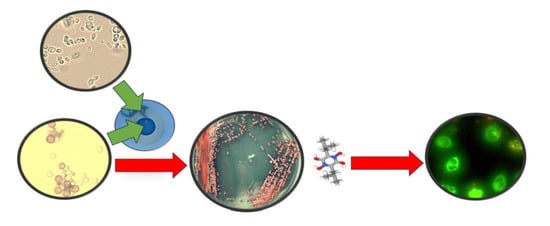
The Metschnikowia pulcherrima Clade as a Model for Assessing Inhibition of Candida spp. and the Toxicity of Its Metabolite, Pulcherrimin
Abstract
1. Introduction
2. Results and Disussion
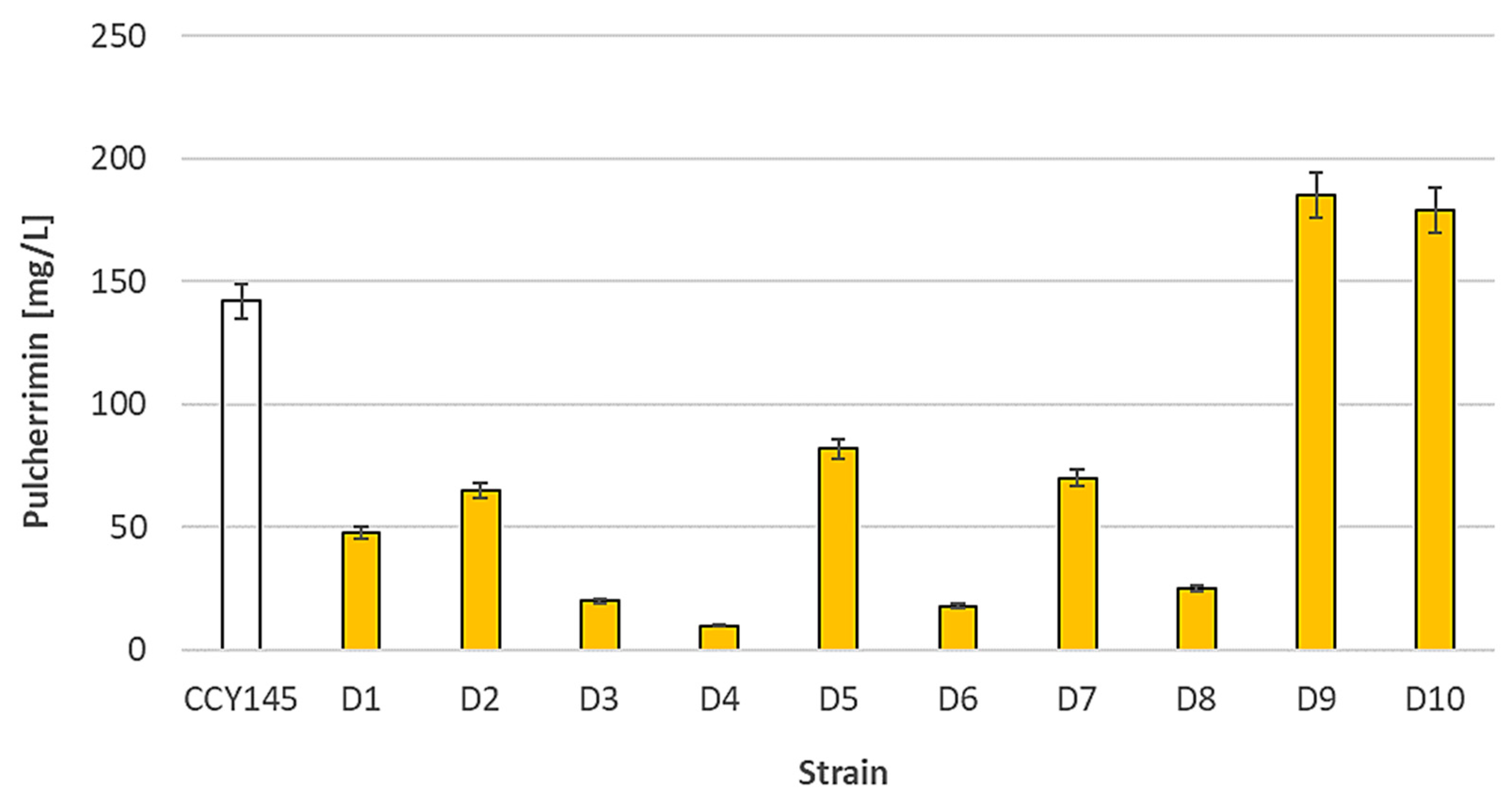
2.1. Pulcherrimin Formation
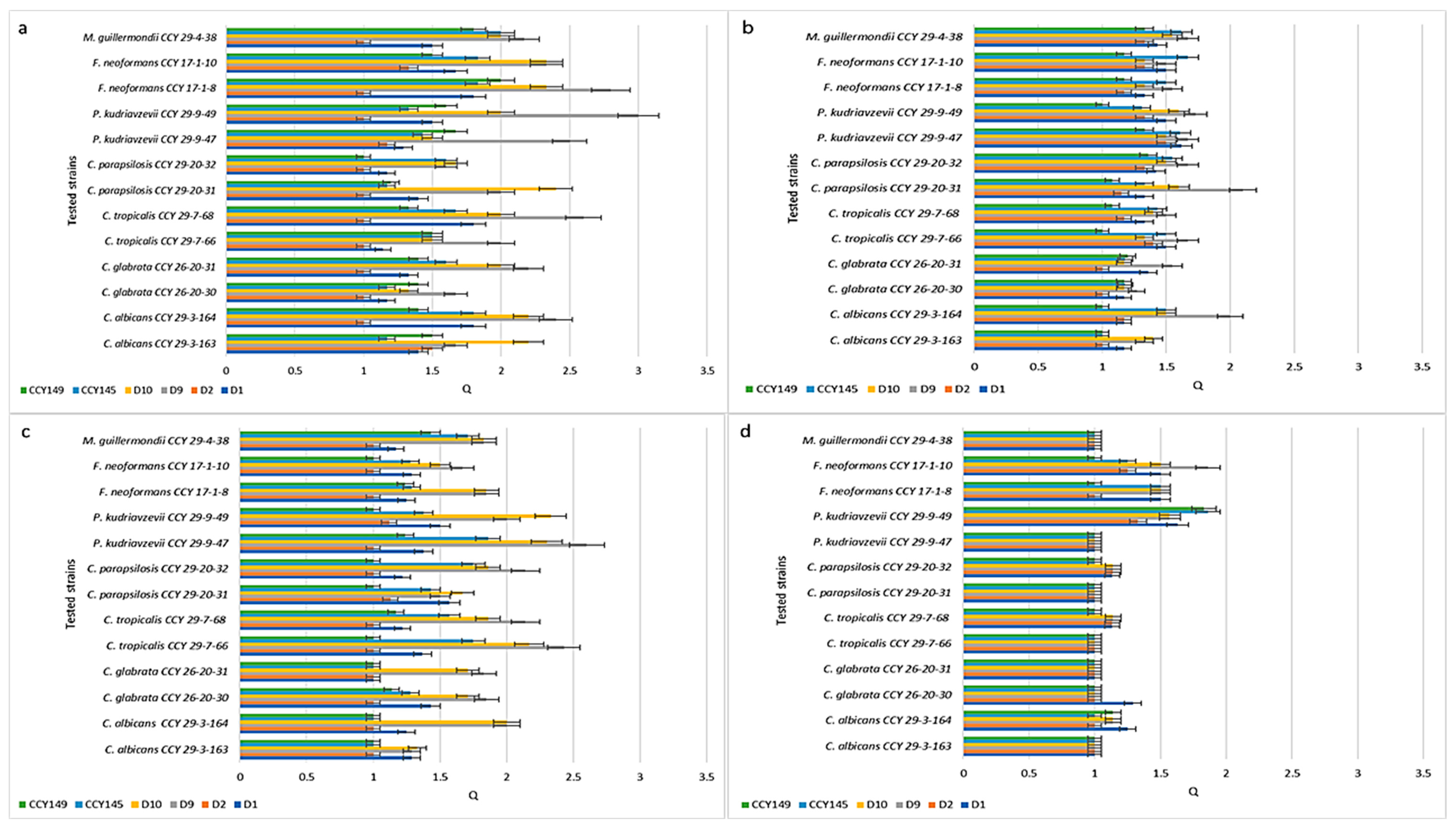
2.2. Inhibition of Candida spp. Strains
2.3. Cytotoxicity of Pulcherrimin
2.4. Genotoxicity
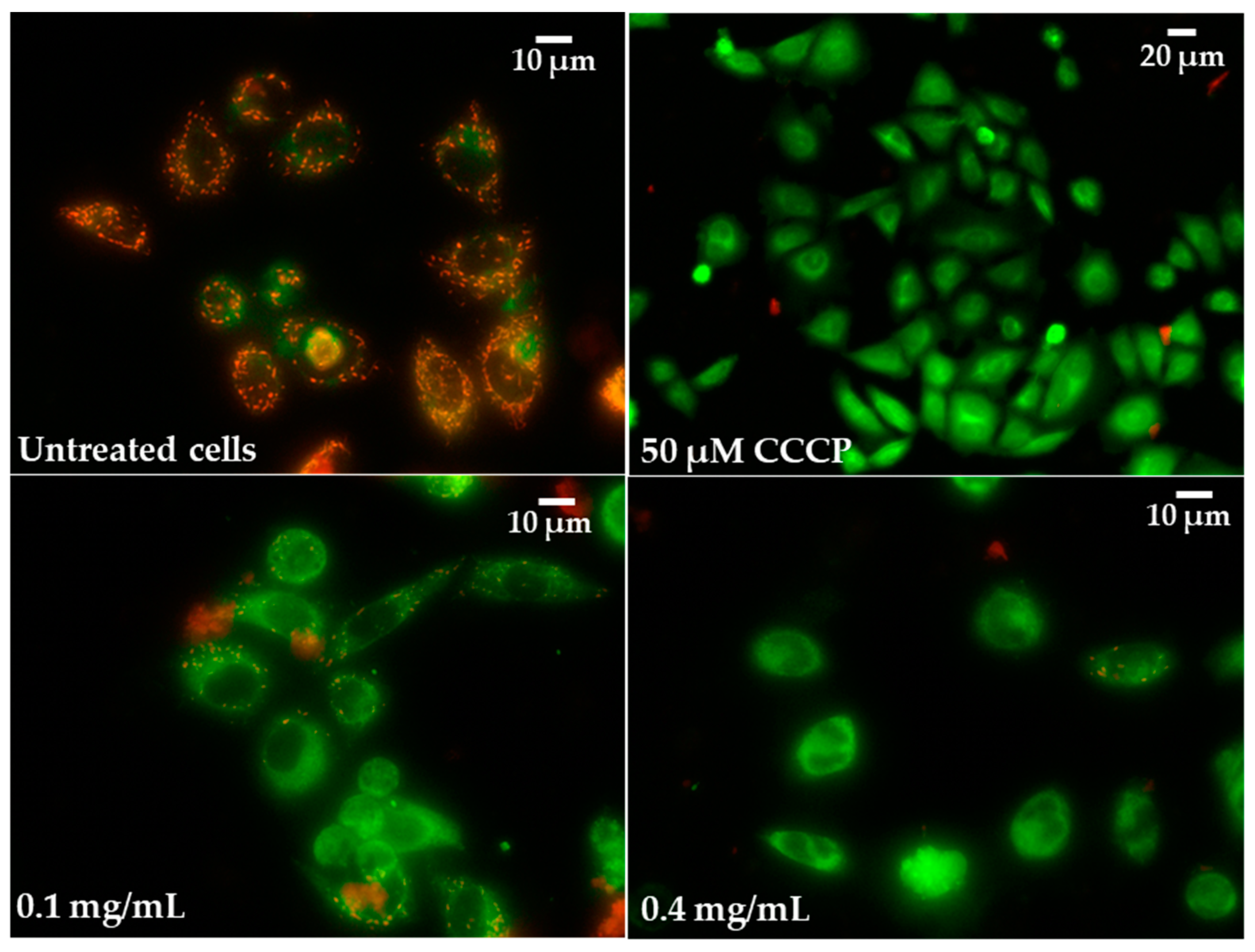
2.5. Oxidative Stress Induction and Influence of Pulcherrimin on Mitochondrial Membrane Potential
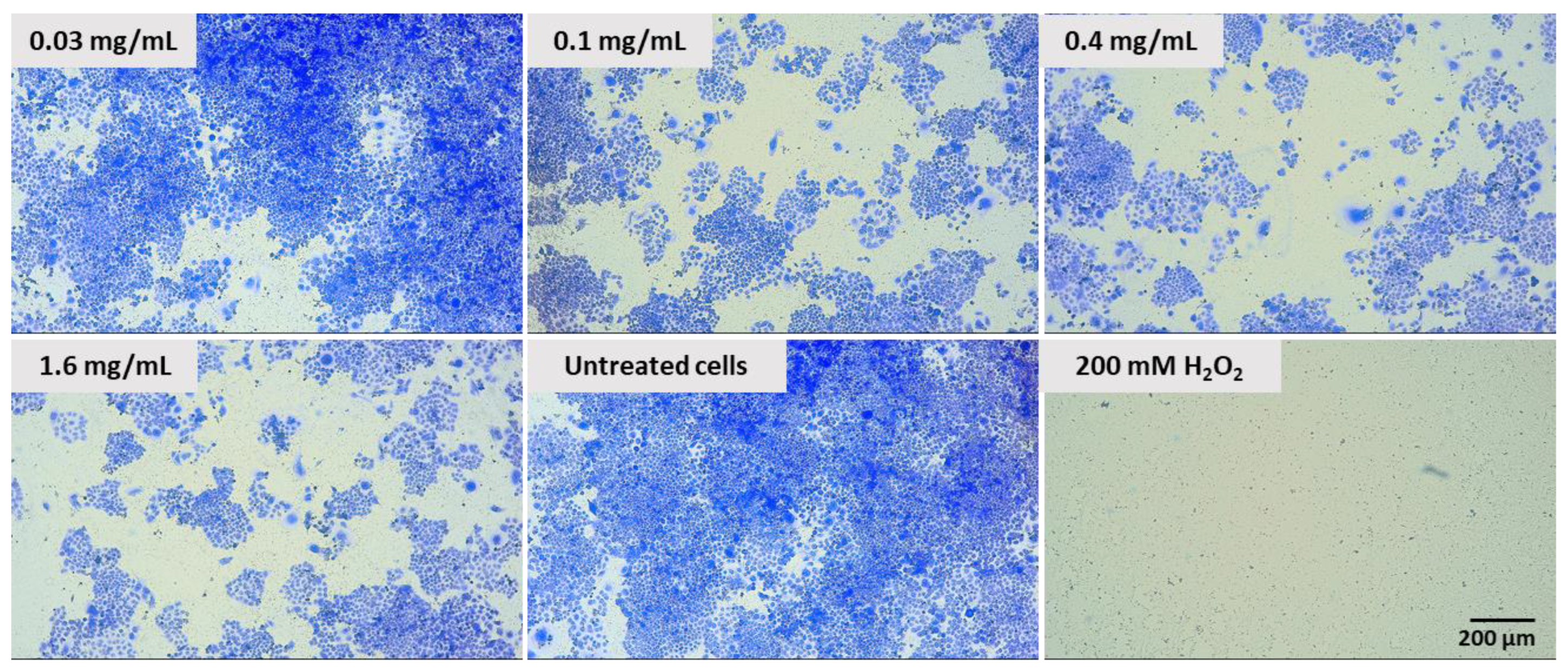
2.6. Effect of Pulcherrimin on Colony Formation
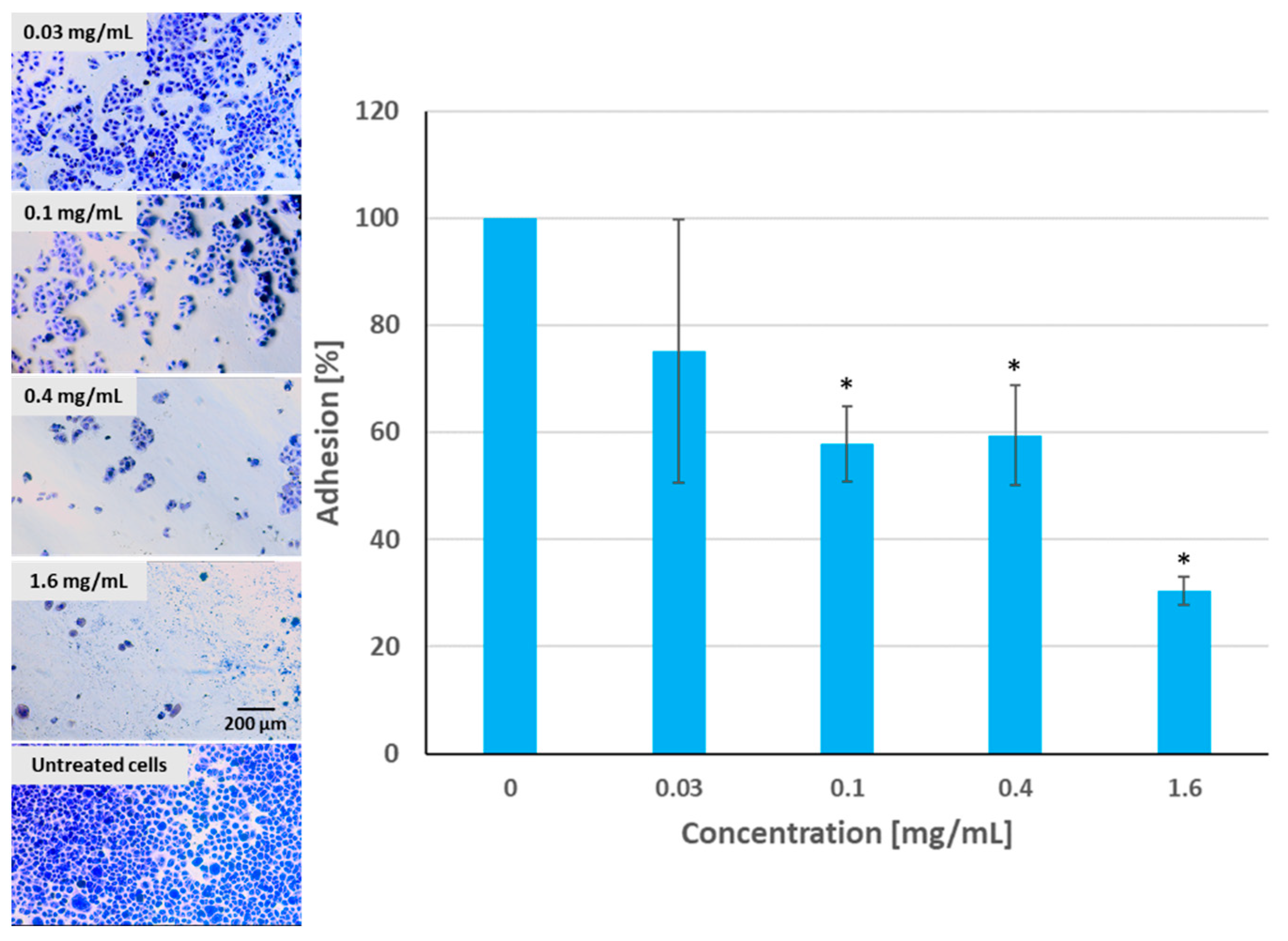
2.7. Adhesion Assay
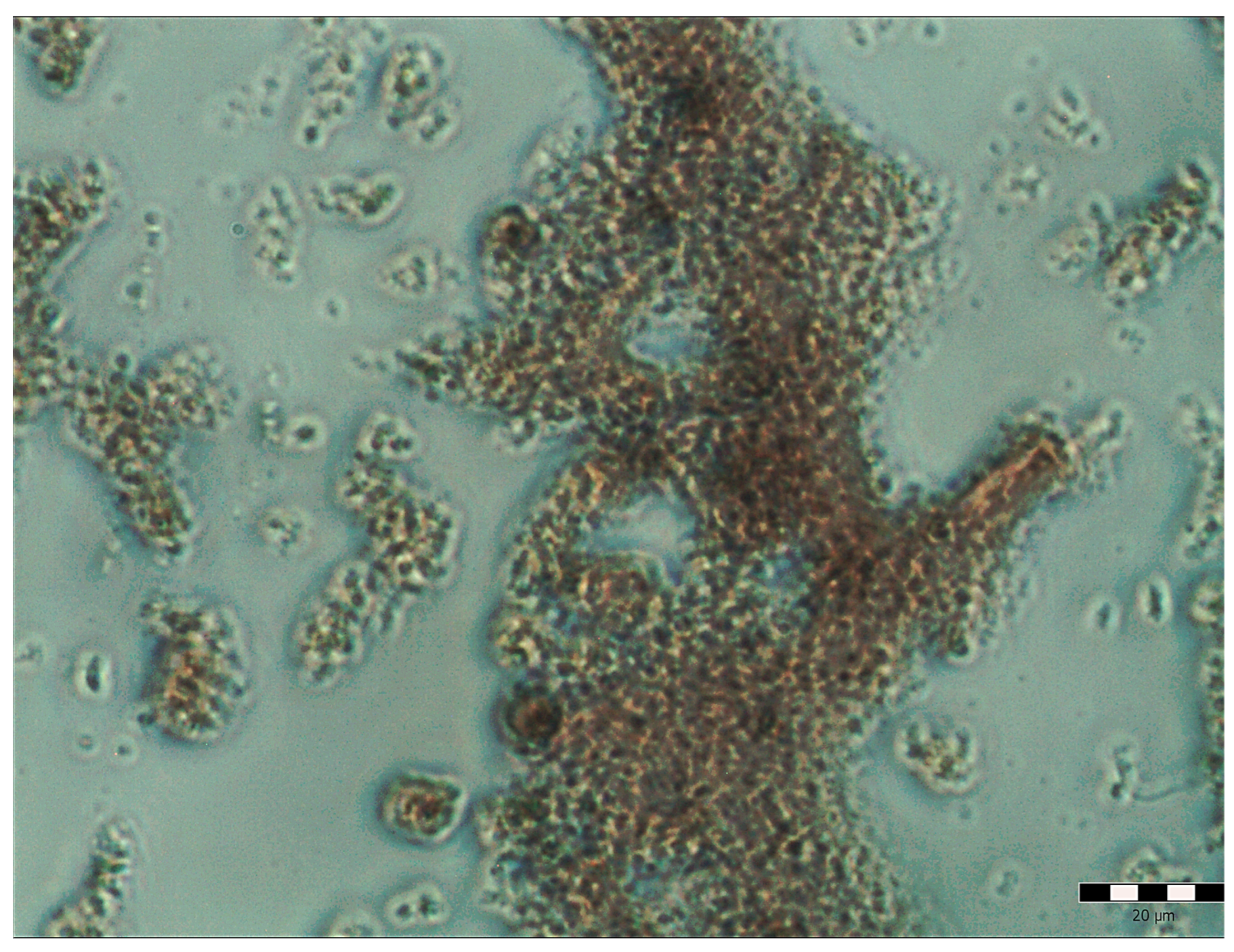
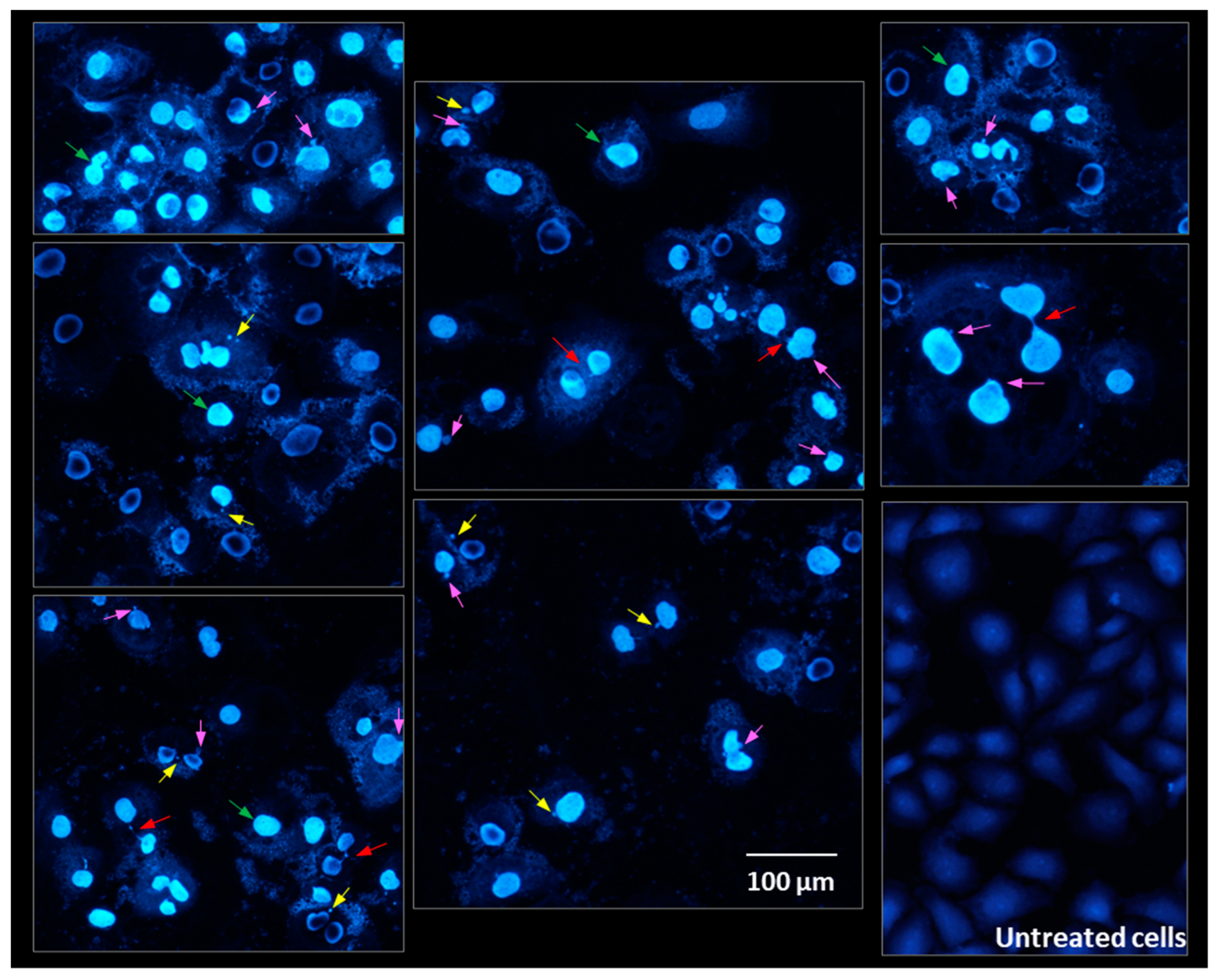
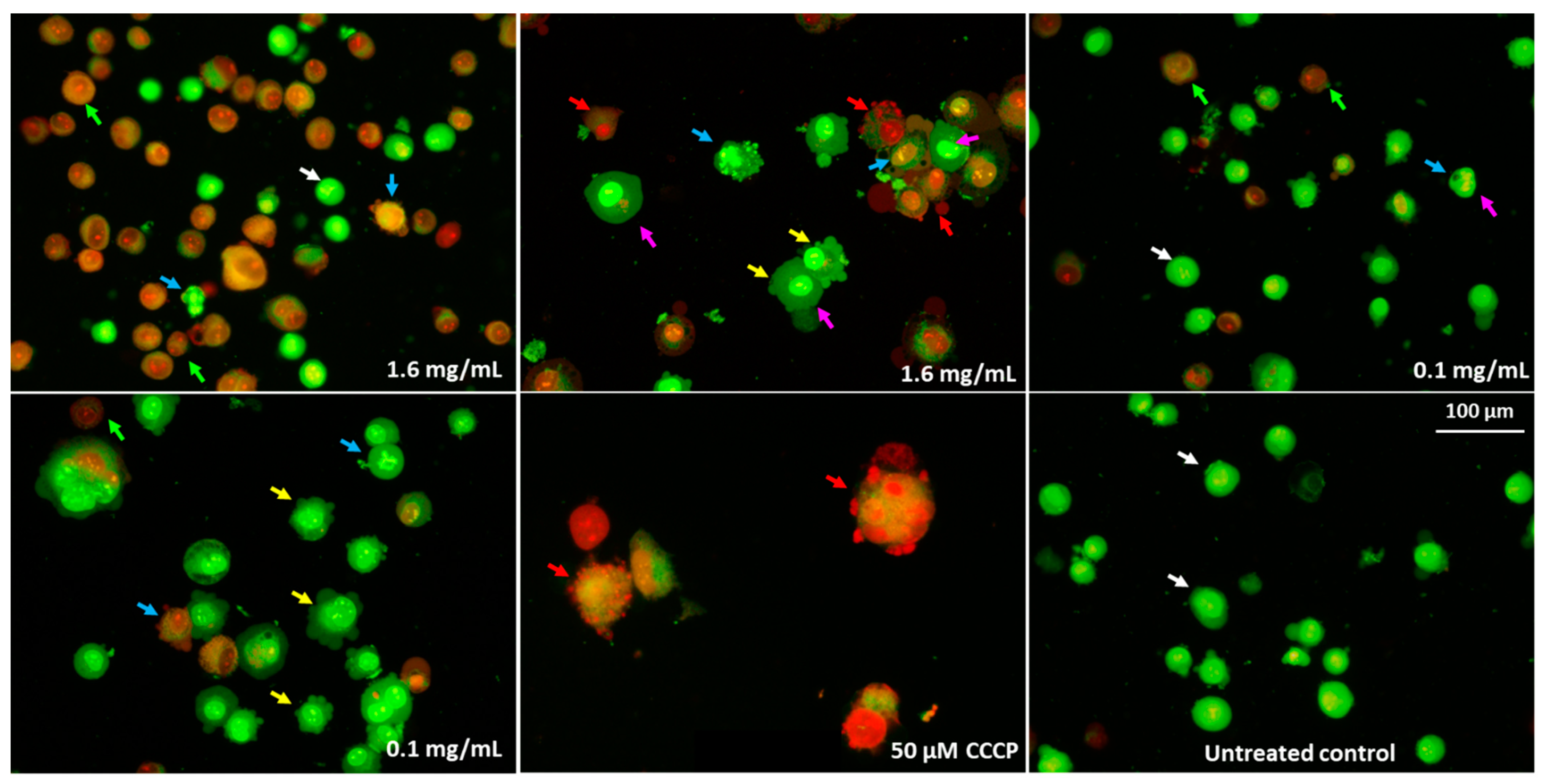
2.8. Cells Morphology Assessment after Fluorescent Staining
3. Materials and Methods
3.1. Yeast Strains
3.2. Antifungal Activity
3.3. Chemicals and Other Materials
3.4. Obtaining Pure Pulcherrimin
3.5. Cell Cultures
3.6. PrestoBlue Assay
