Fused Triazinobenzimidazoles Bearing Heterocyclic Moiety: Synthesis, Structure Investigations, and In Silico and In Vitro Biological Activity
Abstract
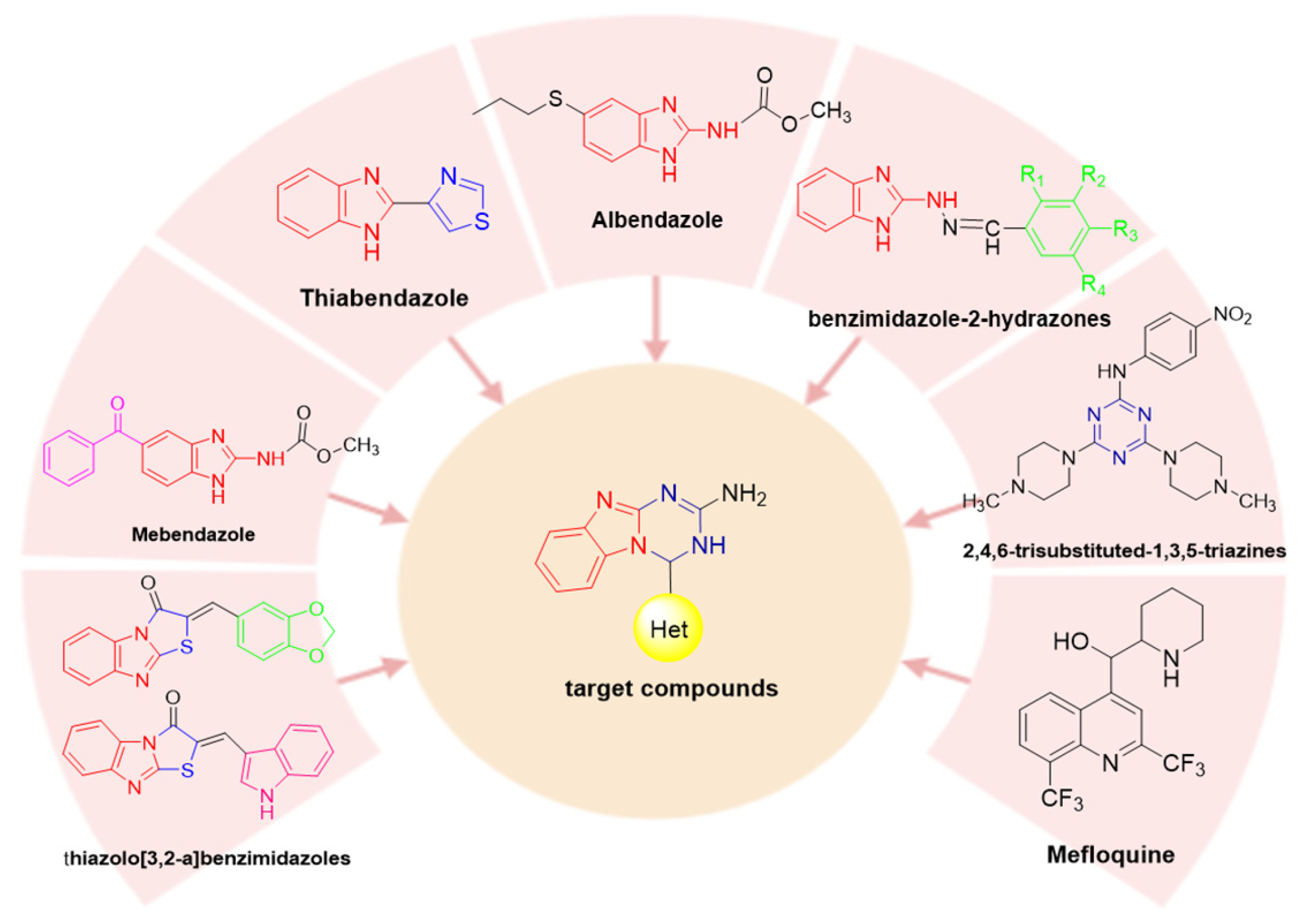
1. Introduction
2. Results and Discussion
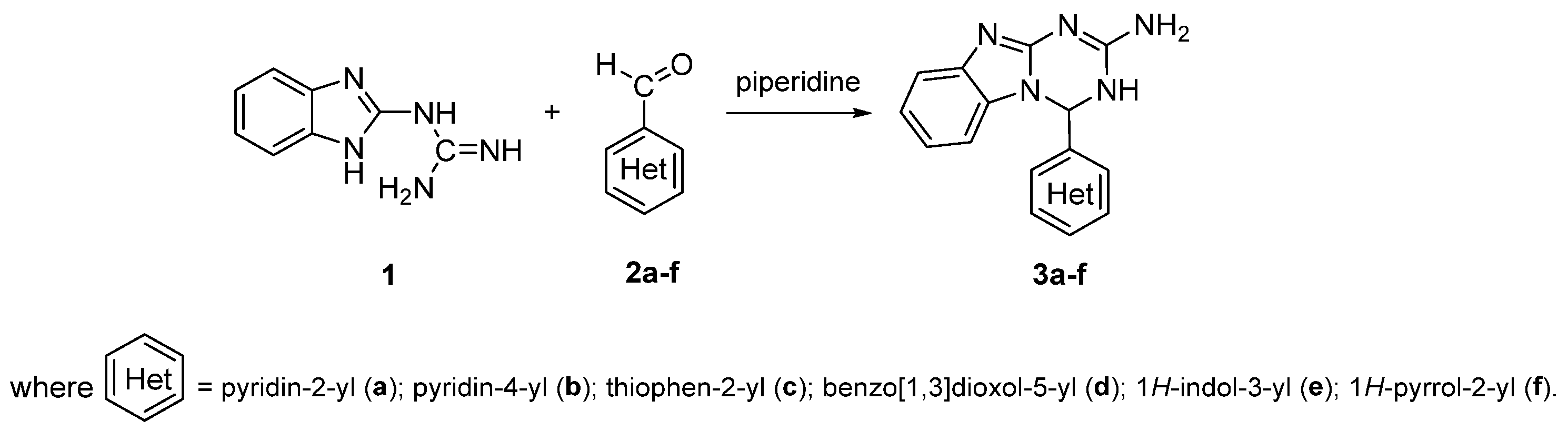
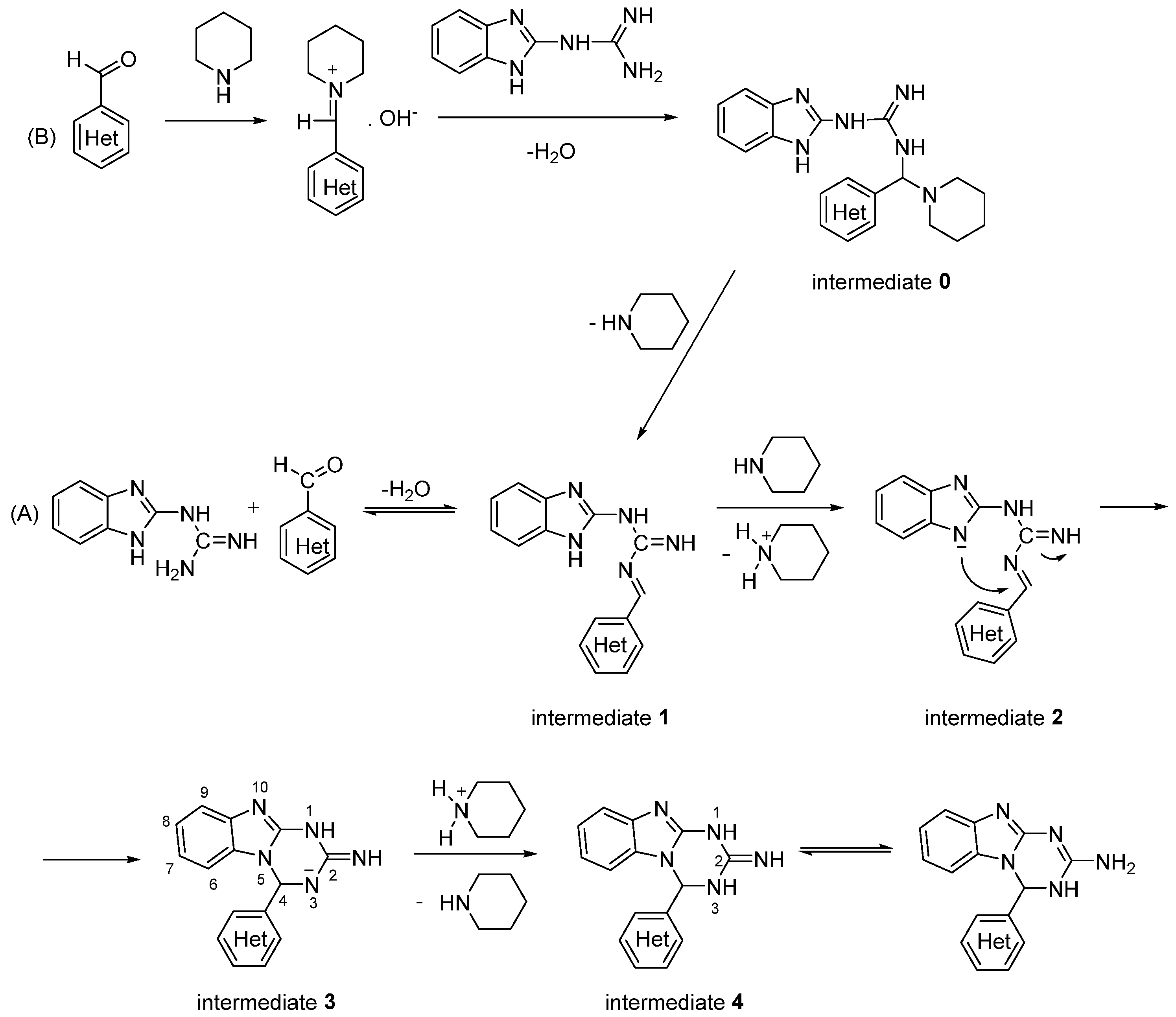
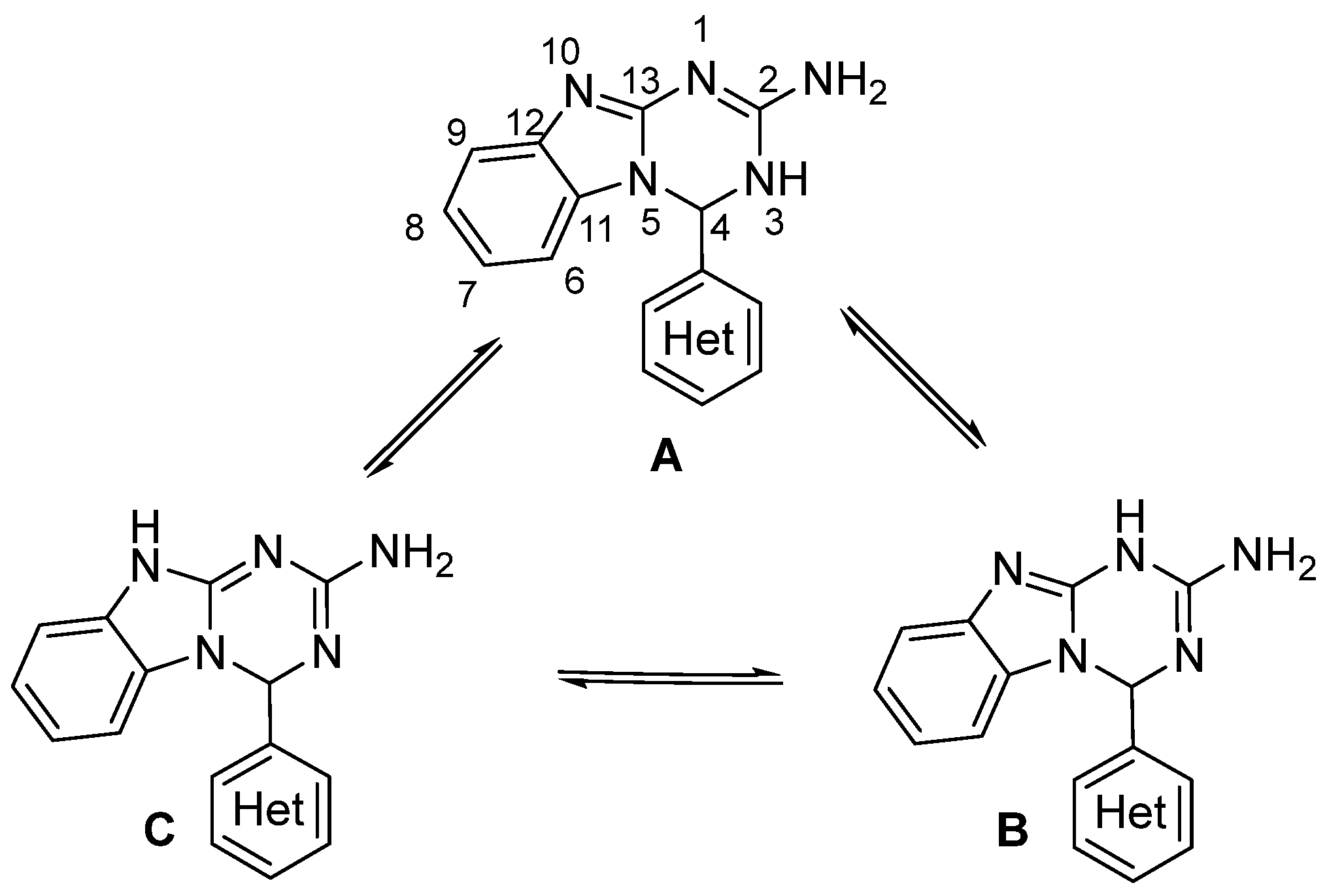
2.1. Synthesis
