Abstract
Pomegranate (Punica granatum L.) is a rich source of polyphenols, including ellagitannins and ellagic acid. The plant is used in traditional medicine, and its purified components can provide anti-inflammatory and antioxidant activity and support of host defenses during viral infection and recovery from disease. Current data show that pomegranate polyphenol extract and its ellagitannin components and metabolites exert their beneficial effects by controlling immune cell infiltration, regulating the cytokine secretion and reactive oxygen and nitrogen species production, and by modulating the activity of the NFκB pathway. In vitro, pomegranate extracts and ellagitannins interact with and inhibit the infectivity of a range of viruses, including SARS-CoV-2. In silico docking studies show that ellagitannins bind to several SARS-CoV-2 and human proteins, including a number of proteases. This warrants further exploration of polyphenol–viral and polyphenol–host interactions in in vitro and in vivo studies. Pomegranate extracts, ellagitannins and ellagic acid are promising agents to target the SARS-CoV-2 virus and to restrict the host inflammatory response to viral infections, as well as to supplement the depleted host antioxidant levels during the stage of recovery from COVID-19.
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a β-coronavirus with a positive-sense ssRNA genome, which is the causative agent of the coronavirus disease (COVID-19). COVID-19 presents a significant health and social burden due to the high risk of severe illness, which may require hospitalization and can lead to mortality and morbidity because of the possible long recovery periods, even after the resolution of the acute respiratory involvement stage.
In the beginning of the pandemic, the medical and scientific community was focused on developing vaccines and therapeutic approaches to manage the acute stages of the disease and to decrease mortality and the spread of the infection. However, with the establishment of effective therapeutic strategies, clinicians started to come across a condition that was later defined as post-COVID-19 syndrome or long COVID. According to the Centers for Disease Control and Prevention, post-COVID-19 is characterized by a variety of symptoms, which may last for weeks or months after the infection is resolved. Post-COVID-19 conditions affect various organ systems and may include general symptoms (fatigue, fever and post-exertion malaise), respiratory and heart symptoms, neurological symptoms and digestive symptoms, as well as other symptoms like joint pain, rash and changes in menstrual cycle (https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html, accessed on 10 February 2023).
Recently published data suggest the use of plant polyphenolic extracts as a promising approach in the prevention of and recovery from COVID-19 [1,2,3,4,5,6]. Pomegranate (Punica granatum, L.) is an edible polyphenol-rich plant that has been used in traditional medicine for centuries. It is a source of several classes of polyphenols, including anthocyanins, flavonols and tannins [7]. Ellagic acid (EA) is a constituent of the hydrolysable ellagitannins (ETs) that are also present in other fruit, nuts and medicinal plants but are abundant in the pomegranate peel extracts (Figure 1). The anti-inflammatory properties of ETs and their ability to act as antioxidants and to mitigate reactive oxygen and nitrogen species (RONS) generation likely contribute to the numerous health benefits attributed to pomegranates.
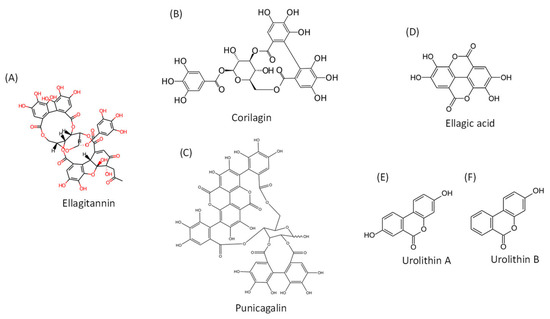
Figure 1.
Chemical structures of ellagitannins, ellagic acid and their metabolites. (A). General chemical structure of ellagitannins; (B). Structure of corilagin; (C). Structure of punicagalin; (D). Structure of ellagic acid; (E). Structure of urolithin A; (F). Structure of urolithin B.
Data from our previous studies, as well as the data available from other sources, support the possibility that pomegranate extract could be used as an anti-inflammatory and antioxidant agent to improve the host immune responses, including those directed against respiratory infections [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Some studies have reported direct interaction of EA and EA-containing polyphenols with viral components and inhibition of viral activity [10,18,25,26]. Other authors have explored the ability of pomegranate polyphenols to modulate the host immune responses to viral infection and concluded that EA and the ETs could be promising agents in preventing and ameliorating damage from COVID-19 [19,27,28,29,30,31].
2. COVID-19 Infection Pathology
The disease progression of COVID-19 is markedly different from that caused by the related coronavirus infections SARS-CoV-1 and MERS, even though SARS-CoV-2 infection also occurs mainly via the respiratory route, and viral entry into the host cells is facilitated by binding to the ACE2 (angiotensin-converting enzyme 2) receptor [31,32,33,34,35,36,37]. With the unfolding of the global COVID-19 pandemic, it has emerged that COVID-19 is not limited to acute infection of the respiratory tract but is a multi-organ disease with pathological changes that may last many months past the acute phase [31,32,38,39,40].
Deregulated neutrophil responses in COVID-19 have been proposed as the cause of the spreading inflammation, leading to severe systemic disease and multi-organ damage [41]. Neutrophil activation is modulated by RONS levels, which, in COVID-19, appear to result from the depletion of host antioxidant defenses [41,42,43]. The cellular effects of the SARS-CoV-1 main protease 3CLpro on human promonocyte cells, such as growth arrest and apoptosis via caspase-3 and caspase-9 activities, have been well characterized. [44] RONS production and signaling through the nuclear factor kappa-light-chain-enhancer (NF-κB) pathway are likely to be associated with SARS-CoV 3CLpro-induced pathology and with SARS-CoV-2 pathology given the involvement of its nonstructural proteins (3CLpro, nsp9, nsp10) in NF-κB signaling [44,45,46].
Oxidative stress has been recognized as a pathogenetic factor in many viral infections [47,48,49]. Previous studies from our group have explored the role of oxidative stress in a model of experimental influenza viral infection induced in ICR mice [28].
Altered redox balance and enhanced production of RONS can induce cell death and the release of virions and thus represents a mode for viral dissemination. On the other hand, oxidative stress is an important trigger for the antiviral immune response [47,48,49,50,51], pointing out the importance of understanding the effects of antioxidants on humoral immunity during infection and immunization. Indeed, Crump and colleagues (2013) showed that antioxidant treatment reduces the expansion of virus-specific antibody-secreting cells through decreased proliferation and increased cell death during the effector phase [51].
If the strength of the immune response is not tightly regulated, it may lead to a cytokine storm and severe inflammation, which affect lung function, as has been observed in the progression of severe COVID-19 [39]. Such deregulated production of cytokines during the cytokine storm may be even more detrimental to lung tissues in respiratory infections than the viruses themselves. Therefore, various agents have been evaluated and are used as remedies to target not only the viruses but also the associated inflammatory immune responses [52,53].
