Abstract
Chemoprevention is one of the ways to fight colorectal cancer, which is a huge challenge in oncology. Numerous pieces of evidence indicate that chronic inflammation in the course of Crohn’s disease or ulcerative colitis (UC) is a significant cancer risk factor. Epidemiologic studies suggest that long-term use of non-steroidal anti-inflammatory drugs (NSAIDs), including mesalazine, has beneficial effects on colitis-associated colorectal cancer. Mesalazine is a first-line therapy for UC and is also widely used for maintaining remission in UC. Data showed that mesalazine has antiproliferative properties associated with cyclooxygenase (COX) inhibition but can also act through COX-independent pathways. This review summarizes knowledge about mesalazine’s molecular mechanisms of action and chemopreventive effect by which it could interfere with colorectal cancer cell proliferation and survival.
1. Introduction
Colorectal cancer (CRC) is one of the most significant healthcare problems worldwide. It is the second most commonly diagnosed cancer in women and the third in men [1,2]. In terms of mortality, it is the second leading cause of cancer-related death in the world [3]. Approximately 25–30% of confirmed colorectal cancers lead to metastasis, and nearly 50% of colorectal cancer patients have recurrence [4,5]. The mainstay of CRC treatment is tumor resection and chemotherapy. Additionally, radiotherapy is used before or after surgery, and in the case of disseminated CRC, targeted therapy such as anti-epidermal growth factor receptor (anti-EGFR) antibodies and anti-vascular endothelial growth factor receptor antibodies (anti-VEGFR) is used [6,7].
There are many risk factors leading to CRC development. We can distinguish between colorectal cancer risk factors depending on lifestyles and unmodified ones regardless of health concerns. An unhealthy lifestyle plays a major role in causing colorectal cancer. Environmental factors, such as overuse of alcohol, a high-calorie diet, lack of exercise, and carcinogens derived from frying foods, play a significant role in the development of CRC [6,7,8,9]. Furthermore, smokers have a 48% higher risk of developing CRC compared to nonsmokers [10]. Conversely, consumption of whole grains, fresh fruits and vegetables, and regular physical activity can decrease the risk. On the other hand, there are many well-known risk factors related to developing colorectal cancer independent of lifestyle. These include hereditary syndromes such as Lynch syndrome and familial polyposis associated with the APC gene mutation [6,11,12].
Inflammatory bowel disease (IBD) significantly increased the risk of CRC developing because of chronic intestinal inflammation, which leads to tumor development [13,14]. Inhibition of inflammatory processes correlates with decreased tumor formation. Numerous studies have indicated that anti-inflammatory drugs cause inhibition of tumor growth and angiogenesis and also increase apoptosis [15,16,17]. Furthermore, Cho et al. [1] show that a healthy lifestyle is associated with a reduced risk of colorectal cancer, regardless of an individual’s genetic risk factors. Due to this information, there has been a recent increase in the amount of ongoing research into the potential cytotoxic and antiproliferative effects of various therapeutic substances, among which mesalazine, an anti-inflammatory drug, can be included. Recent studies indicate that the mechanism of action of mesalazine can also be used in the treatment and prevention of colorectal cancer. This is due to its comprehensive action on the pathways responsible for the development and progression of cancer, i.e., the Wnt/β-catenin pathway and PPARγ, as well as its effect on the cell cycle or inhibition of the proliferation of cancer stem cells, which are the main cause of CRC recurrence. Taken together, the present paper aims to review the molecular basis of mesalazine’s chemopreventive effect in colorectal cancer, which may imply new strategies in the struggle with colorectal cancer. Mesalazine offers the possibility of chemoprevention at the stage of tumor formation and may also contribute to the prevention of its recurrence.
2. Mesalazine—An Old Drug, New Possibilities
The new idea of “drug repurposing”, involves attempts to use known and frequently used drugs for other indications. The main advantage of this approach over the synthesis of de novo drugs is a significant reduction in the costs and time required to implement a new therapy. In addition, a drug that has been available in medicine for a long time is well-established in terms of pharmacotherapy safety, pharmacokinetics, and interactions with other drugs [5,18]. One example with potential use in the treatment of colorectal cancer is mesalazine. Mesalazine, also known as mesalamine or 5-aminosalicylic acid (5-ASA), belongs to the non-steroidal anti-inflammatory drugs (NSAIDs) commonly used to treat inflammatory bowel disease (IBD). It is also used in the chemoprophylaxis of CRC associated with these conditions. Available data indicate that mesalazine also has the potential to inhibit tumor cell proliferation through several pathways [19]. As a medicinal substance, mesalazine is the pharmacologically active part of sulfasalazine, the first compound used to treat ulcerative colitis. Sulfapyridine, on the other hand, is the inactive component of sulfasalazine and is responsible for severe side effects. Consequently, mesalazine is preferred for self-administration [20,21].
Mesalazine is available in several forms, mainly as tablets, suppositories, foams, or as an enema [22]. 5-ASA can also be combined with sulfapyridine, as mentioned earlier, known as sulfasalazine. Two 5-ASA molecules joined by an azo bond are known as olsalazine. Application of 5-ASA in a conjugate form significantly reduces the absorption of the drug in the small intestine, allowing for sufficiently high concentrations in the terminal ileum and colon. The conjugates are cleaved in the colon by azoreductase. About 20–30% of 5-ASA from mesalazine preparations is absorbed in the small intestine and N-acetylated in intestinal epithelial cells and the liver. About 25% of mesalazine is absorbed in the large intestine, and the remainder is excreted unchanged in the feces [23,24]. 5-ASA, compared with other NSAIDs, is relatively well tolerated and has few side effects after use [25,26]. Rarely, interstitial nephritis, alveolitis, and pancreatitis have been observed; however, these side effects are usually reversible after discontinuation of mesalazine therapy [25,26].
IBD includes two main phenotypes: ulcerative colitis (UC) and Crohn’s disease (CD) [27]. Mesalazine is a first-line therapy in UC, resulting in good treatment outcomes in 88% of patients [28]. In addition, it is widely used to maintain remission in UC [29]. The mechanism of 5-ASA’s anti-inflammatory effect is not fully explained; however, existing data indicate that 5-ASA antagonizes pro-inflammatory mediators such as interferon-γ, IL-8, nuclear factor-κB, and tumor necrosis factor-α [29,30,31,32,33]. Mesalazine also inhibits the cyclooxygenase (COX) and lipoxygenase (LOX) pathways, contributing to the inhibition of prostaglandin E2 and leukotriene release [34], which are strongly associated with inflammation. It is also believed that the increase in PPARγ expression in gastrointestinal epithelial cells by mesalazine may be another mechanism of anti-inflammatory action [28]. Mesalazine also has an antioxidant function and is a free radical scavenger [35]. The aforementioned mechanisms result in the conclusion that, as a therapeutic substance, it may prevent intestinal inflammation but also induce mucosal healing [36]. As a compound, it has been extensively studied in vitro [37,38,39]. Numerous clinical trials with mesalazine are also conducted, e.g., in the chemoprevention of diseases related to the large intestine. Examples of such studies are shown in Table 1, generated from the https://clinicaltrials.gov database (accessed on 1 April 2023).