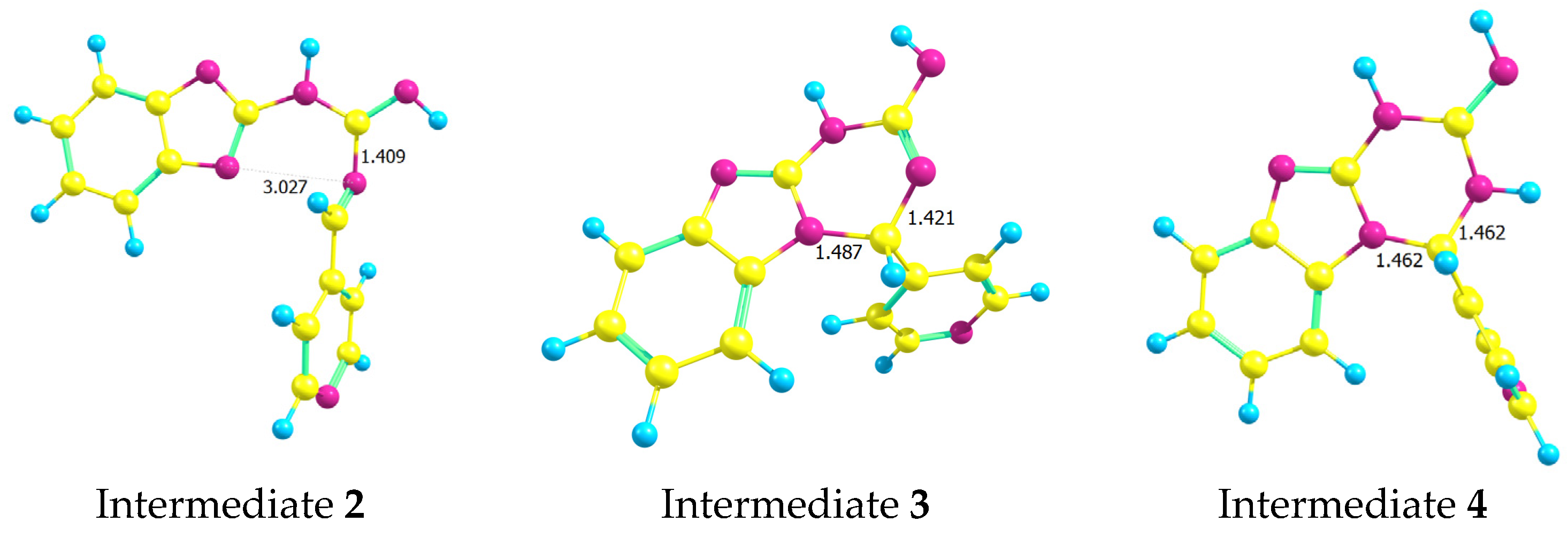
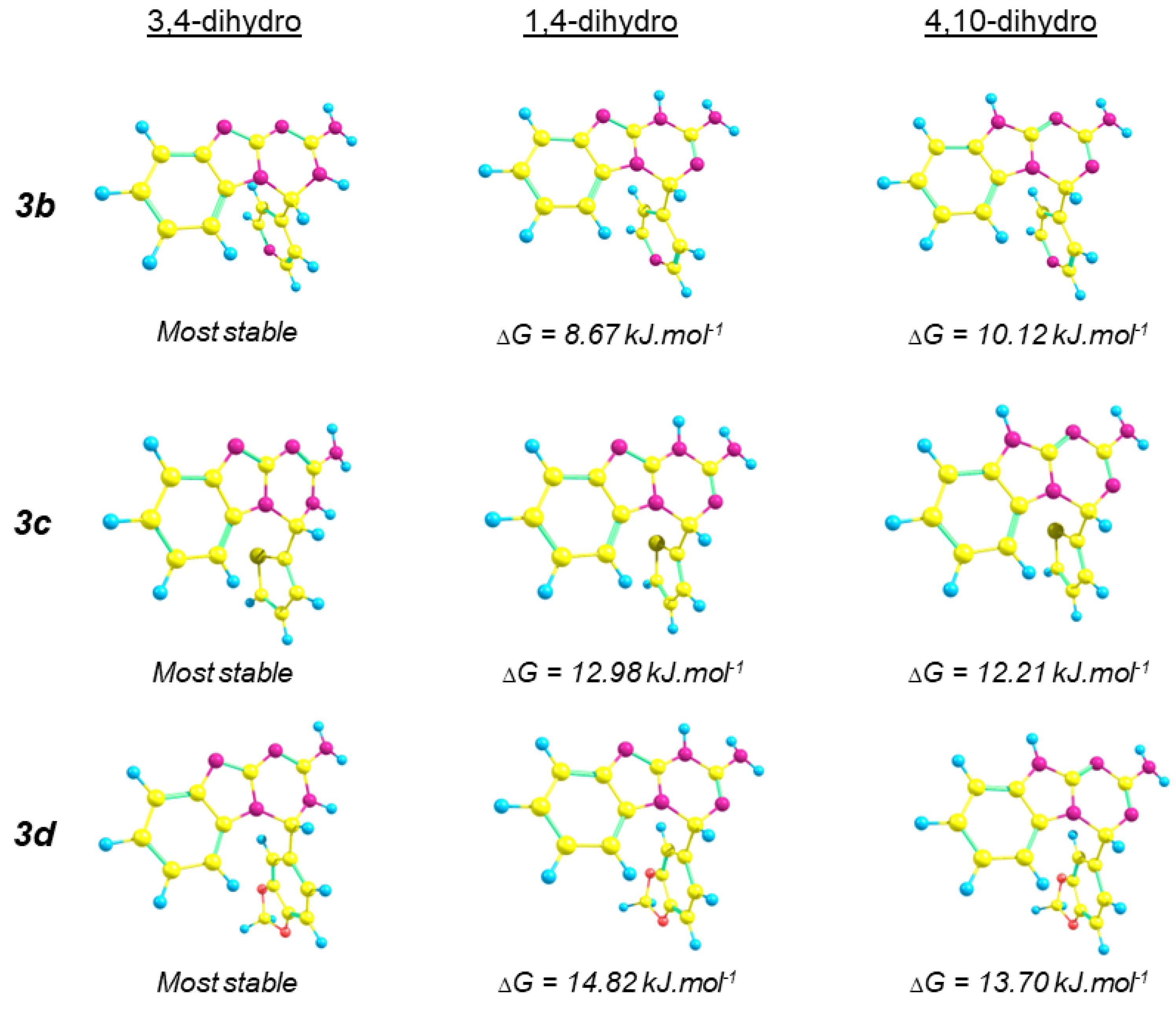
2.2. Computational Methods
2.3. Antihelmintic Activity
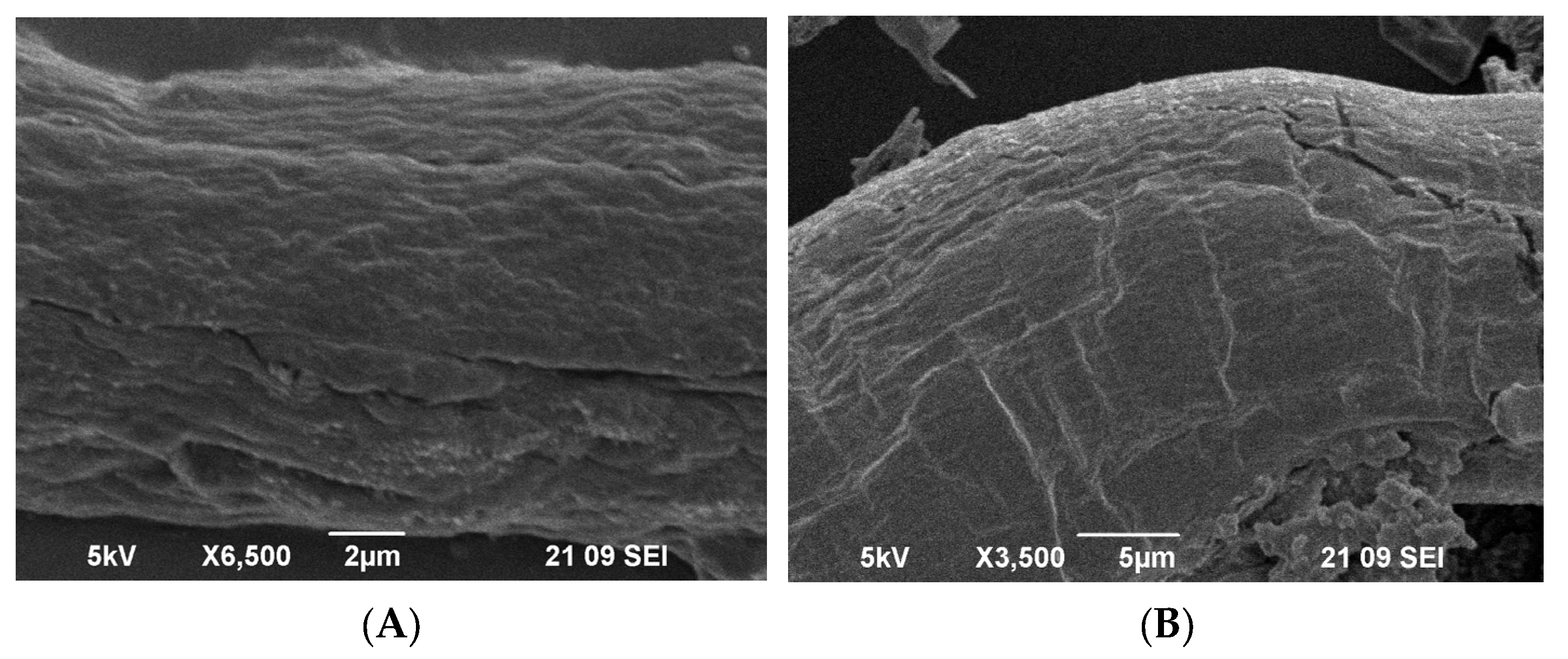
2.4. Scanning Electron Microscopy
2.5. In Vitro Evaluation of Cytotoxicity
2.6. Prediction of the Physico-Chemical Properties and Drug-Likeness
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Compound 3a–f