In support of the involvement of RONS in COVID-19 pathogenesis, the levels of superoxide dismutase 3 (SOD3) in the lungs of elderly patients and children may be a factor for the different disease progression in these age groups [41,42]. It is well known that ageing is associated with a decreased capacity of the cells to maintain their redox balance and to repair subcellular components such as mitochondria through autophagy. This results in chronic low-grade inflammation, or “inflamm-ageing” [31,54,55]. Atherosclerosis, hypertension, obesity, type 2 diabetes, dementia and ageing itself are not only comorbidity factors for worse SARS-CoV-2 infection outcomes but are also emerging as diseases with significant chronic inflammation and ROS involvement [55,56,57,58]. Therefore, proactive control of pro-inflammatory conditions or the limitation of chronic inflammatory conditions may alleviate COVID-19 symptoms and aid recovery. From this point of view, antioxidant supplementation is expected to ameliorate the consequences of infection. Our studies on the subject [19,27,28,29,30] showed the positive role of antioxidant therapy in infected cells and animals, in agreement with other reports [47].
3. Antioxidant and Anti-Inflammatory Activity of Pomegranate Extract
The anti-inflammatory and antioxidant properties of pomegranate are attributed predominantly to the polyphenolic substances present in both the edible and non-edible parts of the plant. These polyphenols are mainly anthocyanins, condensed tannins that give the fruit its brilliant red color and hydrolysable ellagitannins (ETs) [7]. The ETs are regarded as the main contributors to the antioxidant effects of pomegranate extracts, and their concentration is much higher in pomegranate plants compared to other plants [59,60,61]. ETs consist of one or multiple units of EA attached to a sugar or a sugar alcohol core. In pomegranate extract, numerous ET compounds have been identified, the punicalagins (PUN) being the most abundant, and a smaller portion is contributed by their hydrolysis products, punicalin and free EA [22,62,63]. Purified ETs, as well as the pomegranate polyphenol extract itself, have shown good antioxidant and anti-inflammatory activity in a range of experimental systems. Numerous articles have examined their effect on chronic inflammatory conditions, including autoimmune disorders, neurodegenerative conditions, respiratory distress and viral infection. The studies show a general trend of decrease in the levels of pro-inflammatory markers after treatment with plant polyphenol-rich extracts or with their purified components and downstream metabolites [63,64,65,66,67,68,69,70]. The data show that pre-treatment with pomegranate extracts, ETs (corilagin or punicalagin) and urolithin A are associated with anti-inflammatory effects in various tissues [71,72].
4. Antioxidant and Anti-Inflammatory Effects of EA and Its Metabolites
ETs undergo hydrolysis during fruit processing or after ingestion. Therefore, ET-rich plants or plant extracts can be a nutritional source of EA. The resultant EA is further converted to urolithins by the gut flora [63,73]. The urolithins and their conjugates show higher bioavailability compared to the EA precursor and thus can be expected to exert systemic effects [74,75]. However, the human population can be divided into three different metabotypes according to the urolithin profile measured after ingestion of ET-containing foods or extracts, which may result in a high variability of the effects associated with urolithin treatment in vivo [74,75,76,77].
Chronic inflammatory conditions are associated with immune cell invasion of the tissues and often lead to tissue damage, including fibrosis. The ET corilagin and EA have been shown to be able to interfere with hypertrophic scar formation and lung fibrosis by regulating levels of TGF-β1 via activity of lysyl oxidase homolog 2 enzyme (LOXL2) and the remodeling of the extracellular matrix by matrix metalloproteinases (MMPs) [78,79]. EA supports endothelial function not only by directly reducing oxidative stress but also by decreasing the TNF-α-induced endothelial expression of vascular cell adhesion molecule 1 (VCAM1) and intracellular adhesion molecule 1 (ICAM1) [80,81]. A reduction in immune cell invasion was achieved by using pomegranate extract, PUN or urolithin A in the lungs, CNS and other inflammation sites in a variety of rodent model systems. The positive effects of ETs and related metabolites on inhibiting the invasion of CNS tissues with immune cells and the decreased activation of resident immune cells (e.g., microglia) points to the potential benefits of using plant polyphenolic extracts as part of supportive treatment for neuro-inflammation after COVID-19, a serious and long-term complication [32,38].
In addition to infiltrating the inflamed tissues, activated immune cells release pro-inflammatory cytokines (including TNF-α, IL-1β and IL-6) and pro-inflammatory molecules, such as NO, which can also influence chemotaxis. Viral infections are also able to induce the secretion of these molecules [47,50,55,65]. The SARS-CoV-2 proteins nsp9 and nsp10 may stimulate chemotaxis via IL-6 and IL-8 by interfering with NFκB signaling [46,82].
The nuclear factor NFκB has been described as a “matchmaker between inflammation, inflammatory bowel disease, cancer and diabetes” [69], and it is under its regulation that IL-6, TNF-α and IL-1β levels increase in chronic diseases. Viral infection can also be an activator for NFκB. It appears that pomegranate polyphenolic extracts and their components restrict the secretion of pro-inflammatory molecules listed above by reducing NFκB activity [21,22,23,24]. A comparative study testing three ETs (urolithin A, iso-urolithin A and urolithin B), along with their respective glucuronides, on lipopolysaccharide (LPS)-induced inflammation in vitro showed that urolithin A was the most effective in reducing the levels of TNF-alpha, while its glucuronide conjugate did not have any effect [71].
The ability of ETs and EA to regulate cytokine levels may be beneficial to counteract the deregulation of immunity induced by SARS-CoV-2.
The studies demonstrating the antioxidant and anti-inflammatory properties of plant extracts containing ellagitannins or of purified ellagitannins and downstream metabolites (ellagic acid or urolithins) are listed in Table 1.

Table 1.
Antioxidant and anti-inflammatory properties of in vitro and in vivo application of plant extracts containing ellagitannins or application of purified ellagitannins and downstream metabolites (ellagic acid or urolithins). ↑: increased; ↓: decreased; x: counteracted.
6. Binding of Ellagitannins and Ellagic Acid to Components of SARS-CoV-2 and Human Host
In the beginning of the pandemic, the availability of the SARS-CoV-2 genomic information and the crystal structure of several of its proteins and of the human ACE2 receptor allowed for in silico docking studies and screens of phytochemical libraries for appropriate inhibitor molecules. The search identified numerous plant-derived secondary metabolites, among which EA and EA-containing polyphenols were high scoring. The interactions of ETs appear to be mediated primarily by the formation of multiple H-bonds to the amino acids of the viral target protein [25,91,93,96,97,103].
The target proteins in these in silico studies include the non-structural proteins (nsp) of the virus, as well as the spike protein. As our understanding of the SARS-CoV-2 mode of infection and the active sites of the proteins involved has progressed, the predicted docking studies have continued to grow in number and have also been expanded to include human target proteins. Some of these have also been tested in vitro to confirm the computational predictions in binding assays on purified proteins, and the dose-dependent antiviral effect of the purified polyphenols as well as whole pomegranate peel extract against SARS-CoV-2 was supported [92,93,95,96,104].
The receptor binding domain (RBD) in the N’ domain (NTD) of the spike (S) protein of SARS-CoV-2 recognizes the protease site of ACE2 on the surface of human cells. The S and M proteins also gather the viral components during intracellular replication. Thus, the RBD of the S protein has been a target of high interest in the search for plant metabolites with potential antiviral activity. ETs including PUN, corilagin, geraniin, punicalin and EA were identified as binding this viral protein [105]. The interaction was confirmed by an in vitro assay, which showed that punicalin interfered with the S1–ACE2 interaction [88]. Interestingly, in some but not all docking studies, the glycosylated N343 residue of the S1 RBD was identified as interacting with ETs [91,96,105,106].
