Abstract
In recent years, Web of Science has published nearly one hundred reports per year on quinoxalin-2(1H)-ones, which have attracted great interest due to their wide applications in pharmaceutical and materials fields, especially in recyclable heterogeneous catalytic reactions for direct C–H functionalisation. This review summarises for the first time the methods and reaction mechanisms of heterogeneous catalytic reactions of quinoxalin-2(1H)-ones, including six major types of heterogeneous catalysts involved. The heterogeneous reactions of quinoxalin-2(1H)-ones are summarised by classifying different types of catalytic materials (graphitic phase carbon nitride, MOF, COF, ion exchange resin, piezoelectric materials, and microsphere catalysis). In addition, this review discusses the future development of heterogeneous catalytic reactions of quinoxalin-2(1H)-ones, including the construction of C-B/Si/P/RF/X/Se bonds by heterogeneous catalytic reactions, the enrichment of heterogeneous catalysts such as metal oxides, graphene-based composites, doped metal nanoparticles, and molecular sieve-based porous materials, asymmetric synthesis, and other areas. The aim of this review is to contribute to the development of green and sustainable heterogeneous reaction methods for quinoxalin-2(1H)-ones with applications in materials chemistry and pharmacology.
1. Introduction
Catalysis is of vital importance in both fundamental research and industrial applications. At the heart of catalytic science, heterogeneous catalysis has gained widespread attention for its low cost, environmental friendliness, high activity, selectivity, and stability, making catalysts easy to recycle, regenerate, and sustainably produce [1]. As a result, it plays an integral role in the production of over 80% of the world’s chemical products [2]. In the development of high performance heterogeneous catalytic reactions, a wide range of heterogeneous catalysts have been developed, including solid acid catalysts, organic base catalysts, metal catalysts, metal oxide catalysts, complex catalysts, rare earth catalysts, molecular sieve catalysts, biocatalysts, nanocatalysts, single-atom catalysts [3], and more. Ye and Wu et al. [4] recently used microalgal carbon-based magnetic solid acid catalysts to catalyse the production of biodiesel from wet microalgae. Fu et al. [5] used solid acid catalysts integrated with ceramic membranes to facilitate amine-based CO2 desorption. Romero-Zerón et al. [6] reported the preparation of organic base catalysts for biodiesel from animal shells and metal oxides. Heterogeneous catalysts of metals and metal oxides on ordered mesoporous carbon and metal oxide-supported metal catalysts for electrocatalytic oxygen reduction reactions have recently been reported in reviews [7,8]. Progress in research on metal oxide-based biodiesel nanocatalysts has been summarised by Kumar’s group [9]. Bao and his colleagues summarised the metal oxides used for the electrocatalytic reduction of carbon dioxide [10]. Rehman and his colleagues reported the design of a hybrid g-C3N4/ZnO-W/Cox heterojunction photocatalyst for the removal of methylene blue dye [11]. Mono- and multi-nuclear pincer-type Ru(II) complex catalysts and their catalytic applications have been summarised by Yu’s group [12]. Bifunctional hybrid rare earth solid catalysts for the production of biodiesel from acidic palm oil were reported in [13,14], and the application of rare earth-based nanomaterials in electrocatalysis was reviewed. Liu and Song’s research group summarised the research progress on metal molecular sieve catalysts for propane dehydrogenation [15]. The role and application of biocatalysts in cancer drug discovery has recently been reviewed as well [16]. Li and colleagues recently summarised targeted intermetallic nanocatalysts for sustainable biomass and CO2 value addition [17]. Javed and colleagues reported salt-assisted gas–liquid interfacial fluorine doping of metal-free electrocatalysts [18]. Decades of research into these materials have produced many catalysts of great scientific and industrial importance. Heterogeneous catalysis is crucial for the development of organic synthetic methods, especially for the study of multiphase catalysis of parent core structures such as quinoxalin-2(1H)-one, which possess a wide range of biological activities and functionalities. In recent years, heterogeneous catalytic reactions using quinoxalin-2(1H)-one as a substrate have received increasing attention (Scheme 1). A number of ingenious and innovative methods have been developed, showing great promise for this promising compound with many applications.
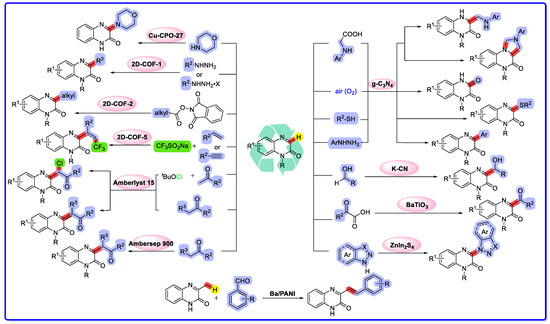
Scheme 1.
Direct C–H functionalization of quinoxalin-2(1H)-ones via heterogeneous catalysis reactions.
Quinoxalin-2(1H)-one is considered to be an important class of nitrogen-containing heterocyclic structural groups found in a variety of biologically active natural products and pharmaceutical compounds (Scheme 2) [19,20,21]. Quinoxalin-2(1H)-ones have a wide range of applications in materials science as well (Scheme 2) [22,23]. The direct C–H functionalisation of quinoxalin-2(1H)-one at the C3 position is the most cost-effective method for the synthesis of quinoxalin-2(1H)-one derivatives, and a large number of studies have appeared in this field in recent years [24,25,26,27,28,29,30,31,32,33,34,35]. A Web of Science search revealed that the number of papers related to quinoxalin-2(1H)-one has increased year by year over the last five years, with a total of 25 in 2018, 57 in 2019, 61 in 2020, 92 in 2021, and 95 in 2022. Thus far, there have been 39 relevant articles published in 2023. In particular, studies on the heterogeneous catalytic reactions of quinoxalin-2(1H)-ones are emerging due to their environmental friendliness, high activity, selectivity, stability, ease of recovery, regeneration, and sustainable production. Despite these achievements, C–H functionalisation studies of quinoxalin-2(1H)-one using heterogeneous catalysts have not yet been reported in a review. A summary of the results of quinoxalin-2(1H)-one in the field of heterogeneous catalytic reaction studies is essential for the development of quinoxalin-2(1H)-one in the field of heterogeneous catalytic reaction studies. Therefore, it is necessary to review them in order to better develop C–H functionalization methods for quinoxalin-2(1H)-one using heterogeneous catalysts. In this paper, for the first time, we review the recent research progress on heterogeneous catalytic reactions for the C–H functionalisation of quinoxalin-2(1H)-one and discuss the corresponding reaction mechanisms. We have classified the heterogeneous catalysts according to their types.
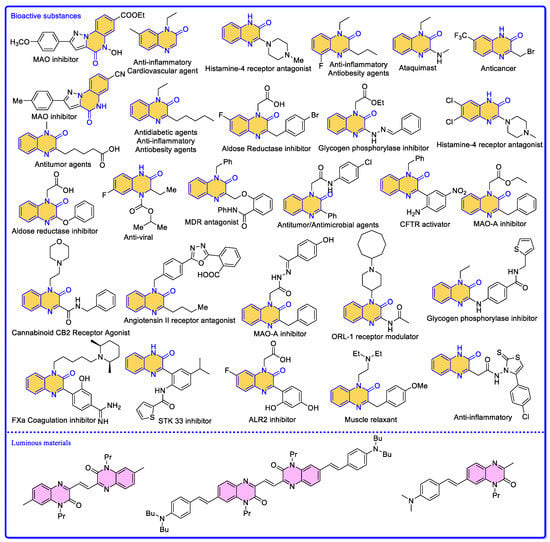
Scheme 2.
Bioactive substances and luminous materials related to quinoxalin-2(1H)-one compounds.
2. Direct C–H Functionalization of Quinoxalin-2(1H)-Ones via Heterogeneous Catalysis
2.1. Graphitic Phase Carbon Nitride as a Heterogeneous Catalyst
Graphite carbon nitride (g-C3N4) is a low cost, easy to prepare, and metal-free semiconductor photocatalyst. g-C3N4 has emerged as a powerful method for various organic transformations as an environmentally friendly, easily recyclable, and efficient heterogeneous photocatalyst [36,37,38,39,40].
In 2020, Yu and colleagues reported the g-C3N4-catalyzed divergent heterogeneous reaction of quinoxalin-2(1H)-one with N-arylglycine under visible light (Scheme 3) [41]. The solvent-controlled reaction resulted in good yields of the products dihydroquinoxalin-2(1H)-ones and tetrahydroimidazo [1,5-a]quinoxalin-4(5H)-ones when the reactions were carried out in DMSO/H2O and EtOH, respectively. A series of substrates were completed and good yields were obtained. The product dihydroquinoxalin-2(1H)-ones was reacted in 80% yield at the gram level. After a series of controlled experiments, it is reasonable to conclude that the reaction medium is the key modulator of this switchable conversion, that is, low concentrations of oxygen in DMSO/H2O produce the hydrogen ammonia methylation product 3a, whereas the cyclization product 4a is favoured when the reaction is carried out in ethanol with high dissolved oxygen concentrations. Recirculation experiments of g-C3N4 were investigated, and the catalytic activity of g-C3N4 remained essentially unchanged in the fifth run after ethyl acetate washing, indicating that this reusable multiphase photocatalyst is stable and reproducible.
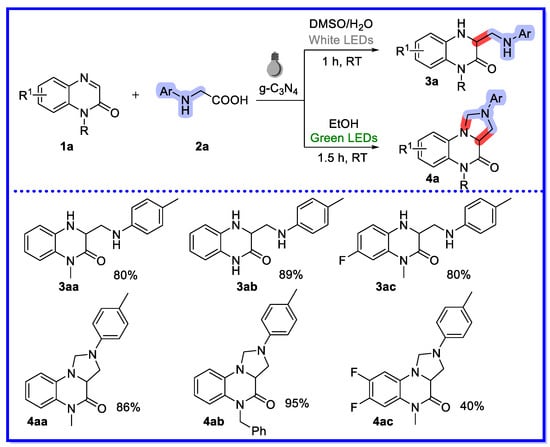
Scheme 3.
g-C3N4-catalyzed divergent heterogeneous reaction.
