Synthesis of Flower-Like Cobalt–Molybdenum Mixed-Oxide Microspheres for Deep Aerobic Oxidative Desulfurization of Fuel
Abstract
1. Introduction
2. Results and Discussion
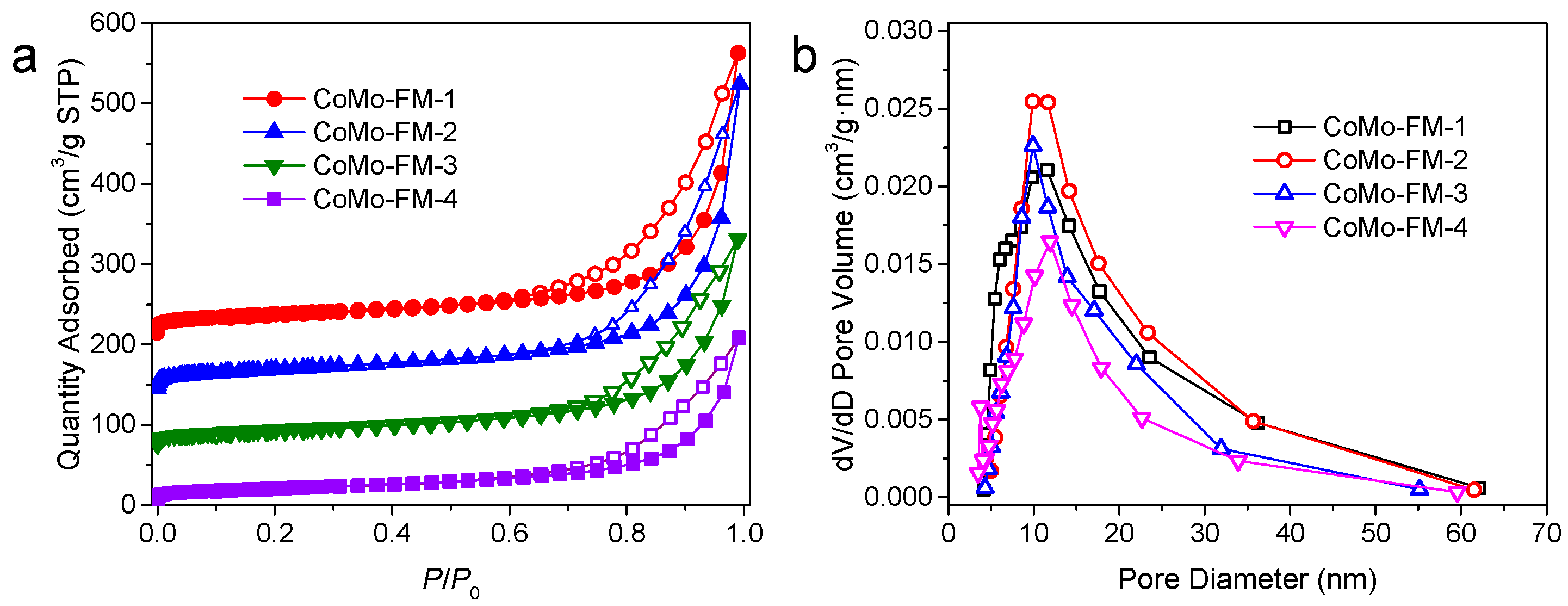
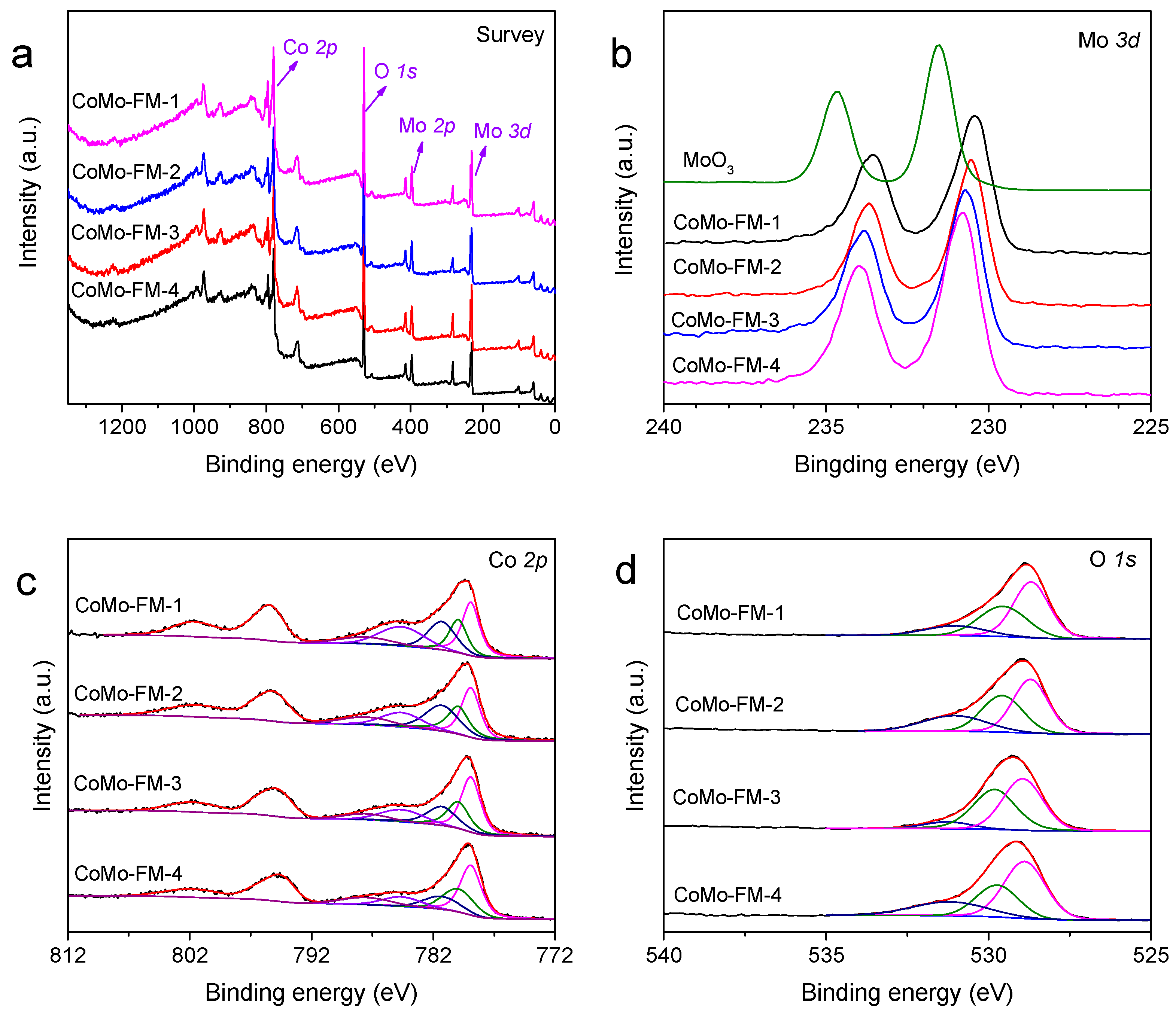
2.1. Synthesis and Characterization of CoMo-FMs
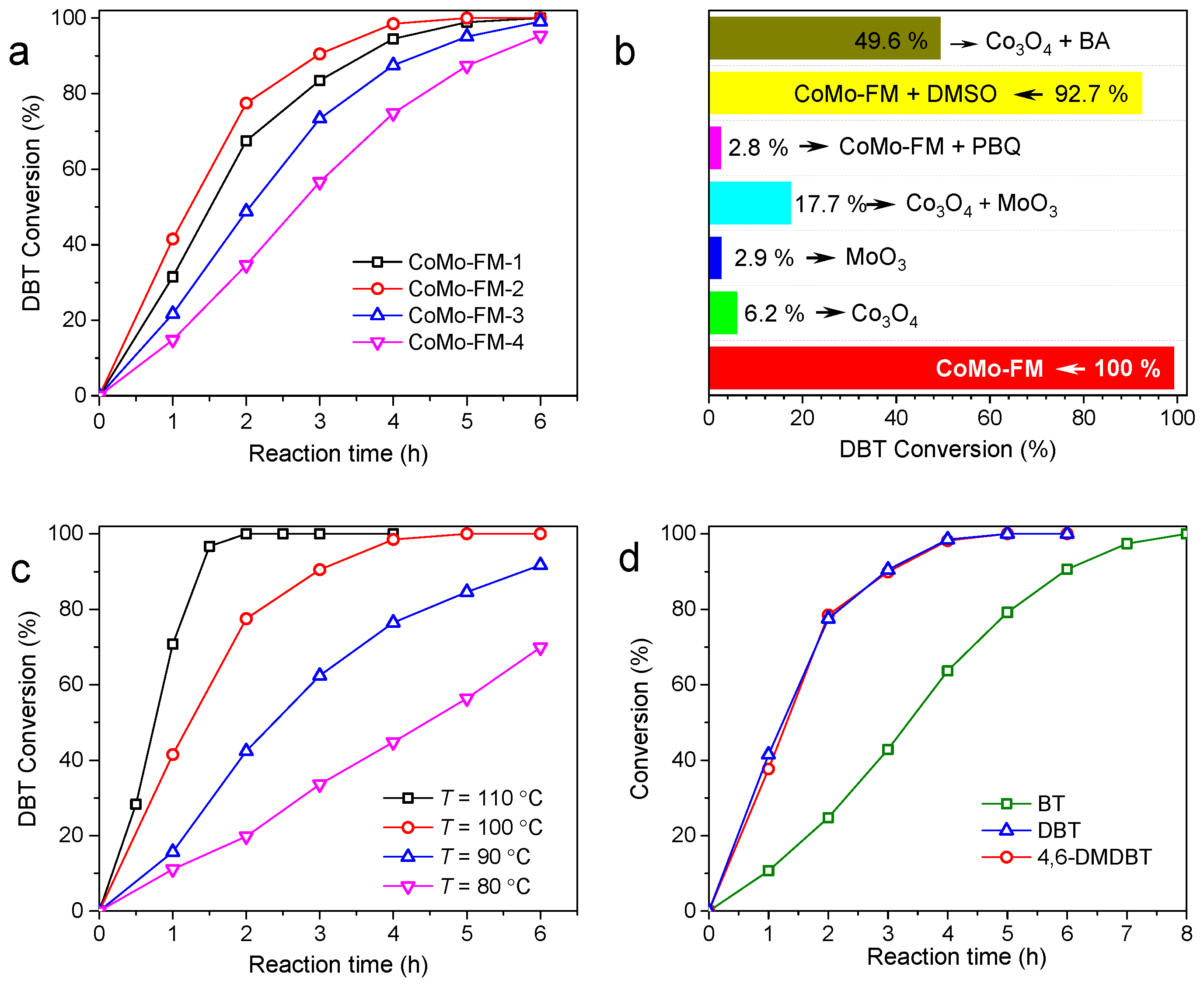
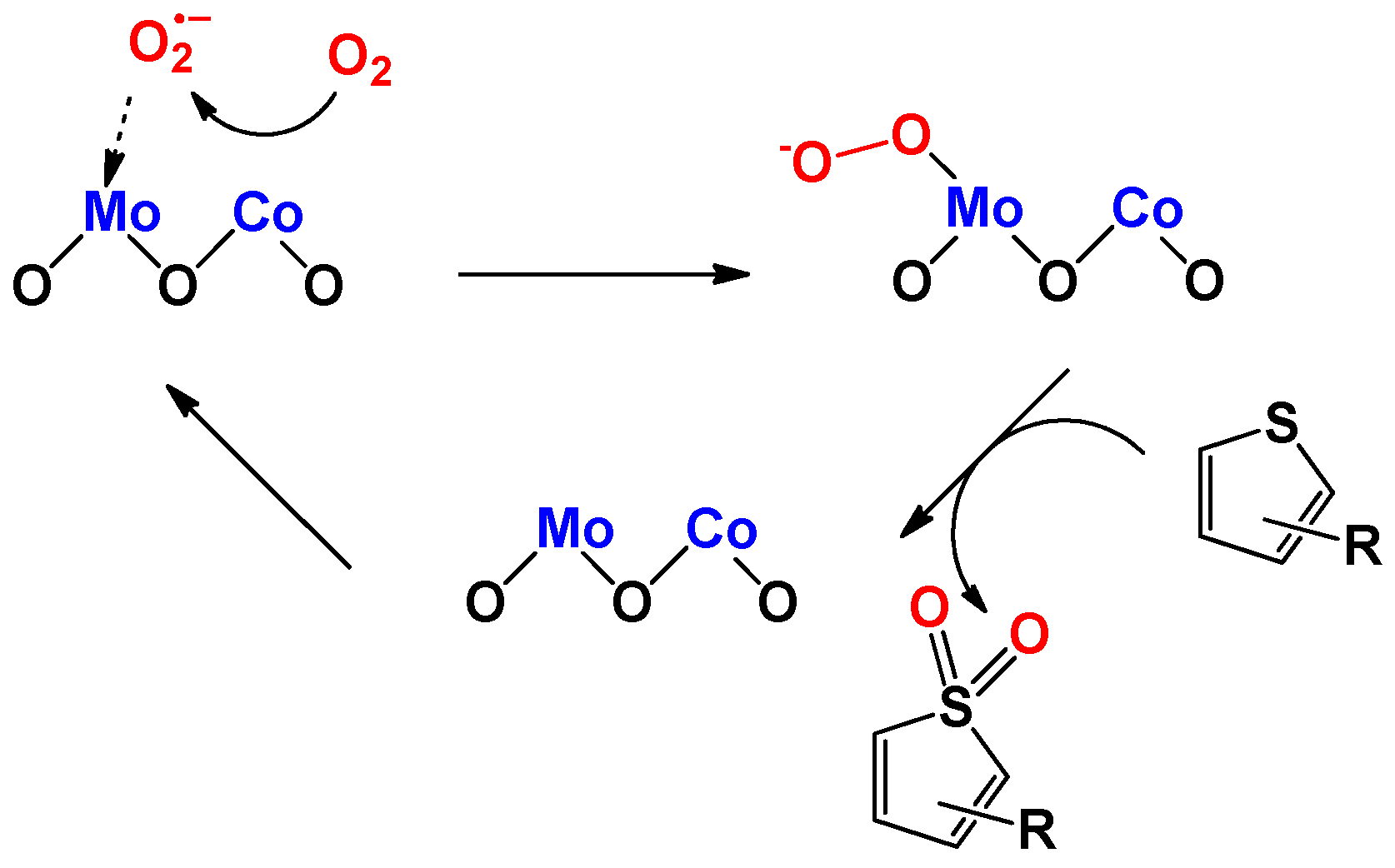
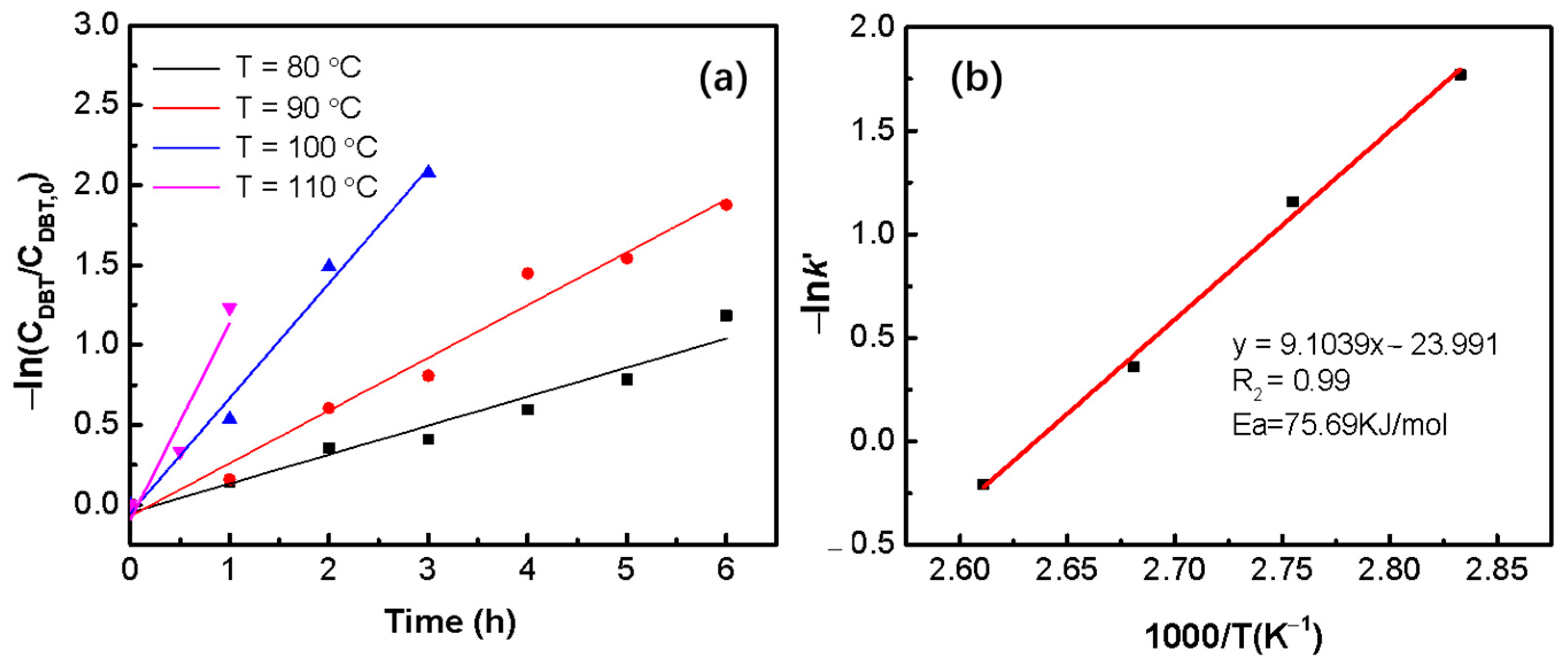
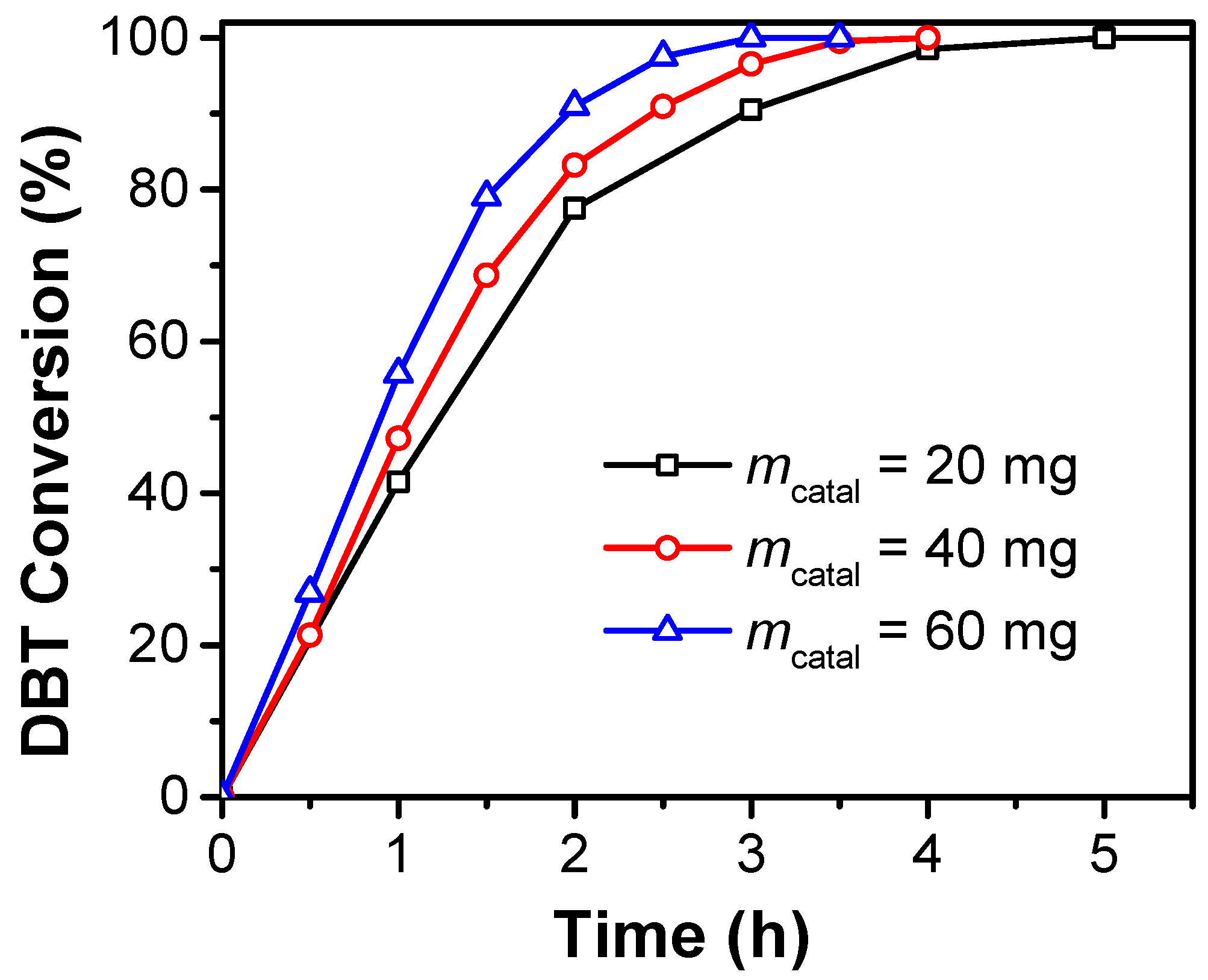
2.2. Catalytic Performances of CoMo-FMs
2.3. Catalytic Activities on Various Sulfides
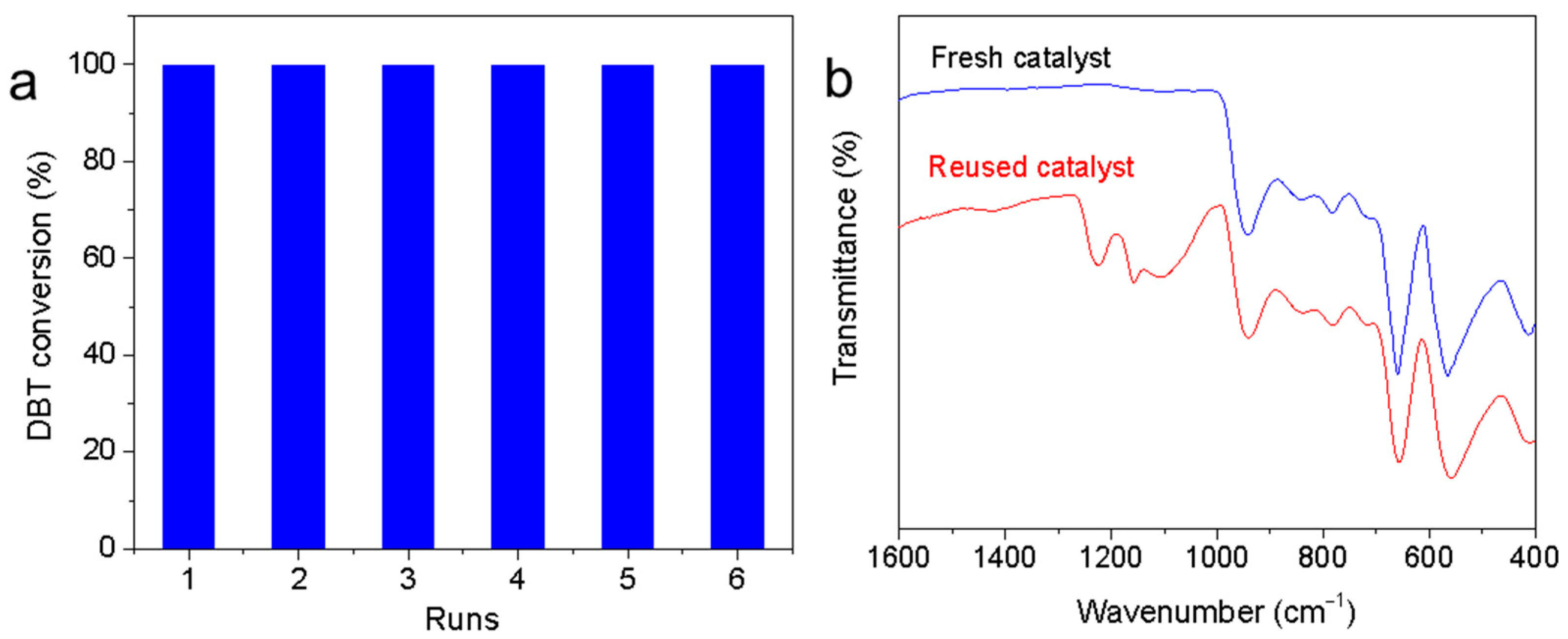
2.4. Reusability of the Catalyst
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of CoMo-FMs
3.3. Test of Catalytic Activity
3.4. Characterization of Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gungor, A. Prediction of SO2 and NOx emissions for low-grade Turkish lignites in CFB combustors. Chem. Eng. J. 2009, 146, 388–400. [Google Scholar] [CrossRef]
- Mahmoudabadi, Z.S.; Rashidi, A.; Tavasoli, A. Synthesis of MoS2 quantum dots as a nanocatalyst for hydrodesulfurization of naphtha: Experimental and DFT study. J. Environ. Chem. Eng. 2020, 8, 103736. [Google Scholar] [CrossRef]
- Jamali, M.A.; Arvani, A.; Amini, M.M. Vanadium containing metal-organic frameworks as highly efficient catalysts for the oxidation of refractory aromatic sulfur compounds. ChemCatChem 2020, 13, 293–303. [Google Scholar] [CrossRef]
- Safa, M.A.; Bouresli, R.; Al-Majren, R.; Al-Shamary, T.; Ma, X.L. Oxidative desulfurization kinetics of refractory sulfur compounds in hydrotreated middle distillates. Fuel 2019, 239, 24–31. [Google Scholar] [CrossRef]
- Zhang, M.T.; Yan, T.T.; Dai, W.L.; Guan, N.J.; Li, L.D. Zeolite stabilized isolated molybdenum species for catalytic oxidative desulfurization. Acta Chim. Sin. 2020, 78, 1404–1410. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, L.; Chao, Y.H.; Pang, J.Y.; Wu, P.W.; Zhang, M.; Zhu, W.S.; Li, H.M. A large number of low coordinated atoms in boron nitride for outstanding adsorptive desulfurization performance. Green Chem. 2016, 18, 3040–3047. [Google Scholar] [CrossRef]
- Caero, L.C.; Hernández, E.; Pedraza, F.; Murrieta, F. Oxidative desulfurization of synthetic diesel using supported catalysts. Catal. Today 2005, 107–108, 564–569. [Google Scholar] [CrossRef]