The SARS-CoV main protease Mpro (3-chymotrypsin-like protease 3CLpro or Nsp5) is a Cys protease that cleaves the viral polyproteins at 11 different sites and is thus a good target for interfering with viral replication [107]. The cleavage site recognized by the protease appears in proteins involved in human innate immunity and may interfere with the immune response to viral infection [45]. Due to the apparent suitability of 3CLpro for drug development, a vast range of studies have included plant polyphenol-3CLpro docking, including PUN, punicalin, corilagin, chebulagic acid and geraniin, as well as EA and urolithins [93,104,106,107,108,109,110,111,112,113,114]. The catalytic dyad of 3CLpro is composed of H41 and C145, and in silico docking showed that EA and its precursors form H-bonds with the catalytic C145 residue [104,106,107,108,114]. Residues in the protease, which also showed up as interacting in many studies, were the nearby position G143 as well as E166 and Q189, all of which comprise part of the substrate-binding site of the protease [45]. On the other hand, in vitro studies by Du et al. (2021) indicated noncompetitive binding of ellagitannins to the viral protease and therefore searched for an allosteric site in the protein molecule, which they identified as a cleft between domains II and III [93].
Bahun and coworkers [104] followed up on their in silico docking studies with in vitro protease inhibition and surface plasmon resonance and calculated a Kd of 311 ± 69 µM for the EA-3CLpro interaction and an IC50 of 11.8 μM. Surface plasmon resonance also revealed strong binding to the main protease, while EA had lower affinity to the enzyme, and the urolithins were weaker inhibitors [113]. In another study, the IC50 of PUN towards 3CLpro 5.7 µM was enhanced in the presence of Zn2+ [92]. Corilagin scored in the top eight molecules in an in vitro fluorescent assay for protease inhibition [115]. A similar assay calculated IC50 9.09 ± 0.87 μM for chebulagic acid and 4.62 ± 0.27 μM for punicalagin [93]. Also, an in vitro study comparing the inhibitory activity of PUN, EA and gallic acid, as well as whole pomegranate peel extract, found 80% inhibition of 3CLpro activity when the pomegranate extract was used at 0.2 mg/mL, and most of this effect was contributed by PUN [116].
In addition to the intensively explored interaction of ETs with 3CLpro, ETs also appear to bind to and interfere with the functioning of some of the remaining 15 non-structural proteins of SARS-CoV-2. EA was the best in silico candidate for nsp9 binding and also showed nsp10 binding energies similar to other tested bioactive components from Moringa oleifera [103]. The two viral proteins nsp9 and nsp10 (methyltransferase) interact with NFκB repressing factor (NKRF) and may interfere with NFκB transcription activity, for which NKRF competes [46]. Nsp15 (endonuclease) and PUN were identified as interacting in silico [110]. A more extensive screen identified punicalin as the lowest binding energy partner for RdRp (nsp12, RNA-dependent RNA polymerase), as well as for nsp9 and PLpro (nsp3, papain-like protease) [1]. In a biosensor-based assay, corilagin was also the best binding partner for RdRp and at 40 μM inhibited the polymerase activity by more than 80% [97]. In a cell-based assay, this activity of corilagin persisted and inhibited infection of Vero cells by HCoV-OC-43 and SARS-CoV-2, indicating a broader coronavirus recognition [97].
The virus also interacts with several host proteases, including transmembrane serine protease 2 (TMPRSS2), furin, cathepsin L and ACE2. These were also tested for interaction with ETs and show additional potential of ETs as host protease inhibitors.
ACE2 acts as a metallocarboxypeptidase that is distant from the ACE2 catalytic site. The ellagitannins PUN, punicalin and EA recognized this human enzyme [105].
Pedunculagin, punicalin, PUN and EA bound in silico to furin at overlapping regions that included the catalytic residues H194, N295 and S368 [105,117]. Furin is a subtilisin-like serine endopeptidase that facilitates the entry of several viruses, including Ebola, HIV, Dengue and influenza, into host cells and activates the spike protein of SARS-CoV-2 by cleaving the spike glycoprotein at the S1 site [117].
Transmembrane protease serine 2 is a membrane-bound enzyme needed for viral entry. It exposes the viral fusion machinery by performing a cleavage on the S protein secondary to furin at the S2’ site and potentially the T1 and T2 sites [118,119]. This protease also facilitates the fusion of influenza A and B viruses with the host cell membrane [118]. The proteolytic processing of the spike protein also releases S1, which can act as an immune decoy [118]. The active site amino acids in TMPRSS2 (Q276, E299, K300, P301, K340, K342, E389, K390, L419, S441, Q438, and W461) and the catalytic triad H296, D345 and S441 interact with the SARS-CoV2 S-protein. They also appear to be recognized by pedunculagin and PUN and by bromhexine and serine protease inhibitors, such as camostat, as agents that inhibit TMPRSS2 and have been considered in COVID-19 treatment [110,117,119]. Camostat and nafamostat act by acetylating the S441 residue, which is also recognized by the ETs [118]. A combination of PUN with 2-deoxy-d-glucose showed the strongest binding energy (−10.8 kcal/mol) to TMPRSS2, more favorable than the established inhibitors bromhexine and camostat (approximately −9 kcal/mol) [110,119]. On the other hand, Surucic et al. [105] identified a different site of TMPRSS2 as the target for camostat, EA and ETs binding (including R87, R91, M97, R405, M404), with lower calculated binding energies. While this may be a potential allosteric site, the authors did not consider the intramembrane region (amino acids 1–145) of TMPRSS2, which is unlikely to be accessible by ellagitannins when the protease is in the plasma membrane [119]. A screen of several thousand plant secondary metabolites identified ellagitannins, including punicalin, pedunculagin and granatin A and B as compounds with good binding energy to TMPRSS2 and an ET metabolite 3′-O-methyl ellagic acid 4-xyloside with somewhat lower binding energy [5].
7. Considerations for the In Vivo Administration of EA-Containing Extracts
The low bioavailability of both EA and ETs after oral ingestion and their extensive biotransformation in the digestive tract makes it unlikely that their antiviral activity in vivo would be directly comparable to what is observed in cultured cells. In vivo exposure to highly concentrated ETs, resembling the conditions used in the studies listed in Table 2, will probably not go beyond the upper respiratory tract. A feasible application method may be lozenges, a nasal spray or microencapsulated pomegranate polyphenolic extract, such as the one used with good results in a murine model of asthma [61,120,121]. EA and the precursor ETs have low bioavailability if ingested but have high potential of being strong antioxidants and anti-inflammatory compounds and may be valuable in inhibiting viral particles if applied directly in the upper respiratory tract.
Nevertheless, the broad antiviral, antioxidant and anti-inflammatory effects of dietary intake of pomegranate extracts cannot be fully explained by direct polyphenols–virus interaction. As evidenced by the comparison of the data in Table 1 and Table 2, the numerous benefits of EA and of the more bioavailable urolithins are probably due to the convergence of poorly understood mechanisms that affect the bioactivity of ETs, resulting in immune cell regulation and signaling. We can also expect that the overall antioxidant and anti-inflammatory properties of ETs may also provide benefits in recovery from long COVID [19,27,28,29,30,34,81].
