A Simple and Practical Bis-N-Heterocyclic Carbene as an Efficient Ligand in Cu-Catalyzed Glaser Reaction
Abstract
1. Introduction
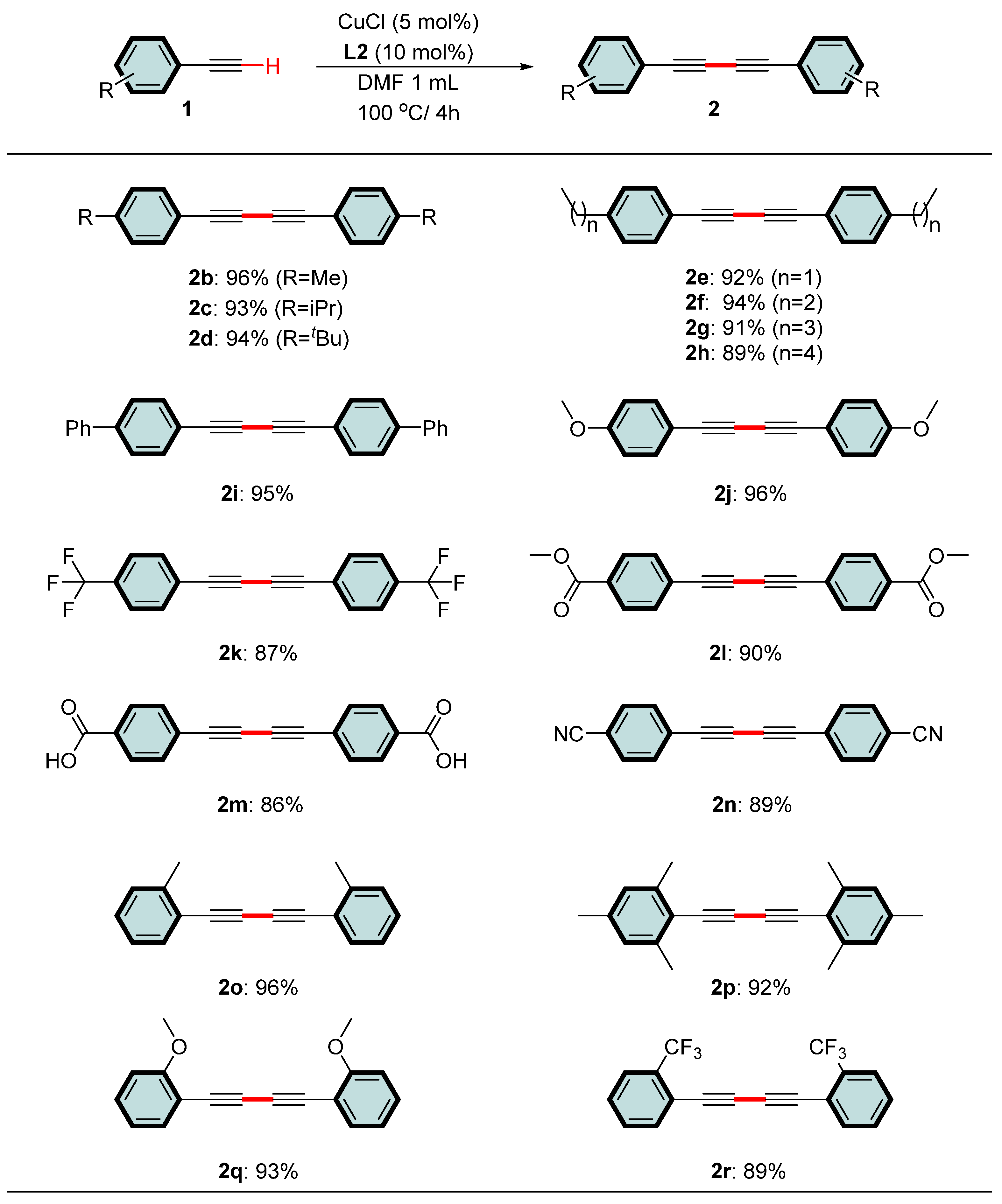
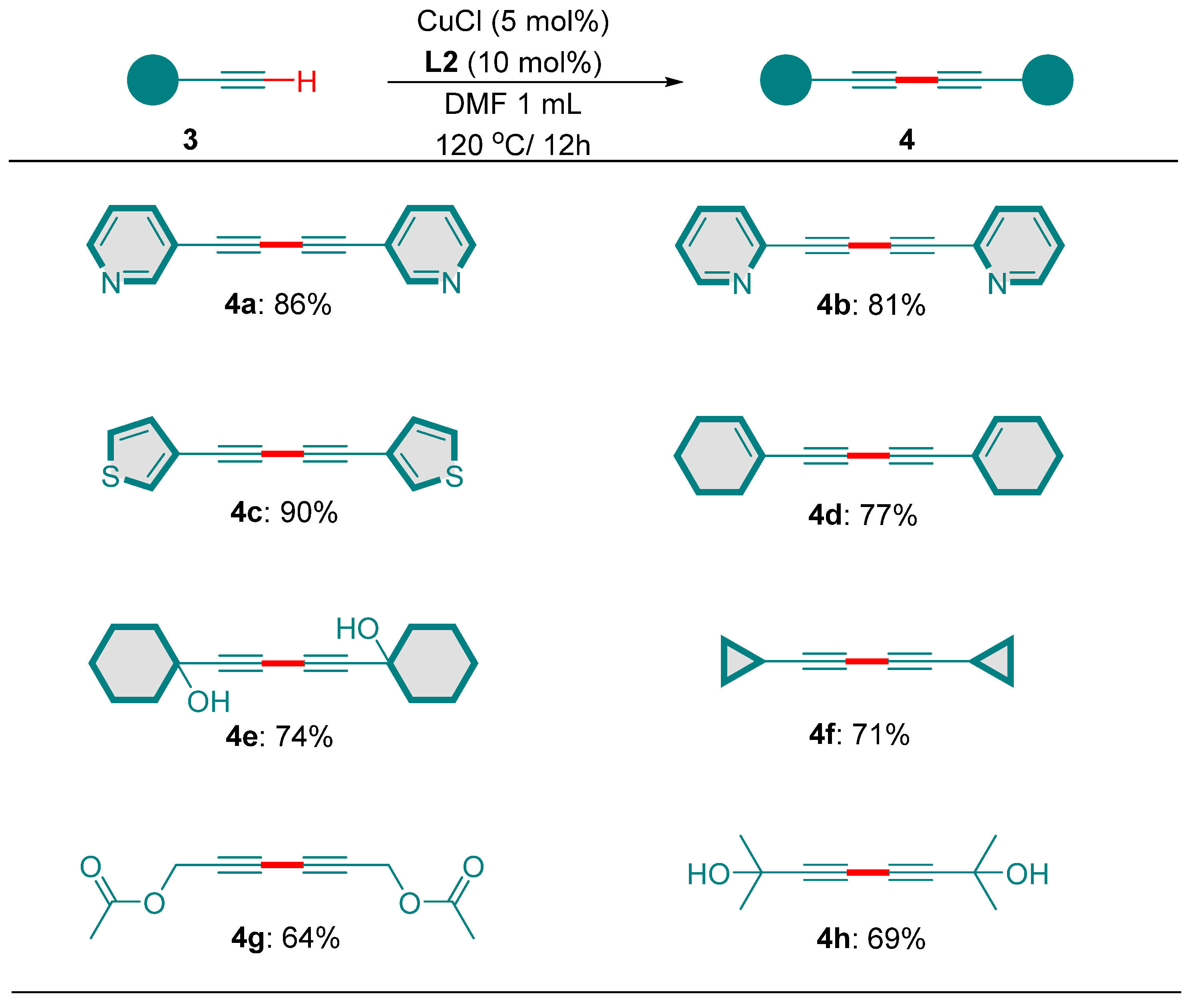
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Typical Experimental Procedure for the Synthesis of 1,3-Diyne
3.3. Characterization Data of the Products
- L1 [56]. White solid. 1H NMR (400 MHz, DMSO-d6) δ 9.60 (s, 2H), 8.12 (s, 2H), 7.83 (s, 2H), 6.81 (s, 2H), 3.91 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 138.03, 124.27, 121.88, 57.79, 36.28.
- L2 [57]. White solid. 1H NMR (400 MHz, DMSO-d6) δ 10.52 (s, 2H), 8.50 (d, J = 17.3 Hz, 4H), 7.86 (d, J = 7.8 Hz, 4H), 7.70 (t, J = 7.6 Hz, 4H), 7.63 (t, J = 7.3 Hz, 2H), 7.01 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δ 137.33, 134.46, 130.28, 130.17, 123.04, 121.92, 121.50, 58.24.
- L3 [57]. White solid. 1H NMR (400 MHz, DMSO-d6) δ 10.22 (s, 2H), 8.56 (s, 2H), 8.25 (s, 2H), 7.65 (d, J = 7.7 Hz, 2H), 7.56 (q, J = 7.4 Hz, 4H), 7.49 (t, J = 7.2 Hz, 2H), 7.05 (s, 2H), 2.31 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 138.79, 133.93, 133.20, 131.76, 130.79, 127.38, 126.31, 124.12, 122.46, 58.08, 17.30.
- L4 [57]. White solid. 1H NMR (400 MHz, DMSO-d6) δ 10.28 (s, 2H), 8.52 (s, 2H), 8.24 (s, 2H), 7.72 (dd, J = 7.9, 1.3 Hz, 2H), 7.66–7.57 (m, 2H), 7.41 (d, J = 8.3 Hz, 2H), 7.21 (t, J = 7.7 Hz, 2H), 7.08 (s, 2H), 3.93 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 151.78, 138.77, 131.88, 125.78, 123.96, 123.03, 122.00, 121.13, 113.42, 57.97, 56.53.
