. Analysis of the Antibacterial Properties of PLP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, N.-C.; Wu, C.-C.; Liu, R.H.; Chai, Y.-C.; Tseng, C.Y. Comparing the functional components, SOD-like activities, antimutagenicity, and nutrient compositions of Phellinus igniarius and Phellinus linteus mushrooms. J. Food Drug Anal. 2016, 24, 343–349. [Google Scholar] [CrossRef]
- Zhu, T.; Kim, S.H.; Chen, C.Y. A medicinal mushroom: Phellinus linteus. Curr. Med. Chem. 2008, 15, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Han, S.; Liu, M.; Guo, T.; Hu, Z.; Zhou, Y.; Luo, F. Polysaccharides from Phellinus linteus: A systematic review of their extractions, purifications, structures and functions. Int. J. Biol. Macromol. 2023, 230, 123–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, X.; Wang, Z.; Li, Z.; Geng, Y. Effects of Pine Pollen Polysaccharides and Sulfated Polysaccharides on Ulcerative Colitis and Gut Flora in Mice. Polymers 2023, 15, 1414. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Shi, H.; Yu, J.; Huang, G.; Huang, H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 2023, 93, 106295. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nomura, K.; Abe, J.; Kajihara, Y. Recent advances on the synthesis of N-linked glycoprotein for the elucidation of glycan functions. Curr. Opin. Chem. Biol. 2023, 73, 102263. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Hao, W. Immunomodulatory function and anti-tumor mechanism of natural polysaccharides: A review. Front. Immunol. 2023, 14, 1147641. [Google Scholar] [CrossRef]
- Chen, H.; Tian, T.; Miao, H.; Zhao, Y.Y. Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: A review. Fitoterapia 2016, 113, 6–26. [Google Scholar] [CrossRef]
- Chakraborty, I.; Sen, I.K.; Mondal, S.; Rout, D.; Bhanja, S.K.; Maity, G.N.; Maity, P. Bioactive polysaccharides from natural sources: A review on the antitumor and immunomodulating activities. Biocatal. Agric. Biotechnol. 2019, 22, 101425. [Google Scholar] [CrossRef]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Jung, G.H.; Kang, J.H. Efficacy of Phellinus linteus (sanghuang) extract for improving immune functions: Study protocol for a randomized, double-blinded, placebo-controlled pilot trial. Medicine 2020, 99, e18829. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Q.; Dong, X.-Y.; Ning, J.-L.; Jin, Y.-X.; Wang, G.-K.; Feng, T. Chemical constituents from the fungus Phellinus baumii and their anti-inflammatory activity. Phytochem. Lett. 2023, 54, 18–22. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wang, S.; Liu, M.; Chen, F.; Yang, W.; Chang, X.; Liu, N.; Zhao, Y.; Wang, J.; Chen, X. Extraction methods, chemical characterizations and biological activities of mushroom polysaccharides: A mini-review. Carbohydr. Res. 2020, 494, 108037. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Zhang, Y.; Li, N. The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: A review. Food Funct. 2021, 12, 1856–1881. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.X.; Zhou, M.; Si, J.; Cui, B.K. Current advances and potential trends of the polysaccharides derived from medicinal mushrooms sanghuang. Front. Microbiol. 2022, 13, 965934. [Google Scholar] [CrossRef]
- Su, C.-H.; Lai, M.-N.; Ng, L.-T. Effects of different extraction temperatures on the physicochemical properties of bioactive polysaccharides from Grifola frondosa. Food Chem. 2017, 220, 400–405. [Google Scholar] [CrossRef]