- Mhemed, H.A.; Gallego, M.M.; Largeau, J.F.; Kordoghli, S.; Zagrouba, F.; Tazerout, M. Gas adsorptive desulfurization of thiophene by spent coffee grounds-derived carbon optimized by response surface methodology: Isotherms and kinetics evaluation. J. Environ. Chem. Eng. 2020, 8, 104036. [Google Scholar] [CrossRef]
- Smolders, S.; Willhammar, T.; Krajnc, A.; Sentosun, K.; Wharmby, M.T.; Lomachenko, K.A.; Bals, S.; Mali, G.; Roeffaers, M.B.J.; De Vos, D.E.; et al. A titanium(iv)-based metal-organic framework featuring defect-rich Ti–O sheets as an oxidative desulfurization catalyst. Angew. Chem. Int. Ed. 2019, 58, 9160–9165. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, Z.H.; Zhang, B.H.; Yang, X.F.; Chang, X.; Zhou, Z.; Wang, D.H.; Zhang, M.H.; Bu, X.H. Synergistic effect of Zr-MOF on phosphomolybdic acid promotes efficient oxidative desulfurization. Appl. Catal. B-Environ. 2019, 256, 117804. [Google Scholar] [CrossRef]
- Baradaran, S.; Sadeghi, M.T. Desulfurization of non-hydrotreated kerosene using hydrodynamic cavitation assisted oxidative desulfurization (HCAOD) process. J. Environ. Chem. Eng. 2020, 8, 103832. [Google Scholar] [CrossRef]
- Qi, Z.Y.; Huang, Z.X.; Wang, H.X.; Li, L.; Ye, C.S.; Qiu, T. In situ bridging encapsulation of a carboxyl-functionalized phosphotungstic acid ionic liquid in UiO-66: A remarkable catalyst for oxidative desulfurization. Chem. Eng. Sci. 2020, 225, 115818. [Google Scholar] [CrossRef]
- Zuo, M.G.; Huang, X.Q.; Li, J.X.; Chang, Q.; Duan, Y.S.; Yan, L.J.; Xiao, Z.Z.; Mei, S.D.; Lu, S.X.; Yao, Y. Oxidative desulfurization in diesel via a titanium dioxide triggered thermocatalytic mechanism. Catal. Sci. Technol. 2019, 9, 2923–2930. [Google Scholar] [CrossRef]
- Dizaji, A.K.; Mokhtarani, B.; Mortaheb, H.R. Deep and fast oxidative desulfurization of fuels using graphene oxide-based phosphotungstic acid catalysts. Fuel 2019, 236, 717–729. [Google Scholar] [CrossRef]
- Kang, M.; Wang, X.; Zhang, J.; Lu, Y.; Chen, X.; Yang, L.; Wang, F. Boosting the photocatalytic oxidative desulfurization of dibenzothiophene by decoration of MWO4 (M=Cu, Zn, Ni) on WO3. J. Environ. Chem. Eng. 2019, 7, 102809. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, P.W.; Chen, L.L.; He, J.; Wu, Y.C.; Wang, C.; Chao, Y.H.; Lu, L.J.; He, M.Q.; Zhu, W.; et al. 3D-printing of integrated spheres as a superior support of phosphotungstic acid for deep oxidative desulfurization of fuel. J. Energy. Chem. 2020, 45, 91–97. [Google Scholar] [CrossRef]