- L5 [49]. White solid. 1H NMR (400 MHz, DMSO-d6) δ 10.62 (s, 2H), 8.81 (s, 2H), 8.60 (s, 1H), 8.25 (s, 2H), 8.07 (s, 2H), 4.05 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 145.23, 144.89, 136.32, 125.00, 119.10, 114.04, 36.51.
- L6 [49]. White solid. 1H NMR (400 MHz, DMSO-d6) δ 10.70 (s, 2H), 8.93 (s, 2H), 8.61 (t, J = 8.1 Hz, 1H), 8.42–8.11 (m, 4H), 4.90 (dt, J = 13.3, 6.6 Hz, 2H), 1.62 (d, J = 6.7 Hz, 12H). 13C NMR (100 MHz, DMSO-d6) δ 145.31, 144.62, 134.73, 121.78, 119.66, 114.30, 53.42, 22.20.
- 1,4-diphenylbuta-1,3-diyne (2a) [24]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.54 (dd, J = 7.8, 1.4 Hz, 4H), 7.44–7.30 (m, 6H). 13C NMR (100 MHz, CDCl3) δ 132.64, 129.35, 128.59, 121.95, 81.70, 74.06.
- 1,4-di-p-tolylbuta-1,3-diyne (2b) [24]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.42 (d, J = 7.9 Hz, 4H), 7.14 (d, J = 7.8 Hz, 4H), 2.37 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 139.63, 132.53, 129.35, 118.95, 81.69, 73.60, 21.76.
- 1,4-bis(4-isopropylphenyl)buta-1,3-diyne (2c) [5]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.45 (d, J = 8.2 Hz, 4H), 7.19 (d, J = 8.1 Hz, 4H), 2.91 (dt, J = 13.8, 6.9 Hz, 2H), 1.25 (d, J = 6.9 Hz, 12H). 13C NMR (100 MHz, CDCl3) δ 150.46, 132.66, 126.75, 119.30, 81.70, 73.56, 34.32, 23.86.