- Jiang, P.; Yuan, L.; Cai, D.; Jiao, L.; Zhang, L. Characterization and antioxidant activities of the polysaccharides from mycelium of Phellinus pini and culture medium. Carbohydr. Polym. 2015, 117, 600–604. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; Van Griensven, L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Yue, F.; Zhang, J.; Xu, J.; Niu, T.; Lü, X.; Liu, M. Effects of monosaccharide composition on quantitative analysis of total sugar content by phenol-sulfuric acid method. Front. Nutr. 2022, 9, 963318. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, R.; Li, L.; Zhang, D.; Liu, Y.; Wu, L.; Zheng, Y. Optimization of Ultrasonic-Assisted Extraction, Characterization, and Antioxidant Activities of Polysaccharides from Sojae Semen Praeparatum. Nat. Prod. Commun. 2021, 16, 1934578X2110206. [Google Scholar] [CrossRef]
- Li, P.; Xue, H.; Xiao, M.; Tang, J.; Yu, H.; Su, Y.; Cai, X. Ultrasonic-Assisted Aqueous Two-Phase Extraction and Properties of Water-Soluble Polysaccharides from Malus hupehensis. Molecules 2021, 26, 2213. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Wang, S.; Qian, G.; Zhu, Q.; Liu, Y.; Wang, W. Optimization of Ultrasonic-assisted Extraction Technology of Sargassum fusiforme Polysaccharides and Evaluation of Their Antioxidant Activity. Food Sci. Technol. Res. 2013, 19, 157–162. [Google Scholar] [CrossRef]
- Sun, J.-R.; Wang, D.-H.; Zhao, J.-F.; Gu, S.-B.; Research, N. Ultrasonic-assisted Extraction, Purification, Antioxidant and Antibacterial Activity of Polysaccharide from Cornus officinalis Leaves. J. Food Nutr. Res. 2020, 8, 496–505. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Dong, C.; Lui, E.M.K.; Ren, G. Optimization of Ultrasonic-Assisted Extraction Process of Polysaccharides from American Ginseng and Evaluation of Its Immunostimulating Activity. J. Integr. Agric. 2014, 13, 2807–2815. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019, 125, 906–908. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.-M.; Xu, S.-Y.; Liu, Y.; Cheong, K.-L. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 145, 1155–1161. [Google Scholar] [CrossRef]
- Cao, M.; Wang, S.; Gao, Y.; Pan, X.; Wang, X.; Deng, R.; Liu, P. Study on physicochemical properties and antioxidant activity of polysaccharides from Desmodesmus armatus. J. Food Biochem. 2020, 44, e13243. [Google Scholar] [CrossRef]
- Chen, X.; Tang, R.; Liu, T.; Dai, W.; Liu, Q.; Gong, G.; Song, S.; Hu, M.; Huang, L.; Wang, Z. Physicochemical properties, antioxidant activity and immunological effects in vitro of polysaccharides from Schisandra sphenanthera and Schisandra chinensis. Int. J. Biol. Macromol. 2019, 131, 744–751. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, Q.; Zeng, X.; Hao, C.; Li, X.; Zhang, L.; Zeng, P. Determination of monosaccharide composition in human serum by an improved HPLC method and its application as candidate biomarkers for endometrial cancer. Front. Oncol. 2022, 12, 6153. [Google Scholar] [CrossRef]
- Ai, Y.; Yu, Z.; Chen, Y.; Zhu, X.; Ai, Z.; Liu, S.; Ni, D. Rapid Determination of the Monosaccharide Composition and Contents in Tea Polysaccharides from Yingshuang Green Tea by Pre-Column Derivatization HPLC. J. Chem. 2016, 2016, 6065813. [Google Scholar] [CrossRef]
- Wu, H.; Li, M.; Yang, X.; Wei, Q.; Sun, L.; Zhao, J.; Shang, H. Extraction optimization, physicochemical properties and antioxidant and hypoglycemic activities of polysaccharides from roxburgh rose (Rosa roxburghii Tratt.) leaves. Int. J. Biol. Macromol. 2020, 165, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Wu, Y.; Kan, L.; Li, Q.; Xie, S.; Ouyang, J. Effects of Ultrasound on the Physicochemical Properties and Antioxidant Activities of Chestnut Polysaccharide. Int. J. Food Eng. 2016, 12, 439–449. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Wang, Y.; Xiong, Z. Effect of drying method on physicochemical properties and antioxidant activities of Hohenbuehelia serotina polysaccharides. Process Biochem. 2016, 51, 1100–1108. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Wu, Y.; Zhao, Y.; Sun, L. Preparation of carboxymethyl chitosan/copper composites and their antibacterial properties. Mater. Res. Bull. 2013, 48, 3411–3419. [Google Scholar] [CrossRef]