- Liu, Y.; Han, L.; Zhang, J.; Yao, R.; Zhan, H.; Yang, H.; Bai, L.; Yang, L.; Wei, D.; Wang, W.; et al. Morphology-controlled construction and aerobic oxidative desulfurization of hierarchical hollow Co–Ni–Mo–O mixed metal-oxide nanotubes. Ind. Eng. Chem. Res. 2020, 59, 6488–6496. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Juliao, D.; Cunha-Silva, L.; Domingues, V.F.; Valenca, R.; Ribeiro, J.C.; de Castro, B.; Balula, S.S. Catalytic oxidative/extractive desulfurization of model and untreated diesel using hybrid based zinc-substituted polyoxometalates. Fuel 2016, 166, 268–275. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Xiao, X.Y.; Li, Y.; Chen, J.Y.; Wang, H.L. Deep desulfurization of liquid fuels with molecular oxygen through graphene photocatalytic oxidation. Appl. Catal. B-Environ. 2017, 209, 98–109. [Google Scholar] [CrossRef]
- Zhu, W.S.; Wang, C.; Li, H.P.; Wu, P.W.; Xun, S.H.; Jiang, W.; Chen, Z.G.; Zhao, Z.; Li, H.M. One-pot extraction combined with metal-free photochemical aerobic oxidative desulfurization in deep eutectic solvent. Green Chem. 2015, 17, 2464–2472. [Google Scholar] [CrossRef]
- Zhong, W.Z.; Liu, M.Q.; Dai, J.; Yang, J.; Mao, L.Q.; Yin, D.L. Synergistic hollow CoMo oxide dual catalysis for tandem oxygen transfer: Preferred aerobic epoxidation of cyclohexene to 1,2-epoxycyclohexane. Appl. Catal. B-Environ. 2018, 225, 180–196. [Google Scholar] [CrossRef]
- Lu, S.Y.; Lu, Y.; Jin, M.; Bao, S.J.; Li, W.Y.; Yu, L. Design and fabrication of highly sensitive and stable biochip for glucose biosensing. Appl. Surf. Sci. 2017, 422, 900–904. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Zhang, J.; Yang, L.; Ma, Z.; Wang, L.; Li, H.; Bai, L.; Wei, D.; Wang, W.; et al. Cellulose nanocrystal shelled with poly(ionic liquid)/polyoxometalate hybrid as efficient catalyst for aerobic oxidative desulfurization. J. Colloid. Interface Sci. 2019, 554, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Y.; Xiao, X.Y.; Chen, J.Y.; Wang, H.L. Electron-hole interactions in choline-phosphotungstic acid boosting molecular oxygen activation for fuel desulfurization. Appl. Catal. B-Environ. 2019, 248, 573–586. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Gohari Derakhshandeh, P.; Leus, K.; Vrielinck, H.; Callens, F.; Schmidt, J.; Savateev, A.; Van Der Voort, P. Metal-free activation of molecular oxygen by covalent triazine frameworks for selective aerobic oxidation. Sci. Adv. 2020, 6, eaaz2310. [Google Scholar] [CrossRef]