3.7. Single Cell Gel Electrophoresis Assay
3.8. Reactive Oxygen Species (ROS) and Hydrogen Peroxide Generation
3.9. Mitochondrial Membrane Potential (MMP)
3.10. Colony Formation Assay
3.11. Adhesion Assay
3.12. Cell Staining with DAPI and AO/PI
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Robledo-Leal, E.; Rivera-Morales, L.G.; Sangorrín, M.P.; González, G.M.; Ramos-Alfano, G.; Adame-Rodriguez, J.M.; Alcocer-Gonzalez, J.M.; Arechiga-Carvajal, E.T.; Rodriguez-Padilla, C. Identification and susceptibility of clinical isolates of Candida spp. to killer toxins. Braz. J. Biol. 2018, 78, 742–749. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and invasive candidiasis. Infect. Dis. Clin. N. Am. 2021, 35, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Kunicka-Styczyńska, A. Typing and virulence factors of food-borne Candida spp. isolates. Int. J. Food Microbiol. 2018, 279, 57–63. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Maske, B.L.; de Carvalho Neto, D.P.; Karp, S.G.; De Dea Lindner, J.; Martin, J.G.P.; de Oliveira Hosken, B.; Soccol, C.R. What is Candida doing in my food? A review and safety alert on its use as starter cultures in fermented foods. Microorganisms 2022, 10, 1855. [Google Scholar] [CrossRef]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Colombo, A.L.; Júnior, J.N.A.; Guinea, J. Emerging multidrug-resistant Candida species. Curr. Opin. Infect. Dis. 2017, 30, 528–538. [Google Scholar] [CrossRef]
- Ksiezopolska, E.; Gabaldon, T. Evolutionary emergence of drug resistance in Candida opportunistic pathogens. Genes 2018, 9, 461. [Google Scholar] [CrossRef]
- Mesini, A.; Mikulska, M.; Giacobbe, D.R.; Del Puente, F.; Gandolfo, N.; Codda, G.; Orsi, A.; Tassinari, F.; Beltramini, S.; Marchese, A. Changing epidemiology of candidaemia: Increase in fluconazole-resistant Candida parapsilosis. Mycoses 2020, 63, 361–368. [Google Scholar] [CrossRef]
- Demirci-Duarte, S.; Arikan-Akdagli, S.; Gülmez, D. Species distribution, azole resistance and related molecular mechanisms in invasive Candida parapsilosis complex isolates: Increase in fluconazole resistance in 21 years. Mycoses 2021, 64, 823–830. [Google Scholar] [CrossRef]