A free radical reaction mechanism has been proposed, as shown in Scheme 4 [41]. Upon irradiation with visible light, the semiconductor g-C3N4 was excited to generate electrons and holes, which play an important role in organic transformations by acting as single electron acceptors and donors, respectively. First, the radical intermediate 5a was formed through a photo-oxidation process by releasing a CO2 molecule and a proton. The radical 5a was then added to the C=N bond of 1a to provide intermediate 7a. Intermediate 7a then underwent a single electron photoreduction to produce the anion 8a. The intermediate anion 8a could be converted into the product 3a after protonation. On the other hand, the oxidation of the radical 5a provided the iminium species 6a. The combination of the iminium species 6a and the anion 8a provided the intermediate 9a, followed by intramolecular cyclization to provide the product 4a through the release of ArNH2. The presence of aromatic amines was confirmed by electrospray ionisation (ESI) mass spectrometry.
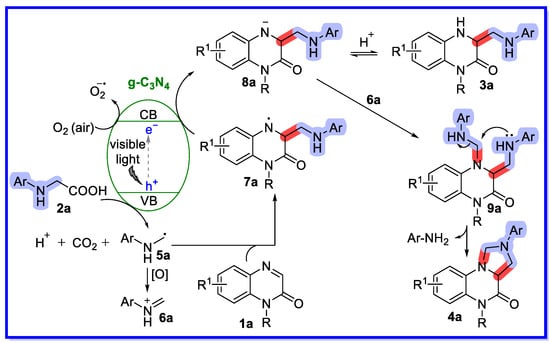
Scheme 4.
Reaction mechanism catalyzed by g-C3N4.
In 2022, Peng and co-workers proposed a heterogeneous visible light-induced direct C–H hydroxylation of quinoxalin-2(1H)-ones (Scheme 5) [42]. The method uses g-C3N4 as a photocatalyst under air conditions and yields a variety of 3-hydroxyquinoxalin-2(1H)-ones by simple washing and filtration. The hydroxylation reaction of quinoxalin-2(1H)-one was studied cyclically under standard conditions. At the end of the reaction, the g-C3N4 photocatalyst was recovered and dried from the reaction mixture by simple filtration and rinsing with the reaction solvent, then directly reused in the next round. The reaction was repeated up to six times with no significant loss of catalytic activity. It is worth noting that the reaction does not proceed smoothly in the absence of thiols or in an N2 environment. This indicates that thiols and oxygen are necessary for the reaction. An identical reaction was carried out in a homogeneous catalytic system using a DL-α-tocopherol methoxy-polyethylene glycol succinate solution (2 wt% water) (TPGS-750-M/H2O) as catalyst. However, this approach has the disadvantage of using large excesses of environmentally unfriendly inorganic strong oxidants and harsh reaction conditions, which generates chemical waste and increases manufacturing costs [43].
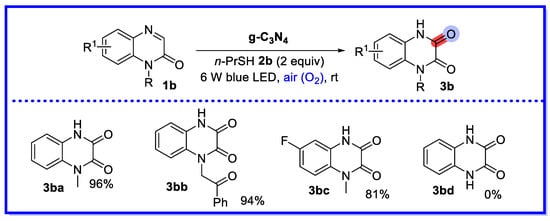
Scheme 5.
Heterogeneous hydroxylation of quinoxalin-2(1H)-one catalyzed by g-C3N4.
Based on controlled experiments and references in the literature, the reaction mechanism shown in Scheme 6 was proposed [42]. First, g-C3N4 is excited by visible light to produce an electron in the conduction band (CB) and a hole in the valence band (VB). The VB hole then gains an electron from the thiol 2b to form the thiyl radical cation 4b through a single electron transfer process. At the same time, an electron from the CB is transferred to O2 (air) to form O2•−, which has been confirmed by EPR analysis using 5,5-dimethyl-1-pyrroline (DMPO) as a spin trapping agent. Next, O2•− abstracts hydrogen from the thiyl radical cation 4b to form HO2• species and thiyl radical 5b. The resulting HO2• can then selectively attack the C-3 position of 1a to form intermediate 7b. Intermediate 7b may undergo homolytic cleavage under visible light irradiation to produce 8b and the hydroxyl radical HO•. The hydroxyl radical HO• adds to the C=N of 1b to provide the nitrogen radical intermediate 9b, which undergoes further oxidation by HO2• or O2 to provide the nitrogen cation intermediate 10b. Deprotonation of intermediate 10b provides intermediate 8b. Finally, intermediate 8b undergoes rapid tautomerisation to provide the more stable product 3b. Meanwhile, the thiyl radical 5b can rapidly dimerise to form disulfides 6b.
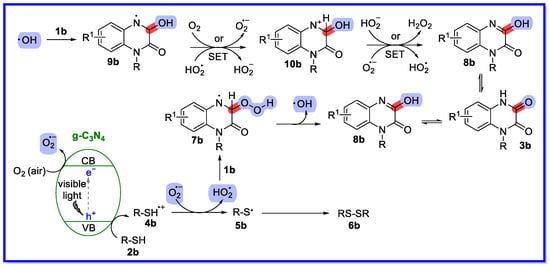
Scheme 6.
Reaction mechanism of hydroxylated quinoxalin-2(1H)-one.
In the same year, Xie et al. reported a sunlight-induced and g-C3N4-catalysed sulfenylation reaction of quinoxalin-2(1H)-ones under air conditions (Scheme 7) [44]. The reactions of this method offer a very attractive and practical way to selectively obtain a wide variety of 3-sulphated quinolin-2(1H)-ones in good to excellent yields. In addition, the heterogeneous catalyst can be easily recycled up to six times while maintaining high catalytic activity. Interestingly, and providing a basis for mechanistic studies, the reaction does not proceed properly under N2 conditions or in the dark. Two reactions of the same type have been reported, with good results obtained with visible light-induced homogeneous catalytic systems using Rhodamine 6G and Rhodamine B as photocatalysts, although in non-recyclable reactions [45,46].
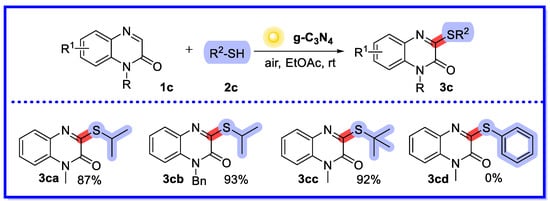
Scheme 7.
g-C3N4-catalysed sulfenylation reaction of quinoxalin-2(1H)-ones.
A possible reaction mechanism has been proposed based on control experiments and related precedents in the literature (Scheme 8) [44]. First, g-C3N4 is excited by visible light irradiation, producing holes in the valence band (VB) and electrons in the conduction band (CB). The holes then acquire an electron from the thiol 2c to form the thiyl radical cation 5c via single electron transfer (SET). At the same time, the electrons in the CB are transferred to O2 (air) to form O2•−. Next, O2•− abstracted hydrogen from the thiyl radical cation 5c forms HO2• species and the thiyl radical 6c, which adds to the C=N of 1a to provide the nitrogen radical intermediate 7c. Intermediate 7c undergoes further oxidation by HO2• or O2 to provide the intermediate 8c. Finally, product 3c is obtained by deprotonation of the nitrogen cation intermediate 7c.
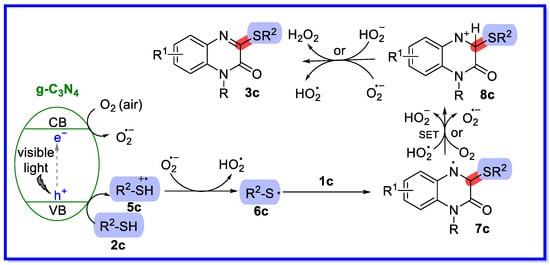
Scheme 8.
Reaction mechanism of the g-C3N4-catalyzed sulfonation of quinolin-2(1H)-ones.
In 2023, He et al. first proposed the formation of 3-arylquinoxaline-2(1H)-ones under blue light irradiation using g-C3N4/NaI semi-heterogeneous bis-catalysed quinoxaline-2(1H)-ones and arylhydrazines (Scheme 9) [47]. Their method yielded a wide variety of 3-arylquinoxalin-2 (1H)-one products in high yields with good functional group tolerance. Similar to the g-C3N4 photocatalyst described above, the desired product was not detected in the absence of molecular oxygen or light conditions. The reaction yield at the gram level was 72%. Cycling experiments with the g-C3N4 catalyst showed that it could be recovered and reused at least five times without significant loss of catalytic activity. In 2017, direct oxidative arylation homogeneous reactions of quinoxaline-2(1H)-ones prepared under air conditions without transition metal iodobenzene promotion were reported using different arylhydrazides as starting materials. In comparison, photocatalytic heterogeneous reactions are more environmentally friendly [48].
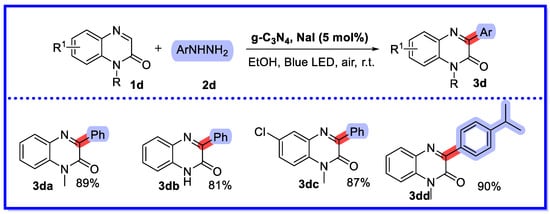
Scheme 9.
g-C3N4/NaI semi-heterogeneous bis-catalysed quinoxaline-2(1H)-ones and arylhydrazines.
A reaction mechanism similar to the one described above for g-C3N4 has been proposed by means of controlled experiments and has been reported in the literature (Scheme 10) [47]. First, blue light irradiation is used to induce charge separation in g-C3N4, producing a photogenerated hole (h+) and a photogenerated electron (e−). The h+ then oxidises the iodide ion to produce the iodine radical, which oxidises 2d to produce the phenyl radical, molecular nitrogen, protons, and the same iodide ion. Simultaneously, molecular oxygen (in air) is reduced by the photogenerated electron to produce O2•−, which further combines with H+ to produce the hydroperoxide radical (HO2•). The phenyl radical region selectively attacks 1d to form the N-centred radical 4d, which undergoes 1,2-H migration to form the C-centred radical 4d. Finally, the O2•− abstracts a hydrogen atom from 5d to provide the terminal product 3d together with H2O2.
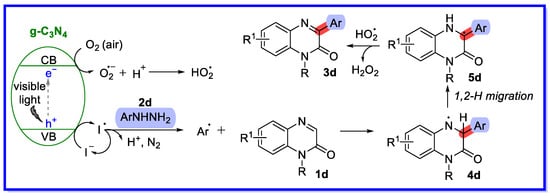
Scheme 10.