- Lin, F.; Wang, D.G.; Jiang, Z.X.; Ma, Y.; Li, J.; Li, R.G.; Li, C. Photocatalytic oxidation of thiophene on BiVO4 with dual co-catalysts Pt and RuO2 under visible light irradiation using molecular oxygen as oxidant. Energy Environ. Sci. 2012, 5, 6400–6406. [Google Scholar] [CrossRef]
- Murata, S.; Murata, K.; Kidena, K.; Nomura, M. A novel oxidative desulfurization system for diesel fuels with molecular oxygen in the presence of cobalt catalysts and aldehydes. Energy Fuels 2004, 18, 116–121. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Gao, L.; Chen, J.; Mao, J.; Xue, Q.; Liu, Y.; Wu, H.; Gao, G.; He, M. Aerobic oxidative desulfurization: A promising approach for sulfur removal from fuels. ChemSusChem 2008, 1, 302–306. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, G.; Zhang, B.; Zhang, X. Oxidation of refractory sulfur compounds with molecular oxygen over a Ce–Mo–O catalyst. Green Chem. 2016, 18, 5273–5279. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, Y.; Wu, H.Y.; Yang, W.S.; Zhu, Q.; Chen, Z.G.; Xun, S.H.; Zhu, W.S.; Li, H.M. Construction of 2D-2D V2O5/BNNS nanocomposites for improved aerobic oxidative desulfurization performance. Fuel 2020, 270, 117498. [Google Scholar] [CrossRef]
- Lu, L.J.; He, J.; Wu, P.W.; Wu, Y.C.; Chao, Y.H.; Li, H.P.; Tao, D.J.; Fan, L.; Li, H.M.; Zhu, W.S. Taming electronic properties of boron nitride nanosheets as metal-free catalysts for aerobic oxidative desulfurization of fuels. Green Chem. 2018, 20, 4453–4460. [Google Scholar] [CrossRef]
- Wu, P.; Yang, S.; Zhu, W.; Li, H.; Chao, Y.; Zhu, H.; Li, H.; Dai, S. Tailoring N-terminated defective edges of porous boron nitride for enhanced aerobic catalysis. Small 2017, 13, 1701857. [Google Scholar] [CrossRef]
- Li, H.; Fu, W.; Yin, J.; Zhang, J.; Li, Y.; Jiang, D.; Lv, N.; Zhu, W. Rational design of the carbon doping of hexagonal boron nitride for oxygen activation and oxidative desulfurization. Phys. Chem. Chem. Phys. 2020, 22, 24310–24319. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.Q.; Wen, G.D.; Ding, Y.X.; Wu, K.H.; Chen, C.M.; Su, D.S. Reduced graphene oxide: A metal-free catalyst for aerobic oxidative desulfurization. Green Chem. 2017, 19, 1175–1181. [Google Scholar] [CrossRef]