- Rodrigues, D.K.B.; Bonfietti, L.X.; Garcia, R.A.; Araujo, M.R.; Rodrigues, J.S.; Gimenes, V.M.F.; Melhem, M.S.C. Antifungal susceptibility profile of Candida clinical isolates from 22 hospitals of São Paulo State, Brazil. Braz. J. Med. Biol. Res. 2021, 54, e10928. [Google Scholar] [CrossRef] [PubMed]
- Yamin, D.; Akanmu, M.H.; Al Mutair, A.; Alhumaid, S.; Rabaan, A.A.; Hajissa, K. Global prevalence of antifungal-resistant Candida parapsilosis: A systematic review and meta-analysis. Trop. Med. Infect. Dis. 2022, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Prigitano, A.; Blasi, E.; Calabrò, M.; Cavanna, C.; Cornetta, M.; Farina, C.; Grancini, A.; Innocenti, P.; Lo Cascio, G.; Nicola, L.; et al. Yeast bloodstream infections in the COVID-19 patient: A Multicenter Italian Study (FiCoV Study). J. Fungi 2023, 9, 277. [Google Scholar] [CrossRef]
- WHO. Fact Sheet: Antimicrobial Resistance 2021. Available online: https://ahpsr.who.int/publications/i/item/global-action-plan-on-antimicrobial-resistance (accessed on 21 April 2023).