Reaction mechanism of g-C3N4/NaI semi-heterogeneous bicatalytic quinoxaline-2(1H)-ones.
In 2022, Yu and his colleagues prepared potassium-modified carbon nitride (K-CN) directly from g-C3N4 at a lower calcination temperature and shorter calcination time, and used K-CN as a heterogeneous photocatalyst to achieve the first hydroxyalkylation of quinoxalin-2(1H)-ones under visible light irradiation using air as an external oxidant. (Scheme 11) [49] After investigating the catalytic performance, a molar ratio of KCl to LiCl of 0.56 and a calcination time of 1.5 h was determined to be the optimum catalyst, and the differences between the optimum catalyst and g-C3N4 were compared using a range of characterisation tools. In order to understand the significantly different catalytic activity observed for K-CN0.56-1.5 in this reaction, the authors performed a comparative study of K-CN0.56-1.5 and g-C3N4. The functional groups in K-CN0.56-1.5 were examined using FT-IR spectroscopy; two peaks located at 805 cm−1 and 2177 cm−1 were similar to the FT-IR spectra of g-C3N4, while two other peaks at 914 cm−1 and 993 cm−1 were associated with the addition of K+ and the presence of electron-absorbing nitrile groups. Notably, in the spectrum of K-CN0.56-1.5 the band belonging to N-H is weaker than that of g-C3N4, suggesting that the K+ is linked to the N atom. The XRD spectra of K-CN0.56-1 samples were similar to the other K-CN. The XPS results were in agreement with the FT-IR analysis, confirming that K+ was successfully doped. The morphology of K-CN0.56-1. was compared with that of g-C3N4 by SEM, with g-C3N4 showing an aggregated stacked lamellar structure and K-CN0.56-1 showing an aggregated granular lamellar structure. In addition, the transient photocurrent (TPC) response confirmed the high photocatalytic activity of K-CN0.56-1. Using optimal reaction conditions, a number of substrates were screened, providing moderate to excellent yields. It is worth noting that mono-alcohols can complete the reaction, whereas diols are not available as corresponding products. The cycling experiments showed a constant loss of potassium ions from the K-CN catalyst. With the addition of two equivalents of KF, a yield of 76% was obtained in the second recovery test; however, the yield of the desired product dropped significantly in the third run, probably due to the continuous loss of potassium ions.
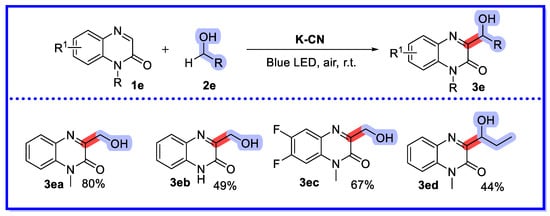
Scheme 11.
K-CN used as heterogeneous photocatalyst for hydroxyalkylation of quinoxalin-2(1H)-ones.
On the basis of the experimental results and the literature to date, a plausible mechanism is proposed in Scheme 12 [49]. First, the K-ion-activated MeOH reacts with the hole in the valence band (VB) of the excited photocatalyst to form the radical 4e, with the loss of a proton through a single electron transfer (SET) process. Meanwhile, O2 is reduced by the electron in the conduction band (CB) to form O2•−, which combines with the proton to form HO2•. Subsequently, the radical 4e is added to the C=N double bond of 1e to provide the nitrogen-centred radical 5e, which undergoes 1,2-H migration to access radical 6e. Finally, the hydrogen atom transfer (HAT) process between radical 6e and HO2• produces the target product 3e and H2O2.
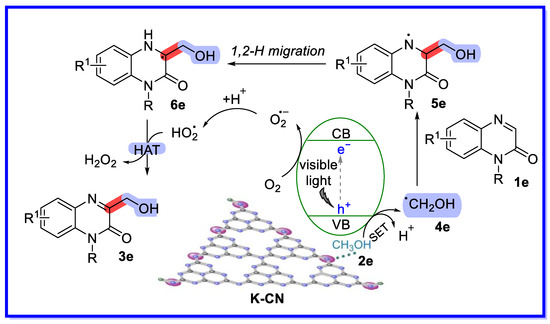
Scheme 12.
Mechanism of quinoxalin-2(1H)-one hydroxyl alkylation.
2.2. MOF/COF as Heterogeneous Catalysts
Metal-organic frameworks (MOFs) have turned up as intriguing series of crystalline materials, consisting of metal cations interconnected by multifunctionalised organic ligands during a self-assembly procedure [50,51,52]. Potential applications of these networks have expanded from gas capture and storage to other domains such as catalysis, conductivity, chemical sensors, optoelectronics, biomedical imaging, drug storage, and drug delivery [53,54].
After being first reported by Yaghi and colleagues in 2005, nine covalent organic frameworks (COFs) have received a great deal of attention in terms of synthesis, structure, fundamental properties, energy storage, and catalytic applications [55,56,57]. COFs have emerged as a new class of non-homogeneous photocatalysts for visible light-driven chemical conversions due to their high thermal and chemical stability, large surface area, and excellent optical properties. They have the inherent advantage of easy catalyst recovery over existing homogeneous photocatalysts, which is essential for practical applications in continuous chemistry [58,59,60].
In 2017, Phan’s group synthesised copper-based MOF Cu-CPO-27 for a recyclable heterogeneous catalytic reaction in which quinoxalin-2(1H)-ones undergo C–H amination with amines (Scheme 13) [61]. Alkylamines and benzylamines can be used as a source of amines, while aromatic amines do not react properly as a source of amines. A series of 3-aminoquinoxalin-2(1H)-ones were synthesised using molecular oxygen as the oxidant, and high yields were obtained. Fifteen substrates were reported with yields ranging from 42–89%. The authors compared the reactivity of Cu-CPO-27 with other MOFs for this reaction, and Cu-CPO-27 showed higher activity. At the end of the reaction there was no significant change in yield after five iterations of the reaction by washing and drying the catalyst. In addition, the recycled framework catalyst was characterised by FT-IR and XRD. The FT-IR spectrum of the recycled Cu-MOF showed an absorption band comparable to that of the new framework. The XRD analysis showed that the crystallinity of the catalyst remained constant during the experiment, although slight differences were detected in the repurposed framework. A copper-catalysed homogeneous reaction method for C-3 amination oxidation of quinoxalin-2(1H)-ones using primary or secondary amines as the nitrogen source has been reported as well. Compared with the above reactions, this synthetic strategy uses less catalyst, the heterogeneous catalysts are recyclable, and each has its own properties [62].
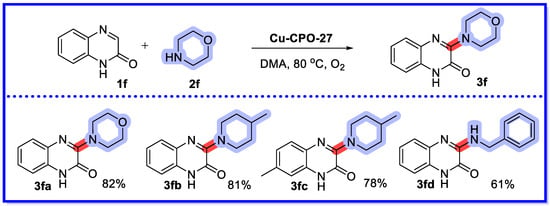
Scheme 13.
Cu-CPO-27 catalysis of the C–H amination of quinoxalin-2(1H)-ones.
A possible reaction mechanism can be deduced from controlled experiments and literature reports (Scheme 14) [61]. Initially, deprotonation of quinoxalin-2(1H)-one (1f) forms the pyridinocopper(II) species. Subsequent oxidation of copper (II) by oxygen produces copper (III) species, which undergo nucleophilic substitution with morpholine to form aminocopper (III) species. Reductive elimination follows the single electron oxidation to regenerate the original catalyst. There is doubt as to the mechanism of this reaction. We think it unlikely that Cu3+ appears in the catalytic cycle, while it is not clear where the hydrogen disappears from in this scheme.
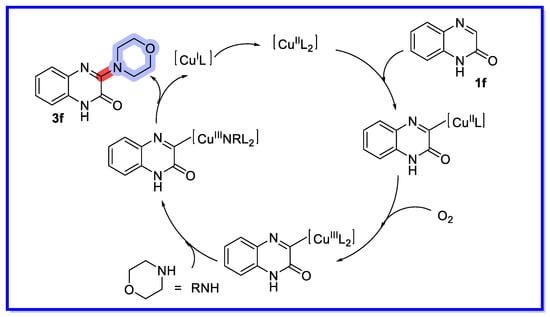
Scheme 14.
Proposed mechanism for the direct C–H amination reaction.
In 2019, Yang’s group reported a visible light-driven arylation and alkylation reaction of quinoxalin-2(1H)-ones with hydrazines, using a hydrazine-based two-dimensional covalent organic framework (2D-COF-1) as a non-homogeneous photocatalyst (Scheme 15) [63]. Due to its excellent photocatalytic properties, good chemical stability, and heterogeneous nature, this method is characterised by high efficiency, good functional group tolerance, easy scalability, and reusable catalysts. Twenty-three substrates were reported, with yields ranging from 41–85%. Importantly, this provides an alternative method for rapid access to a variety of C3 arylated or alkylated quinoxalin-2(1H)-ones in a more environmentally friendly and sustainable manner. Note that the reaction cannot take place under an argon atmosphere, which means that oxygen in the air may play a key role in the current photocatalytic cycle. Photocatalyst recycling experiments showed that 2D-COF-1 maintained high photocatalytic activity after six runs.
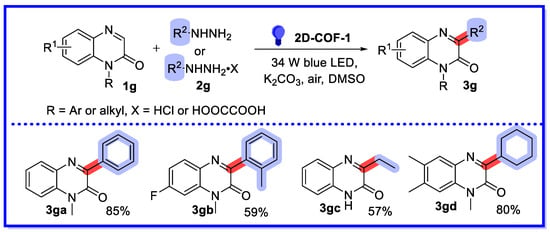
Scheme 15.
Visible light-driven arylation and alkylation of quinoxalin-2(1H)-ones.
A possible heterogeneous visible light-accelerated C3 arylation and alkylation reaction pathway has been suggested by radical inhibition experiments, calculation of apparent quantum efficiency (450 nm) values, and other relevant reports (Scheme 16) [63]. First, 2D-COF-1 is excited by the blue LED irradiation and subsequently reduces the oxygen to form a singlet oxygen radical and a superoxide radical anion, which oxidises the hydrazine 2g with the loss of nitrogen and water to provide the nucleophilic cyclohexyl radical 4g. Next, it reacts with the electron-deficient 1g to provide the radical intermediate 5g. The 1,2-H shift of radical 5g then provides the carbon radical intermediate 6g. Intermediate 6g undergoes single electron transfer (SET) with 2D-COF-1+ to provide the carbon cation intermediate 7g. This is finally converted by deprotonation to the desired product 3g.