Table 1.
Clinical trials with the use of mesalazine in the chemoprevention of diseases related to the large intestine.
3. Chronic Inflammation Triggers CRC Development
The first suggestion that inflammation might be associated with cancer development comes from the nineteenth-century physician Rudolf Virchow [40]. Since then, numerous data confirmed that chronic inflammation may stimulate tumor initiation, promotion, and progression so-called “cancer-promoting inflammation’’ [41]. IBDs are chronic inflammatory illnesses that are commonly associated with a higher risk of colorectal cancer development. Although the exact reason for IBD is still elusive, it is generally accepted that IBD is associated with external environmental factors, immunological abnormalities, and genetic susceptibility of the host or intestinal microbiota [42]. Long-term inflammation may lead to colitis-associated cancer (CAC), wherein the severity and time of active disease correlate with the risk of CAC development. Chronic inflammation of IBD patients reveals a higher level of pro-inflammatory cytokines. Especially levels of IL-1, IL-6, IL-8, IL-12, and tumor necrosis factor α (TNF-α) are increased in inflamed mucosa [43,44]. Intestinal cytokine network disruption might trigger colorectal cancer development. For instance, IL-6 is a pro-inflammatory cytokine that exerts a pro-tumorigenic effect by activating the Janus kinases (JAK) and signal transducers and transcription activators (STATs), which are strongly associated with tumorigenic processes [17,45]. Interestingly, chronic inflammation and cancer development work on positive feedback mechanisms. Inflammation predisposes cancer development, but newly emerging tumors sustain an inflammatory process that stimulates its progression. During chronic inflammation, different inflammatory mediators are released, which activate transcriptional factors [15]. This is well described by two pathways connecting inflammation and cancer: an extrinsic and an intrinsic pathway. The extrinsic pathway is related to inflammatory conditions that predispose to developing cancer. Intrinsic ones rely on genetic alteration, which is the reason for inflammation and cancer development. Both intrinsic and extrinsic pathways result in the activation of transcriptional factors such as Nuclear Factor-κB (NF-κB), STAT3, and hypoxia-inducible factor 1α (HIF1α) [46,47], as shown in Figure 1.
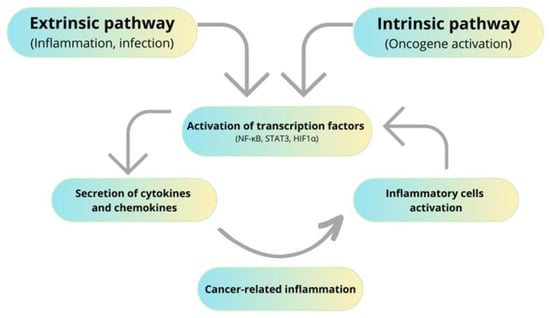
Figure 1.
Influence of inflammation on cancer development.
NFκB is a nuclear transcription factor involved in inflammation, carcinogenesis, proliferation, and apoptosis. It is also responsible for DNA damage by reactive oxygen species (ROS) secretion [17]. STAT3 is a transcription factor that stimulates apoptosis inhibitors and angiogenesis inducers. It is also involved in regulating the cell cycle, thus playing a significant role in tumor development and progression. Likewise, HIF1α is a transcriptional factor promoting cell proliferation and survival [48]. Beyond the influence of chronic inflammatory processes on cell proliferation, they also participate in the generation of ROS and reactive nitrogen species (RNS), epithelial-mesenchymal transition (EMT), angiogenesis, and metastasis [17]. Furthermore, COX-2-overexpressing cells produce large amounts of vascular endothelial growth factor (VEGF). VEGF is involved in angiogenesis, which is needed by cancer patients to provide large amounts of nutrients and also to promote metastasis [49].
Since chronic inflammation could be an important trigger for CRC development, targeting the eicosanoids pathway seems to be a good strategy for cancer prevention. There is strong data about aspirin’s chemopreventive effects in CRC [50,51]. Aspirin—acetylsalicylic acid—belongs to non-steroidal anti-inflammatory drugs, whose mechanism of action relies on the inhibition of cyclooxygenase 1 (COX1) and cyclooxygenase 2 (COX2). Early studies about aspirin’s chemopreventive action in CRC come from the 1990s [52]. Since then, many studies have been conducted that have proved that aspirin causes a reduction in CRC risk, but only after continuous and long-term use. Ma et al. [50] summarized these studies, prepared a meta-analysis of randomized controlled trial data, and showed that aspirin reduces the risk of mortality and the recurrence of CRC, which are significant problems in the struggle with CRC [53]. Unfortunately, this beneficial chemopreventive effect was also correlated with an increased risk of bleeding [2].
4. Chemopreventive Effect of Mesalazine on CRC
Recent studies suggest that, besides aspirin, long-term 5-ASA treatment reduces the risk of CRC developing in patients with IBD [54,55,56]. The first clinical findings about the chemopreventive role of 5-ASA come from 1994. Pinczowski et al. [54] showed that sulfasalazine significantly decreased the risk of CRC in patients with ulcerative colitis. Qiu et al. [36] conducted a systematic review with a meta-analysis to evaluate the effects of 5-ASA on the risk of colorectal cancer in patients with ulcerative colitis and Crohn’s disease. As a result, they showed that 5-ASA has a chemopreventive effect on CRC in IBD patients, with more benefits for UC patients than CD patients. To ensure effective treatment for reducing CRC risk in IBD patients, a mesalazine maintenance dosage of ≥1.2 g/day is needed. The European Crohn’s and Colitis Organization has recommended oral 5-ASA administration as chemoprevention in colitis-associated cancer (UC, CD).
Many in vitro studies show that mesalazine inhibits the growth and enhances apoptosis of CRC cell lines [37,38,39]. Moreover, studies conducted using animal models show that mesalazine inhibits tumor growth [57,58,59]. Notably, mesalazine treatment reduces the rate of proliferation of tumor cells without affecting the proliferation of normal epithelial cells [58,59]. Bus et al. [60] showed that topical administration of mesalazine for two weeks at a dosage of 4 g a day resulted in induced apoptosis in colorectal cancer cells, whereas it has no effect on the normal mucosa. 5-ASA’s chemopreventive mechanisms of action include interfering with the Wnt/β-catenin pathway, inhibition of cyclooxygenase and lipoxygenase mediators, antioxidant function, activation of the PPARγ pathway, and interfering with the EGFR pathway [61,62]. The chemical structure and mechanisms of action of mesalazine in CRC chemoprevention are shown in Figure 2.