- Horváth, E.; Sipiczki, M.; Csoma, H.; Miklós, I. Assaying the effect of yeasts on growth of fungi associated with disease. BMC Microbiol. 2020, 20, 320. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.M.; Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Salgueiro, L. Unveiling the bioactive potential of the essential oil of a Portuguese endemism, Santolina impressa. J. Ethnopharmacol. 2019, 244, 112120. [Google Scholar] [CrossRef]
- Shishido, T.K.; Humisto, A.; Jokela, J.; Liu, L.; Wahlsten, M.; Tamrakar, A.; Fewer, D.P.; Permi, P.; Andreote, A.P.; Fiore, M.F.; et al. Antifungal compounds from cyanobacteria. Mar. Drugs 2015, 13, 2124–2140. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, N.A.; Perveen, K. Susceptibility of fluconazole-resistant Candida albicans to thyme essential oil. Microorganisms 2021, 9, 2454. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia pulcherrima and related pulcherrimin-producing yeasts: Fuzzy species boundaries and complex antimicrobial antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef]
- Janakiev, T.; Berić, T.; Stević, T.; Stanković, S.; Bačić, J.; Majstorović, H.; Fira, D.; Dimkić, I. The microbiome of the ‘Williams’ pear variety grown in the organic orchard and antifungal activity by the autochthonous bacterial and yeast isolates. Microorganisms 2022, 10, 1282. [Google Scholar] [CrossRef]
- Pawlikowska, E.; James, S.A.; Breierova, E.; Antolak, H.; Kregiel, D. Biocontrol capability of local Metschnikowia sp. isolates. Antonie Van Leeuwenhoek 2019, 112, 1425–1445. [Google Scholar] [CrossRef]
- Ferrocino, I.; Ponzo, V.; Pellegrini, M.; Goitre, I.; Papurello, M.; Franciosa, I.; D’Eusebio, C.; Ghigo, E.; Cocolin, L.; Bo, S. Mycobiota composition and changes across pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 2022, 12, 9192. [Google Scholar] [CrossRef]
- Mažeika, K.; Šiliauskas, L.; Skridlaitė, G.; Matelis, A.; Garjonytė, R.; Paškevičius, A.; Melvydas, V. Features of iron accumulation at high concentration in pulcherrimin-producing Metschnikowia yeast biomass. J. Biol. Inorg. Chem. 2021, 26, 299–311. [Google Scholar] [CrossRef]
- Kluyver, A.J.; Walt, J.P.; Triet, A.J. Pulcherrimin, the pigment of Candida pulcherrima. Proc. Natl. Acad. Sci. USA 1953, 39, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M. Metschnikowia strains isolated from botrytized grapes antagonize fungal and bacterial growth by iron depletion. Appl. Environ. Microbiol. 2006, 72, 6716–6724. [Google Scholar] [CrossRef] [PubMed]
- Türkel, S.; Enerb, B. Isolation and characterization of new Metschnikowia pulcherrima strains as producers of the antimicrobial pigment pulcherrimin. Z. Für Nat. C 2009, 5–6, 405–410. [Google Scholar] [CrossRef]
- Sipiczki, M. When barcoding fails: Genome chimerization (admixing) and reticulation obscure phylogenetic and taxonomic relationships. Mol. Ecol. Resour. 2022, 22, 1762–1785. [Google Scholar] [CrossRef]
- Lachance, M.A. Metschnikowia: Half tetrads, a regicide and the fountain of youth. Yeast 2016, 33, 563–574. [Google Scholar] [CrossRef]
- Ünlü, G.; Nielsen, B.; Ionita, C. Production of antilisterial bacteriocins from lactic acid bacteria in dairy-based media: A comparative study. Probiotics Antimicrob. Proteins 2015, 7, 259–274. [Google Scholar] [CrossRef]