- Wang, K. Mutation Breeding of Bacteriocin-like Substance-Producing Lactococcus Lactis NFL and the Optimization of Determinationmethod of the Antimicrobial Activity of Bacteriocin-like Substance. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2015. [Google Scholar]
- Zhao, C.; Liao, Z.; Wu, X.; Liu, Y.; Liu, X.; Lin, Z.; Huang, Y.; Liu, B. Isolation, Purification, and Structural Features of a Polysaccharide from Phellinus linteus and Its Hypoglycemic Effect in Alloxan-Induced Diabetic Mice. J. Food Sci. 2014, 79, H1002–H1010. [Google Scholar] [CrossRef]


| Run | Independent Variables | Response | ||
|---|---|---|---|---|
| A/(min) | B/(g/mL) | C/(°C) | Yield/(%) | |
| 1 | 1 | 1 | 0 | 10.79 |
| 2 | 0 | 0 | 0 | 12.82 |
| 3 | 0 | 0 | 0 | 12.99 |
| 4 | 0 | 1 | 1 | 11.27 |
| 5 | 0 | −1 | 1 | 10.84 |
| 6 | −1 | 1 | 0 | 10.83 |
| 7 | 0 | −1 | −1 | 9.98 |
| 8 | 0 | 0 | 0 | 12.9 |
| 9 | −1 | 0 | −1 | 9.9 |
| 10 | 0 | 0 | 0 | 13.1 |
| 11 | 0 | 0 | 0 | 12.97 |
| 12 | 1 | 0 | 1 | 10.35 |
| 13 | −1 | −1 | 0 | 9.72 |
| 14 | 1 | 0 | −1 | 10.61 |
| 15 | −1 | 0 | 1 | 10.77 |
| 16 | 1 | −1 | 0 | 10.06 |
| 17 | 0 | 1 | −1 | 11.36 |
| Source | Sum of Squares | Df | Mean Square | F Value | p-Value Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 23.76 | 9 | 2.64 | 392.81 | <0.0001 | ** |
| A | 0.044 | 1 | 0.044 | 6.47 | 0.0384 | * |
| B | 1.67 | 1 | 1.67 | 247.79 | <0.0001 | ** |
| C | 0.24 | 1 | 0.24 | 35.42 | 0.0006 | ** |
| AB | 0.036 | 1 | 0.036 | 5.37 | 0.0536 | |
| AC | 0.32 | 1 | 0.32 | 47.50 | 0.0002 | ** |
| BC | 0.23 | 1 | 0.23 | 33.57 | 0.0007 | ** |
| A2 | 9.86 | 1 | 9.86 | 1467.53 | <0.0001 | ** |
| B2 | 4.87 | 1 | 4.87 | 724.67 | <0.0001 | ** |
| C2 | 4.36 | 1 | 4.36 | 649.26 | <0.0001 | ** |
| Residual | 0.047 | 7 | 0.007 | |||
| Lack of fit | 0.003 | 3 | 0.001 | 0.10 | 0.9550 | |
| Pure Error | 0.044 | 4 | 0.011 | |||
| Cor Total | 23.81 | 16 | ||||
| R2 | 0.9980 |
| Samples | Man | Clc-A | Glc | Gal | Xyl | Ara | Fuc |
|---|---|---|---|---|---|---|---|
| EPS | 1.9 | 0.8 | 93.6 | 1.8 | -- | 1.8 | -- |
| IPS | 5.4 | 0.8 | 87.6 | 4.2 | 0.6 | -- | 1.4 |
| Independent Variable | Coded Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Ultrasonic time (A) (min) | 10 | 20 | 30 |
| Solid–liquid ratio (B) (g/mL) | 1:30 | 1:40 | 1:50 |
| Ultrasonic temperature (C) (°C) | 50 | 60 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, N.; Zhang, W.; Hu, D.; Lin, G.; Wang, J.; Xue, F.; Wang, Q.; Zhao, H.; Dou, X.; Zhang, L. Study on Extraction, Physicochemical Properties, and Bacterio-Static Activity of Polysaccharides from Phellinus linteus. Molecules 2023, 28, 5102. https://doi.org/10.3390/molecules28135102
Gao N, Zhang W, Hu D, Lin G, Wang J, Xue F, Wang Q, Zhao H, Dou X, Zhang L. Study on Extraction, Physicochemical Properties, and Bacterio-Static Activity of Polysaccharides from Phellinus linteus. Molecules. 2023; 28(13):5102. https://doi.org/10.3390/molecules28135102
Chicago/Turabian StyleGao, Nengbin, Weijia Zhang, Dianjie Hu, Guo Lin, Jingxuan Wang, Feng Xue, Qian Wang, Hongfei Zhao, Xin Dou, and Lihong Zhang. 2023. "Study on Extraction, Physicochemical Properties, and Bacterio-Static Activity of Polysaccharides from Phellinus linteus" Molecules 28, no. 13: 5102. https://doi.org/10.3390/molecules28135102
APA StyleGao, N., Zhang, W., Hu, D., Lin, G., Wang, J., Xue, F., Wang, Q., Zhao, H., Dou, X., & Zhang, L. (2023). Study on Extraction, Physicochemical Properties, and Bacterio-Static Activity of Polysaccharides from Phellinus linteus. Molecules, 28(13), 5102. https://doi.org/10.3390/molecules28135102