- 1,4-bis(4-(tert-butyl)phenyl)buta-1,3-diyne (2d) [24]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.45 (t, J = 10.7 Hz, 4H), 7.39–7.31 (m, 4H), 1.32 (s, 18H). 13C NMR (100 MHz, CDCl3) δ 152.71, 132.40, 125.61, 118.98, 81.65, 73.63, 35.06, 31.25.
- 1,4-bis(4-ethylphenyl)buta-1,3-diyne (2e) [45]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.45 (d, J = 8.0 Hz, 4H), 7.17 (d, J = 8.0 Hz, 4H), 2.67 (q, J = 7.6 Hz, 4H), 1.24 (t, J = 7.6 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 145.88, 132.63, 128.16, 119.18, 81.71, 73.61, 29.06, 15.37.
- 1,4-bis(4-propylphenyl)buta-1,3-diyne (2f) [43]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 8.1 Hz, 4H), 7.14 (d, J = 8.1 Hz, 4H), 2.82–2.41 (m, 4H), 1.83–1.52 (m, 4H), 0.94 (t, J = 7.3 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 144.37, 132.54, 128.76, 119.20, 81.73, 73.64, 38.19, 24.40, 13.89.
- 1,4-bis(4-butylphenyl)buta-1,3-diyne (2g) [25]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 8.1 Hz, 4H), 7.15 (d, J = 8.2 Hz, 4H), 2.78–2.47 (m, 4H), 1.60 (dt, J = 12.9, 7.5 Hz, 4H), 1.35 (dq, J = 14.6, 7.3 Hz, 4H), 0.93 (t, J = 7.3 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 144.60, 132.55, 128.70, 119.15, 81.73, 73.63, 35.83, 33.45, 22.45, 14.05.
- 1,4-bis(4-pentylphenyl)buta-1,3-diyne (2h) [24]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 8.0 Hz, 4H), 7.15 (d, J = 8.0 Hz, 4H), 2.98–2.39 (m, 4H), 1.78–1.52 (m, 4H), 1.32 (dt, J = 10.6, 3.3 Hz, 8H), 0.90 (t, J = 6.8 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 144.63, 132.55, 128.69, 119.14, 81.73, 73.64, 36.11, 31.58, 30.99, 22.65, 14.14.
- 1,4-di([1,1′-biphenyl]-4-yl)buta-1,3-diyne (2i) [5]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.64–7.53 (m, 12H), 7.45 (t, J = 7.5 Hz, 4H), 7.37 (t, J = 7.3 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 141.74, 140.41, 132.70, 129.02, 127.87, 127.20, 127.15, 121.13, 83.70, 77.88.
- 1,4-bis(4-methoxyphenyl)buta-1,3-diyne (2j) [24]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.46 (d, J = 8.9 Hz, 4H), 6.85 (d, J = 8.8 Hz, 4H), 3.82 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 160.39, 134.18, 114.28, 114.11, 81.38, 73.10, 55.48.
- 1,4-bis(4-(trifluoromethyl)phenyl)buta-1,3-diyne (2k) [5]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.63 (q, J = 8.5 Hz, 8H). 13C NMR (100 MHz, CDCl3) δ 132.96, 131.27 (d, J = 33.0 Hz), 125.61 (d, J = 3.7 Hz), 125.31 (d, J = 22.8 Hz), 122.49, 81.12, 75.79.
- dimethyl 4,4′-(buta-1,3-diyne-1,4-diyl)dibenzoate (2l) [24]. White solid. Rf = 0.7 (10% ethyl acetate/n-hexane) 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 8.4 Hz, 4H), 7.59 (d, J = 8.4 Hz, 4H), 3.93 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 166.41, 132.62, 130.72, 129.73, 126.28, 82.00, 76.42, 52.50.
- 4,4′-(buta-1,3-diyne-1,4-diyl)dibenzoic acid (2m) [58]. White solid. Rf = 0.6 (ethyl acetate). 1H NMR (400 MHz, CDCl3) δ 8.06 (d, J = 8.2 Hz, 4H), 7.58 (d, J = 8.2 Hz, 4H), 3.26 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 170.03, 132.33, 130.19, 80.63.
- 4,4′-(buta-1,3-diyne-1,4-diyl)dibenzonitrile (2n) [43]. White solid. Rf = 0.4 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.74–7.52 (m, 8H). 13C NMR (100 MHz, CDCl3) δ 132.82, 132.17, 127.16, 118.39, 112.50, 82.01, 81.67.
- 1,4-di-o-tolylbuta-1,3-diyne (2o) [36]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 7.6 Hz, 2H), 7.25 (dt, J = 17.1, 7.3 Hz, 4H), 7.16 (t, J = 7.4 Hz, 2H), 2.51 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 141.78, 133.06, 129.71, 129.24, 125.80, 121.88, 81.29, 77.67, 20.88.
- 1,4-dimesitylbuta-1,3-diyne (2p) [59]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 6.87 (s, 4H), 2.41 (s, 12H), 2.28 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 140.96, 138.25, 127.71, 119.09, 84.64, 81.52, 21.45, 21.02.
- 1,4-bis(2-methoxyphenyl)buta-1,3-diyne (2q) [36]. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 7.6 Hz, 2H), 7.32 (t, J = 7.9 Hz, 2H), 6.90 (dd, J = 15.5, 8.0 Hz, 4H), 3.89 (s, 6H). 13C NMR (100 MHz, Chloroform-d) δ 161.45, 134.49, 130.65, 120.61, 111.40, 110.80, 78.78, 78.10, 55.93.
- 1,4-bis(2-(trifluoromethyl)phenyl)buta-1,3-diyne (2r) [24]. White solid. White solid. Rf = 0.6 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.70 (t, J = 8.3 Hz, 4H), 7.50 (dt, J = 22.3, 7.5 Hz, 4H). 13C NMR (100 MHz, CDCl3) δ 135.29, 131.63, 129.27, 126.20 (q, J = 4.9 Hz), 124.75, 122.04, 119.90 (d, J = 2.8 Hz), 78.85, 78.75.