8. Conclusions
In silico and in vitro studies show that EA and ETs bind to several components of the SARS-CoV-2 virus with different affinity. This complementary effect may explain the higher antiviral activity of whole pomegranate extract relative to its isolated components. Other flavonoids also interact with the same SARS-CoV-2 targets and are present in ET-containing plants. These synergistic effects deserve further exploration in vivo. While the SARS-CoV-2 virus can lead to acute and prolonged disease by deregulating host immunity, triggering systemic inflammatory responses, and depleting intrinsic antioxidants, the EA-containing polyphenols may be promising candidates in countering all the above. Pomegranate extracts, containing a multitude of EA-derivatives, appear to act through different mechanisms to maintain endothelia integrity, to restrict inflammatory cell activation and tissue invasion and to supplement the antioxidant systems in the body. Supplementation with these bioactive compounds may therefore be especially beneficial to individuals with high risk for severe COVID-19 progression, e.g., ageing, type 2 diabetes, cardiovascular pathology, atherosclerosis and neurodegenerative diseases.
Author Contributions
Conceptualization, R.A., S.A., F.N. and L.T.; writing—original draft preparation R.A., S.A., A.S. and S.D.; writing—review and editing, R.K., F.N., P.F., M.C.P., S.M. and K.M.; supervision, R.K., F.N. and L.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Leon, V.N.O.; Manzano, J.A.H.; Pilapil, D.Y.H.; Fernandez, R.A.T.; Ching, J.K.A.R.; Quimque, M.T.J.; Agbay, J.C.M.; Notarte, K.I.R.; Macabeo, A.P.G. Anti-HIV reverse transcriptase plant polyphenolic natural products with in silico inhibitory properties on seven non-structural proteins vital in SARS-CoV-2 pathogenesis. J. Genet. Eng. Biotechnol. 2021, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Piri, S.; Majnooni, M.B.; Farzaei, M.H.; Echeverría, J. Targeting Neurological Manifestations of Coronaviruses by Candidate Phytochemicals: A Mechanistic Approach. Front. Pharm. 2020, 11, 621099. [Google Scholar] [CrossRef] [PubMed]
- Santhi, V.P.; Masilamani, P.; Sriramavaratharajan, V.; Murugan, R.; Gurav, S.S.; Sarasu, V.P.; Parthiban, S.; Ayyanar, M. Therapeutic potential of phytoconstituents of edible fruits in combating emerging viral infections. J. Food Biochem. 2021, 45, e13851. [Google Scholar] [CrossRef] [PubMed]
- David, A.B.; Diamant, E.; Dor, E.; Barnea, A.; Natan, N.; Levin, L.; Chapman, S.; Mimran, L.C.; Epstein, E.; Zichel, R.; et al. Identification of SARS-CoV-2 Receptor Binding Inhibitors by In Vitro Screening of Drug Libraries. Molecules 2021, 26, 3213. [Google Scholar] [CrossRef] [PubMed]
- Puttaswamy, H.; Gowtham, H.G.; Ojha, M.D.; Yadav, A.; Choudhir, G.; Raguraman, V.; Kongkham, B.; Selvaraju, K.; Shareef, S.; Gehlot, P.; et al. In silico studies evidenced the role of structurally diverse plant secondary metabolites in reducing SARS-CoV-2 pathogenesis. Sci. Rep. 2020, 10, 20584. [Google Scholar] [CrossRef]
- Ali, S.; Alam, M.; Khatoon, F.; Fatima, U.; Elasbali, A.M.; Adnan, M.; Islam, A.; Hassan, M.I.; Snoussi, M.; De Feo, V. Natural products can be used in therapeutic management of COVID-19: Probable mechanistic insights. Biomed. Pharm. 2022, 147, 112658. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- Lim, S.K.; Othman, R.; Yusof, R.; Heh, C.H. Rational drug discovery: Ellagic acid as a potent dual-target inhibitor against hepatitis C virus genotype 3 (HCV G3) NS3 enzymes. Chem. Biol. Drug Des. 2021, 97, 28–40. [Google Scholar] [CrossRef]
- AbouAitah, K.; Allayh, A.K.; Wojnarowicz, J.; Shaker, Y.M.; Swiderska-Sroda, A.; Lojkowski, W. Nanoformulation Composed of Ellagic Acid and Functionalized Zinc Oxide Nanoparticles Inactivates DNA and RNA Viruses. Pharmaceutics 2021, 13, 2174. [Google Scholar] [CrossRef]
- Arunkumar, J.; Rajarajan, S. Study on antiviral activities, drug-likeness and molecular docking of bioactive compounds of Punica granatum L. to Herpes simplex virus—2 (HSV-2). Microb. Pathog. 2018, 118, 301–309. [Google Scholar] [CrossRef]
- Živković, I.; Šavikin, K.; Živković, J.; Zdunić, G.; Janković, T.; Lazić, D.; Radin, D. Antiviral Effects of Pomegranate Peel Extracts on Human Norovirus in Food Models and Simulated Gastrointestinal Fluids. Plant Foods Hum. Nutr. 2021, 76, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.-T.; Karimi, A.; Shahrani, M.; Hashemi, L.; Ghaffari-Goosheh, M.-S. Anti-Influenza Virus Activity and Phenolic Content of Pomegranate (Punica granatum L.) Peel Extract and Fractions. Avicenna J. Med. Biotechnol. 2019, 11, 285–291. [Google Scholar] [PubMed]
- Yang, Y.; Xiu, J.; Zhang, L.; Qin, C.; Liu, J. Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Phytomedicine 2012, 20, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.H.; Kown, T.Y.; Oh, G.T.; Park, W.F.; Park, S.I.; Park, S.K.; Lee, Y.I. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen. Antivir. Res. 2006, 72, 100–106. [Google Scholar] [CrossRef]
- Houston, D.M.J.; Robins, B.; Bugert, J.J.; Denyer, S.P.; Heard, C.M. In vitro permeation and biological activity of punicalagin and zinc (II) across skin and mucous membranes prone to Herpes simplex virus infection. Eur. J. Pharm. Sci. 2017, 96, 99–106. [Google Scholar] [CrossRef]
- Karimi, A.; Moradi, M.T.; Rabiei, M.; Alidadi, S. In vitro anti-adenoviral activities of ethanol extract, fractions, and main phenolic compounds of pomegranate (Punica granatum L.) peel. Antivir. Chem. Chemother. 2020, 28. [Google Scholar] [CrossRef]
- Salles, T.S.; Meneses, M.D.F.; Caldas, L.A.; Sá-Guimarães, T.E.; de Oliveira, D.M.; Ventura, J.A.; Azevedo, R.C.; Kuster, R.M.; Soares, M.R.; Ferreira, D.F. Virucidal and antiviral activities of pomegranate (Punica granatum) extract against the mosquito-borne Mayaro virus. Parasites Vectors 2021, 14, 433. [Google Scholar] [CrossRef]