- Gomez-Paricio, A.; Santiago-Portillo, A.; Navalon, S.; Concepcion, P.; Alvaro, M.; Garcia, H. MIL-101 promotes the efficient aerobic oxidative desulfurization of dibenzothiophenes. Green Chem. 2016, 18, 508–515. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Yang, H.; Dong, Y.; Liu, Y.; Yang, L.; Wei, D.; Wang, W.; Bai, L.; Chen, H. Efficient aerobic oxidative desulfurization over Co–Mo–O bimetallic oxide catalysts. Catal. Sci. Technol. 2019, 9, 2915–2922. [Google Scholar] [CrossRef]
- Sun, L.L.; Su, T.; Xu, J.J.; Hao, D.M.; Liao, W.P.; Zhao, Y.C.; Ren, W.Z.; Deng, C.L.; Lu, H.Y. Aerobic oxidative desulfurization coupling of Co polyanion catalysts and p-TsOH-based deep eutectic solvents through a biomimetic approach. Green Chem. 2019, 21, 2629–2634. [Google Scholar] [CrossRef]
- Jiang, W.; Xiao, J.; Dong, L.; Wang, C.; Li, H.P.; Luo, Y.P.; Zhu, W.S.; Li, H.M. Polyoxometalate-based poly(ionic liquid) as a precursor for superhydrophobic magnetic carbon composite catalysts toward aerobic oxidative desulfurization. ACS Sustain. Chem. Eng. 2019, 7, 15755–15761. [Google Scholar] [CrossRef]
- Tang, N.F.; Jiang, Z.X.; Li, C. Oxidation of refractory sulfur-containing compounds with molecular oxygen catalyzed by vanadoperiodate. Green Chem. 2015, 17, 817–820. [Google Scholar] [CrossRef]
- Lü, H.; Ren, W.; Liao, W.; Chen, W.; Li, Y.; Suo, Z. Aerobic oxidative desulfurization of model diesel using a B-type anderson catalyst [(C18H37)2N(CH3)2]3Co(OH)6Mo6O18·3H2O. Appl. Catal. B-Environ. 2013, 138–139, 79–83. [Google Scholar] [CrossRef]
- Shafiq, I.; Shafique, S.; Akhter, P.; Ishaq, M.; Yang, W.; Hussain, M. Recent breakthroughs in deep aerobic oxidative desulfurization of petroleum refinery products. J. Clean. Prod. 2021, 294, 125731. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, W.; Wei, Y.; Wang, C.; Fu, Y.; Gao, Y.; Zhu, L.; Zhu, W.; Li, H. Aerobic oxidative desulfurization by nanoporous tungsten oxide with oxygen defects. ACS Appl. Nano. Mater. 2021, 4, 1085–1093. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.Y.; Fan, H.X.; Yang, Z.F.; Feng, J.; Li, W.Y. A comprehensive review on oxidative desulfurization catalysts targeting clean energy and environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Kim, S.O.; Sastri, C.V.; Seo, M.S.; Kim, J.; Nam, W. Dioxygen activation and catalytic aerobic oxidation by a mononuclear nonheme iron(ii) complex. J. Am. Chem. Soc. 2005, 127, 4178–4179. [Google Scholar] [CrossRef]