- 1,4-di(pyridin-3-yl)buta-1,3-diyne (4a) [24]. White solid. White solid. Rf = 0.4 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 8.77 (s, 2H), 8.60 (d, J = 3.9 Hz, 2H), 7.93–7.70 (m, 2H), 7.30 (dd, J = 7.6, 5.0 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 153.12, 149.48, 139.69, 123.31, 119.07, 79.27.
- 1,4-di(pyridin-2-yl)buta-1,3-diyne (4b) [20]. White solid. White solid. Rf = 0.4 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 8.62 (d, J = 4.6 Hz, 2H), 7.69 (td, J = 7.7, 1.4 Hz, 2H), 7.54 (d, J = 7.8 Hz, 2H), 7.29 (dd, J = 7.0, 5.5 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 150.53, 142.06, 136.35, 128.56, 123.93, 81.04, 73.35.
- 1,4-di(thiophen-3-yl)buta-1,3-diyne (4c) [24]. White solid. White solid. Rf = 0.4 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 7.59 (s, 2H), 7.37–7.24 (m, 2H), 7.21–7.03 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 131.37, 130.31, 125.74, 121.04, 76.70, 73.66.
- 1,4-di(cyclohex-1-en-1-yl)buta-1,3-diyne (4d) [25]. White solid. White solid. Rf = 0.5 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 6.53–6.06 (m, 2H), 2.35–1.95 (m, 8H), 1.66–1.53 (m, 8H). 13C NMR (100 MHz, CDCl3) δ 138.23, 120.10, 82.84, 60.55, 28.83, 26.01, 22.27, 21.45.
- 1,1′-(buta-1,3-diyne-1,4-diyl)bis(cyclohexan-1-ol) (4e) [24]. White solid. Rf = 0.4 (ethyl acetate) 1H NMR (400 MHz, CDCl3) δ 2.14 (s, 2H), 1.88 (dd, J = 9.9, 5.8 Hz, 4H), 1.67 (dq, J = 11.4, 5.4, 4.9 Hz, 4H), 1.61–1.45 (m, 10H), 1.32–1.11 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 87.89, 72.18, 68.61, 39.86, 25.20, 23.21.
- 1,4-dicyclopropylbuta-1,3-diyne (4f) [20]. Colorless oil. White solid. Rf = 0.5 (n-hexane). 1H NMR (400 MHz, CDCl3) δ 1.24 (ddp, J = 11.4, 5.4, 3.0, 2.5 Hz, 2H), 0.90–0.64 (m, 8H). 13C NMR (100 MHz, CDCl3) δ 87.87, 63.51, 8.26, −0.67.
- hexa-2,4-diyne-1,6-diyl diacetate (4g) [20]. Colorless oil. Rf = 0.6 (ethyl acetate). 1H NMR (400 MHz, CDCl3) δ 4.63 (s, 4H), 2.06 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 170.14, 77.74, 74.88, 51.97, 20.68.
- 2,7-dimethylocta-3,5-diyne-2,7-diol (4h) [20]. Colorless oil. Rf = 0.4 (ethyl acetate) 1H NMR (400 MHz, CDCl3) δ 2.13 (s, 2H), 1.49 (s, 12H). 13C NMR (100 MHz, CDCl3) δ 88.91, 70.23, 65.05, 31.35.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, X.; Liu, L.; Huang, T.; Tang, Z.; Li, C.; Li, W.; Zhang, T.; Li, Z.; Chen, T. Palladium-Catalyzed Decarbonylative Sonogashira Coupling of Terminal Alkynes with Carboxylic Acids. Org. Lett. 2021, 23, 3304–3309. [Google Scholar] [CrossRef]
- Jin, L.; Hao, W.; Xu, J.; Sun, N.; Hu, B.; Shen, Z.; Mo, W.; Hu, X. N-Heterocyclic carbene copper-catalyzed direct alkylation of terminal alkynes with non-activated alkyl triflates. Chem. Commun. 2017, 53, 4124–4127. [Google Scholar] [CrossRef]
- Shi Shun, A.L.K.; Tykwinski, R.R. Synthesis of Naturally Occurring Polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef]
- Yang, B.; Lu, S.; Wang, Y.; Zhu, S. Diverse synthesis of C2-linked functionalized molecules via molecular glue strategy with acetylene. Nat. Commun. 2022, 13, 1858. [Google Scholar] [CrossRef]
- Morri, A.K.; Thummala, Y.; Ghosh, S.; Doddi, V.R. Urea-Promoted Metal-Free Homolytic Alkynyl Substitution (HAS): Metal-Free C−C Coupling of Alkynyl Bromides Formed In Situ from 1,1-Dibromoalkenes. ChemistrySelect 2021, 6, 2387–2393. [Google Scholar] [CrossRef]
- Sagadevan, A.; Pampana, V.K.K.; Hwang, K.C. Copper Photoredox Catalyzed A3′ Coupling of Arylamines, Terminal Alkynes, and Alcohols through a Hydrogen Atom Transfer Process. Angew. Chem. Int. Ed. 2019, 58, 3838–3842. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhao, C.-Q.; Han, L.-B. Hydrophosphorylation of Alkynes Catalyzed by Palladium: Generality and Mechanism. J. Am. Chem. Soc. 2018, 140, 3139–3155. [Google Scholar] [CrossRef]