- Li, P.; Du, R.; Chen, Z.; Wang, Y.; Zhan, P.; Liu, X.; Kang, D.; Chen, Z.; Zhao, X.; Wang, L.; et al. Punicalagin is a neuraminidase inhibitor of influenza viruses. J. Med. Virol. 2021, 93, 3465–3472. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Stefanova, T.; Krumova, E.; Tancheva, L. Protective effect of polyphenol-rich extract on acute lung injury in influenza virus infected mice. Biotechnol. Biotechnol. Equip. 2009, 23, 1355–1359. [Google Scholar] [CrossRef]
- Cui, Q.; Du, R.; Anantpadma, M.; Schafer, A.; Hou, L.; Tian, J.; Davey, R.A.; Cheng, H.; Rong, L. Identification of ellagic acid from plant rhodiola rosea l. as an anti-ebola virus entry inhibitor. Viruses 2018, 10, 152. [Google Scholar] [CrossRef]
- Pavlova, E.L.; Simeonova, L.S.; Gegova, G.A. Combined efficacy of oseltamivir, isoprinosine and ellagic acid in influenza A(H3N2)-infected mice. Biomed. Pharmacother. 2018, 98, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Haidari, M.; Ali, M.; Ward Casscells, S.; Madjid, M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine 2009, 16, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kwon, M.J.; Yoo, J.Y.; Choi, H.J.; Ahn, Y.J. Antiviral activity and possible mode of action of ellagic acid identified in Lagerstroemia speciosa leaves toward human rhinoviruses. BMC Complement. Altern. Med. 2014, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Acquadro, S.; Civra, A.; Cagliero, C.; Marengo, A.; Rittà, M.; Francese, R.; Sanna, C.; Bertea, C.; Sgorbini, B.; Lembo, D.; et al. Punica granatum Leaf Ethanolic Extract and Ellagic Acid as Inhibitors of Zika Virus Infection. Planta Med. 2020, 86, 1363–1374. [Google Scholar] [CrossRef]
- Lin, L.T.; Chen, T.Y.; Chung, C.Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.C.; Wang, G.H.; Lin, C.C.; Richardson, C.D. Hydrolyzable Tannins (Chebulagic Acid and Punicalagin) Target Viral Glycoprotein-Glycosaminoglycan Interactions to Inhibit Herpes Simplex Virus 1 Entry and Cell-to-Cell Spread. J. Virol. 2011, 85, 4386–4398. [Google Scholar] [CrossRef]
- Reddy, B.U.; Mullick, R.; Kumar, A.; Sudha, G.; Srinivasan, N.; Das, S. Small molecule inhibitors of HCV replication from Pomegranate. Sci. Rep. 2014, 4, 5411. [Google Scholar] [CrossRef]
- Abarova, S.; Tancheva, L.; Nikolov, R.; Serkedjieva, J.; Pavlova, E.; Bramanti, A.; Nicoletti, F.; Tzvetkov, N.T. Preventive effect of a polyphenol-rich extract from Geranium sanguineum L. On hepatic drug metabolism in influenza infected mice. Sci. Pharm. 2020, 88, 45. [Google Scholar] [CrossRef]
- Tancheva, L. Drug metabolism and oxidative stress during Influenza Virus Infection. In Experimental Approaches for Antioxidant Protection; Publisher Eagle: Silistra, Bulgaria, 2019; p. 111. [Google Scholar]
- Serkedjieva, J.; Krumova, E.; Stefanova, T.; Tancheva, L. Pulmonary protection of a plant polyphenol extract in influenza virus-infected mice. J. Infect. 2009, 59, S426. [Google Scholar] [CrossRef]
- Encheva, E.; Tancheva, L.; Abarova, S.; Serkedjieva, J.; Ivantcheva, S. Modulation of hepatic drug metabolism in influenza infected mice. Preventive effect of polyphenols isolated from Geranium sanguineum L. Toxicol. Lett. 2010, 196, 723. [Google Scholar] [CrossRef]
- Moreno Fernández-Ayala, D.J.; Navas, P.; López-Lluch, G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp. Gerontol. 2020, 142, 111147. [Google Scholar] [CrossRef]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 2021, 31, 100683. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Wu, L.A.; Wang, Q.; Qi, J.; Gao, G.F. Cell entry by SARS-CoV-2. Trends Biochem. Sci. 2021, 46, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Basile, M.S.; Cavalli, E.; McCubrey, J.; Hernández-Bello, J.; Muñoz-Valle, J.F.; Fagone, P.; Nicoletti, F. The PI3K/Akt/mTOR pathway: A potential pharmacological target in COVID-19. Drug Discov. Today 2022, 27, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Islam, T.; Shahjaman, M.; Islam, M.R.; Lombardo, S.D.; Bramanti, P.; Ciurleo, R.; Bramanti, A.; Tchorbanov, A.; Fisicaro, F.; et al. Discovering common pathogenetic processes between COVID-19 and diabetes mellitus by differential gene expression pattern analysis. Brief. Bioinform. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, E.; Petralia, M.C.; Basile, M.S.; Bramanti, A.; Bramanti, P.; Nicoletti, F.; Spandidos, D.A.; Shoenfeld, Y.; Fagone, P. Transcriptomic analysis of COVID-19 lungs and bronchoalveolar lavage fluid samples reveals predominant B cell activation responses to infection. Int. J. Mol. Med. 2020, 46, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Fagone, P.; Ciurleo, R.; Lombardo, S.D.; Iacobello, C.; Palermo, C.I.; Shoenfeld, Y.; Bendtzen, K.; Bramanti, P.; Nicoletti, F. Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies. Autoimmun. Rev. 2020, 19, 102571. [Google Scholar] [CrossRef]
- Tancheva, L.; Petralia, M.C.; Miteva, S.; Dragomanova, S.; Solak, A.; Kalfin, R.; Lazarova, M.; Yarkov, D.; Ciurleo, R.; Cavalli, E.; et al. Emerging neurological and psychobiological aspects of COVID-19 infection. Brain Sci. 2020, 10, 852. [Google Scholar] [CrossRef]
- Harrison, A.G.; Lin, T.; Wang, P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol. 2020, 41, 1100–1115. [Google Scholar] [CrossRef]
- Cavalli, E.; Bramanti, A.; Ciurleo, R.; Tchorbanov, A.I.; Giordano, A.; Fagone, P.; Belizna, C.; Bramanti, P.; Shoenfeld, Y.; Nicoletti, F. Entangling COVID-19 associated thrombosis into a secondary antiphospholipid antibody syndrome: Diagnostic and therapeutic perspectives (Review). Int. J. Mol. Med. 2020, 46, 903–912. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef] [PubMed]
- Fratta Pasini, A.M.; Stranieri, C.; Cominacini, L.; Mozzini, C. Potential Role of Antioxidant and Anti-Inflammatory Therapies to Prevent Severe SARS-CoV-2 Complications. Antioxidants 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Lin, K.H.; Hsieh, T.H.; Shiu, S.Y.; Li, J.Y. Severe acute respiratory syndrome coronavirus 3C-like protease-induced apoptosis. FEMS Immunol. Med. Microbiol. 2006, 46, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Hameedi, M.A.T.; Prates, E.; Garvin, M.R.; Mathews, I.I.; Amos, B.K.; Demerdash, O.; Bechthold, M.; Iyer, M.; Rahighi, S.; Kneller, D.W.; et al. Structural and functional characterization of NEMO cleavage by SARS-CoV-2 3CLpro. Nat. Commun. 2022, 13, 5285. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, M.; Tian, X.; Liu, C.; Wang, X.; Yang, X.; Wu, P.; Xiao, Z.; Qu, Y.; Yin, Y.; et al. Virus-host interactome and proteomic survey of PMBCs from COVID-19 patients reveal potential virulence factors influencing SARS-CoV-2 pathogenesis. bioRxiv 2020, 2, 99–112.e7. [Google Scholar] [CrossRef]