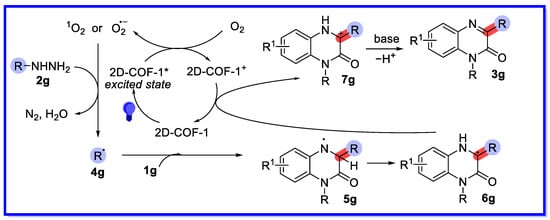
Scheme 16.
Mechanisms of arylation and alkylation of quinoxalin-2(1H)-ones driven by visible light.
In 2021, Yang’s group described a visible light-driven decarboxylative alkylation reaction of heterocycles, including quinoxalin-2(1H)-one, catalysed by an olefin-linked covalent organic framework (2D-COF-2) instead of the commonly used noble metal complexes and organic dyes (Scheme 17) [64]. A variety of alkylated heterocycles were selectively and efficiently synthesised under non-homogeneous reaction conditions. Twenty-seven quinoxalin-2(1H)-one substrates were reported, with yields ranging from 61–97%. 2D-COF-2 relies on the super-stability of the olefin chains to maintain its basic structure and photoactivity under strongly acidic conditions. Furthermore, the structure, porosity, and crystallinity of the catalysts were confirmed by 13C solid-state NMR spectroscopy, Fourier transform infrared (FT-IR) spectroscopy, N2 adsorption measurements, and powder X-ray diffraction (PXRD). In addition, its potential for industrialisation in recycling experiments, functionalisation of bioactive molecules, and scale-up reactions has been demonstrated. Homogeneous photocatalysts for the same type of reaction have been limited to noble metal complexes and organic dyes, and the heterogeneous approach is advantageous from a green and sustainable chemistry perspective.
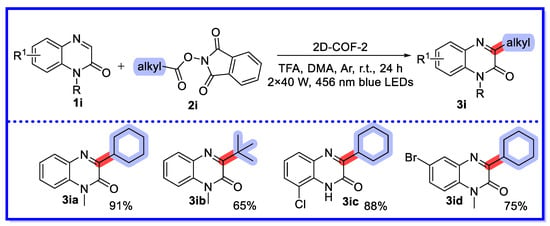
Scheme 17.
Visible light-driven decarboxylative alkylation reaction of heterocycles.
Combining controlled experiments and their inherent optoelectronic properties, a preliminary mechanism can be proposed (Scheme 18) [64]. Under visible light irradiation, the excited 2D-COF-2 can directly reduce the NHPI ester 2i by single electron transfer to form alkyl radicals with loss of CO2. The alkyl radical then attacks the low electron density site on isoquinoline 1i, which is activated by TFA, resulting in the formation of the radical cation intermediate 4i. Intermediate 4i is then oxidised by 2D-COF-2+ to provide the alkylated isoquinoline cation 5i with photocatalyst regeneration. Finally, the desired product 3i is obtained by deprotonation.
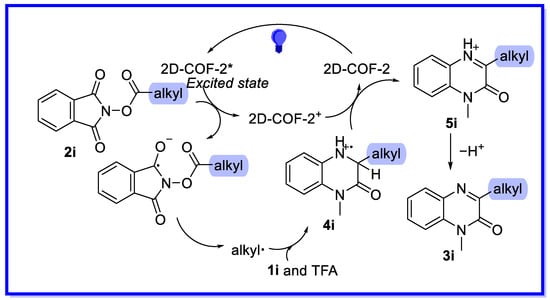
Scheme 18.
Mechanism of visible light-driven decarboxylative alkylation of heterocycles.
In 2022, Yang and colleagues reported a visible light-driven heterogeneous catalytic three-component cascade reaction of alkene or alkyne, CF3SO2Na, and quinoxalin-2(1H)-one (Scheme 19) [65]. In this reaction, two-dimensional imine-linked covalent organic frameworks (2D-COF-5) were used as heterogeneous photocatalysts. A wide range of C3 trifluoroalkyl and trifluoroalkenyl quinoxalin-2(1H)-one derivatives were prepared in satisfactory yields. Thirty-four substrates were reported, with yields ranging from 37–83%. The catalytic efficiency, basic structure, crystallinity, and microscopic appearance remained unchanged after eight cycles, although the BET surface area decreased. Particularly surprising was the high Z/E selectivity of the reaction of quinoxalin-2(1H)-one with alkynes due to the mild reaction conditions. The Z/E ratio was higher than 10:1, demonstrating good Z selectivity. The same type of reaction has been reported using 4CzIPN as a homogeneous photocatalyst and a cascade reaction initiated by visible light irradiation. Although considerable progress has been made in the synthesis of homogeneous reactions, there is much room for improvement in terms of sustainability [66].
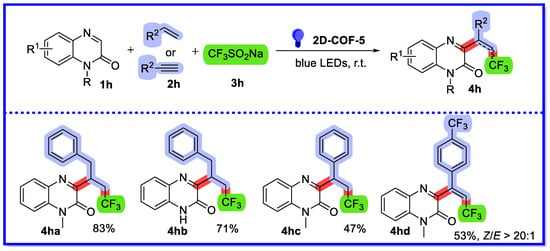
Scheme 19.
Visible light-driven heterogeneous catalytic three-component reaction of quinoxalin-2(1H)-one.
Based on controlled experiments and previous related studies, a plausible response mechanism for this heterogeneous visible light-induced three-component system is outlined in Scheme 20 [65]. First, 2D-COF-5* is excited by blue LED irradiation and activates oxygen in air to 1O2 via energy transfer (ET). Then, the singlet oxygen oxidises CF3SO2Na to produce the CF3 radical 5h, with simultaneous loss of SO2. Simultaneously, O2•− is produced via single electron transfer (SET). Subsequently, the CF3 radical is trapped by the olefin to produce the carbon radical intermediate 6h. Intermediate 6h then undergoes regioselective addition at the C3 position of quinoxalin-2(1H)-one 1h to provide the free radical intermediate 7h. Finally, hydrogen atom abstraction (HAT) occurs between intermediate 7h and O2•− to provide the desired product 4h.
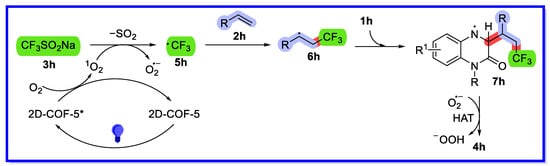
Scheme 20.
Three-component reaction mechanism of visible light-driven heterogeneous catalytic quinoxalin-2(1H)-one.
2.3. Ion-Exchange Resins as Heterogeneous Catalysts
Ion exchange resin catalysts have gained much attention because of their low cost, metal-free composition, easy product purification, recyclability, and reusability [67,68,69].
In 2021, Zhang’s group demonstrated a non-homogeneous synthetic method for sunlight-catalysed multicomponent transformations in water using quinoxalin-2(1H)-one as a reactant for the bifunctionalisation of methyl ketones (Scheme 21) [70]. In this transformation, the ion exchange resin Amberlyst 15 was used as a multiphase catalyst due to its recyclability and reusability. This method provides a green and sustainable route for the synthesis of various alpha-bifunctionalised ketones in medium to high yields. It should be noted that when using an unsymmetrical aliphatic ketone such as 2-pentanone as the starting material, a small amount of product 8ibb is formed. The authors suggest two possible reasons for this result. On the one hand, although more substituents favour enolisation, the greater the site resistance is not favourable for the reaction. On the other hand, compounds 8ib and 8ibb are competitive, and compound 8ib can be further converted to the final product 3ib. Thus, according to the Le Chatelier’s principle, the formation of compound 8ib is more favourable, making compound 3ib the main product. In addition, the higher yields of the aromatic ketone conversion products with the addition of Triton X-100 as surfactant may be due to the increased water solubility of the aromatic ketones by the surfactant. After completion of the conversion, the mixture was extracted directly with ethyl acetate, while the aqueous phase containing the catalyst was used to facilitate the reaction by direct addition of the starting material. Cycling experiments showed that a significant loss of catalytic activity was observed in the third cycle. However, reprocessing the catalytic system (which had been reused four times) with a simple washing method allowed the catalyst to be reactivated with better catalytic activity.
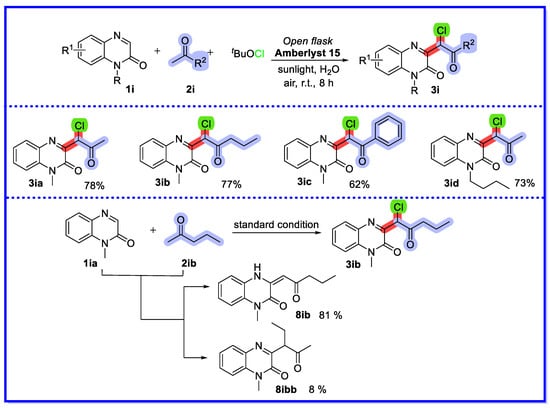
Scheme 21.
Sunlight-induced heterogeneous catalytic multicomponent reactions of quinoxalin-2(1H)-ones.
Based on previous reports and the results of control experiments, a reasonable mechanism has been proposed (Scheme 22) [70]. First, protonation of substrate 1a with protonic acid provides an iminium ion 4i, which reacts with the enol form 5i to provide the intermediate 6i. Intermediate 6i is then converted by deprotonation to the Mannich-type product 7i. Meanwhile, the starting material 1i (or 3i) is excited by sunlight to form the excited species 1i* (or 3i*), which acts as a photocatalyst to undergo an energy transfer (ET) process with triplet oxygen (3O2) to provide singlet oxygen (1O2) together with reformation of the ground state 1i. The Mannich-type product 7i is oxidised to compound 8i by an oxidative dehydrogenation process enabled by singlet oxygen (1O2). The chlorine radical (Cl•) generated by the homolysis of tBuOCl then attacks compound 8i to provide intermediate 9i. The final product 3i is obtained by oxidation of the intermediate 9i in the presence of tert-butoxy (tBuO•). The corresponding product can act as a photosensitiser to produce singlet oxygen (1O2).
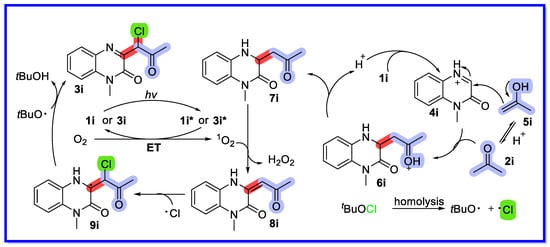
Scheme 22.