- Bosmans, L.; De Bruijn, I.; De Mot, R.; Rediers, H.; Lievens, B. Agar composition affects in vitro screening of biocontrol activity of antagonistic microorganisms. J. Microbiol. Methods 2016, 127, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Lemos-Carolino, M.; Madeira-Lopes, A.; Van Uden, N. The temperature profile of the pathogenic yeast Candida albicans. Z. Für Allg. Mikrobiol. 1982, 22, 705–709. [Google Scholar] [CrossRef]
- Pawlikowska, E.; Kolesińska, B.; Nowacka, M.; Kregiel, D. A new approach to producing high yields of pulcherrimin from Metschnikowia yeasts. Fermentation 2020, 6, 114. [Google Scholar] [CrossRef]
- Nawaz, A.; Mäkinen, A.; Pärnänen, P.; Meurman, J.H. Proteolytic activity of non-albicans Candida and Candida albicans in oral cancer patients. New Microbiol. 2018, 41, 296–301. [Google Scholar]
- Athira, S.; Mann, B.; Sharma, R.; Pothuraju, R.; Bajaj, R.K. Preparation and characterization of iron-chelating peptides from whey protein: An alternative approach for chemical iron fortification. Food Res. Int. 2021, 141, 110133. [Google Scholar] [CrossRef] [PubMed]
- Dubrovinsky, L.; Dubrovinskaia, N.; Langenhorst, F.; Dobson, D.; Rubie, D.; Gessmann, C.; Abrikosov, I.A.; Johansson, B.; Baykov, V.I.; Vitos, L.; et al. Iron-silica interaction at extreme conditions and the electrically conducting layer at the base of Earth’s mantle. Nature 2003, 422, 58–61. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.C. The structure of pulcherriminic acid. Can. J. Chem. 1963, 41, 165–172. [Google Scholar] [CrossRef]
- Vysoka, M.; Szotkowski, M.; Slaninova, E.; Dzuricka, L.; Strecanska, P.; Blazkova, J.; Marova, I. Oleaginous yeast extracts and their possible effects on human health. Microorganisms 2023, 2, 492. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Nowacka, M.; Rygala, A.; Vadkertiová, R. Biological activity of pulcherrimin from the Metschnikowia pulcherrima clade. Molecules 2022, 27, 1855. [Google Scholar] [CrossRef]
- Smith, I.M.; Baker, A.; Arneborg, N.; Jespersen, L. Non-Saccharomyces yeasts protect against epithelial cell barrier disruption induced by Salmonella enterica subsp. enterica serovar Typhimurium. Lett. Appl. Microbiol. 2015, 61, 491–497. [Google Scholar] [CrossRef]
- Wilson, D. A tale of two yeasts: Saccharomyces cerevisiae as a therapeutic against candidiasis. Virulence 2017, 8, 15–17. [Google Scholar] [CrossRef]
- Kántor, A.; Hutková, J.; Petrová, J.; Hleba, L.; Kačániová, M. Antimicrobial activity of pulcherrimin pigment produced by Metschnikowia pulcherrima against various yeast species. J. Microbiol. Biotechnol. Food Sci. 2015, 5, 282–285. [Google Scholar] [CrossRef]
- Steglińska, A.; Kołtuniak, A.; Berłowska, J.; Czyżowska, A.; Szulc, J.; Cieciura-Włoch, W.; Okrasa, M.; Kręgiel, D.; Gutarowska, B. Metschnikowia pulcherrima as a biocontrol agent against potato (Solanum tuberosum) pathogens. Agronomy 2022, 12, 2546. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Bavanilatha, M.; Narendrakumar, G.; Samrot, A.V. Bioactivity of pulcherrimin isolated from Bacillus subtilis SU-10 grown in FeSO4 rich medium. Int. J. Future Biotechnol. 2012, 1, 1–4. [Google Scholar]
- Shahat, A.S. Antioxidant and anticancer activities of yeast grown on commercial media. Int. J. Biol. Chem. Sci. 2017, 11, 2442–2455. [Google Scholar] [CrossRef]