3.2.2. Compound Data
2-Amino-4-(pyridine-2-yl)-3,4-dihydro[1,3,5]triazino[1,2-a]benzimidazole (3a)
2-Amino-4-(pyridine-4-yl)-3,4-dihydro[1,3,5]triazino[1,2-a]benzimidazole (3b)
2-Amino-4-(thiophen-2-yl)-3,4-dihydro[1,3,5]triazino[1,2-a]benzimidazole (3c)
2-Amino-4-(benzo[1,3]dioxol-5-yl)-3,4-dihydro[1,3,5]triazino[1,2-a]benzimidazole (3d)
2-Amino-4-(1H-indol-3-yl)-3,4-dihydro[1,3,5]triazino[1,2-a]benzimidazole (3e)
2-Amino-4-(1H-pyrrol-2-yl)-3,4-dihydro[1,3,5]triazino[1,2]benzimidazole (3f)
3.3. Computational Methods
3.4. Anthelmintic Study
3.5. Cytotoxicity Study
3.5.1. Preparation of Cell Cultures
3.5.2. Cell Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rainova, I.; Harizanov, R.; Kaftandjiev, I.; Tsvetkova, N.; Mikov, O.; Kaneva, E. Human Parasitic Diseases in Bulgaria in between 2013–2014. Balk. Med. J. 2018, 35, 61–67. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Trichinellosis. In ECDC. Annual Epidemiological Report for 2020; ECDC: Stockholm, Sweden, 2022; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Trichinellosis-AER_2020_Final.pdf (accessed on 25 October 2022).