- Wachs, I.E. Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal. Today 2005, 100, 79–94. [Google Scholar] [CrossRef]
- Lu, S.Y.; Li, S.W.; Jin, M.; Gao, J.C.; Zhang, Y. Greatly boosting electrochemical hydrogen evolution reaction over Ni3S2 nanosheets rationally decorated by Ni3Sn2S2 quantum dots. Appl. Catal. B-Environ. 2020, 267, 118675. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, M.; Meng, X.; Miao, Y.; Zhang, D.J.M.C. Physics Needle-like comoo with multi-modal porosity for pseudocapacitors. Mater. Chem. Phys. 2017, 198, 258–265. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Yang, Y.; Zhang, S.; Zhu, H.; Zhu, X.; Xing, H.; Zhang, Y.; Huang, B.; Guo, S.; et al. Co3O4/Fe0.33Co0.66P interface nanowire for enhancing water oxidation catalysis at high current density. Adv. Mater. 2018, 30, e1803551. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, J.; Ma, Z.; Xu, H.; Yang, H.; Yang, L.; Bai, L.; Wei, D.; Wang, W.; Chen, H. Preparation of co-mo-o ultrathin nanosheets with outstanding catalytic performance in aerobic oxidative desulfurization. Chem. Commun. 2019, 55, 13995–13998. [Google Scholar] [CrossRef]
- Yang, H.W.; Bai, L.J.; Wei, D.L.; Yang, L.X.; Wang, W.X.; Chen, H.; Niu, Y.Z.; Xue, Z.X. Ionic self-assembly of poly(ionic liquid)-polyoxometalate hybrids for selective adsorption of anionic dyes. Chem. Eng. J. 2019, 358, 850–859. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Zhang, J.; Wu, N.; Liu, G.; Chen, H.; Yuan, C.; Liu, X. Optimizing electronic structure of porous Ni/MoO2 heterostructure to boost alkaline hydrogen evolution reaction. J. Colloid Interface Sci. 2022, 627, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.F.; Xu, J.; Guo, Y.; Liu, J.Y. Ultrathin, polycrystalline, two-dimensional Co3O4 for low-temperature CO oxidation. ACS Catal. 2019, 9, 2558–2567. [Google Scholar] [CrossRef]
- Yu, M.Q.; Jiang, L.X.; Yang, H.G. Ultrathin nanosheets constructed CoMoO4 porous flowers with high activity for electrocatalytic oxygen evolution. Chem. Commun. 2015, 51, 14361–14364. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Pereira, M.F.R.; Orfao, J.J.M.; Figueiredo, J.L. The role of lattice oxygen on the activity of manganese oxides towards the oxidation of volatile organic compounds. Appl. Catal. B-Environ. 2010, 99, 353–363. [Google Scholar] [CrossRef]