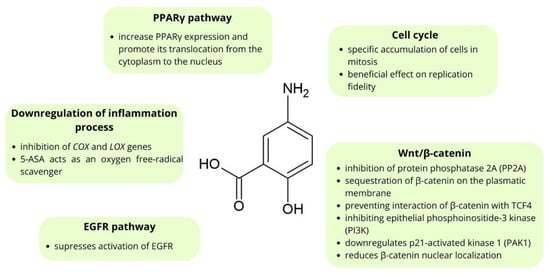
Figure 2.
The main points of molecular chemoprevention by mesalazine.
5. Mesalazine’s Anticancer Mechanisms of Action
5.1. Mesalazine’s Effect on Cell Cycle
Experimental data indicate that 5-ASA reduces the survival and growth of CRC cells through the modulation of different replication checkpoints [63]. Reinacher-Schick et al. [64] showed that mesalazine inhibits the proliferation of colon cancer cells, probably through a specific accumulation of cells in mitosis. HT-29 cells treated with mesalazine accumulated in the G2/M phase. This mechanism differs from that of other NSAIDs (indomethacin and sulindac), which induce a robust G1. Additionally, they showed that mesalazine induced apoptosis in colon cancer cells, possibly through the activation of caspase-3. This examination elucidated that the effect of apoptosis was seen in no more than 10% of the tested sample, which is less than in other NSAIDs. However, Stolfi et al. [65] showed that mesalazine leads to the accumulation of CRC cells in the S phase. This effect was associated with the ubiquitination and proteasome-dependent degradation of CDC25A, which is known to regulate the G1/S transition and S phase progression.
In addition, mesalazine has a beneficial effect on replication fidelity. Gasche et al. [66] demonstrated that mesalazine improves replication fidelity independent of post-replication mismatch repair. They showed that mesalazine at the 5.0 mmol/L level reduced the mutation rate in the mismatch repair-deficient HCT 116 cell line. It proves that mesalazine can reduce the rate of mutation, which is correlated with a decreased speed of tumor progression.
5.2. Inhibition “Inflammogenesis of Cancer” and ROS Scavenging
Numerous studies indicate that chronic inflammation is a significant cancer risk factor [17,67]. Mesalazine is a weak COX and LOX inhibitor. Some existing data suggest that Mesalazine’s chemopreventive mechanism of action is due to its anti-inflammatory properties. Pharmacological inhibition of COX-2 can prevent CRC development, possibly by inducing apoptosis, reducing cell proliferation, or modulating angiogenesis [54,64]. Stolfi et al. [51] conducted interesting research using the HT-115 CRC cell line with functionally active COX-2 and the colorectal adenocarcinoma cell line with COX-deficient (DLD-1). In effect, treatment of HT-115 cells with mesalazine causes a reduction in prostaglandin E2 levels, which is correlated with decreased proliferation of these cells. Interestingly, DLD-1 cell lines treated with mesalazine also showed blocking of cell growth, but through different mechanisms of action [54,68]. Moreover, the inflammatory process often generates large amounts of ROS [69]. Numerous data indicate that 5-ASA acts as an oxygen-free-radical scavenger [70,71,72]. Oxidized 5-ASA regenerates using endogenous compounds like cysteine, glutathione, or ascorbic acid. Therefore, the drug is preserved and able to act even in tissues undergoing oxidative stress [73].
5.3. Mesalazine Affects the Wnt/β-Catenin Pathway
The 5-ASA antineoplastic mechanism of action in CRC is strongly related to suppressing the Wnt/β-catenin pathway [74]. Mutation of the suppressor gene adenomatous polyposis coli (APC) is a common cause of genetic changes leading to the development of colorectal cancer by nuclear accumulation of β-catenin. The product of the APC gene is a protein that is important in creating the destruction complex responsible for the regulation of the level of β-catenin. The degradation complex consists of axin, APC, casein kinase 1, and glycogen synthase kinase 3 (GSK3β). β-catenin is a protein that plays a key role in the Wnt/β-catenin pathway [75,76,77]. This pathway is important in regulating embryonic development, cell growth and differentiation, cell fate, and self-renewal of stem cells [78]. In the absence of a ligand, this complex is responsible for the phosphorylation of β-catenin. Then, phosphorylated β-catenin is ubiquitylated by ligase and degraded by the proteasome [75]. On the other hand, when a ligand is present, it binds to Frizzled receptors, which leads to the inactivation of GSK3β. Inactivated complex blocks β-catenin phosphorylation, allowing accumulation in the cytoplasm. β-catenin then translocates into the cell nucleus, where it activates the transcription factor TCF/LEF (T-cell factor/lymphocyte enhance factor), which increases cell proliferation by activating the transcription of target genes encoding proteins such as c-Myc, cyclin D1, and CD44 [75,79]. Mutations resulting in upregulated Wnt/β-catenin signaling have been demonstrated to be sufficient for early adenoma formation [74,80].
Recently, there have been many new reports on the effect of mesalazine on the Wnt/β-catenin pathway. Furthermore, these studies show that mesalazine interacts with this signaling pathway in different ways [81,82,83,84,85,86,87,88].
Bos et al. [81] showed that mesalazine inhibits the Wnt/β-catenin pathway via inhibition of protein phosphatase 2A (PP2A). Treatment with mesalazine leads to increased phosphorylation of PP2A, which is related to decreased PP2A enzymatic activity. This effect led to increased β-catenin phosphorylation and then degradation; thus, mesalazine treatment reduced the expression of Wnt/β-catenin target genes. Furthermore, 5-ASA can also act by inducing phosphorylation of β-catenin at threonine 41 and serine 45, leading in the same way to its degradation by the proteasome.