- Wu, X.; Wei, B.; Hu, R.; Tang, B.Z. Polycouplings of Alkynyl Bromides and Sulfonamides toward Poly(ynesulfonamide)s with Stable Csp–N Bonds. Macromolecules 2017, 50, 5670–5678. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, X.; Cheng, X.; Qin, A.; Sun, J.Z.; Tang, B.Z. Polymerization of 1-chloro-2-benzaldehyde-acetylene using an NHC-Pd/AgOTf catalyst and post-polymerization modification. Polym. Chem. 2017, 8, 5546–5553. [Google Scholar] [CrossRef]
- Haque, A.; Al-Balushi, R.A.; Al-Busaidi, I.J.; Khan, M.S.; Raithby, P.R. Rise of Conjugated Poly-ynes and Poly(Metalla-ynes): From Design Through Synthesis to Structure–Property Relationships and Applications. Chem. Rev. 2018, 118, 8474–8597. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.Z.; Tang, B.Z. Poly(disubstituted acetylene)s: Advances in polymer preparation and materials application. Prog. Polym. Sci. 2018, 79, 98–120. [Google Scholar]
- Wei, J.; Liu, M.; Ye, X.; Zhang, S.; Sun, E.; Shan, C.; Wojtas, L.; Shi, X. Facile synthesis of diverse hetero polyaromatic hydrocarbons (PAHs) via the styryl Diels–Alder reaction of conjugated diynes. Org. Chem. Front. 2022, 9, 4301–4308. [Google Scholar]
- Day, D.P.; Chan, P.W.H. Gold-Catalyzed Cycloisomerizations of 1,n-Diyne Carbonates and Esters. Adv. Synth. Catal. 2016, 358, 1368–1384. [Google Scholar] [CrossRef]
- Shi, W.; Lei, A. 1,3-Diyne chemistry: Synthesis and derivations. Tetrahedron Lett. 2014, 55, 2763–2772. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar Chattopadhyay, S. Transition-Metal-Free Synthesis of Symmetrical 1,4-Diarylsubstituted 1,3-Diynes by Iodine-Mediated Decarboxylative Homocoupling of Arylpropiolic Acids. Tetrahedron Lett. 2022, 102, 153908. [Google Scholar]
- Bakhoda, A.; Okoromoba, O.E.; Greene, C.; Boroujeni, M.R.; Bertke, J.A.; Warren, T.H. Three-Coordinate Copper(II) Alkynyl Complex in C–C Bond Formation: The Sesquicentennial of the Glaser Coupling. J. Am. Chem. Soc. 2020, 142, 18483–18490. [Google Scholar] [CrossRef]
- Albrecht, F.; Rey, D.; Fatayer, S.; Schulz, F.; Pérez, D.; Peña, D.; Gross, L. Intramolecular Coupling of Terminal Alkynes by Atom Manipulation. Angew. Chem. Int. Ed. 2020, 59, 22989–22993. [Google Scholar] [CrossRef]
- Tang, S.; Li, L.; Ren, X.; Li, J.; Yang, G.; Li, H.; Yuan, B. Metallomicelle catalyzed aerobic tandem desilylation/Glaser reaction in water. Green Chem. 2019, 21, 2899–2904. [Google Scholar]
- Xu, D.; Sun, Q.; Quan, Z.; Wang, X.; Sun, W. Cobalt-Catalyzed Dimerization and Homocoupling of Terminal Alkynes. Asian J. Org. Chem. 2018, 7, 155–159. [Google Scholar]
- Zhang, S.; Liu, X.; Wang, T. An Efficient Copper-Catalyzed Homocoupling of Terminal Alkynes to Give Symmetrical 1,4-Disubstituted 1,3-Diynes. Adv. Synth. Catal. 2011, 353, 1463–1466. [Google Scholar]
- Singh, F.V.; Amaral, M.F.Z.J.; Stefani, H.A. Synthesis of symmetrical 1,3-diynes via homocoupling reaction of n-butyl alkynyltellurides. Tetrahedron Lett. 2009, 50, 2636–2639. [Google Scholar] [CrossRef]
- Maji, M.S.; Pfeifer, T.; Studer, A. Oxidative Homocoupling of Aryl, Alkenyl, and Alkynyl Grignard Reagents with TEMPO and Dioxygen. Angew. Chem. Int. Ed. 2008, 47, 9547–9550. [Google Scholar] [CrossRef]
- Nishihara, Y.; Ikegashira, K.; Hirabayashi, K.; Ando, J.-I.; Mori, A.; Hiyama, T. Coupling Reactions of Alkynylsilanes Mediated by a Cu(I) Salt: Novel Syntheses of Conjugate Diynes and Disubstituted Ethynes. J. Org. Chem. 2000, 65, 1780–1787. [Google Scholar] [CrossRef]