- Khomich, O.A.; Kochetkov, S.N.; Bartosch, B.; Ivanov, A.V. Redox biology of respiratory viral infections. Viruses 2018, 10, 392. [Google Scholar] [CrossRef]
- Camini, F.C.; da Silva Caetano, C.C.; Almeida, L.T.; de Brito Magalhães, C.L. Implications of oxidative stress on viral pathogenesis. Arch. Virol. 2017, 162, 907–917. [Google Scholar] [CrossRef]
- Beck, M.A.; Handy, J.; Levander, O.A. The Role of Oxidative Stress in Viral Infections. Ann. N. Y. Acad. Sci. 2000, 917, 906–912. [Google Scholar] [CrossRef]
- Checconi, P.; De Angelis, M.; Marcocci, M.E.; Fraternale, A.; Magnani, M.; Palamara, A.T.; Nencioni, L. Redox-modulating agents in the treatment of viral infections. Int. J. Mol. Sci. 2020, 21, 4084. [Google Scholar] [CrossRef]
- Crump, K.E.; Langston, P.K.; Rajkarnikar, S.; Grayson, J.M. Antioxidant treatment regulates the humoral immune response during acute viral infection. J. Virol. 2013, 87, 2577–2586. [Google Scholar] [CrossRef]
- Mendonca, P.; Soliman, K.F.A. Flavonoids Activation of the Transcription Factor Nrf2 as a Hypothesis Approach for the Prevention and Modulation of SARS-CoV-2 Infection Severity. Antioxidants 2020, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Nabar, N.R.; Huang, N.N.; Kehrl, J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- Ganji, R.; Reddy, P.H. Impact of COVID-19 on Mitochondrial-Based Immunity in Aging and Age-Related Diseases. Front. Aging Neurosci. 2021, 12, 614650. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, S.; Baldassari, S.; Ailuno, G.; Zuccari, G.; Drava, G.; Petretto, A.; Cossu, V.; Marini, C.; Alfei, S.; Florio, T.; et al. Two Novel PET Radiopharmaceuticals for Endothelial Vascular Cell Adhesion Molecule-1 (VCAM-1) Targeting. Pharmaceutics 2021, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Rumbaugh, J.A.; Nath, A. Viruses and the Brain: From Inflammation to Dementia. Clin. Sci. 2006, 110, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Savoia, C.; Schiffrin, E.L. Inflammation in Hypertension. Curr. Opin. Nephrol. Hypertens. 2006, 15, 152–158. [Google Scholar] [CrossRef]
- Benchagra, L.; Berrougui, H.; Islam, M.O.; Ramchoun, M.; Boulbaroud, S.; Hajjaji, A.; Fulop, T.; Ferretti, G.; Khalil, A. Antioxidant effect of moroccan pomegranate (Punica granatum L. sefri variety) extracts rich in punicalagin against the oxidative stress process. Foods 2021, 10, 2219. [Google Scholar] [CrossRef]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef]
- Braidy, N.; Selvaraju, S.; Essa, M.M.; Vaishnav, R.; Al-Adawi, S.; Al-Asmi, A.; Al-Senawi, H.; Abd Alrahman Alobaidy, A.; Lakhtakia, R.; Guillemin, G.J. Neuroprotective effects of a variety of pomegranate juice extracts against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxid. Med. Cell Longev. 2013, 2013, 685909. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; BiBi, J.; Kamboh, A.A.; Arain, M.A.; Shah, Q.A.; Alagawany, M.; El-Hack, M.E.A.; Abdel-Latif, M.A.; Yatoo, M.I.; et al. The Promising Pharmacological Effects and Therapeutic/Medicinal Applications of Punica Granatum L. (Pomegranate) as a Functional Food in Humans and Animals. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 24–38. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, N.A.; Nahar, P.P.; Ma, H.; Eid, A.; Wei, Z.; Meschwitz, S.; Zawia, N.H.; Slitt, A.L.; Seeram, N.P. Pomegranate ellagitannin-gut microbial-derived metabolites, urolithins, inhibit neuroinflammation in vitro. Nutr. Neurosci. 2019, 22, 185–195. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, J.F.F.; Garreto, D.V.; Da Silva, M.C.P.; Fortes, T.S.; De Oliveira, R.B.; Nascimento, F.R.F.; Da Costa, F.B.; Grisotto, M.A.G.; Nicolete, R. Therapeutic potential of biodegradable microparticles containing Punica granatum L. (pomegranate) in murine model of asthma. Inflamm. Res. 2013, 62, 971–980. [Google Scholar] [CrossRef]
- Guo, Y.J.; Zhao, L.; Li, X.F.; Mei, Y.W.; Zhang, S.L.; Tao, J.Y.; Zhou, Y.; Dong, J.H. Effect of Corilagin on anti-inflammation in HSV-1 encephalitis and HSV-1 infected microglias. Eur. J. Pharmacol. 2010, 635, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Husari, A.; Khayat, A.; Bitar, H.; Hashem, Y.; Rizkallah, A.; Zaatari, G.; El Sabban, M. Antioxidant activity of pomegranate juice reduces acute lung injury secondary to hyperoxia in an animal model. BMC Res. Notes 2014, 7, 664. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wei, D.; Fu, Z.; Li, D.; Tan, Y.; Xu, T.; Zhou, J.; Zhang, T. Punicalagin Ameliorates Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome in Mice. Inflammation 2015, 38, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.X.; Li, X.; Deng, S.Y.; Zhao, L.; Zhang, Y.Y.; Deng, X.; Han, B.; Yu, J.; Li, Y.; Wang, Z.Z.; et al. Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor. EBioMedicine 2021, 64, 103227. [Google Scholar] [CrossRef]
- Abdelazeem, K.N.M.; Kalo, M.Z.; Beer-Hammer, S.; Lang, F. The gut microbiota metabolite urolithin A inhibits NF-κB activation in LPS stimulated BMDMs. Sci. Rep. 2021, 11, 7117. [Google Scholar] [CrossRef]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W.; Yang, M.; Hou, C. Pomegranate peel polyphenols inhibits inflammation in LPS-induced RAW264.7 macrophages via the suppression of TLR4/NF-κB pathway activation. Food Nutr. Res. 2019, 63, 3392. [Google Scholar] [CrossRef]
- Bobowska, A.; Granica, S.; Filipek, A.; Melzig, M.F.; Moeslinger, T.; Zentek, J.; Kruk, A.; Piwowarski, J.P. Comparative studies of urolithins and their phase II metabolites on macrophage and neutrophil functions. Eur. J. Nutr. 2021, 60, 1957–1972. [Google Scholar] [CrossRef]