Parenti et al. [82] consider the involvement of μ-protocadherin in mediating the effect of 5-ASA on the Wnt/β-catenin pathway. Mucin and cadherin-like protein (MUCDHL), also called cadherin-related family member 5 (CDHR5), belong to the cadherin-related family, which showed the strongest stimulation in expression profiling of all cadherins after 5-ASA treatment of colon cancer cells. MUCDHL gene encoding μ-protocadherin is silenced during the carcinogenesis of CRC [83]. MUCDHL in CRC is up-regulated by mesalazine, which is further reported to confirm that mesalazine inhibits the Wnt/β-catenin signaling pathway. In a previous study, Parenti et al. [84] showed that induction of expression μ-protocadherin by mesalazine is regulated by the Kruppel-like factor 4 (KLF4)—transcription factor. KLF4 is able to inhibit Wnt signaling by preventing the interaction of β-catenin with TCF4. Additionally, researchers showed that mesalazine induces the expression of μ-protocadherin, leading to the sequestration of β-catenin on the plasmatic membrane [82].
Brown et al. [85] found another way of interacting 5-ASA with the Wnt/β-catenin pathway that relies on inhibiting epithelial phosphoinositide-3 kinase, which is related to enhanced expression of Wnt/β-catenin target genes. Further investigations showed that 5-ASA demonstrates antioxidant properties, which confirm increased levels of antioxidant catalase after 5-ASA treatment [85]. Additionally, 5-ASA decreased the inactive phosphatase and tensin homolog (PTEN), which is a major negative regulator of PI3K. H2O2 leads to the inactivation of PTEN expression, whereas the antioxidant properties of 5-ASA increase PTEN activity. This data suggests that the antioxidant properties of 5-ASA may be the predominant mechanism for 5-ASA chemoprevention.
In another study regarding the influence of mesalazine on Wnt signaling, Khare et al. [87] showed that administration of mesalazine in mice downregulates p21-activated kinase 1 (PAK1), a serine/threonine kinase required for the full activation of Wnt/β-catenin signaling.
Munding et al. [88] revealed the inhibitory effect of mesalazine on the Wnt/β-catenin pathway through in vivo observations. β-catenin/TCF signaling activity was significantly reduced, probably through decreased β-catenin levels. Moreover, 5-ASA also reduced β-catenin nuclear localization, changing the expression of β-catenin target genes. The effect of mesalazine on the Wnt/β-catenin pathway is shown in Figure 3.
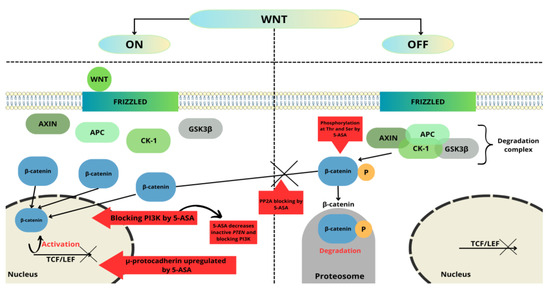
Figure 3.
5-ASA’s major checkpoints in the Wnt/β-catenin pathway. This figure was created using the Servier Medical Art Commons Attribution 3.0 Unported Licence (http://smart.servier.com (accessed 4 May 2023)).
5.4. Mesalazine Influences Homeostasis and Intestinal Healing
E-cadherin is the main mediator of cell-cell adhesion, which is important to maintain epithelial integrity. E-cadherin is a transmembrane molecule whose intracellular domain interacts with catenin, mainly β-catenin, which enhances adhesion properties. Data showed that disorders in maintaining the homeostasis of the intestinal epithelium are related both to the initiation and progression of CRC [83]. Impaired expression of E-cadherin is implicated in chronic gut inflammation and colorectal cancer [89]. In a study conducted by Losi et al. [83] on colon cancer cell lines, they demonstrated the effect of mesalazine on the expression level of µ-protocadherin, which interacts with the β-catenin-related pathway. The results suggest that an increase in the expression of this molecule may therefore have an inhibitory effect on the process of colon cancer carcinogenesis.
Furthermore, β-catenin forms a complex that maintains cell-cell adhesion by interacting with the intracellular domain of E-cadherin. Loss of E-cadherin leads to releasing β-catenin into the cytoplasm, from where it penetrates the cell nucleus, resulting in abnormal proliferation [75]. Khare et al. [87,89] showed that 5-ASA is the factor leading to increased membranous expression of E-cadherin and β-catenin. These findings testify about the mucosal healing possibilities of 5-ASA through enhanced cell-cell adhesion, which is often a disorder in both UC and colon cancer.
5.5. Mesalazine’s Effect on PPARγ Pathway and EGFR
Mesalazine is a ligand for peroxisome proliferator-activated receptor (PPAR) transcription factors, highly expressed in the colon, which play an important role in cell differentiation and proliferation [90,91]. An increase in cytoplasmic PPARγ and loss of nuclear one were observed in malignancy [86]. It has been shown that ligands capable of binding to PPARs and activating them, especially PPARγ and PPARα forms, influence the differentiation and induction of apoptosis of neoplastic cells. As a result, these ligands are potential drugs in anti-cancer and chemopreventive CRC therapy [92,93].
Rousseaux et al. [92] showed that 5-ASA increased PPARγ expression and promoted its translocation from the cytoplasm to the nucleus. Furthermore, PPARγ can retain β-catenin in the cytosol and reduce TCF transcriptional activity [93]. Activation of PPAR also enhances the proteasomal degradation of β-catenin [94].
Epidermal growth factor receptor (EGFR) is a protein whose activation leads to stimulating mitogenic and pro-survival signals [95]. It is strongly related to the pathogenesis of CRC and is also overexpressed in CRC. Mesalazine suppresses the activation of EGFR by inhibiting its phosphorylation. Mesalazine also leads to enhanced activity of the protein tyrosine phosphatases (PTPs), which negatively control EGFR activation [62].
5.6. Mesalazine Inhibits Proliferation of Colorectal Cancer Stem Cells
The theory of cancer stem cells (CSCs) describes that cancer cells are hierarchically organized. CSCs are a small subpopulation representing approximately 0.1–10% of all tumor cells that have the capacity to self-renew and differentiate into all types of cells in colon cancer. CSCs are involved in tumor initiation, maintenance, metastasis, and cancer recurrences [4,96,97,98]. Furthermore, these cells are responsible for chemotherapy and radiotherapy resistance. Many signaling pathways are dysregulated in CSCs; moreover, Hedgehog (Hh), Notch, and Wnt/β-catenin potentially regulate tumorigenesis in CSCs [99,100]. A recent study conducted by Dixon et al. [74] showed that 5-ASA suppresses β-catenin transcriptional activity, a key signaling pathway in stem cells. 5-ASA also inhibits the growth of adenoma cells and their stemness, which was demonstrated by repressing the expression of the stem cell marker LGR5 and protein CD133. Moreover, 5-ASA blocked the formation of adenoma-derived spheroids.