Mechanism of sunlight-induced heterogeneous catalytic multicomponent reactions of quinoxalin-2(1H)-ones.
In the same year, the Zhang group reported a reusable sulfonic ion exchange resin (Amberlyst 15)-facilitated photocatalytic approach for the direct enolization of quinoxalin-2(1H)-ones in water (Scheme 23) [71]. The recoverable aqueous phase reaction was carried out under milder conditions, providing a green and practical route for the production of various (Z)-amino ketones containing a 3,4-dihydroquinoxalin-2(1H)-one skeleton. This method successfully combines the heterogeneous Mannich reaction with photocatalysis. At the end of the reaction, the organic layer containing the product was purified by extracting the reaction mixture with ethyl acetate. The starting material was then added directly and the aqueous phase was used to catalyse the conversion. A slight loss of catalytic activity was observed in the second run, and a significant loss of catalytic activity was observed in the third and fourth runs. Reprocessing the catalysts after three or four runs regenerated the catalysts with better catalytic activity.
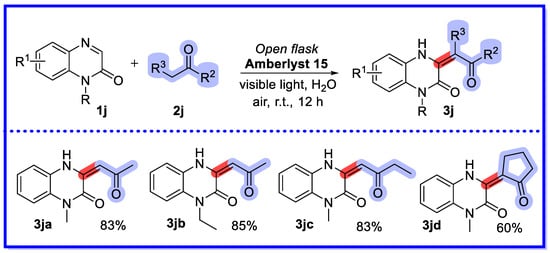
Scheme 23.
Enolization of quinoxalin-2(1H)-ones in water.
Based on mechanistic studies and previous reports, a plausible mechanism for this reaction is proposed in Scheme 24 [71]. In this conversion, Amberlyst 15 acts as a protonic acid to catalyse a Mannich-type reaction. First, acetone 2j is converted to the enol form 4j, which is then attacked by the protonated imine 5j to provide the intermediate 6j. The Mannich-type product 7j is then formed by deprotonation of intermediate 6j. Finally, the reaction undergoes two SET processes with the singlet oxygen 1O2 to provide the target product together with H2O2 (detected by hydrogen peroxide test paper). Intermediate 7j and the resulting product 3j can be used as photocatalysts to facilitate the photocatalytic cycle.
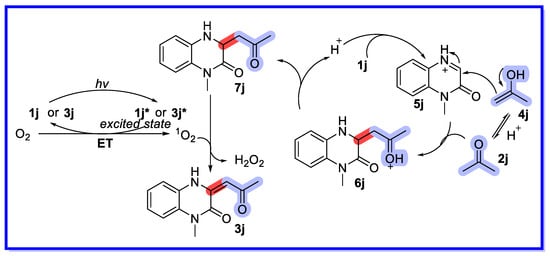
Scheme 24.
Mechanism of enolization of quinoxalin-2(1H)-ones in water.
In 2022, Li’s group (Scheme 25) [72] published a similar reaction method to that of Zhang [71], that is, a sunlight-promoted recyclable ion exchange resin (Ambersep 900)-catalysed strategy for the α-heteroarylation of ketones with quinoxalin-2(1H)-ones in the aqueous phase. In particular, good regioselectivity was observed in this reaction, suggesting that the ketone of the secondary carbon was the active site. The method was well adapted to the substrate, and a variety of α-heteroarylated products of ketones were synthesised. The recoverability of the catalytic system of the method were essentially similar to those previously reported by Zhang [71]. The authors used the reaction of methyl ketone and quinoxalin-2(1H)-ones, and the product 7k was isolated with 76% yield, while the target product 8k was not detected. It may have been the case that the energy required to form compound 7k was less than that required for compound 8k.
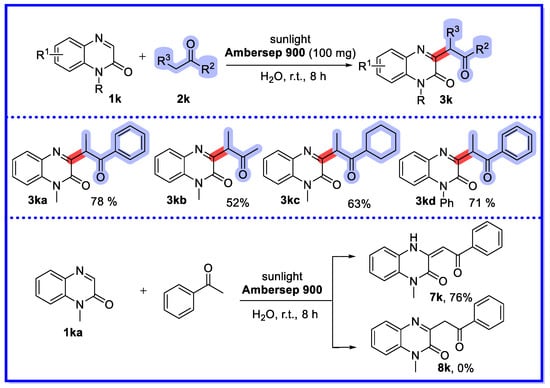
Scheme 25.
Ambersep 900-catalysed strategy for α-heteroarylation of ketones with quinoxalin-2(1H)-ones.
Based on previous reports and mechanism studies, the same authors proposed a plausible mechanism for the α-functionalisation of ketones (Scheme 26). First, the starting material 2k is converted to the enol form 4k under alkaline conditions, then substrate 2k reacts with 4k in a Mannich-type reaction to provide the intermediate 5k. Then, protonation of 5k with H2O provides the Mannich product 6k. Meanwhile, compound 1k or 3k is activated by sunlight to provide the excited species 1k* or 3k*, which can act as a photocatalyst to produce singlet oxygen from triplet oxygen via energy transfer (ET). Finally, the reaction proceeds through an SET process with singlet oxygen to produce the target product 3k and generate H2O2, which can be detected using H2O2 test paper.
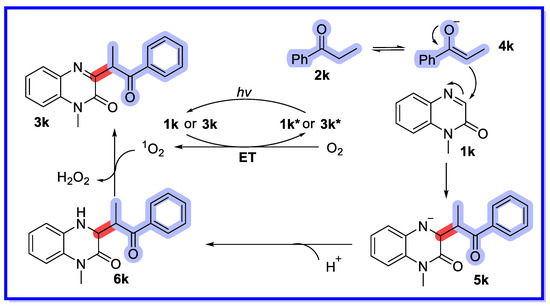
Scheme 26.
Mechanism of Ambersep 900-catalysed strategy.
The selectivity of the reaction of quinoxalin-2(1H)-one, another asymmetric aliphatic ketone, with ion-exchange resins is highly dependent on the type of ion exchange resin as well as on the stability of the enolisation and the site resistance. Amberlyst 15 reacts readily with ketones with few substituents at the α-position to form 3j, whereas Ambersep 900 reacts readily with ketones with many substituents at the α-position to form 3k.
2.4. Other Heterogeneous Catalysts
In 2022, Kapoor et al. used barium chloride-embedded polyaniline (Ba/PANI) nanocomposites as heterogeneous mesoporous nanocatalysts for the preparation of bioactive (E)-3-substituted styrylquinoxalin-2(1H)-ones from 3-methylquinoxalin-2(1H)-one and substituted aldehydes under solvent-free conditions (Scheme 27) [73]. The results showed that the catalytic activity of the Ba/PANI nanocatalysts was significantly higher than that of BaCl2 and PANI; moreover, the catalytic activity was easily recoverable, and remained high after five repeated uses. The TON for the template substrate was 28.68 and the TOF was 191.2 h−1. The authors characterized the synthesized barium-containing nanocomposites using XRD, FTIR, SEM, HR-TEM, EDX, ICP-MS, UV-Vis, TGA, N2 adsorption-desorption isotherm (BET), and XPS. TEM analysis confirmed that the average grain size of the barium chloride nanoparticles in the composites was 13–14 nm. BET analysis confirmed that the mesoporous structure of the Ba/PANI nanocomposites played an important role in their catalytic efficiency. XRD and HRTEM analyses showed that the nanocomposites were polycrystalline in nature. ICP-MS analysis confirmed that 22.51 wt% of Ba was loaded on PANI. This method has the advantages of high yield, wide range of substrates, short reaction time, easy preparation, and simple handling of the catalysts.
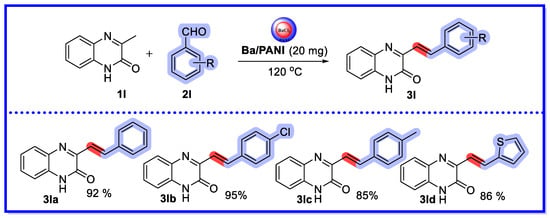
Scheme 27.
Ba/PANI nanocomposites as heterogeneous mesoporous nanocatalysts.
A mechanism for the synthesis of 3-substituted styrylquinoxalin-2(1H)-ones can be proposed based on reports in the literature (Scheme 28) [73]. Ba/PANI acts as a Lewis acid and activates the carbonyl group of the aldehyde (2l), thereby facilitating the nucleophilic attack of 3-methylquinoxalin-2(1H)-one (1l) through the intermediate 4l to provide the intermediate 5l. Finally, 5l is dehydrated to provide the desired product 3l.
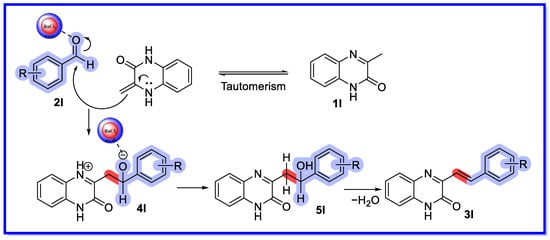
Scheme 28.
Mechanism of Ba/PANI nanocomposites as heterogeneous mesoporous nanocatalysts.
In 2023, Yuan’s group developed the first piezochemically-driven decarboxylation coupling of C–H bonds (Scheme 29) [74]. Stirring BaTiO3 by ball milling converts mechanical energy into electrical potential, leading to the production of benzoyl radicals via a single electron transfer pathway similar to photocatalytic reactions. This mechanical redox BaTiO3-catalysed synthesis of C3 acylated quinoxalin-2(1H)-ones using α-oxocarboxylic acids as acylation reagents is a solvent-free strategy with short reaction times and simple operation, and has promising applications in large-scale chemical production. In addition, BaTiO3 can be reused three times under optimal reaction conditions without significant loss of product yield. To further investigate the piezoelectric effect, the authors carried out SEM and XRD analyses of BaTiO3 samples from the first, second, third, and fourth cycles. SEM showed that after the first cycle BaTiO3 grains began to break down into smaller sizes with irregularities on the grain surface. The size of the secondary grains continued to decrease. After the third cycle, the size of these loose soft agglomerates increased significantly. XRD showed that the diffraction peak of BaTiO3 decreased with an increasing number of cycles, while the lattice parameters did not change significantly. This was in agreement with the SEM results. Hu and colleagues reported a reaction of the same type involving the silver-catalysed decarboxylation of α-oxocarboxylic acids with quinoxalin-2(1H)-ones to provide 3-acylquinoxalin-2(1H)-one derivatives. Compared to this reaction, the ball milling reaction is more environmentally friendly, as it is solvent-free [75].