- Yu, Y.R.; Qi, Y.F. Progress in Treatment and Prevention of Trichinellosis. J. Infect. Dis. Ther. 2015, 3, 251. [Google Scholar] [CrossRef]
- Priotti, J.; Codina, A.V.; Leonardi, D.; Vasconi, M.D.; Hinrichsen, L.I.; Lamas, M.C. Albendazole microcrystal formulations based on chitosan and cellulose derivatives: Physicochemical characterization and in vitro parasiticidal activity in Trichinella spiralis adult worms. AAPS PharmSciTech 2017, 18, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-González, A.; De Armas-Serra, C.; Criado-Fornelio, A.; Casado-Escribano, N.; Rodríguez-Caabeiro, F.; Díez, J.C. Preliminary characterization and interaction of tubulin from Trichinella spiralis larvae with benzimidazole derivatives. Vet. Parasitol. 1991, 39, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Urbizu, L.; Confalonieri, A.; Sánchez Bruni, S.; Lanusse, C.; Alvarez, L.I. Nematodicidal Activity of Flubendazole and Its Reduced Metabolite on a Murine Model of Trichinella spiralis Infection. Chemotherapy 2012, 58, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Mavrova, A.T.; Anichina, K.K.; Vuchev, D.I.; Tsenov, J.A.; Kondeva, M.S.; Micheva, M.K. Synthesis and antitrichinellosis activity of some 2-substituted-[1,3]thiazolo[3,2-a]benzimidazol-3(2H)-ones. Bioorg. Med. Chem. 2005, 13, 5550–5559. [Google Scholar] [CrossRef] [PubMed]
- Mavrova, A.T.; Denkova, P.; Tsenov, Y.A.; Anichina, K.K.; Vutchev, D.I. Syn-thesis and antitrichinellosis activity of some bis(benzimidazol-2-yl)amines. Bioorg. Med. Chem. 2007, 15, 6291–6297. [Google Scholar] [CrossRef] [PubMed]
- Mavrova, A.T.; Anichina, K.K.; Vuchev, D.I.; Tsenov, J.A.; Denkova, P.S.; Kondeva, M.S.; Micheva, M.K. Antihelminthic activity of some newly synthesized 5(6)-(un)substituted-1H-benzimidazol-2-ylthioacetylpiperazine derivatives. Eur. J. Med. Chem. 2006, 41, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Anichina, K.; Argirova, M.; Tzoneva, R.; Uz-unova, V.; Mavrova, A.; Vuchev, D.; Popova-Daskalova, G.V.; Fratev, F.; Guncheva, M.; Yancheva, D. 1H-benzimidazole-2-yl hydrazones as tubulin-targeting agents: Synthesis, structural characterization, anthelmintic activity and antiproliferative activity against MCF-7 breast carcinoma cells and molecular docking studies. Chem. Biol. Interact. 2021, 345, 109540. [Google Scholar] [CrossRef]
- Singh, S.; Mandal, M.K.; Masih, A.; Saha, A.; Ghosh, S.K.; Bhat, H.R.; Singh, U.P. 1,3,5-Triazine: A versatile pharmacophore with diverse biological activities. Arch. Pharm. 2021, 354, e2000363. [Google Scholar] [CrossRef]
- Dolzhenko, A.V.; Chui, W.K. Synthesis of Ethyl 6-Aryl-4-oxo-4,6-dihydro-1(12)(13)H-pyrimido-[2′,1′:4,5][1,3,5]triazino[1,2-a]benzimidazole-3-carboxylates. J. Heterocycl. Chem. 2006, 43, 1513–1521. [Google Scholar] [CrossRef]
- Dolzhenko, A.V.; Chui, W.K. Synthesis and Biological activity of 1,3,5-triazino[1,2-a]benzimidazole-2-amines. Pharm. Chem. J. 2007, 41, 470–471. [Google Scholar] [CrossRef]
- Agarwal, A.; Srivastava, K.; Puri, S.K.; Chauhan, P.M.S. Syntheses of 2,4,6-trisubstituted triazines as antimalarial agents. Bioorg. Med. Chem. Lett. 2005, 15, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.R.; Singh, U.P.; Gahtori, P.; Ghosh, S.K.; Gogoi, K.; Prakash, A.; Singh, R.K. Synthesis, Docking, In Vitro and In Vivo Antimalarial Activity of Hybrid 4-aminoquinoline–1,3,5-triazine Derivatives against Wild and Mutant Malaria Parasites. Chem. Biol. Drug Des. 2015, 86, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.M.; Diab, T.M. Therapeutic efficacy of Albendazole and Mefloquine alone or in combination against early and late stages of Trichinella spiralis infection in mice. Helminthologia 2021, 58, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yeh, P.J. Suppressive drug combinations and their potential to combat antibiotic resistance. J. Antibiot. 2017, 70, 1033–1042. [Google Scholar] [CrossRef]