6. Conclusions
Over the last few decades, extensive research has been performed to indicate that mesalazine is effective in preventing CRC. The analyzed data showed that mesalazine has anti-neoplastic properties related both to its ability to inhibit COX enzymes and through COX-independent pathways. Importantly, these findings highlighted that the anticancer effect was not associated with changes in normal epithelial cells. Furthermore, mesalazine has an inhibitory effect on colon cancer stem cells, which are often involved in CRC recurrence. These observations, together with mesalazine’s mucosal healing properties, indicate that mesalazine could be a potential candidate to support patients after chemotherapy and reduce the number of cancer relapses. Further studies will be necessary to evaluate the potential effect on colon cancer stem cells and the possibilities of designing novel chemoprevention programs.
Author Contributions
Conceptualization, B.S.-M. and J.S.; literature review, M.M. and J.S.; writing—original draft preparation and review and editing, all the authors; visualization, M.M.; supervision, B.S.-M.; funding acquisition, J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Górnośląsko-Zagłębiowską Metropolis as part of the implementation of the project entitled “Support for scientific activity of doctoral students and employees at the doctoral level of the Silesian Medical University in Katowice” implemented within the framework of the Metropolitan Science Support Fund program for 2022–2024 (Grant Agreement No. RW/45/2023 dated 9 May 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available on request and with regulations.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Cho, Y.A.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Genetic risk score, combined lifestyle factors and risk of colorectal cancer. Cancer Res. Treat. 2019, 51, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Katona, B.W.; Weiss, J.M. Chemoprevention of colorectal cancer. Gastroenterology 2020, 158, 368–388. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Bhatt, L.K.; Johnston, T.P.; Prabhavalkar, K.S. Colon cancer stem cells: Potential target for the treatment of colorectal cancer. Cancer Biol. Ther. 2019, 20, 1068–1082. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Scapozza, L.; Ruiz i Altaba, A. Drug repurposing in oncology: Compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochim. Biophys. Acta BBA-Rev. Cancer 2019, 1871, 434–454. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Balchen, V.; Simon, K. Colorectal cancer development and advances in screening. Clin. Interv. Aging 2016, 11, 967–976. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Nasioulas, G.; Kosmidis, P.A. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009, 29, 2727–2737. [Google Scholar]
- Burnett-Hartman, A.N.; Lee, J.K.; Demb, J.; Gupta, S. An update on the epidemiology, molecular characterization, diagnosis, and screening strategies for early-onset colorectal cancer. Gastroenterology 2021, 160, 1041–1049. [Google Scholar] [CrossRef]
- Chen, X.; Jansen, L.; Guo, F.; Hoffmeister, M.; Chang-Claude, J.; Brenner, H. Smoking, genetic predisposition, and colorectal cancer risk. Clin. Transl. Gastroenterol. 2021, 12, e00317. [Google Scholar] [CrossRef]
- Kemp Bohan, P.M.; Mankaney, G.; Vreeland, T.J.; Chick, R.C.; Hale, D.F.; Cindass, J.L.; Hickerson, A.T.; Ensley, D.C.; Sohn, V.; Clifton, G.T.; et al. Chemoprevention in familial adenomatous polyposis: Past, present and future. Fam. Cancer. 2021, 20, 23–33. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal cancer and nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Kim, E.R. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 2014, 20, 9872. [Google Scholar] [CrossRef]
- Stidham, R.; Higgins, P. Colorectal cancer in inflammatory bowel disease. Clin. Colon Rectal Surg. 2018, 31, 168–178. [Google Scholar]
- Maniewska, J.; Jeżewska, D. Non-steroidal anti-inflammatory drugs in colorectal cancer chemoprevention. Cancers 2021, 13, 594. [Google Scholar] [CrossRef]
- Wesselink, E.; van Baar, H.; van Zutphen, M.; Tibosch, M.; Kouwenhoven, E.A.; Keulen, E.T.P.; Kok, D.E.; van Halteren, H.K.; Breukink, S.O.; de Wilt, J.H.W.; et al. Inflammation is a mediating factor in the association between lifestyle and fatigue in colorectal cancer patients. Cancers 2020, 12, 3701. [Google Scholar] [CrossRef]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef]
- Hua, Y.; Dai, X.; Xu, Y.; Xing, G.; Liu, H.; Lu, T.; Chen, Y.; Zhang, Y. Drug repositioning: Progress and challenges in drug discovery for various diseases. Eur. J. Med. Chem. 2022, 234, 114239. [Google Scholar] [CrossRef]
- Lyakhovich, A.; Gasche, C. Systematic review: Molecular chemoprevention of colorectal malignancy by mesalazine. Aliment Pharmacol. Ther. 2010, 31, 202–209. [Google Scholar] [CrossRef]
- Berends, S.E.; Strik, A.S.; Löwenberg, M.; D’Haens, G.R.; Mathôt, R.A.A. Clinical pharmacokinetic and pharmacodynamic considerations in the treatment of ulcerative colitis. Clin. Pharmacokinet. 2019, 58, 15–37. [Google Scholar] [CrossRef]
- Cunliffe, R.N.; Scott, B.B. Review article: Monitoring for drug side-effects in inflammatory bowel disease. Aliment Pharmacol. Ther. 2002, 16, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Kucharzik, T.; Koletzko, S.; Kannengiesser, K.; Dignass, A. Ulcerative colitis-diagnostic and therapeutic algorithms. Dtsch. Arztebl. Int. 2020, 117, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Lück, H.; Kinzig, M.; Jetter, A.; Fuhr, U.; Sörgel, F. Mesalazine pharmacokinetics and NAT2 phenotype. Eur. J. Clin. Pharmacol. 2009, 65, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Campregher, C.; Gasche, C. Aminosalicylates. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.C.; Peppercorn, M.A. The risks and the benefits of mesalazine as a treatment for ulcerative colitis. Expert. Opin. Drug Saf. 2007, 6, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.; Parkes, M. Systematic review: The use of mesalazine in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 23, 841–855. [Google Scholar] [CrossRef]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef]