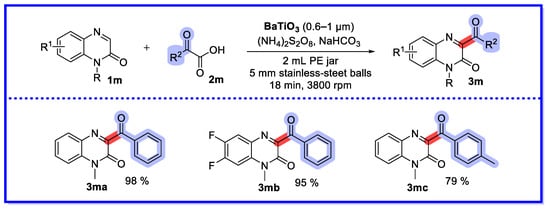
Scheme 29.
Piezochemically-driven decarboxylation coupling of C–H bonds.
A plausible mechanism can be proposed, shown in Scheme 30 [74]. Initially, the mechanical force introduced by ball milling agitates the piezoelectric material BaTiO3, creating a transient electrochemical potential. The highly polarised BaTiO3 reduces (NH4)2S2O8 via single electron transfer (SET), leading to the formation of a free sulphate radical (SO4•−). The free sulphate radical is an even more aggressive oxidant than the persulphate anion. The presence of a base can accelerate the decomposition of persulphate into sulphate free radicals. The strong oxidant SO4•− then accepts a single electron from the 2-oxo-2-phenylacetic acid anion, resulting in the formation of a sulphate anion (SO42−) and corresponding benzoyl radical, with extrusion of CO2 as a byproduct. Addition of the benzoyl radical to quinoxalin-2(1H)-one 1m leads to the nitrogen radical intermediate 4m, which undergoes a 1,2-hydrogen shift to provide the carbon radical intermediate 5m. Further oxidation of 5m by holes present in the stirred piezocatalyst BaTiO3 yields the carbon cation intermediate 6m. Finally, deprotonation of 6m yields the desired product 3m.
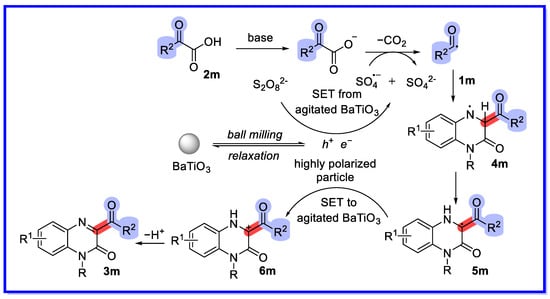
Scheme 30.
Mechanism of piezochemically-driven decarboxylation coupling of C–H bonds.
In 2022, Yu and Chen’s group synthesised a series of indium zinc sulphide (ZnIn2S4) in different solvents using a solvothermal method (Scheme 31) [76]. ZnIn2S4 prepared in ethanol (ZIS-1) was characterised as having the highest photocurrent density. The direct C–H azoation of N-heterocyclic compounds (including quinoxalin-2(1H)-ones, quinazoline, and 2H-benzo[b]-[1,4]oxazin-2-ones) was achieved in an air atmosphere under 456 nm blue light, using ZIS-1 as a photocatalyst. More importantly, the ZIS-1 microsphere photocatalyst was stable during the catalytic process and could be used at least five times with no change in reactivity. The authors used PXRD to characterise the physical phases and crystal structures of the three synthesised ZnIn2S4 samples. The PXRD spectra of the three samples were almost identical, which is in agreement with previous reports. The UV-vis diffuse reflectance spectra of the synthesised ZnIn2S4 showed a significant broadening of the absorption band in the range of 400–500 nm, which favours light absorption in the visible region. The three catalysts consist of flower-like microspheres of about 4–5 μm in size. The microspheres consist of many interwoven nanosheets, and it can be seen that ZIS-1 appears to be somewhat looser than the other two catalysts. Further characterisation of the recovered ZIS-1 photocatalyst showed that the peaks and intensities of the recovered ZIS-1 remained almost unchanged compared to the newly synthesised ZIS-1. In addition, the recovered SEM showed that the surface morphology of the catalyst remained unchanged during the catalytic process, indicating that the photocatalyst ZIS-1 was stable during the catalytic process.
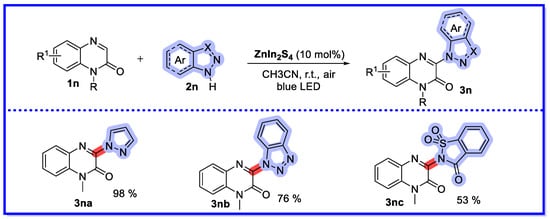
Scheme 31.
The direct C–H azoation of N-heterocyclic compounds.
Based on the experimental results, the reaction mechanism in Scheme 32 was proposed [76]. First, ZIS-1 absorbs photons and produces electrons in the conduction band (CB) and causes holes in the valence band (VB). The positive oxidation potential makes it suitable for reaction with pyrazole (2n) to produce an N-centred pyrazol-1-yl radical cation 4n via single electron transfer (SET). At the same time, the electrons in CB are transferred to O2 (air) by SET, producing a superoxide radical (O2•−). Then, 4n is deprotonated by O2•− to form the hydrogen peroxide radical (HO2•) and the N-radical intermediate 5n. The N-radical intermediate 5n is then incorporated into the C=N bond of quinoxalin-2(1H)-one 1n to provide the intermediate 6n, followed by 1,2-H migration to provide the carbon-centred radical intermediate 7n. Next, 7n is oxidised by HO2 via the SET pathway to produce the radical cation intermediate 8n. Finally, the cation 8n undergoes deprotonation to produce the desired product 3n, while hydrogen peroxide (H2O2) is formed and can be detected by KI.
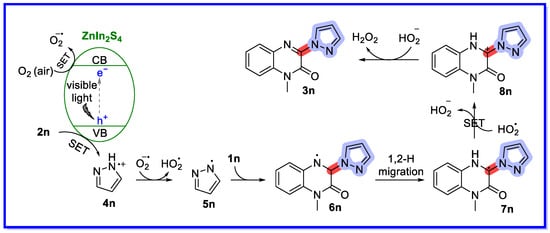
Scheme 32.
Mechanism of C–H azoation of N-heterocyclic compounds.
3. Summary and Outlook
The direct C–H functionalisation of quinoxalin-2(1H)-ones via heterogeneous catalytic reactions is a green and sustainable chemical reaction, a simple and effective approach that has attracted increasing interest from synthetic chemists and holds great promise for medicinal chemistry and functional materials. This paper reviews the heterogeneous catalytic reactions reported in recent years for the C–H functionalisation of quinoxalin-2(1H)-ones via different heterogeneous catalysts (e.g., graphitic phase carbon nitride, MOF, COF, ion exchange resins, piezoelectric materials, and microspheres) and shows the mechanisms and representative substrates of the mainly radical-based reactions in order to provide a more in-depth and rapid understanding of these reactions. At the same time, it can provide a reference for the further development of heterogeneous catalytic reactions of quinoxalin-2(1H)-ones.
Despite these outstanding achievements, relatively few heterogeneous catalytic reactions of quinoxalin-2(1H)-ones have been reported, and further development of heterogeneous catalytic reactions of quinoxalin-2(1H)-ones is essential. First, the types of heterogeneous catalytic reactions of quinoxalin-2(1H)-ones are very homogeneous, mainly involving the formation of C-C, C-N, C-S, and C-O bonds. In particular, the C-B/SI/P/RF/X/SE bond at the C3 position of quinoxalin-2(1H)-ones has not been completed, and is expected to be completed by the heterogeneous catalytic reaction of quinoxalin-2(1H)-ones. Second, the heterogeneous catalytic reactions of quinoxalin-2(1H)-ones use relatively few types of heterogeneous catalysts, such as metal oxides; graphene-based composites, doped metal nanoparticles, molecular sieve-based porous materials, single-atom catalysts, etc., have not yet been involved, and the types of heterogeneous catalysts that have been used to enantiosequence the reactions, e.g., piezoelectric materials, are very homogeneous as well and have only completed the acylation reactions. Third, asymmetric synthesis has been a research topic in organic equations; it is challenging to complete asymmetric catalytic reactions with quinoxalin-2(1H)-ones as substrates, and the introduction of chiral centres via inhomogeneous catalytic reactions of quinoxalin-2(1H)-ones is a concise route. Fourth, the importance of green chemistry metrics such as TOF and TON based on heterogeneous catalysts requires further elaboration. In summary, we hope that the review provided in this paper will be useful for research in this area. Heterogeneous catalytic reactions of quinoxalin-2(1H)-ones provide an atomically economical, environmentally friendly, and sustainable approach for the preparation of a wide range of functionalized quinoxalin-2(1H)-ones, with promising applications in materials science and medicinal chemistry.