- Upadhyay, T.K.; Trivedi, R.; Khan, F.; Al-Keridis, L.A.; Pandey, P.; Sharangi, A.B.; Alshammari, N.; Abdullah, N.M.; Yadav, D.K.; Saeed, M. In vitro elucidation of antioxidant, antiproliferative, and apoptotic potential of yeast-derived β-1,3-glucan particles against cervical cancer cells. Front. Oncol. 2022, 12, 942075. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-San Millan, A.; Gamir, J.; Farran, I.; Larraya, L.; Veramendi, J. Identification of new antifungal metabolites produced by the yeast Metschnikowia pulcherrima involved in the biocontrol of postharvest plant pathogenic fungi. Postharvest Biol. Technol. 2022, 192, 111995. [Google Scholar] [CrossRef]
- Arnaouteli, S.; Matoz-Fernandez, D.A.; Porter, M.; Kalamara, M.; Abbott, J.; MacPhee, C.E.; Davidson, F.A.; Stanley-Wall, N.R. Pulcherrimin formation controls growth arrest of the Bacillus subtilis biofilm. Proc. Natl. Acad. Sci. USA 2019, 116, 13553–13562. [Google Scholar] [CrossRef]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Baskic, D.; Popovic, S.; Ristic, P.; Arsenijevic, N. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int. 2006, 30, 924–932. [Google Scholar] [CrossRef]
- Salim, L.; Mohan, S.; Othman, R.; Abdelwahab, S.; Kamalidehghan, B.; Sheikh, B.; Ibrahim, M. Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules 2013, 18, 11219–11240. [Google Scholar] [CrossRef]


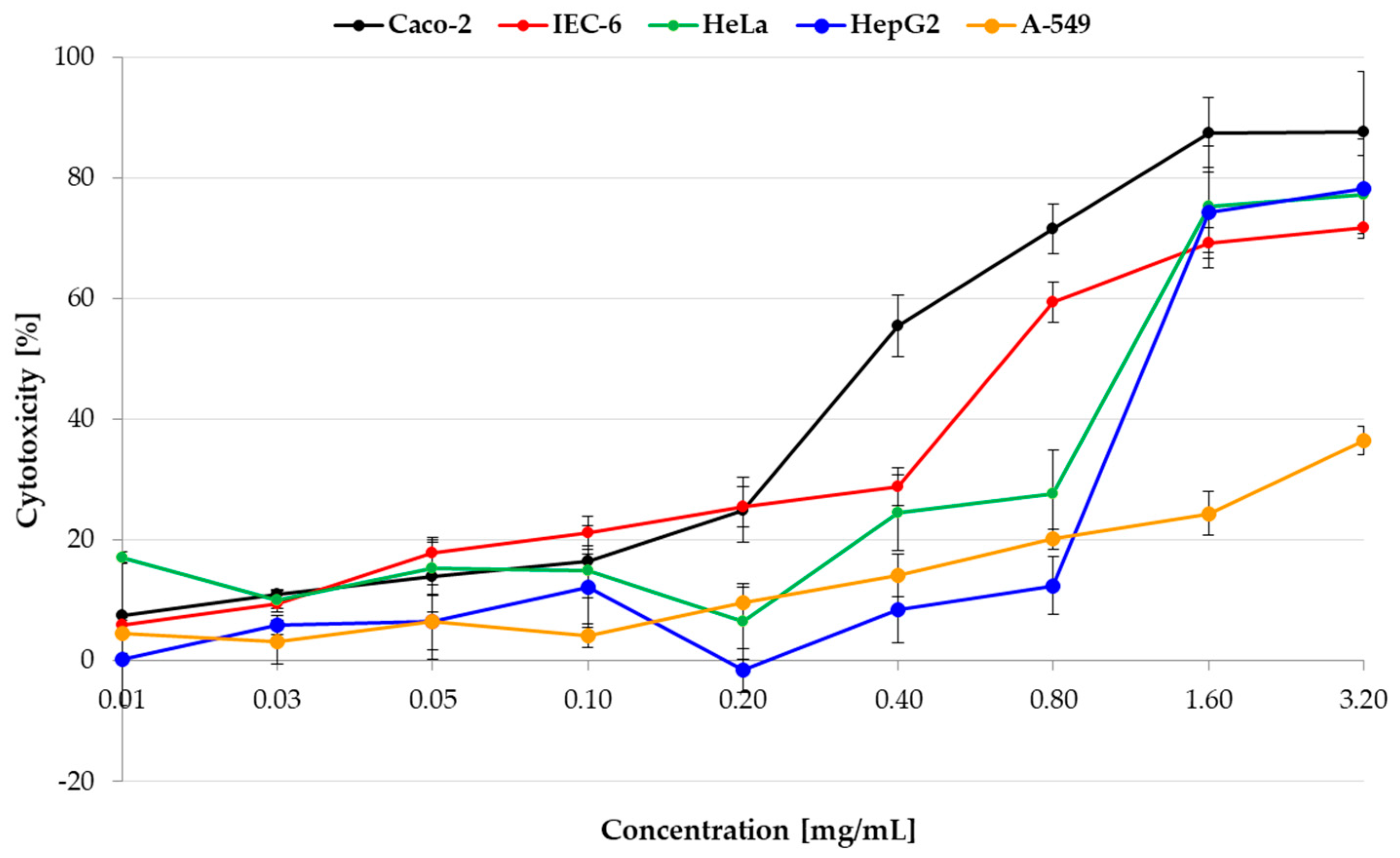

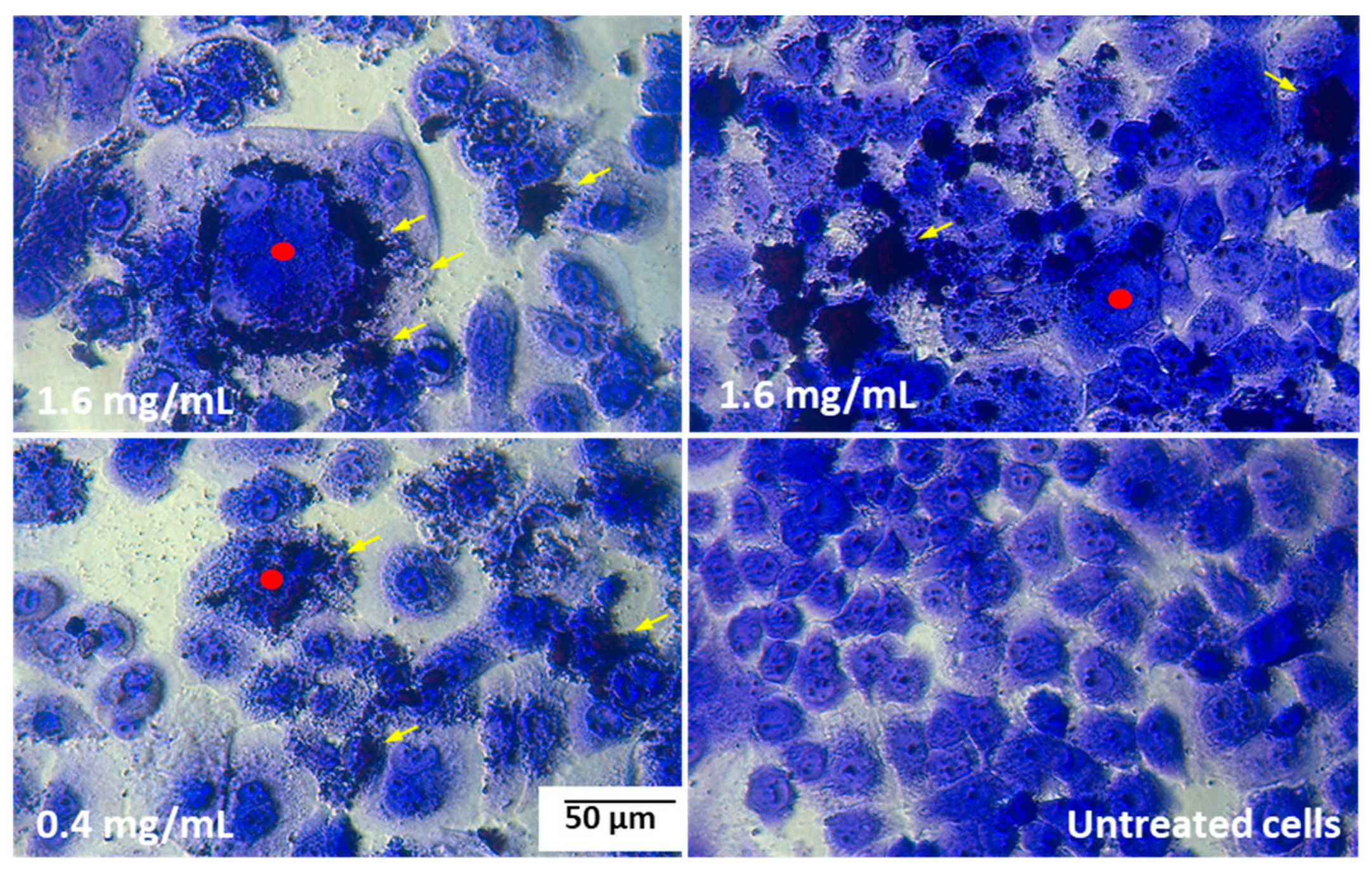
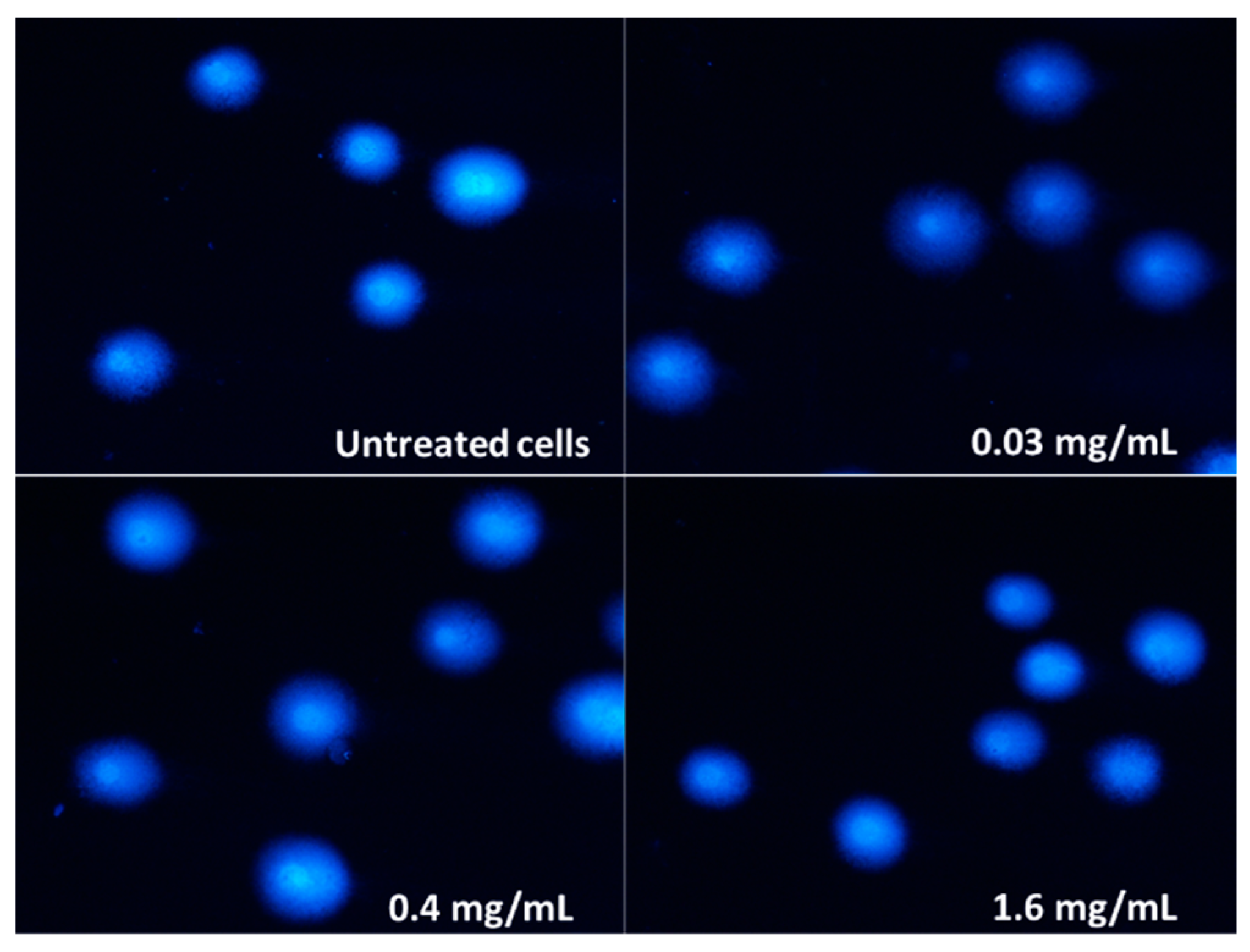
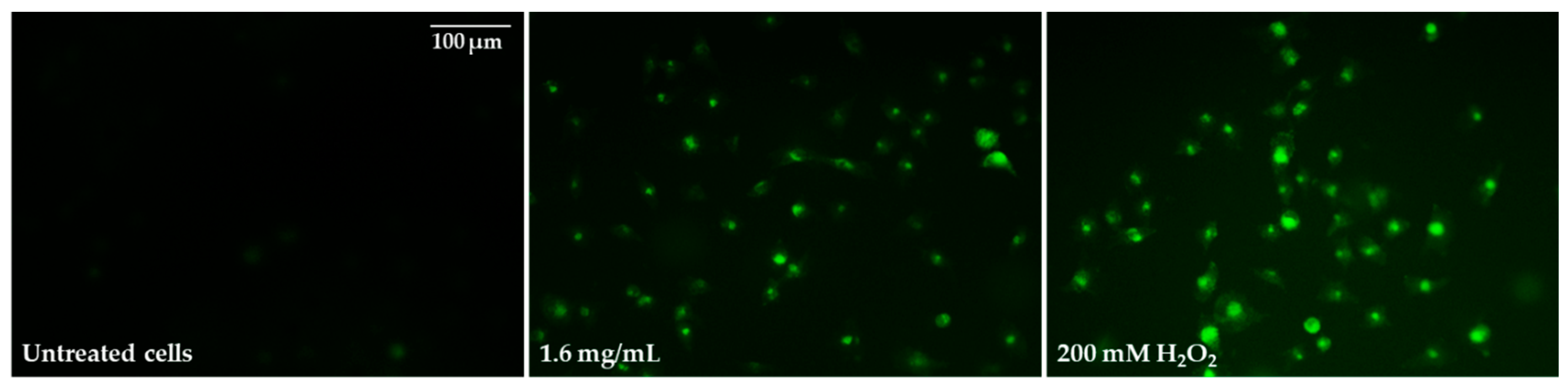
| Cell Line | IC50 [mg/mL] | Sequence of the Cytotoxicity |
|---|---|---|
| A-549 | not detected | 5—the weakest |
| Caco-2 | 0.36 | 1—the strongest |
| HeLa | 1.16 | 3 |
| HepG2 | 1.27 | 4 |
| IEC-6 | 0.67 | 2 |
| Concentration [mg/mL] | DNA Damage [%] ± S.E.M. |
|---|---|
| 0.01 | 4.84 ± 4.60 |
| 0.03 | 8.06 ± 2.15 |
| 0.1 | 6.33 ± 1.15 |
| 0.4 | 7.34 ± 1.33 |
| 1.6 | 7.09 ± 1.49 |
| Concentration of Pulcherrimin [mg/mL] | Average Fluorescence Intensity [%] ± SD |
|---|---|
| Negative control (unexposed cells) | 100 ± 3.9 |
| 0.03 | 109 ± 2.6 |
| 0.1 | 114 ± 2.5 |
| 0.4 | 125 ± 2.5 * |
| 1.6 | 222 ± 3.4 * |
| 200 mM H2O2 (positive control) | 362 ± 3.9 * |
| Concentration of Pulcherrimin [mg/mL] | H2O2 Production [µM] ± SD |
|---|---|
| Negative control (unexposed cells) | 1.2 ± 1.9 |
| 0.03 | 6.5 ± 2.3 * |
| 0.1 | 7.5 ± 1.8 * |
| 0.4 | 7.5 ± 2.1 * |
| 1.6 | 7.2 ± 2.3 * |
| 10 mM H2O2 (positive control) | 21.5 ± 2.9 * |
| Concentration of Pulcherrimin [mg/mL] | Mitochondrial Membrane Potential [%] ± SD |
|---|---|
| Negative control (unexposed cells) | 100 ± 2.3 |
| 0.03 | 88 ± 2.3 * |
| 0.1 | 83 ± 2.1 * |
| 0.4 | 77 ± 2.2 * |
| 1.6 | 59 ± 1.9 * |
| CCCP (positive control, 50 µM) | 43 ± 1.9 * |
| No | Metschnikowia Clade | Symbol | Origin |
|---|---|---|---|
| 1 | M. sinensis D1 | LOCK 1135 | apple fruits, Poland |
| 2 | M. andauensis D2 | LOCK 1136 | apple fruits, Poland |
| 3 | M. sinensis D3 | LOCK 1137 | raspberry fruits, Poland |
| 4 | M. andauensis D4 | LOCK 1138 | raspberry fruits, Poland |
| 5 | M. andauensis D5 | LOCK 1139 | grape berries, Poland |
| 6 | M. andauensis D6 | LOCK 1140 | grape berries, Poland |
| 7 | M. andauensis D7 | LOCK 1141 | red currant berries, Poland |
| 8 | M. andauensis D8 | LOCK 1142 | red currant berries, Poland |
| 9 | M. sinensis D9 | LOCK 1143 | strawberry fruits, Poland |
| 10 | M. sinensis D10 | LOCK 1144 | strawberry flowers, Poland |
| 11 | M. pulcherrima CCY145 | CCY 29-2-145 | grape berries, Slovakia |
| 12 | M. pulcherrima CCY149 | CCY 29-2-149 | grape berries, Slovakia |
| No | Candida and non-Candida strains * | Symbol | Origin |
| 1 * | C. albicans | CCY 29-3-163 | blood, Slovakia |