- Mishra, N.; Singh, S.K.; Singh, A.S.; Agrahari, A.K.; Tiwari, V.K. Glycosyl Triazole Ligand for Temperature-Dependent Competitive Reactions of Cu-Catalyzed Sonogashira Coupling and Glaser Coupling. J. Org. Chem. 2021, 86, 17884–17895. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, A.S.; Mishra, N.; Agrahari, A.K.; Tiwari, V.K. Benzotriazole as an Efficient Ligand in Cu-Catalyzed Glaser Reaction. ACS Omega 2019, 4, 2418–2424. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Wang, Y.-H.; Liu, J.; Xu, M.-C.; Zhang, X.-M. Efficient and environmentally friendly Glaser coupling of terminal alkynes catalyzed by multinuclear copper complexes under base-free conditions. RSC Adv. 2016, 6, 28653–28657. [Google Scholar] [CrossRef]
- Radhika, S.; Harry, N.A.; Neetha, M.; Anilkumar, G. Recent trends and applications of the Cadiot–Chodkiewicz reaction. Org. Biomol. Chem. 2019, 17, 9081–9094. [Google Scholar] [CrossRef] [PubMed]
- Knutson, P.C.; Fredericks, H.E.; Ferreira, E.M. Synthesis of 1,3-Diynes via Cadiot–Chodkiewicz Coupling of Volatile, in Situ Generated Bromoalkynes. Org. Lett. 2018, 20, 6845–6849. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, X.; Sun, N.; Liu, Y. Gold-Catalyzed Cadiot–Chodkiewicz-type Cross-Coupling of Terminal Alkynes with Alkynyl Hypervalent Iodine Reagents: Highly Selective Synthesis of Unsymmetrical 1,3-Diynes. Angew. Chem. Int. Ed. 2017, 56, 6994–6998. [Google Scholar] [CrossRef]
- Glaser, C. Beiträge zur Kenntniss des Acetenylbenzols. Ber. Der Dtsch. Chem. Ges. 1869, 2, 422–424. [Google Scholar] [CrossRef]
- Kaldhi, D.; Vodnala, N.; Gujjarappa, R.; Kabi, A.K.; Nayak, S.; Malakar, C.C. Transition-metal-free variant of Glaser- and Cadiot-Chodkiewicz-type Coupling: Benign access to diverse 1,3-diynes and related molecules. Tetrahedron Lett. 2020, 61, 151775. [Google Scholar] [CrossRef]
- Lampkowski, J.S.; Uthappa, D.M.; Halonski, J.F.; Maza, J.C.; Young, D.D. Application of the Solid-Supported Glaser–Hay Reaction to Natural Product Synthesis. J. Org. Chem. 2016, 81, 12520–12524. [Google Scholar] [CrossRef]
- Sindhu, K.S.; Anilkumar, G. Recent advances and applications of Glaser coupling employing greener protocols. RSC Adv. 2014, 4, 27867–27887. [Google Scholar] [CrossRef]
- Jia, X.; Yin, K.; Li, C.; Li, J.; Bian, H. Copper-catalyzed oxidative alkyne homocoupling without palladium, ligands and bases. Green Chem. 2011, 13, 2175–2178. [Google Scholar] [CrossRef]
- Hsiao, T.-H.; Wu, T.-L.; Chatterjee, S.; Chiu, C.-Y.; Lee, H.M.; Bettucci, L.; Bianchini, C.; Oberhauser, W. Palladium acetate complexes bearing chelating N-heterocyclic carbene (NHC) ligands: Synthesis and catalytic oxidative homocoupling of terminal alkynes. J. Organomet. Chem. 2009, 694, 4014–4024. [Google Scholar] [CrossRef]
- Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Homocoupling reaction of terminal alkynes catalyzed by a reusable cationic 2,2′-bipyridyl palladium(II)/CuI system in water. Green Chem. 2009, 11, 269–274. [Google Scholar] [CrossRef]
- Han, J.-F.; Guo, P.; Chen, L.; Ye, K.-Y. Cobalt-Catalyzed Glaser-type Homocoupling Reaction. Synthesis 2022, 54, 1989–1995. [Google Scholar] [CrossRef]
- Krafft, M.E.; Hirosawa, C.; Dalal, N.; Ramsey, C.; Stiegman, A. Cobalt-catalyzed homocoupling of terminal alkynes: Synthesis of 1,3-diynes. Tetrahedron Lett. 2001, 42, 7733–7736. [Google Scholar] [CrossRef]
- Das, U.K.; Jena, R.K.; Bhattacharjee, M. Synthesis, structure and catalytic properties of [Ru(dppp)2(CH3CN)Cl][BPh4] and isolation of catalytically active [Ru(dppp)2Cl][BPh4]: Ruthenium catalysed alkyne homocoupling and tandem alkyne–azide cycloaddition. RSC Adv. 2014, 4, 21964–21970. [Google Scholar] [CrossRef]