Author Contributions
Conceptualization, writing—review and editing, Q.Y. and Y.L.; validation, H.W. and X.W.; supervision, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 22162017), the Key Research Projects of Henan Higher Education Institutions (CN) (Grant No. 21A350001), Liupanshui Normal College High-level Talent Research Initiation Projects (Grant No. LPSSYKYJJ202314), Scientific Research Projects of Liupanshui Normal College (Grant No. Lpssyzxxm202301), Top Science and Technology Talents Project of Guizhou Education Department ([2020]038), State Key Laboratory of Materials-Oriented Chemical Engineering (No. KL-MCE-22B01), Guizhou Provincial Key Laboratory of Coal Clean Utilization ([2020]2001), Guizhou Provincial Science and Technology Planning Project of China (Grant No. QKHPTRC[2019]5907), Natural Science Foundation of Guizhou Province ([2021]063) and Youth Talent Growth Project of Educational Department of Guizhou Province ([2022]043).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef]
- Alsudairy, Z.; Brown, N.; Campbell, A.; Ambus, A.; Brown, B.; Smith-Petty, K.; Li, X. Covalent organic frameworks in heterogeneous catalysis: Recent advances and future perspective. Mater. Chem. Front. 2023, in press. [CrossRef]
- Shah, S.S.A.; Najam, T.; Bashir, M.S.; Peng, L.; Nazir, M.A.; Javed, M.S. Single-atom catalysts for next-generation rechargeable batteries and fuel cells. Energy Storage Mater. 2022, 45, 301–322. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Q.; Sun, X.; Zhang, Y.; Cai, Q.; Deng, W.; Rao, S.; Wu, X.; Ye, Q. Conversion of wet microalgae to biodiesel with microalgae carbon based magnetic solid acid catalyst. Energy Convers. Manag. 2023, 286, 117022. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, H.; Fu, K.; Chen, X.; Qiu, M.; Fan, Y. Integration of solid acid catalyst and ceramic membrane to boost amine-based CO2 desorption. Energy 2023, 274, 127329. [Google Scholar] [CrossRef]
- Saetang, R.; Altamirano, M.; Romero-Zerón, L.B. Formulation of an Organic Base Catalyst with Animal Shells and Metal Oxide for Biodiesel Production. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 1690–1700. [Google Scholar] [CrossRef]
- Gao, M.; Wang, L.; Yang, Y.; Sun, Y.; Zhao, X.; Wan, Y. Metal and Metal Oxide Supported on Ordered Mesoporous Carbon as Heterogeneous Catalysts. ACS Catal. 2023, 13, 4060–4090. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Zhang, Y.; Wang, H.; Fei, H.; Liu, R.; Kong, H.; Gao, R.; Zhao, S.; Liu, T.; et al. Metal Oxide-Supported Metal Catalysts for Electrocatalytic Oxygen Reduction Reaction: Characterization Methods, Modulation Strategies, and Recent Progress. Small Methods 2023, e2201714. [Google Scholar] [CrossRef]
- Joshi, N.C.; Gururani, P.; Bhatnagar, P.; Kumar, V.; Vlaskin, M.S. Advances in Metal Oxide-based Nanocatalysts for Biodiesel Production: A Review. ChemBioEng Rev. 2023, 10, 258–271. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Sufyan Javed, M.; Najam, T.; Molochas, C.; Khan, N.A.; Nazir, M.A.; Xu, M.; Tsiakaras, P.; Bao, S.-J. Metal oxides for the electrocatalytic reduction of carbon dioxide: Mechanism of active sites, composites, interface and defect engineering strategies. Coord. Chem. Rev. 2022, 471, 214716. [Google Scholar] [CrossRef]
- Malik, M.; Ibrahim, S.M.; Nazir, M.A.; Tahir, A.A.; Tufail, M.K.; Shah, S.S.A.; Anum, A.; Wattoo, M.A.; Rehman, A.u. Engineering of a Hybrid g-C3N4/ZnO-W/Cox Heterojunction Photocatalyst for the Removal of Methylene Blue Dye. Catalysts 2023, 13, 813. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wu, K.; Wang, Q.; Yu, Z. Mono- and multinuclear pincer-type Ru(II) complex catalysts and their catalytic applications. Inorg. Chim. Acta 2023, 551, 121458. [Google Scholar] [CrossRef]
- Duangdee, B.; Rattanaphra, D.; Nuchdang, S.; Thanapimmetha, A.; Saisriyoot, M.; Srinophakun, P. Bifunctional mixed rare earth solid catalyst for biodiesel production from acid palm oil. J. Rare Earths 2023, 41, 240–249. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; He, M.; Yuan, X.; Fang, Z.; Li, Z. Rare earth-based nanomaterials in electrocatalysis. Coord. Chem. Rev. 2023, 489, 215204. [Google Scholar] [CrossRef]
- Song, S.; Sun, Y.; Yang, K.; Fo, Y.; Ji, X.; Su, H.; Li, Z.; Xu, C.; Huang, G.; Liu, J.; et al. Recent Progress in Metal-Molecular Sieve Catalysts for Propane Dehydrogenation. ACS Catal. 2023, 13, 6044–6067. [Google Scholar] [CrossRef]
- Sengupta, S.; Das, P.; Sharma, S.; Shukla, M.K.; Kumar, R.; Kumar Tonk, R.; Pandey, S.; Kumar, D. Role and Application of Biocatalysts in Cancer Drug Discovery. Catalysts 2023, 13, 250. [Google Scholar] [CrossRef]
- Feng, S.; Geng, Y.; Liu, H.; Li, H. Targeted Intermetallic Nanocatalysts for Sustainable Biomass and CO2 Valorization. ACS Catal. 2022, 12, 14999–15020. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Nazir, M.A.; Wu, Y.; Ali, H.; Rehman, A.U.; Rahman, M.M.; Imran, M.; Javed, M.S. Salt-assisted gas-liquid interfacial fluorine doping: Metal-free defect-induced electrocatalyst for oxygen reduction reaction. Mol. Catal. 2021, 514, 111878. [Google Scholar] [CrossRef]
- El-Hawash, S.A.M.; Habib, N.S.; Kassem, M.A. Synthesis of Some New Quinoxalines and 1,2,4-Triazolo[4,3-a]-quinoxalines for Evaluation of in vitro Antitumor and Antimicrobial Activities. Arch. Pharm. 2006, 339, 564–571. [Google Scholar] [CrossRef]
- Liu, R.; Huang, Z.; Murray, M.G.; Guo, X.; Liu, G. Quinoxalin-2(1H)-One Derivatives as Inhibitors Against Hepatitis C Virus. J. Med. Chem. 2011, 54, 5747–5768. [Google Scholar] [CrossRef]
- Qin, X.; Hao, X.; Han, H.; Zhu, S.; Yang, Y.; Wu, B.; Hussain, S.; Parveen, S.; Jing, C.; Ma, B.; et al. Design and Synthesis of Potent and Multifunctional Aldose Reductase Inhibitors Based on Quinoxalinones. J. Med. Chem. 2015, 58, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Burganov, T.I.; Katsyuba, S.A.; Islamova, L.N.; Fazleeva, G.M.; Sharipova, S.M.; Kalinin, A.A.; Monari, A.; Assfeld, X. To what extent are the photophysical properties of quinoxaline- and quinoxalinone-based chromophores predictable? Dyes Pigm. 2019, 170, 107580. [Google Scholar] [CrossRef]
- Gerasimova, T.P.; Burganov, T.I.; Katsyuba, S.A.; Kalinin, A.A.; Islamova, L.N.; Fazleeva, G.M.; Ahmadeev, B.S.; Mustafina, A.R.; Monari, A.; Assfeld, X.; et al. Halochromic luminescent quinoxalinones as a basis for pH-sensing in organic and aqueous solutions. Dyes Pigm. 2021, 186, 108958. [Google Scholar] [CrossRef]
- Ke, Q.; Yan, G.; Yu, J.; Wu, X. Recent advances in the direct functionalization of quinoxalin-2(1H)-ones. Org. Biomol. Chem. 2019, 17, 5863–5881. [Google Scholar] [CrossRef]
- Sun, K.; Xiao, F.; Yu, B.; He, W.-M. Photo-/electrocatalytic functionalization of quinoxalin-2(1H)-ones. Chin. J. Catal. 2021, 42, 1921–1943. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Wu, M.; Lei, Y.-Z. Recent Developments in Direct C&H Functionalization of Quinoxalin-2(1H)-Ones via Multi-Component Tandem Reactions. Molecules 2023, 28, 2513. [Google Scholar]
- Azev, Y.A.; Kodess, M.I.; Ezhikova, M.A.; Ermakova, O.g.S.; Berseneva, V.S.; Bakulev, V.A. Reactions of quinoxalin-2-one with β-diketones: A new approach to 6a,7-dihydro-5H-pyrido[1,2-a]quinoxaline-6,8-diones. Mendeleev Commun. 2017, 27, 97–98. [Google Scholar] [CrossRef]
- Azev, Y.A.; Koptyaeva, O.S.; Mkrtchan, A.A.; Pospelova, T.A. Features of -C-C- coupling of quinoxaline-2-one with ethyl acetoacetate under acid catalysis. Chim. Techno Acta 2022, 9, 20229103. [Google Scholar] [CrossRef]
- Rusinov, G.L.; Slepukhin, P.A.; Charushin, V.N.; Dyachenko, O.A.; Kazheva, O.N.; Chekhlov, A.N.; Verbitsky, E.V.; Kodess, M.I.; Chupakhin, O.N. Chemistry of O- and C-adducts derived from 1,4-diazinium salts: The use of tetrahydropyrazines in the synthesis of condensed systems. Mendeleev Commun. 2006, 16, 26–29. [Google Scholar] [CrossRef]
- Utepova, I.A.; Chupakhin, O.N.; Trestsova, M.A.; Musikhina, A.A.; Kucheryavaya, D.A.; Charushin, V.N.; Rempel, A.A.; Kozhevnikova, N.S.; Valeeva, A.A.; Mikhaleva, A.I.; et al. Direct functionalization of the C–H bond in (hetero)arenes: Aerobic photoinduced oxidative coupling of azines with aromatic nucleophiles (SNH-reactions) in the presence of a CdS/TiO2 photocatalyst. Russ. Chem. Bull. 2016, 65, 445–450. [Google Scholar] [CrossRef]
- Dutysheva, E.A.; Utepova, I.A.; Trestsova, M.A.; Anisimov, A.S.; Charushin, V.N.; Chupakhin, O.N.; Margulis, B.A.; Guzhova, I.V.; Lazarev, V.F. Synthesis and approbation of new neuroprotective chemicals of pyrrolyl- and indolylazine classes in a cell model of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 222, 113577. [Google Scholar] [CrossRef] [PubMed]