- Rodriguez, E.M.; Marquez, G.; Tena, M.; Alvarez, P.M.; Beltran, F.J. Determination of main species involved in the first steps of TiO2 photocatalytic degradation of organics with the use of scavengers: The case of ofloxacin. Appl. Catal. B-Environ. 2015, 178, 44–53. [Google Scholar] [CrossRef]
- Panganamala, R.V.; Sharma, H.M.; Heikkila, R.E.; Geer, J.C.; Cornwell, D.G. Role of hydroxyl radical scavengers dimethyl sulfoxide, alcohols and methional in the inhibition of prostaglandin biosynthesis. Prostaglandins 1976, 11, 599–607. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, D.; Yu, X.; Li, Y.; Wang, X.; Yang, W. Deep oxidative desulfurization catalyzed by (NH4)5H6PV8Mo4O40 using molecular oxygen as an oxidant. Fuel Process. Technol. 2017, 160, 136–142. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Z.; Ding, Q.; Zhang, Y.; Wu, X.; Sun, L.; Du, J. Oxidative desulfurization of dibenzothiophene with molecular oxygen using cobalt and copper salen complexes encapsulated in nay zeolite. Catal. Today 2019, 339, 105–112. [Google Scholar] [CrossRef]
- Li, X.; Gu, Y.; Chu, H.; Ye, G.; Zhou, W.; Xu, W.; Sun, Y. MFM-300(V) as an active heterogeneous catalyst for deep desulfurization of fuel oil by aerobic oxidation. Appl. Catal. A–Gen. 2019, 584, 117152. [Google Scholar] [CrossRef]
- Yu, X.; Shi, M.; Yan, S.; Wang, H.; Wang, X.; Yang, W. Designation of choline functionalized polyoxometalates as highly active catalysts in aerobic desulfurization on a combined oxidation and extraction procedure. Fuel 2017, 207, 13–21. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, G.; Zeng, D.; Tang, Y.; Wang, M.; Li, Y. Oxidative desulfurization of diesel fuel using amphiphilic quaternary ammonium phosphomolybdate catalysts. Fuel Process. Technol. 2009, 90, 1538–1542. [Google Scholar] [CrossRef]

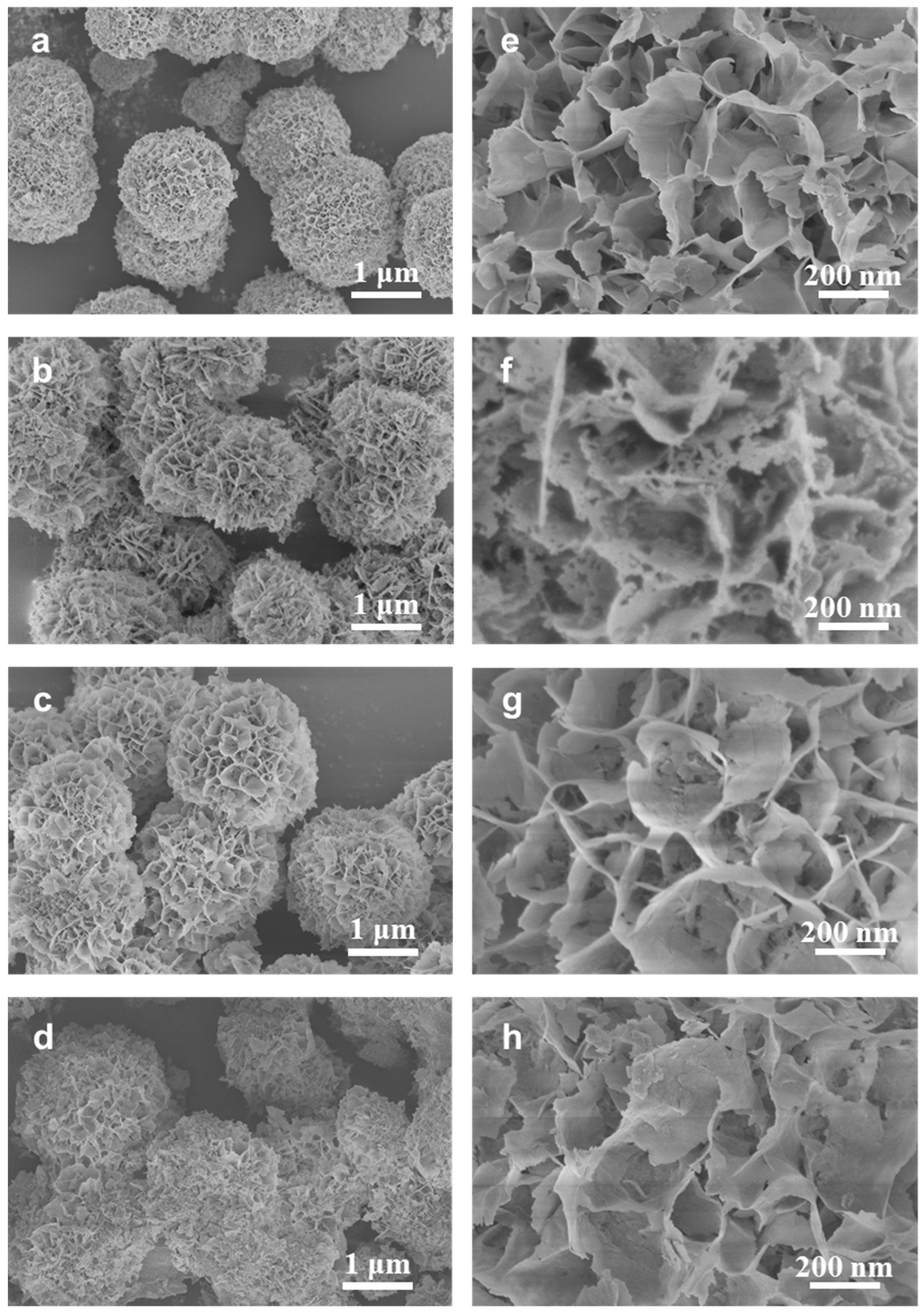
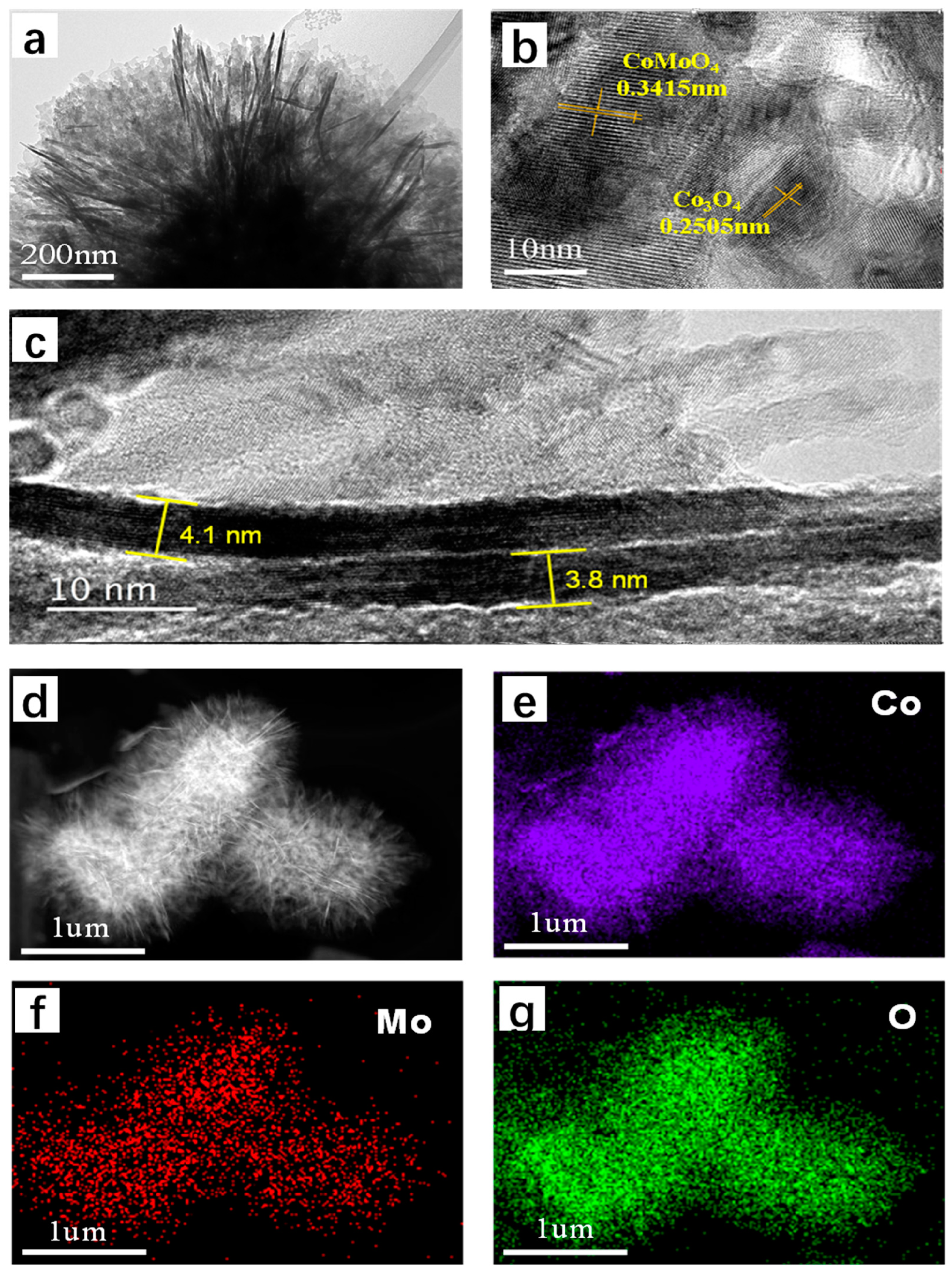
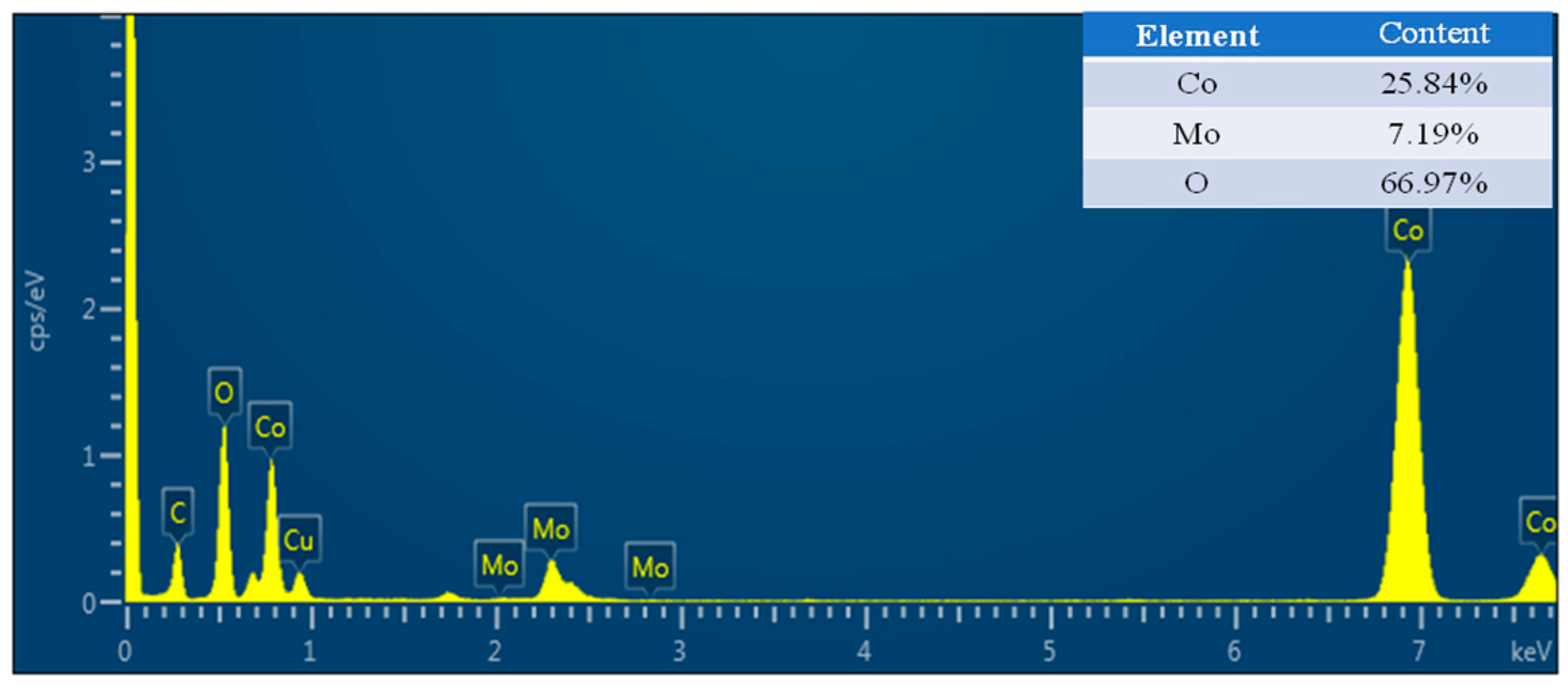
| Sample | Elemental Percentage (atm.%) | Mo/Co Ratio | |
|---|---|---|---|
| Co | Mo | ||
| CoMo-MF-1 | 29.22% | 4.42% | 0.151 |
| CoMo-MF-2 | 25.93% | 6.87% | 0.265 |
| CoMo-MF-3 | 22.88% | 8.51% | 0.372 |
| CoMo-MF-4 | 21.36% | 10.43% | 0.488 |
| Sample | Specific Pore Volume /cm3 g−1 | Specific Surface Area /m2 g−1 | Mean Pore Size /nm |
|---|---|---|---|
| CoMo-FM-1 | 0.55 | 97.48 | 17.48 |