- Mo, G.; Tian, Z.; Li, J.; Wen, G.; Yang, X. Silver-catalyzed Glaser coupling of alkynes. Appl. Organomet. Chem. 2015, 29, 231–233. [Google Scholar] [CrossRef]
- Boronat, M.; Laursen, S.; Leyva-Pérez, A.; Oliver-Meseguer, J.; Combita, D.; Corma, A. Partially oxidized gold nanoparticles: A catalytic base-free system for the aerobic homocoupling of alkynes. J. Catal. 2014, 315, 6–14. [Google Scholar] [CrossRef]
- Alonso, F.; Yus, M. Heterogeneous Catalytic Homocoupling of Terminal Alkynes. ACS Catal. 2012, 2, 1441–1451. [Google Scholar] [CrossRef]
- Thangarasu, A.K.; Yadhukrishnan, V.O.; Krishnakumar, K.A.; Varma, S.S.; Lankalapalli, R.S. Cu(I)-azidopyrrolo [3,2-d]pyrimidine Catalyzed Glaser–Hay Reaction under Mild Conditions. ACS Org. Inorg. Au 2022, 2, 3–7. [Google Scholar] [CrossRef]
- Györke, G.; Dancsó, A.; Volk, B.; Hunyadi, D.; Szalóki, I.; Milen, M. Copper-Containing Mineral Mediated Glaser Coupling of Terminal Alkynes. ChemistrySelect 2022, 7, e202200480. [Google Scholar] [CrossRef]
- Wang, B.; Gao, L.; Zheng, G. Leaf-like CuO nanosheets on rGO as an efficient heterogeneous catalyst for Csp-Csp homocoupling of terminal alkynes. Catal. Commun. 2021, 150, 106260. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Chen, J.; Gong, H. A Mild CuCl-catalyzed Glaser-type Homocoupling Reaction: Air Oxidation and Room Temperature. Chem. Lett. 2014, 44, 129–131. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-Y.; Lan, X.-B.; Xu, C.; Yao, H.-G.; Li, T.; Liu, F.-S. Rigid hindered N-heterocyclic carbene palladium precatalysts: Synthesis, characterization and catalytic amination. Org. Chem. Front. 2019, 6, 3292–3299. [Google Scholar] [CrossRef]
- Lan, X.-B.; Ye, Z.; Liu, J.; Huang, M.; Shao, Y.; Cai, X.; Liu, Y.; Ke, Z. Sustainable and Selective Alkylation of Deactivated Secondary Alcohols to Ketones by Non-bifunctional Pincer N-heterocyclic Carbene Manganese. ChemSusChem 2020, 13, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-H.; Lan, X.-B.; Song, A.X.; Rahman, M.M.; Xu, C.; Huang, F.-D.; Szostak, R.; Szostak, M.; Liu, F.-S. Buchwald-Hartwig Amination of Coordinating Heterocycles Enabled by Large-but-Flexible Pd-BIAN-NHC Catalysts**. Chem. Eur. J. 2022, 28, e202103341. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.-S.; Szostak, M. BIAN-NHC Ligands in Transition-Metal-Catalysis: A Perfect Union of Sterically Encumbered, Electronically Tunable N-Heterocyclic Carbenes? Chem. Eur. J. 2021, 27, 4478–4499. [Google Scholar] [CrossRef]
- Ma, B.-B.; Lan, X.-B.; Shen, D.-S.; Liu, F.-S.; Xu, C. Direct C-H bond (Hetero)arylation of thiazole derivatives at 5-position catalyzed by N-heterocyclic carbene palladium complexes at low catalyst loadings under aerobic conditions. J. Organomet. Chem. 2019, 897, 13–22. [Google Scholar] [CrossRef]
- Lan, X.-B.; Li, Y.; Li, Y.-F.; Shen, D.-S.; Ke, Z.; Liu, F.-S. Flexible Steric Bulky Bis(Imino)acenaphthene (BIAN)-Supported N-Heterocyclic Carbene Palladium Precatalysts: Catalytic Application in Buchwald–Hartwig Amination in Air. J. Org. Chem. 2017, 82, 2914–2925. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.-B.; Chen, F.-M.; Ma, B.-B.; Shen, D.-S.; Liu, F.-S. Pd-PEPPSI Complexes Bearing Bulky [(1,2-Di-(tert-butyl)acenaphthyl] (DtBu-An) on N-Heterocarbene Backbones: Highly Efficient for Suzuki–Miyaura Cross-Coupling under Aerobic Conditions. Organometallics 2016, 35, 3852–3860. [Google Scholar] [CrossRef]