- Dolzhenko, A.V.; Chui, W.-K.; Dolzhenko, A.V.; Chan, L.-W. Synthesis and biological activity of fluorinated 2-amino-4-aryl-3,4-dihydro[1,3,5]triazino[1,2-a]benzimidazoles. J. Fluor. Chem. 2005, 126, 759–763. [Google Scholar] [CrossRef]
- Hranjec, M.; Pavlović, G.; Karminski-Zamola, G. Synthesis, crystal structure determination and antiproliferative activity of novel 2-amino-4-aryl-4,10-dihydro[1,3,5]triazino[1,2-a]benzimidazoles. J. Mol. Struct. 2012, 1007, 242–251. [Google Scholar] [CrossRef]
- Barve, I.J.; Chen, C.-H.; Kao, C.-H.; Sun, C.-M. Regioselective Piperidine-Catalyzed Tandem Imination–Isocyanate Annulation to Fused Tricyclic Triazines. ACS Comb. Sci. 2014, 16, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Argirova, M.A.; Georgieva, M.K.; Hristova-Avakumova, N.G.; Vuchev, D.I.; Popova-Daskalova, G.V.; Anichina, K.K.; Yancheva, D.Y. New 1H-benzimidazole-2-yl hydrazones with combined antiparasitic and antioxidant activity. RSC Adv. 2021, 11, 39848–39868. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yao, J.; Liu, K.; Yang, W.; Wang, G.; Shi, C.; Jiang, Y.; Wang, J.; Kang, Y.; Wang, D.; et al. Sanguinarine has anthelmintic activity against the enteral and parenteral phases of Trichinella infection in experimentally infected mice. Acta Trop. 2020, 201, 105226. [Google Scholar] [CrossRef] [PubMed]
- Gottstein, B.; Pozio, E.; Nockler, K. Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef]
- Codina, A.V.; García, A.; Leonardi, D.; Vasconi, M.D.; Di Masso, R.J.; Lamas, M.C.; Hinrichsen, L.I. Efficacy of albendazole:β-cyclodextrin citrate in the parenteral stage of Trichinella spiralis infection. Int. J. Biol. Macromol. 2015, 77, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Bughdadi, F.A. Ultrastractural studies on the parasitic worm Trichinella Spiralis. J. Taibah Univ. Sci. 2010, 3, 33–38. [Google Scholar] [CrossRef]
- ElGhannam, M.; Dar, Y.; ElMehlawy, M.H.; Mokhtar, F.A.; Bakr, L. Eugenol; Effective Anthelmintic Compound against Foodborne Parasite Trichinella spiralis Muscle Larvae and Adult. Pathogens 2023, 12, 127. [Google Scholar] [CrossRef] [PubMed]
- El-Wakil, E.S.; Abdelmaksoud, H.F.; AbouShousha, T.S.; Ghallab, M.M.I. Evaluation of Annona muricata (Graviola) leaves activity against experimental trichinellosis: In vitro and in vivo studies. J. Helminthol. 2021, 95, e53. [Google Scholar] [CrossRef] [PubMed]
- Dziduch, K.; Greniuk, D.; Wujec, M. The Current Directions of Searching for Antiparasitic Drugs. Molecules 2022, 27, 1534. [Google Scholar] [CrossRef]
- Campbell, W.C.; Rew, R.S. Chemotherapy of Parasitic Diseases; Springer: New York, NY, USA, 1986; pp. 3–21. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef]
- Di, L.; Fish, P.V.; Mano, T. Bridging solubility between drug discovery and development. Drug Discov. Today 2012, 17, 486–495. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. (Eds.) Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian-basis sets for molecular calculations. 1. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Raghavachari, K.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. 20. Basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