| 2 | C. albicans | CCY 29-3-164 | tonsils, Slovakia (resistant to antimycotics) |
| 3 | C. albicans | CCY 29-7-065 | plum fruits, Slovakia |
| 4 | C. glabrata | CCY 26-20-3 | mushroom, fruiting body, Czech Republic |
| 5 | C. glabrata | CCY 26-20-30 | sputum, Slovakia (resistant to antimycotics) |
| 6 | C. glabrata | CCY 26-20-31 | urine, Slovakia |
| 7 | Meyerozyma guilliermondii (syn. Candida guilliermondii) | CCY 29-4-38 | sputum, unknown |
| 8 | Meyerozyma guilliermondii (syn. Candida guilliermondii) | CCY 29-4-39 | peach tree, blossom, Slovakia |
| 9 | Meyerozyma guilliermondii (syn. Candida guilliermondii) | CCY 39-23-6 | apple tree, leaves, Slovakia |
| 10 | Pichia kudriavzevii (syn. Candida krusei) | CCY 29-9-47 | mouth, Slovakia (resistant to antimycotics) |
| 11 | Pichia kudriavzevii (syn. Candida krusei) | CCY 29-9-49 | urine, Slovakia |
| 12 | Pichia kudriavzevii (syn. Candida krusei) | CCY 29-9-50 | soil adjacent to apricot tree, Slovakia |
| 13 | C. tropicalis | CCY 29-7-64 | plum tree, blossom, Slovakia |
| 14 | C. tropicalis | CCY 29-7-66 | urine, Slovakia (resistant to antimycotics) |
| 15 | C. tropicalis | CCY 29-7-68 | mouth, Slovakia |
| 16 | C. parapsilosis | CCY 29-20-29 | apricot tree, blossom, Slovakia |
| 17 | C. parapsilosis | CCY 29-20-31 | tongue, Slovakia |
| 18 | C. parapsilosis | CCY 29-20-32 | trachea, Slovakia (resistant to antimycotics) |
| 19 | Filobasidiella neoformans var. bacillispora (syn. Cryptococcus neoformans) | CCY 17-1-8 | clinical strain, Japan |
| 20 | Filobasidiella neoformans var. bacillispora (syn. Cryptococcus neoformans) | CCY 17-1-10 | clinical strain, Japan |
| Methylene Blue Agar Variants | Symbol | Composition |
|---|---|---|
| YED-methylene blue agar | YED-MB | 0.3% yeast extract (Merck), 0.5%glucose (Merck), 2% agar (Merck), 0.002% methylene blue (Merck), pH = 4.5 |
| YEPD-methylene blue agar | YEPD-MB | 0.3% yeast extract, 0.5%glucose, 1% peptone, 2% agar, 0.002% methylene blue, pH = 4.5 |
| YEDSi-methylene blue agar | YEDSi-MB | 0.3% yeast extract, 0.5%glucose, 2% agar, 0.1% sodium silicate (Lachema), 0.002% methylene blue, pH = 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kregiel, D.; Czarnecka-Chrebelska, K.H.; Schusterová, H.; Vadkertiová, R.; Nowak, A. The Metschnikowia pulcherrima Clade as a Model for Assessing Inhibition of Candida spp. and the Toxicity of Its Metabolite, Pulcherrimin. Molecules 2023, 28, 5064. https://doi.org/10.3390/molecules28135064
Kregiel D, Czarnecka-Chrebelska KH, Schusterová H, Vadkertiová R, Nowak A. The Metschnikowia pulcherrima Clade as a Model for Assessing Inhibition of Candida spp. and the Toxicity of Its Metabolite, Pulcherrimin. Molecules. 2023; 28(13):5064. https://doi.org/10.3390/molecules28135064
Chicago/Turabian StyleKregiel, Dorota, Karolina H. Czarnecka-Chrebelska, Hana Schusterová, Renáta Vadkertiová, and Adriana Nowak. 2023. "The Metschnikowia pulcherrima Clade as a Model for Assessing Inhibition of Candida spp. and the Toxicity of Its Metabolite, Pulcherrimin" Molecules 28, no. 13: 5064. https://doi.org/10.3390/molecules28135064
APA StyleKregiel, D., Czarnecka-Chrebelska, K. H., Schusterová, H., Vadkertiová, R., & Nowak, A. (2023). The Metschnikowia pulcherrima Clade as a Model for Assessing Inhibition of Candida spp. and the Toxicity of Its Metabolite, Pulcherrimin. Molecules, 28(13), 5064. https://doi.org/10.3390/molecules28135064