- Sehgal, P.; Colombel, J.F.; Aboubakr, A.; Narula, N. Systematic review: Safety of mesalazine in ulcerative colitis. Aliment Pharmacol. Ther. 2018, 47, 1597–1609. [Google Scholar] [CrossRef]
- Ham, M.; Moss, A.C. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev. Clin. Pharmacol. 2012, 5, 113–123. [Google Scholar] [CrossRef]
- Tursi, A.; Scarpignato, C.; Strate, L.L.; Lanas, A.; Kruis, W.; Lahat, A.; Danese, S. Colonic diverticular disease. Nat. Rev. Dis. Primers 2020, 6, 20. [Google Scholar] [CrossRef]
- Oliveira, L.; Cohen, R.D. Maintaining remission in ulcerative colitis--role of once daily extended-release mesalamine. Drug Des. Devel. Ther. 2011, 5, 111–116. [Google Scholar]
- Subramanian, S.; Rhodes, J.M.; Hart, C.A.; Tam, B.; Roberts, C.L.; Smith, S.L.; Corkill, J.E.; Winstanley, C.; Virji, M.; Campbell, B.J. Characterization of epithelial IL-8 response to inflammatory bowel disease mucosal E. coli and its inhibition by mesalamine. Inflamm. Bowel Dis. 2008, 14, 162–175. [Google Scholar] [CrossRef]
- Yan, F.; Polk, D.B. Aminosalicylic acid inhibits IκB kinase α phosphorylation of IκBα in mouse intestinal epithelial cells. J. Biol. Chem. 1999, 274, 36631–36636. [Google Scholar] [CrossRef]
- Sonu, I.; Lin, M.V.; Blonski, W.; Lichtenstein, G.R. Clinical pharmacology of 5-ASA compounds in inflammatory bowel disease. Gastroenterol. Clin. N. Am. 2010, 39, 559–599. [Google Scholar] [CrossRef]
- Iacucci, M.; de Silva, S.; Ghosh, S. Mesalazine in inflammatory bowel disease: A trendy topic once again? Can. J. Gastroenterol. 2010, 24, 127–133. [Google Scholar] [CrossRef]
- Qiu, X.; Ma, J.; Wang, K.; Zhang, H. Chemopreventive effects of 5-aminosalicylic acid on inflammatory bowel disease-associated colorectal cancer and dysplasia: A systematic review with meta-analysis. Oncotarget 2017, 8, 1031–1045. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef]
- Goethel, A.; Croitoru, K.; Philpott, D.J. The interplay between microbes and the immune response in inflammatory bowel disease: Interplay between NOD2, microbiota and immune response in IBD. J. Physiol. 2018, 596, 3869–3882. [Google Scholar] [CrossRef]
- Mitselou, A.; Grammeniatis, V.; Varouktsi, A.; Papadatos, S.S.; Katsanos, K.; Galani, V. Proinflammatory cytokines in irritable bowel syndrome: A comparison with inflammatory bowel disease. Intest. Res. 2020, 18, 115–120. [Google Scholar] [CrossRef]
- Borowczak, J.; Szczerbowski, K.; Maniewski, M.; Kowalewski, A.; Janiczek-Polewska, M.; Szylberg, A.; Marszałek, A.; Szylberg, Ł. The role of inflammatory cytokines in the pathogenesis of colorectal carcinoma-recent findings and review. Biomedicines 2022, 10, 1670. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Candido, J.; Hagemann, T. Cancer-related inflammation. J. Clin. Immunol. 2013, 33, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Bae, S.H.; Jeong, J.W.; Kim, S.H.; Kim, K.W. Hypoxia-inducible factor (HIF-1)α: Its protein stability and biological functions. Exp. Mol. Med. 2004, 36, 1–12. [Google Scholar] [CrossRef]
- Cianchi, F.; Cuzzocrea, S.; Vinci, M.C.; Messerini, L.; Comin, C.E.; Navarra, G.; Perigli, G.; Centorrino, T.; Marzocco, S.; Lenzi, E.; et al. Heterogeneous expression of cyclooxygenase-2 and inducible nitric oxide synthase within colorectal tumors: Correlation with tumor angiogenesis. Dig. Liver Dis. 2010, 42, 20–27. [Google Scholar] [CrossRef]
- Stark, L.A.; Din, F.V.N.; Zwacka, R.M.; Dunlop, M.G. Aspirin-induced activation of the NF-κB signaling pathway: A novel mechanism for aspirin-mediated apoptosis in colon cancer cells. FASEB J. 2001, 15, 1273–1275. [Google Scholar] [CrossRef]
- Flossmann, E.; Rothwell, P.M. Effect of aspirin on long-term risk of colorectal cancer: Consistent evidence from randomised and observational studies. Lancet 2007, 369, 1603–1613. [Google Scholar] [CrossRef]
- Mereto, E.; Frencia, L.; Ghia, M. Effect of aspirin on incidence and growth of aberrant crypt foci induced in the rat colon by 1,2-dimethylhydrazine. Cancer Lett. 1994, 76, 5–9. [Google Scholar] [CrossRef]
- Ma, S.; Han, T.; Sun, C.; Cheng, C.; Zhang, H.; Qu, G.; Bhan, C.; Yang, H.; Guo, Z.; Yan, Y.; et al. Does aspirin reduce the incidence, recurrence, and mortality of colorectal cancer? A meta-analysis of randomized clinical trials. Int. J. Colorectal Dis. 2021, 36, 1653–1666. [Google Scholar] [CrossRef]
- Stolfi, C.; Fina, D.; Caruso, R.; Caprioli, F.; Sarra, M.; Fantini, M.C.; Rizzo, A.; Pallone, F.; Monteleone, G. Cyclooxygenase-2-dependent and -independent inhibition of proliferation of colon cancer cells by 5-aminosalicylic acid. Biochem. Pharmacol. 2008, 75, 668–676. [Google Scholar] [CrossRef]
- Eaden, J. Review article: The data supporting a role for aminosalicylates in the chemoprevention of colorectal cancer in patients with inflammatory bowel disease. Aliment Pharmacol. Ther. 2003, 18, 15–21. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Eaden, J.; Steinhart, A.H.; Munkholm, P.; Gordon, P.H. Cancer prevention in inflammatory bowel disease and the chemoprophylactic potential of 5-aminosalicylic acid. Inflamm. Bowel Dis. 2002, 8, 356–361. [Google Scholar] [CrossRef]