| Compound | Het | Concentration 50 (100) µg/mL in µM | Efficacy (%) b after 24 h | Efficacy (%) b after 48 h | Efficacy (%) b after 72 h | ||
|---|---|---|---|---|---|---|---|
| 50 µg/mL | 100 µg/mL | 50 µg/mL | 100 µg/mL | 50 µg/mL | |||
| 3a | pyridin-2-yl | 0.189 (0.378) | 37.21 ± 0.16 | 44.0 ± 0.86 | 46.78 ± 1.04 | 51.43 ± 2.87 | 72.75 ± 2.01 |
| 3b | pyridin-4-yl | 0.189 (0.378) | 21.33 ± 0.47 | 30.84 ± 0.27 | 28.92 ± 0.80 | 35.2 ± 3.50 | 65.50 ± 1.16 |
| 3c | thiophen-2-yl | 0.186 (0.371) | 58.41 ± 0.22 | 71.54 ± 0.63 | 85.74 ± 1.25 | 100.00 ± 0.00 | 100.00 ± 0.00 |
| 3d | benzo[1,3]dioxol-5-yl | 0.163 (0.325) | 42.10 ± 1.17 | 51.48 ± 3.21 | 52.41 ± 0.39 | 58.24 ± 0.73 | 95.01 ± 0.43 |
| 3e | 1H-indol-3-yl | 0.165 (0.331) | 31.54 ± 0.57 | 39.80 ± 0.37 | 36.88 ± 0.58 | 47.00 ± 2.71 | 80.24 ± 1.65 |
| 3f | 1H-pyrrol-2-yl | 0.198 (0.396) | 49.90 ± 2.15 | 63.02 ± 1.85 | 72.81 ± 0.52 | 95.74 ± 1.57 | 100.00 ± 0.00 |
| Alb. d | 0.188 (0.377) | 10.62 ± 0.51 | 10.91 ± 0.25 | 14.60 ± 0.27 | 15.72 ± 0.52 | NT c | |
| Iverm. e | 0.057 (0.114) | 45.38 ± 0.72 | 49.00 ± 0.18 | 62.23 ± 0.12 | 78.83 ± 1.15 | NT c | |
| Compound | MW a | TPSA b | Natoms c | HBA d | HBD e | Nviol f | RotB g | logP h |
|---|---|---|---|---|---|---|---|---|
| 3a | 264.29 | 81.12 | 20 | 3 | 2 | 0 | 1 | 0.91 |
| 3b | 264.29 | 81.12 | 20 | 3 | 2 | 0 | 1 | 0.92 |
| 3c | 269.32 | 96.47 | 19 | 2 | 2 | 0 | 1 | 1.64 |
| 3d | 307.31 | 86.69 | 23 | 4 | 2 | 0 | 1 | 1.51 |
| 3e | 302.33 | 84.02 | 23 | 2 | 3 | 0 | 1 | 1.84 |
| 3f | 252.27 | 84.02 | 19 | 2 | 3 | 0 | 1 | 0.90 |
| Albendazole | 265.33 | 92.31 | 18 | 3 | 2 | 0 | 6 | 1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anichina, K.; Georgiev, N.; Lumov, N.; Vuchev, D.; Popova-Daskalova, G.; Momekov, G.; Cherneva, E.; Mihaylova, R.; Mavrova, A.; Atanasova-Vladimirova, S.; et al. Fused Triazinobenzimidazoles Bearing Heterocyclic Moiety: Synthesis, Structure Investigations, and In Silico and In Vitro Biological Activity. Molecules 2023, 28, 5034. https://doi.org/10.3390/molecules28135034
Anichina K, Georgiev N, Lumov N, Vuchev D, Popova-Daskalova G, Momekov G, Cherneva E, Mihaylova R, Mavrova A, Atanasova-Vladimirova S, et al. Fused Triazinobenzimidazoles Bearing Heterocyclic Moiety: Synthesis, Structure Investigations, and In Silico and In Vitro Biological Activity. Molecules. 2023; 28(13):5034. https://doi.org/10.3390/molecules28135034
Chicago/Turabian StyleAnichina, Kameliya, Nikolai Georgiev, Nikolay Lumov, Dimitar Vuchev, Galya Popova-Daskalova, Georgi Momekov, Emiliya Cherneva, Rositsa Mihaylova, Anelia Mavrova, Stela Atanasova-Vladimirova, and et al. 2023. "Fused Triazinobenzimidazoles Bearing Heterocyclic Moiety: Synthesis, Structure Investigations, and In Silico and In Vitro Biological Activity" Molecules 28, no. 13: 5034. https://doi.org/10.3390/molecules28135034
APA StyleAnichina, K., Georgiev, N., Lumov, N., Vuchev, D., Popova-Daskalova, G., Momekov, G., Cherneva, E., Mihaylova, R., Mavrova, A., Atanasova-Vladimirova, S., Piroeva, I., & Yancheva, D. (2023). Fused Triazinobenzimidazoles Bearing Heterocyclic Moiety: Synthesis, Structure Investigations, and In Silico and In Vitro Biological Activity. Molecules, 28(13), 5034. https://doi.org/10.3390/molecules28135034