- Li, W.; Huang, M.; Liu, J.; Huang, Y.-L.; Lan, X.-B.; Ye, Z.; Zhao, C.; Liu, Y.; Ke, Z. Enhanced Hydride Donation Achieved Molybdenum Catalyzed Direct N-Alkylation of Anilines or Nitroarenes with Alcohols: From Computational Design to Experiment. ACS Catal. 2021, 11, 10377–10382. [Google Scholar] [CrossRef]
- Lan, X.-B.; Ye, Z.; Huang, M.; Liu, J.; Liu, Y.; Ke, Z. Nonbifunctional Outer-Sphere Strategy Achieved Highly Active α-Alkylation of Ketones with Alcohols by N-Heterocyclic Carbene Manganese (NHC-Mn). Org. Lett. 2019, 21, 8065–8070. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Li, Y.; Liu, J.; Shu, S.; Liu, Y.; Ke, Z. Room temperature N-heterocyclic carbene manganese catalyzed selective N-alkylation of anilines with alcohols. Chem. Commun. 2019, 55, 6213–6216. [Google Scholar] [CrossRef]
- Colazzo, L.; Sedona, F.; Moretto, A.; Casarin, M.; Sambi, M. Metal-Free on-Surface Photochemical Homocoupling of Terminal Alkynes. J. Am. Chem. Soc. 2016, 138, 10151–10156. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Li, Q.; Song, T.; Yang, Y. Facile Fabrication of the Cu-N-C Catalyst with Atomically Dispersed Unsaturated Cu-N2 Active Sites for Highly Efficient and Selective Glaser–Hay Coupling. ACS Appl. Mater. Interfaces 2020, 12, 27210–27218. [Google Scholar] [CrossRef]

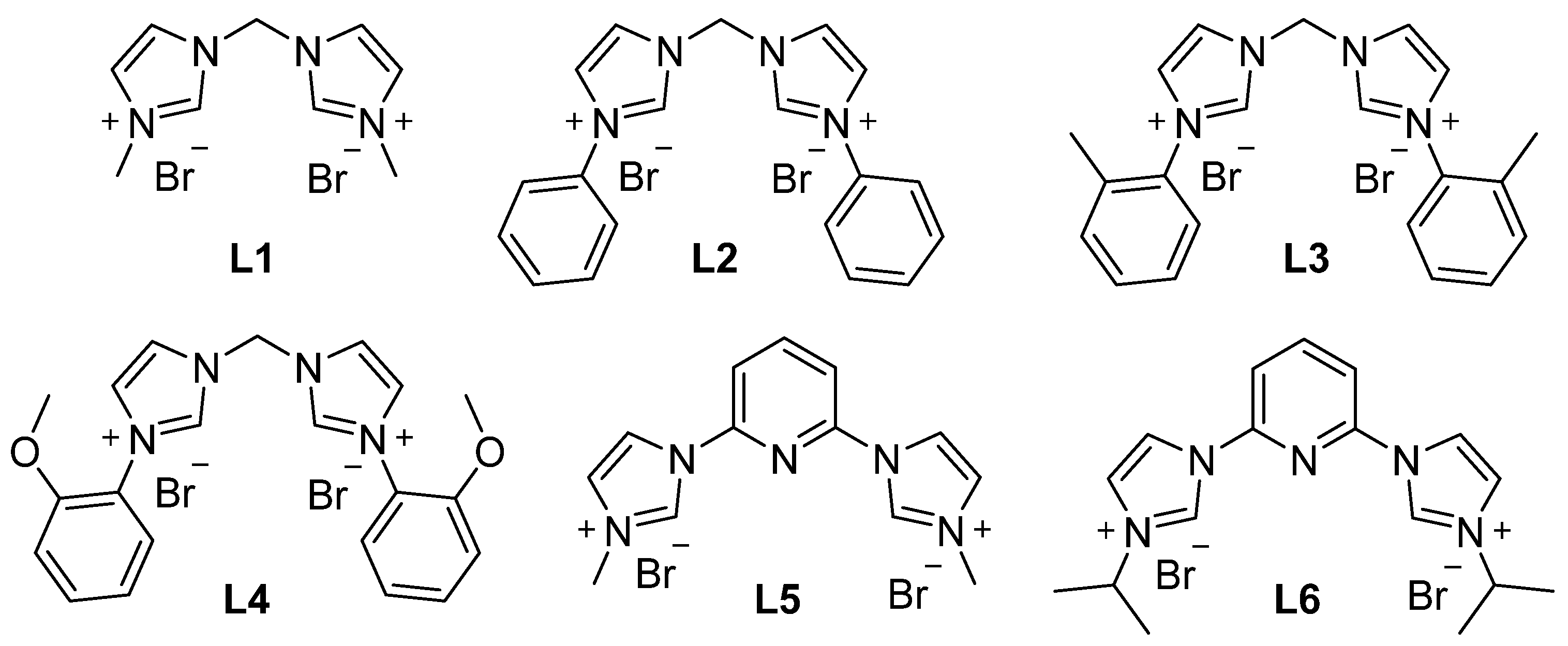

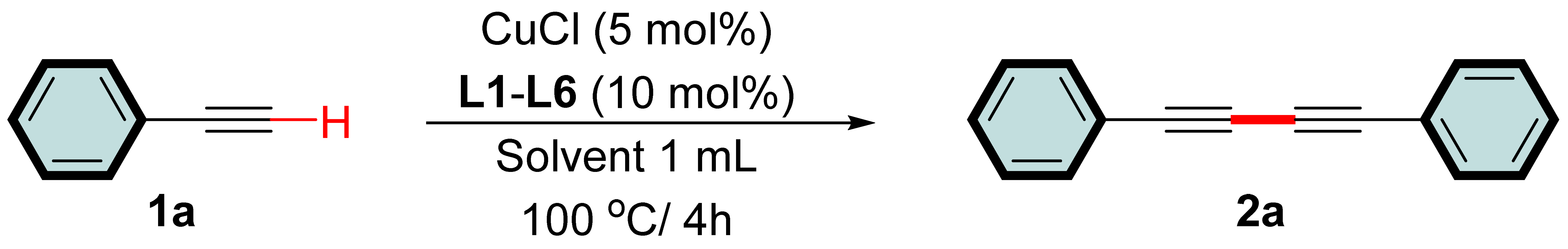 | ||||||
|---|---|---|---|---|---|---|
| Entry | [Cu] | Ligand | Solvent | Temperature (°C) | Time (h) | Yield(%) [b] |
| 1 | CuCl | L1 | DME | 120 | 12 | 78 |
| 2 | CuCl | L2 | DME | 120 | 12 | 86 |
| 3 | CuCl | L3 | DME | 120 | 12 | 34 |
| 4 | CuCl | L4 | DME | 120 | 12 | 42 |
| 5 | CuCl | L5 | DME | 120 | 12 | 49 |
| 6 | CuCl | L6 | DME | 120 | 12 | 47 |
| 7 | CuCl | L2 | 1,4-dioxane | 120 | 12 | trace |
| 8 | CuCl | L2 | DMF | 120 | 12 | 95 |
| 9 | CuCl | L2 | DMAc | 120 | 12 | 93 |
| 10 | CuCl | L2 | toluene | 120 | 12 | 92 |
| 11 | CuCl | L2 | xylene | 120 | 12 | 88 |
| 12 | CuCl | L2 | DMF | 100 | 12 | 94 |
| 13 | CuCl | L2 | DMF | 80 | 12 | 89 |
| 14 | CuCl | L2 | DMF | 100 | 8 | 95 |
| 15 | CuCl | L2 | DMF | 100 | 6 | 93 |
| 16 | CuCl | L2 | DMF | 100 | 4 | 94 |
| 17 | CuCl | L2 | DMF | 80 | 4 | 86 |
| 18 | CuCl | L2 | DMF | 60 | 4 | 66 |
| 19 | CuCl | L2 | DMF | 40 | 4 | 37 |
| 20 | CuCl | ----- | DMF | 100 | 4 | 61 |
| 21 | ----- | L2 | DMF | 100 | 4 | 0 |
| 22 | ----- | ----- | DMF | 100 | 4 | 0 |
| 23 | CuCl | L2 | DMF | 60 | 4 | 67 |
| 24 | CuCl | ----- | DMF | 60 | 4 | 28 |
| 25 | ----- | L2 | DMF | 60 | 4 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhu, Y.; Luo, J.; Zhu, Z.; Zhao, L.; Zeng, X.; Li, D.; Chen, J.; Lan, X. A Simple and Practical Bis-N-Heterocyclic Carbene as an Efficient Ligand in Cu-Catalyzed Glaser Reaction. Molecules 2023, 28, 5083. https://doi.org/10.3390/molecules28135083
Liu J, Zhu Y, Luo J, Zhu Z, Zhao L, Zeng X, Li D, Chen J, Lan X. A Simple and Practical Bis-N-Heterocyclic Carbene as an Efficient Ligand in Cu-Catalyzed Glaser Reaction. Molecules. 2023; 28(13):5083. https://doi.org/10.3390/molecules28135083
Chicago/Turabian StyleLiu, Jie, Yao Zhu, Jun Luo, Ziyi Zhu, Lin Zhao, Xiaoyan Zeng, Dongdong Li, Jun Chen, and Xiaobing Lan. 2023. "A Simple and Practical Bis-N-Heterocyclic Carbene as an Efficient Ligand in Cu-Catalyzed Glaser Reaction" Molecules 28, no. 13: 5083. https://doi.org/10.3390/molecules28135083
APA StyleLiu, J., Zhu, Y., Luo, J., Zhu, Z., Zhao, L., Zeng, X., Li, D., Chen, J., & Lan, X. (2023). A Simple and Practical Bis-N-Heterocyclic Carbene as an Efficient Ligand in Cu-Catalyzed Glaser Reaction. Molecules, 28(13), 5083. https://doi.org/10.3390/molecules28135083