| CoMo-FM-2 | 0.59 | 103.46 | 17.55 |
| CoMo-FM-3 | 0.4 | 82.95 | 15.82 |
| CoMo-FM-4 | 0.32 | 72.81 | 14.01 |
| Sample | Mo 3d5/2 | O1s | ||
|---|---|---|---|---|
| Olat | Oad | Waterad | ||
| CoMo-FM-1 | 230.44 | 528.68 (51.34%) | 529.55 (34.59%) | 531.01 (14.07%) |
| CoMo-FM-2 | 230.48 | 528.71 (44.54%) | 529.57 (33.44%) | 531.03 (22.02%) |
| CoMo-FM-3 | 230.61 | 528.89 (48.94%) | 529.73 (43.67%) | 531.19 (7.39%) |
| CoMo-FM-4 | 230.78 | 528.94 (50.43%) | 529.79 (27.65%) | 531.25 (21.92%) |
| Entry | Catalyst | Substrate | Oxidant | Reaction Conditions a | Conversion | Ref. |
|---|---|---|---|---|---|---|
| 1 | CoMo-FM-3 | DBT | Air | 100 °C, 20 mg/20 g, 4 h | 100% | This work |
| 2 | (NH4)5H6PV8Mo4O40 | DBT | O2 | 100 °C, 20 mg, 6 h | 88.4% | [57] |
| 3 | r-GO | DBT | O2 | 140 °C, 5 mg/25 mL, 4 h | 100% | [34] |
| 4 | MoOx/MC-600 | DBT | O2 | 120 °C, 10 mg, 8 h | 100% | [38] |
| 5 | Ce–Mo–O | DBT | O2 | 100 °C, 100 mg, 6 h | 100% | [29] |
| 6 | (Cu-Co)(salen)Y | DBT | O2 | 100 °C, 200 mg, 4 h | 97.6% | [58] |
| 7 | MFM-300(V) | DBT | O2 | 120 °C, 0.75 g/L, 5 h | 99.6% | [59] |
| 8 | ChxNa5-xIMo6O24 | DBT | O2 | 100 °C, 0.1 mmol/6 mL, 5 h | 100% | [60] |
| 9 | MIL-101 | DBT | O2 | 120 °C, 500 mg/L, 15 h | 100% | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Tong, R.; Wang, J.; Zhang, L.; Wang, Y.; Lou, Y.; Wang, X. Synthesis of Flower-Like Cobalt–Molybdenum Mixed-Oxide Microspheres for Deep Aerobic Oxidative Desulfurization of Fuel. Molecules 2023, 28, 5073. https://doi.org/10.3390/molecules28135073
Cao X, Tong R, Wang J, Zhang L, Wang Y, Lou Y, Wang X. Synthesis of Flower-Like Cobalt–Molybdenum Mixed-Oxide Microspheres for Deep Aerobic Oxidative Desulfurization of Fuel. Molecules. 2023; 28(13):5073. https://doi.org/10.3390/molecules28135073
Chicago/Turabian StyleCao, Xinxiang, Ruijian Tong, Jingyuan Wang, Lan Zhang, Yulan Wang, Yan Lou, and Xiaomeng Wang. 2023. "Synthesis of Flower-Like Cobalt–Molybdenum Mixed-Oxide Microspheres for Deep Aerobic Oxidative Desulfurization of Fuel" Molecules 28, no. 13: 5073. https://doi.org/10.3390/molecules28135073
APA StyleCao, X., Tong, R., Wang, J., Zhang, L., Wang, Y., Lou, Y., & Wang, X. (2023). Synthesis of Flower-Like Cobalt–Molybdenum Mixed-Oxide Microspheres for Deep Aerobic Oxidative Desulfurization of Fuel. Molecules, 28(13), 5073. https://doi.org/10.3390/molecules28135073