- Pinczowski, D.; Ekbom, A.; Baron, J.; Yuen, J.; Adami, H.O. Risk factors for colorectal cancer in patients with ulcerative colitis: A case-control study. Gastroenterology 1994, 107, 117–120. [Google Scholar] [CrossRef]
- Schwab, M.; Reynders, V.; Loitsch, S.; Shastri, Y.M.; Steinhilber, D.; Schröder, O.; Stein, J. PPARgamma is involved in mesalazine-mediated induction of apoptosis and inhibition of cell growth in colon cancer cells. Carcinogenesis 2008, 29, 1407–1414. [Google Scholar] [CrossRef]
- Baan, B.; Dihal, A.A.; Hoff, E.; Bos, C.L.; Voorneveld, P.W.; Koelink, P.J.; Wildenberg, M.E.; Muncan, V.; Heijmans, J.; Verspaget, H.W.; et al. 5-Aminosalicylic acid inhibits cell cycle progression in a phospholipase D dependent manner in colorectal cancer. Gut 2012, 61, 1708–1715. [Google Scholar] [CrossRef]
- Koelink, P.J.; Mieremet-Ooms, M.A.; Corver, W.E.; Wolanin, K.; Hommes, D.W.; Lamers, C.B.; Verspaget, H.W. 5-aminosalicylic acid interferes in the cell cycle of colorectal cancer cells and induces cell death modes. Inflamm. Bowel Dis. 2010, 16, 379–389. [Google Scholar] [CrossRef]
- Brown, W.A.; Farmer, K.C.; Skinner, S.A.; Malcontenti-Wilson, C.; Misajon, A.; O’Brien, P.E. 5-aminosalicylic acid and olsalazine inhibit tumor growth in a rodent model of colorectal cancer. Dig. Dis. Sci. 2000, 45, 7. [Google Scholar] [CrossRef]
- Ikeda, I.; Tomimoto, A.; Wada, K.; Fujisawa, T.; Fujita, K.; Yonemitsu, K.; Nozaki, Y.; Endo, H.; Takahashi, H.; Yoneda, M.; et al. 5-aminosalicylic acid given in the remission stage of colitis suppresses colitis-associated cancer in a mouse colitis model. Clin. Cancer Res. 2007, 13, 6527–6531. [Google Scholar] [CrossRef]
- Bus, P.J.; Nagtegaal, I.D.; Verspaget, H.W.; Lamers, C.B.; Geldof, H.; Van Krieken, J.H.; Griffioen, G. Mesalazine-induced apoptosis of colorectal cancer: On the verge of a new chemopreventive era? Aliment Pharmacol. Ther. 1999, 13, 1397–1402. [Google Scholar] [CrossRef]
- Sebastian, S.; Hernández, V.; Myrelid, P.; Kariv, R.; Tsianos, E.; Toruner, M.; Marti-Gallostra, M.; Spinelli, A.; van der Meulen-de Jong, A.E.; Yuksel, E.S.; et al. Colorectal cancer in inflammatory bowel disease: Results of the 3rd ECCO pathogenesis scientific workshop (I). J. Crohns Colitis 2014, 8, 5–18. [Google Scholar] [CrossRef]
- Stolfi, C.; De Simone, V.; Pallone, F.; Monteleone, G. Mechanisms of action of non-steroidal anti-inflammatory drugs (NSAIDs) and mesalazine in the chemoprevention of colorectal cancer. Int. J. Mol. Sci. 2013, 14, 17972–17985. [Google Scholar] [CrossRef] [PubMed]
- Fina, D.; Franchi, L.; Caruso, R.; Peluso, I.; Naccari, G.C.; Bellinvia, S.; Testi, R.; Pallone, F.; Monteleone, G. 5-aminosalicylic acid enhances anchorage-independent colorectal cancer cell death. Eur. J. Cancer 2006, 42, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Reinacher-Schick, A. Mesalazine causes a mitotic arrest and induces caspase-dependent apoptosis in colon carcinoma cells. Carcinogenesis 2003, 24, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Fina, D.; Caruso, R.; Caprioli, F.; Fantini, M.C.; Rizzo, A.; Sarra, M.; Pallone, F.; Monteleone, G. Mesalazine negatively regulates CDC25A protein expression and promotes accumulation of colon cancer cells in S phase. Carcinogenesis 2008, 29, 1258–1266. [Google Scholar] [CrossRef]
- Gasche, C.; Goel, A.; Natarajan, L.; Boland, C.R. Mesalazine improves replication fidelity in cultured colorectal cells. Cancer Res. 2005, 65, 3993–3997. [Google Scholar] [CrossRef]
- Huang, H.; Huang, Y.; Chen, Y.; Luo, Z.; Zhang, Z.; Sun, R.; Wan, Z.; Sun, J.; Lu, B.; Zhang, L.; et al. A novel immunochemotherapy based on targeting of cyclooxygenase and induction of immunogenic cell death. Biomaterials 2021, 270, 120708. [Google Scholar] [CrossRef]
- Stolfi, C.; Pellegrini, R.; Franzè, E.; Pallone, F.; Monteleone, G. Molecular basis of the potential of mesalazine to prevent colorectal cancer. World J. Gastroenterol. 2008, 14, 4434. [Google Scholar] [CrossRef]
- El-Kenawi, A.; Ruffell, B. Inflammation, ROS, and mutagenesis. Cancer Cell 2017, 32, 727–729. [Google Scholar] [CrossRef]
- Joshi, R.; Kumar, S.; Unnikrishnan, M.; Mukherjee, T. Free radical scavenging reactions of sulfasalazine, 5-aminosalicylic acid and sulfapyridine: Mechanistic aspects and antioxidant activity. Free Radic. Res. 2005, 39, 1163–1172. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Speisky, H.; Lissi, E. Antioxidant effect of 5-amino salicylic acid on copper-mediated LDL oxidation. Biol. Res. 2007, 40, 155–162. [Google Scholar] [CrossRef]
- Nandi, J.; Saud, B.; Zinkievich, J.M.; Palma, D.T.; Levine, R.A. 5-aminosalicylic acid improves indomethacin-induced enteropathy by inhibiting iNOS transcription in rats. Dig. Dis. Sci. 2008, 53, 123–132. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Rocco, C.; Lissi, E.; Carrasco, C.; Squella, J.A.; Nuñez-Vergara, L.; Speisky, H. Reaction of 5-aminosalicylic acid with peroxyl radicals: Protection and recovery by ascorbic acid and amino acids. Pharm. Res. 2005, 22, 1642–1648. [Google Scholar] [CrossRef]
- Dixon, S.W.; Collard, T.J.; Mortensson, E.M.H.; Legge, D.N.; Chambers, A.C.; Greenhough, A.; Creed, T.J.; Williams, A.C. 5-Aminosalicylic acid inhibits stem cell function in human adenoma-derived cells: Implications for chemoprophylaxis in colorectal tumorigenesis. Br. J. Cancer 2021, 124, 1959–1969. [Google Scholar] [CrossRef]
- Matly, A.; Quinn, J.A.; McMillan, D.C.; Park, J.H.; Edwards, J. The relationship between β-catenin and patient survival in colorectal cancer systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103337. [Google Scholar] [CrossRef]
- Koziński, K.; Dobrzyń, A. Wnt signaling pathway—its role in regulation of cell metabolism. Postepy Hig. Med. Dosw. 2013, 67, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signaling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef]