- Busto, R.; Serna, J.; Perianes-Cachero, A.; Quintana-Portillo, R.; García-Seisdedos, D.; Canfrán-Duque, A.; Paino, C.L.; Lerma, M.; Casado, M.E.; Martín-Hidalgo, A.; et al. Ellagic acid protects from myelin-associated sphingolipid loss in experimental autoimmune encephalomyelitis. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2018, 1863, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Esselun, C.; Theyssen, E.; Eckert, G.P. Effects of Urolithin A on Mitochondrial Parameters in a Cellular Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2021, 22, 8333. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative stress, antioxidant capabilities, and bioavailability: Ellagic acid or urolithins? Antioxidants 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; García-Villalba, R.; González-Sarrías, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramírez-De-Molina, A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef]
- Selma, M.V.; González-Sarrías, A.; Salas-Salvadó, J.; Andrés-Lacueva, C.; Alasalvar, C.; Örem, A.; Tomás-Barberán, F.A.; Espín, J.C. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: Comparison between normoweight, overweight-obesity and metabolic syndrome. Clin. Nutr. 2018, 37, 897–905. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Z.; Zhao, D.; Han, D. Corilagin alleviates hypertrophic scars via inhibiting the transforming growth factor (TGF)-β/Smad signal pathway. Life Sci. 2021, 277, 119483. [Google Scholar] [CrossRef]
- Wei, Y.; Kim, T.J.; Peng, D.H.; Duan, D.; Gibbons, D.L.; Yamauchi, M.; Jackson, J.R.; Le Saux, C.J.; Calhoun, C.; Peters, J.; et al. Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J. Clin. Investig. 2017, 127, 3675–3688. [Google Scholar] [CrossRef]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 99, 715–722. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, B.; Zhou, K.; Chen, M.; Wang, M.; Jia, Y.; Song, Y.; Li, Y.; Wen, A. Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: Role of Nrf2 activation. Int. J. Cardiol. 2014, 175, 508–514. [Google Scholar] [CrossRef]
- Anderson, M.; Turchi, J. Targeting of Non-Structural Protein 9 as a Novel Therapeutic Target for the Treatment of SARS-CoV-2. Proc. IMPRS 2020, 3, 24502. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Jilma-Stohlawetz, P.; Rios, J.; Hingorani, L.; Derendorf, H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J. Agric. Food Chem. 2006, 54, 8956–8961. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Ma, H.; Liu, W.; Niesen, D.B.; Shah, N.; Crews, R.; Rose, K.N.; Vattem, D.A.; Seeram, N.P. Pomegranate’s Neuroprotective Effects against Alzheimer’s Disease Are Mediated by Urolithins, Its Ellagitannin-Gut Microbial Derived Metabolites. ACS Chem Neurosci. 2016, 7, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Yang, Q.; Harada, M.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Pomegranate flower extract diminishes cardiac fibrosis in Zucker diabetic fatty rats: Modulation of cardiac endothelin-1 and nuclear factor-kappaB pathways. J. Cardiovasc. Pharmacol. 2005, 46, 856–862. [Google Scholar] [CrossRef]
- Tancheva, L.P.; Lazarova, M.I.; Alexandrova, A.V.; Dragomanova, S.T.; Nicoletti, F.; Tzvetanova, E.R.; Hodzhev, Y.K.; Kalfin, R.E.; Miteva, S.A.; Mazzon, E.; et al. Neuroprotective Mechanisms of Three Natural Antioxidants on a Rat Model of Parkinson’s Disease: A Comparative Study. Antioxidants 2020, 9, 49. [Google Scholar] [CrossRef]
- Bachoual, R.; Talmoudi, W.; Boussetta, T.; Braut, F.; El-Benna, J. An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem. Toxicol. 2011, 49, 1224–1228. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, B.S.; Kim, K.S.; Lee, S.; Shin, K.S.; Lim, J.S. Immune-suppressive activity of punicalagin via inhibition of NFAT activation. Biochem. Biophys. Res. Commun. 2008, 371, 799–803. [Google Scholar] [CrossRef]
- Romier, B.; Van De Walle, J.; During, A.; Larondelle, Y.; Schneider, Y.J. Modulation of signalling nuclear factor-κB activation pathway by polyphenols in human intestinal Caco-2 cells. Br. J. Nutr. 2008, 100, 542–551. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Suručić, R.; Travar, M.; Petković, M.; Tubić, B.; Stojiljković, M.P.; Grabež, M.; Šavikin, K.; Zdunić, G.; Škrbić, R. Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: In silico and in vitro studies. Bioorganic. Chem. 2021, 114, 105145. [Google Scholar] [CrossRef]
- Saadh, M.J.; Almaaytah, A.M.; Alaraj, M.; Dababneh, M.F.; Sa’adeh, I.; Aldalaen, S.M.; Kharshid, A.M.; Alboghdadly, A.; Hailat, M.; Khaleel, A.; et al. Punicalagin and zinc (II) ions inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Cooper, L.; Chen, Z.; Lee, H.; Rong, L.; Cui, Q. Discovery of chebulagic acid and punicalagin as novel allosteric inhibitors of SARS-CoV-2 3CLpro. Antivir. Res. 2021, 190, 105075. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Chen, T.Y.; Lin, S.C.; Chung, C.Y.; Lin, T.C.; Wang, G.H.; Anderson, R.; Lin, C.C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013, 13, 187. [Google Scholar] [CrossRef]
- Kim, Y.S.; Chung, H.S.; Noh, S.G.; Lee, B.; Chung, H.Y.; Choi, J.G. Geraniin inhibits the entry of SARS-CoV-2 by blocking the interaction between spike protein RBD and human ACE2 receptor. Int. J. Mol. Sci. 2021, 22, 8604. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Chen, R.H.; Hamdoun, S.; Coghi, P.; Ng, J.P.L.; Zhang, D.W.; Guo, X.; Xia, C.; Law, B.Y.K.; Wong, V.K.W. Corilagin prevents SARS-CoV-2 infection by targeting RBD-ACE2 binding. Phytomedicine 2021, 87, 153591. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yi, D.; Lei, X.; Zhao, J.; Zhang, Y.; Cui, X.; Xiao, X.; Jiao, T.; Dong, X.; Zhao, X.; et al. Corilagin inhibits SARS-CoV-2 replication by targeting viral RNA-dependent RNA polymerase. Acta Pharm. Sin. B 2021, 11, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Promsong, A.; Chuenchitra, T.; Saipin, K.; Tewtrakul, S.; Panichayupakaranant, P.; Satthakarn, S.; Nittayananta, W. Ellagic acid inhibits HIV-1 infection in vitro: Potential role as a novel microbicide. Oral Dis. 2018, 24, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Kang, E.H.; Lee, Y.I. A flavonoid from medicinal plants blocks hepatitis B virus-e antigen secretion in HBV-infected hepatocytes. Antivir. Res. 2005, 67, 163–168. [Google Scholar] [CrossRef]
- Giordano, D.; Facchiano, A.; Carbone, V. Food Plant Secondary Metabolites Antiviral Activity and Their Possible Roles in SARS-CoV-2 Treatment: An Overview. Molecules 2023, 8, 2470. [Google Scholar] [CrossRef]
- Antoine, T.E.; Park, P.J.; Shukla, D. Glycoprotein targeted therapeutics: A new era of anti-herpes simplex virus-1 therapeutics. Rev. Med. Virol. 2013, 23, 194–208. [Google Scholar] [CrossRef]
- Roa-Linares, V.C.; Escudero-Flórez, M.; Vicente-Manzanares, M.; Gallego-Gómez, J.C. Host Cell Targets for Unconventional Antivirals against RNA Viruses. Viruses 2023, 15, 776. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Hassan, S.H.; Al-Sehemi, A.G.; Shakir, H.A.; Khan, M.; Irfan, M.; Iqbal, J. Exploring the new potential antiviral constituents of Moringa oliefera for SARS-CoV-2 pathogenesis: An in silico molecular docking and dynamic studies. Chem. Phys. Lett. 2021, 767, 138379. [Google Scholar] [CrossRef] [PubMed]
- Bahun, M.; Jukić, M.; Oblak, D.; Kranjc, L.; Bajc, G.; Butala, M.; Bozovičar, K.; Bratkovič, T.; Podlipnik, Č.; Poklar Ulrih, N. Inhibition of the SARS-CoV-2 3CL(pro) main protease by plant polyphenols. Food Chem. 2022, 373, 131594. [Google Scholar] [CrossRef] [PubMed]
- Suručić, R.; Tubić, B.; Stojiljković, M.P.; Djuric, D.M.; Travar, M.; Grabež, M.; Šavikin, K.; Škrbić, R. Computational study of pomegranate peel extract polyphenols as potential inhibitors of SARS-CoV-2 virus internalization. Mol. Cell Biochem. 2021, 476, 1179–1193. [Google Scholar] [CrossRef]
- Arokiyaraj, S.; Stalin, A.; Kannan, B.S.; Shin, H. Geranii Herba as a Potential Inhibitor of SARS-CoV-2 Main 3CL(pro), Spike RBD, and Regulation of Unfolded Protein Response: An In Silico Approach. Antibiotics 2020, 9, 863. [Google Scholar] [CrossRef]
- Umar, A.K.; Zothantluanga, J.H.; Aswin, K.; Maulana, S.; Sulaiman Zubair, M.; Lalhlenmawia, H.; Rudrapal, M.; Chetia, D. Antiviral phytocompounds “ellagic acid” and “(+)-sesamin” of Bridelia retusa identified as potential inhibitors of SARS-CoV-2 3CL pro using extensive molecular docking, molecular dynamics simulation studies, binding free energy calculations, and bioactivity prediction. Struct. Chem. 2022, 33, 1445–1465. [Google Scholar] [CrossRef]
- Pandey, A.K.; Verma, S. An in-silico evaluation of dietary components for structural inhibition of SARS-CoV-2 main protease. J. Biomol. Struct. Dyn. 2022, 40, 136–142. [Google Scholar] [CrossRef]
- Falade, V.A.; Adelusi, T.I.; Adedotun, I.O.; Abdul-Hammed, M.; Lawal, T.A.; Agboluaje, S.A. In silico investigation of saponins and tannins as potential inhibitors of SARS-CoV-2 main protease (M(pro)). In Silico Pharmacol. 2021, 9, 9. [Google Scholar] [CrossRef]
- Gupta, A.; Chauhan, S.S.; Gaur, A.S.; Parthasarathi, R. Computational screening for investigating the synergistic regulatory potential of drugs and phytochemicals in combination with 2-deoxy-D-glucose against SARS-CoV-2. Struct. Chem. 2022, 33, 2179–2193. [Google Scholar] [CrossRef]
- Adelusi, T.I.; Oyedele, A.Q.K.; Monday, O.E.; Boyenle, I.D.; Idris, M.O.; Ogunlana, A.T.; Ayoola, A.M.; Fatoki, J.O.; Kolawole, O.E.; David, K.B.; et al. Dietary polyphenols mitigate SARS-CoV-2 main protease (Mpro)–Molecular dynamics, molecular mechanics, and density functional theory investigations. J. Mol. Struct. 2022, 1250, 131879. [Google Scholar] [CrossRef]
- Monteiro, N.; Monteiro, V.; Lima, L.; Karolline, A.; Machado, R. In Silico Prediction of Inhibitory Potential of a Punicalagin β-anomer Against SARS-CoV-2 Main Protease (Mpro). Quimica Nova 2021, 45, 1230–1235. [Google Scholar] [CrossRef]
- Li, H.; Xu, F.; Liu, C.; Cai, A.; Dain, J.A.; Li, D.; Seeram, N.P.; Cho, B.P.; Ma, H. Inhibitory Effects and Surface Plasmon Resonance-Based Binding Affinities of Dietary Hydrolyzable Tannins and Their Gut Microbial Metabolites on SARS-CoV-2 Main Protease. J. Agric. Food Chem. 2021, 69, 12197–12208. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, I.; Zhu, W.; Mohammed, H.H.H.; Dutta, K.; Li, C. Tannins inhibit SARS-CoV-2 through binding with catalytic dyad residues of 3CLpro: An in silico approach with 19 structural different hydrolysable tannins. J. Food Biochem. 2020, 44, e13412. [Google Scholar] [CrossRef] [PubMed]
- Loschwitz, J.; Jäckering, A.; Keutmann, M.; Olagunju, M.; Eberle, R.J.; Coronado, M.A.; Olubiyi, O.O.; Strodel, B. Novel inhibitors of the main protease enzyme of SARS-CoV-2 identified via molecular dynamics simulation-guided in vitro assay. Bioorg. Chem. 2021, 111, 104862. [Google Scholar] [CrossRef] [PubMed]
- Tito, A.; Colantuono, A.; Pirone, L.; Pedone, E.; Intartaglia, D.; Giamundo, G.; Conte, I.; Vitaglione, P.; Apone, F. Pomegranate Peel Extract as an Inhibitor of SARS-CoV-2 Spike Binding to Human ACE2 Receptor (in vitro): A Promising Source of Novel Antiviral Drugs. Front. Chem. 2021, 9, 638187. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, S.; Sahoo, S.K. Virtual screening by targeting proteolytic sites of furin and TMPRSS2 to propose potential compounds obstructing the entry of SARS-CoV-2 virus into human host cells. J. Tradit. Complement. Med. 2022, 12, 6–15. [Google Scholar] [CrossRef]
- Fraser, B.J.; Beldar, S.; Seitova, A.; Hutchinson, A.; Mannar, D.; Li, Y.; Kwon, D.; Tan, R.; Wilson, R.P.; Leopold, K.; et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 2022, 18, 963–971. [Google Scholar] [CrossRef]
- Vankadari, N.; Ketavarapu, V.; Mitnala, S.; Vishnubotla, R.; Reddy, D.N.; Ghosal, D. Structure of Human TMPRSS2 in Complex with SARS-CoV-2 Spike Glycoprotein and Implications for Potential Therapeutics. J. Phys. Chem. Lett. 2022, 13, 5324–5333. [Google Scholar] [CrossRef]
- Vora, A.; Londhe, V.; Pandita, N. Herbosomes Enhance the in Vivo Antioxidant Activity and Bioavailability of Punicalagins from Standardized Pomegranate Extract. J. Funct. Foods 2015, 12, 540–548. [Google Scholar] [CrossRef]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Appl. Sci. 2020, 10, 3353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).