- Caspi, M.; Wittenstein, A.; Kazelnik, M.; Shor-Nareznoy, Y.; Rosin-Arbesfeld, R. Therapeutic targeting of the oncogenic Wnt signaling pathway for treating colorectal cancer and other colonic disorders. Adv. Drug Deliv. Rev. 2021, 169, 118–136. [Google Scholar] [CrossRef]
- Lu, D.; Cottam, H.B.; Corr, M.; Carson, D.A. Repression of beta-catenin function in malignant cells by nonsteroidal antiinflammatory drugs. Proc. Natl. Acad. Sci. USA 2005, 102, 18567–18571. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, X.; Chen, D.; Zhao, F.; Wang, W. Therapeutic potential of targeting the Wnt/β-catenin signaling pathway in colorectal cancer. Biomed. Pharmacother. 2019, 110, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.L.; Diks, S.H.; Hardwick, J.C.H.; Walburg, K.V.; Peppelenbosch, M.P.; Richel, D.J. Protein phosphatase 2A is required for mesalazine-dependent inhibition of Wnt/ -catenin pathway activity. Carcinogenesis 2006, 27, 2371–2382. [Google Scholar] [CrossRef]
- Parenti, S.; Ferrarini, F.; Zini, R.; Montanari, M.; Losi, L.; Canovi, B.; Ferrari, S.; Grande, A. Mesalazine inhibits the beta-catenin signalling pathway acting through the upregulation of mu-protocadherin gene in colo-rectal cancer cells. Aliment Pharmacol. Ther. 2010, 31, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Losi, L.; Parenti, S.; Ferrarini, F.; Rivasi, F.; Gavioli, M.; Natalini, G.; Ferrari, S.; Grande, A. Down-regulation of μ-protocadherin expression is a common event in colorectal carcinogenesis. Hum. Pathol. 2011, 42, 960–971. [Google Scholar] [CrossRef]
- Parenti, S.; Montorsi, L.; Fantini, S.; Mammoli, F.; Gemelli, C.; Atene, C.G.; Losi, L.; Frassineti, C.; Calabretta, B.; Tagliafico, E.; et al. KLF4 mediates the effect of 5-ASA on the β-catenin pathway in colon cancer cells. Cancer Prev. Res. 2018, 11, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.B.; Lee, G.; Managlia, E.; Grimm, G.R.; Dirisina, R.; Goretsky, T.; Cheresh, P.; Blatner, N.R.; Khazaie, K.; Yang, G.Y.; et al. Mesalamine inhibits epithelial beta-catenin activation in chronic ulcerative colitis. Gastroenterology 2010, 138, 595–605. [Google Scholar] [CrossRef]
- Managlia, E.; Katzman, R.B.; Brown, J.B.; Barrett, T.A. Antioxidant properties of mesalamine in colitis inhibit phosphoinositide 3-kinase signaling in progenitor cells. Inflamm. Bowel Dis. 2013, 19, 2051–2060. [Google Scholar] [CrossRef]
- Khare, V.; Lyakhovich, A.; Dammann, K.; Lang, M.; Borgmann, M.; Tichy, B.; Pospisilova, S.; Luciani, G.; Campregher, C.; Evstatiev, R.; et al. Mesalamine modulates intercellular adhesion through inhibition of p-21 activated kinase-1. Biochem. Pharmacol. 2013, 85, 234–244. [Google Scholar] [CrossRef]
- Munding, J.; Ziebarth, W.; Pox, C.P.; Ladigan, S.; Reiser, M.; Hüppe, D.; Brand, L.; Schmiegel, W.; Tannapfel, A.; Reinacher-Schick, A.C. The influence of 5-aminosalicylic acid on the progression of colorectal adenomas via the β-catenin signaling pathway. Carcinogenesis 2012, 33, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Lang, M.; Dammann, K.; Campregher, C.; Lyakhovich, A.; Gasche, C. Modulation of N-glycosylation by mesalamine facilitates membranous E-cadherin expression in colon epithelial cells. Biochem. Pharmacol. 2014, 87, 312–320. [Google Scholar] [CrossRef]
- Desreumaux, P.; Ghosh, S. Review article: Mode of action and delivery of 5-aminosalicylic acid—New evidence. Aliment Pharmacol. Ther. 2006, 24, 2–9. [Google Scholar] [CrossRef]
- Stolfi, C.; Pallone, F.; Monteleone, G. Colorectal cancer chemoprevention by mesalazine and its derivatives. J. Biomed. Biotechnol. 2012, 2012, 7247238. [Google Scholar] [CrossRef]
- Rousseaux, C.; Lefebvre, B.; Dubuquoy, L.; Lefebvre, P.; Romano, O.; Auwerx, J.; Metzger, D.; Wahli, W.; Desvergne, B.; Naccari, G.C.; et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J. Exp. Med. 2005, 201, 1205–1215. [Google Scholar] [CrossRef]
- Fujisawa, T.; Nakajima, A.; Fujisawa, N.; Takahashi, H.; Ikeda, I.; Tomimoto, A.; Yonemitsu, K.; Nakajima, N.; Kudo, C.; Wada, K.; et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) suppresses colonic epithelial cell turnover and colon carcinogenesis through inhibition of the beta-catenin/T cell factor (TCF) pathway. J. Pharmacol. Sci. 2008, 106, 627–638. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Zuo, Y.; Farmer, S.R. Functional interaction between peroxisome proliferator-activated receptor γ and β-catenin. Mol. Cell Biol. 2006, 26, 5827–5837. [Google Scholar] [CrossRef]
- Jorissen, R. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp. Cell Res. 2003, 284, 31–53. [Google Scholar] [CrossRef]
- Das, P.K.; Islam, F.; Lam, A.K. The roles of cancer stem cells and therapy resistance in colorectal carcinoma. Cells 2020, 9, 1392. [Google Scholar] [CrossRef] [PubMed]
- Leng, Z.; Tao, K.; Xia, Q.; Tan, J.; Yue, Z.; Chen, J.; Xi, H.; Li, J.; Zheng, H. Krüppel-like factor 4 acts as an oncogene in colon cancer stem cell-enriched spheroid cells. PLoS ONE 2013, 8, e56082. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.H.; Wilson, B.J.; Gold, J.S.; Frank, N.Y. Clinical implications of colorectal cancer stem cells in the age of single-cell omics and targeted therapies. Gastroenterology 2021, 160, 1947–1960. [Google Scholar] [CrossRef]
- Zhu, G.X.; Gao, D.; Shao, Z.Z.; Chen, L.; Ding, W.J.; Yu, Q.F. Wnt/β-catenin signaling: Causes and treatment targets of drug resistance in colorectal cancer (Review). Mol. Med. Rep. 2020, 23, 105. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting notch, hedgehog, and wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).