- Sonam; Shinde, V.N.; Rangan, K.; Kumar, A. Selectfluor-Mediated Regioselective C-3 Alkoxylation, Amination, Sulfenylation, and Selenylation of Quinoxalin-2(1H)-ones. J. Org. Chem. 2023, 88, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, P.; Dong, T.; Tsui, G.C.; Liao, S. Radical Fluorosulfonyl Heteroarylation of Unactivated Alkenes with Quinoxalin-2(1H)-ones and Related N-Heterocycles. Org. Lett. 2023, 25, 1088–1093. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Yuan, S.; Yu, X.; Liu, L.; Li, J. Direct Alkylation of Quinoxalinones with Boracene-Based Alkylborate under Visible Light Irradiation. J. Org. Chem. 2023, 88, 6218–6226. [Google Scholar] [CrossRef]
- Li, Y.; Song, G.-T.; Tang, D.-Y.; Xu, Z.-G.; Chen, Z.-Z. Acid-Promoted Direct C–H Carbamoylation at the C-3 Position of Quinoxalin-2(1H)-ones with Isocyanide in Water. ACS Omega 2023, 8, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Tang, Y.; Fan, L.; Lefebvre, Q.; Hou, H.; Rueping, M. Heterogeneous Visible-Light Photoredox Catalysis with Graphitic Carbon Nitride for α-Aminoalkyl Radical Additions, Allylations, and Heteroarylations. ACS Catal. 2018, 8, 9471–9476. [Google Scholar] [CrossRef]
- Savateev, A.; Ghosh, I.; König, B.; Antonietti, M. Photoredox Catalytic Organic Transformations using Heterogeneous Carbon Nitrides. Angew. Chem. Int. Ed. 2018, 57, 15936–15947. [Google Scholar] [CrossRef]
- Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Science 2019, 365, 360–366. [Google Scholar] [CrossRef]
- Xiao, Y.; Tian, G.; Li, W.; Xie, Y.; Jiang, B.; Tian, C.; Zhao, D.; Fu, H. Molecule Self-Assembly Synthesis of Porous Few-Layer Carbon Nitride for Highly Efficient Photoredox Catalysis. J. Am. Chem. Soc. 2019, 141, 2508–2515. [Google Scholar] [CrossRef]
- Geng, P.; Tang, Y.; Pan, G.; Wang, W.; Hu, J.; Cai, Y. A g-C3N4-based heterogeneous photocatalyst for visible light mediated aerobic benzylic C–H oxygenations. Green Chem. 2019, 21, 6116–6122. [Google Scholar] [CrossRef]
- Si, Y.-F.; Chen, X.-L.; Fu, X.-Y.; Sun, K.; Song, X.; Qu, L.-B.; Yu, B. Divergent g-C3N4-catalyzed Reactions of Quinoxalin-2(1H)-ones with N-Aryl Glycines under Visible Light: Solvent-Controlled Hydroaminomethylation and Annulation. ACS Sustain. Chem. Eng. 2020, 8, 10740–10746. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Xie, Q.-X.; Chen, Y.-D.; Zhou, J.-Y.; Peng, S. Visible-Light-Induced Recyclable g-C3N4 Catalyzed C–H Hydroxylation of Quinoxalin-2(1H)-ones. Synthesis 2022, 55, 443–450. [Google Scholar] [CrossRef]
- Peng, S.; Hu, D.; Hu, J.-L.; Lin, Y.-W.; Tang, S.-S.; Tang, H.-S.; He, J.-Y.; Cao, Z.; He, W.-M. Metal-Free C3 Hydroxylation of Quinoxalin-2(1H)-ones in Water. Adv. Synth. Catal. 2019, 361, 5721–5726. [Google Scholar] [CrossRef]
- Peng, S.; Liu, J.; Yang, L.-H.; Xie, L.-Y. Sunlight Induced and Recyclable g-C3N4 Catalyzed C–H Sulfenylation of Quinoxalin-2(1H)-Ones. Molecules 2022, 27, 5044. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, P.; Zhao, T.; Ren, Q.; Li, J. (Thio)etherification of Quinoxalinones under Visible-Light Photoredox Catalysis. Adv. Synth. Catal. 2019, 361, 5371–5382. [Google Scholar] [CrossRef]
- Xie, L.-Y.; Chen, Y.-L.; Qin, L.; Wen, Y.; Xie, J.-W.; Tan, J.-X.; Huang, Y.; Cao, Z.; He, W.-M. Visible-light-promoted direct C–H/S–H cross-coupling of quinoxalin-2(1H)-ones with thiols leading to 3-sulfenylated quinoxalin-2(1H)-ones in air. Org. Chem. Front. 2019, 6, 3950–3955. [Google Scholar] [CrossRef]
- Song, H.-Y.; Jiang, J.; Wu, C.; Hou, J.-C.; Lu, Y.-H.; Wang, K.-L.; Yang, T.-B.; He, W.-M. Semi-heterogeneous g-C3N4/NaI dual catalytic C–C bond formation under visible light. Green Chem. 2023, 25, 3292–3296. [Google Scholar] [CrossRef]
- Paul, S.; Ha, J.H.; Park, G.E.; Lee, Y.R. Transition Metal-Free Iodosobenzene-Promoted Direct Oxidative 3-Arylation of Quinoxalin-2(H)-ones with Arylhydrazines. Adv. Synth. Catal. 2017, 359, 1515–1521. [Google Scholar] [CrossRef]
- Wu, S.-J.; Shi, Y.; Sun, K.; Yuan, X.-Y.; Tang, S.; Yu, B. Potassium doping carbon nitride: Dramatically enhanced photocatalytic properties for hydroxyalkylation of quinoxalin-2(1H)-ones with alcohol under air atmosphere. J. Catal. 2022, 415, 87–94. [Google Scholar] [CrossRef]
- Claes, B.; Boudewijns, T.; Muchez, L.; Hooyberghs, G.; Van der Eycken, E.V.; Vanderleyden, J.; Steenackers, H.P.; De Vos, D.E. Smart Metal–Organic Framework Coatings: Triggered Antibiofilm Compound Release. ACS Appl. Mater. Interfaces 2017, 9, 4440–4449. [Google Scholar] [CrossRef]
- Andrzejewski, M.; Katrusiak, A. Piezochromic Porous Metal–Organic Framework. J. Phys. Chem. Lett. 2017, 8, 279–284. [Google Scholar] [CrossRef]
- González Miera, G.; Bermejo Gómez, A.; Chupas, P.J.; Martín-Matute, B.; Chapman, K.W.; Platero-Prats, A.E. Topological Transformation of a Metal–Organic Framework Triggered by Ligand Exchange. Inorg. Chem. 2017, 56, 4576–4583. [Google Scholar] [CrossRef]
- Hu, L.; Lin, X.-M.; Mo, J.-T.; Lin, J.; Gan, H.-L.; Yang, X.-L.; Cai, Y.-P. Lead-Based Metal–Organic Framework with Stable Lithium Anodic Performance. Inorg. Chem. 2017, 56, 4289–4295. [Google Scholar] [CrossRef]
- Lemaire, P.C.; Zhao, J.; Williams, P.S.; Walls, H.J.; Shepherd, S.D.; Losego, M.D.; Peterson, G.W.; Parsons, G.N. Copper Benzenetricarboxylate Metal–Organic Framework Nucleation Mechanisms on Metal Oxide Powders and Thin Films formed by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2016, 8, 9514–9522. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Lohse, M.; Bein, T. Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xia, L.; Liu, X. Covalent organic frameworks (COFs): Perspectives of industrialization. CrystEngComm 2018, 20, 1613–1634. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Q.; Aguila, B.; Ma, S. Opportunities of Covalent Organic Frameworks for Advanced Applications. Adv. Sci. 2019, 6, 1801410. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, H.; Hooshmand, S.E.; Varma, R.S. Covalent organic frameworks and multicomponent reactions: An endearing give-and-take relationship. Org. Chem. Front. 2022, 9, 4178–4191. [Google Scholar] [CrossRef]
- Hoang, T.T.; To, T.A.; Cao, V.T.T.; Nguyen, A.T.; Nguyen, T.T.; Phan, N.T.S. Direct oxidative CH amination of quinoxalinones under copper-organic framework catalysis. Catal. Commun. 2017, 101, 20–25. [Google Scholar] [CrossRef]
- Li, Y.; Gao, M.; Wang, L.; Cui, X. Copper-catalysed oxidative amination of quinoxalin-2(1H)-ones with aliphatic amines. Org. Biomol. Chem. 2016, 14, 8428–8432. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, S.; Bu, X.; Wang, Y.; Yu, J.; Yang, X. Covalent Organic Frameworks: A Sustainable Photocatalyst toward Visible-Light-Accelerated C3 Arylation and Alkylation of Quinoxalin-2(1H)-ones. Chem. Eur J. 2020, 26, 369–373. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.; Bu, X.; Wang, Y.; Yang, X. An ultrastable olefin-linked covalent organic framework for photocatalytic decarboxylative alkylations under highly acidic conditions. Catal. Sci. Technol. 2021, 11, 4272–4279. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Cui, Y.; Liu, M.; Bu, X.; Tian, H.; Yang, X. A covalent organic framework-catalyzed visible-light-induced three-component cascade synthesis of trifluoroalkyl and trifluoroalkenyl quinoxalin-2(1H)-one derivatives. New J. Chem. 2022, 46, 20412–20418. [Google Scholar] [CrossRef]
- Meng, N.; Lv, Y.; Liu, Q.; Liu, R.; Zhao, X.; Wei, W. Visible-light-induced three-component reaction of quinoxalin-2(1H)-ones, alkenes and CF3SO2Na leading to 3-trifluoroalkylated quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2021, 32, 258–262. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Russo, P.A.; Antunes, M.M.; Neves, P.; Wiper, P.V.; Fazio, E.; Neri, F.; Barreca, F.; Mafra, L.; Pillinger, M.; Pinna, N.; et al. Solid acids with SO3H groups and tunable surface properties: Versatile catalysts for biomass conversion. J. Mater. Chem. A 2014, 2, 11813–11824. [Google Scholar] [CrossRef]
- Chen, F.; Shi, L.; Yao, J.; Wang, Y.; Zhang, D.; Zhu, W.; Liu, Z. A highly efficient sulfonic acid resin for liquid-phase carbonylation of dimethoxymethane. Catal. Sci. Technol. 2018, 8, 580–590. [Google Scholar] [CrossRef]
- Xu, J.; He, L.; Liang, C.; Yue, X.; Ouyang, Y.; Zhang, P. Multicomponent Bifunctionalization of Methyl Ketones Enabled by Heterogeneous Catalysis and Solar Photocatalysis in Water. ACS Sustain. Chem. Eng. 2021, 9, 13663–13671. [Google Scholar] [CrossRef]
- Xu, J.; Huang, L.; He, L.; Ni, Z.; Shen, J.; Li, X.; Chen, K.; Li, W.; Zhang, P. A combination of heterogeneous catalysis and photocatalysis for the olefination of quinoxalin-2(1H)-ones with ketones in water: A green and efficient route to (Z)-enaminones. Green Chem. 2021, 23, 2123–2129. [Google Scholar] [CrossRef]
- He, L.; Liang, C.; Ouyang, Y.; Li, L.; Guo, Y.; Zhang, P.; Li, W. α-Functionalization of ketones promoted by sunlight and heterogeneous catalysis in the aqueous phase. Org. Biomol. Chem. 2022, 20, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Gupta, A.; Kapoor, K.K. Unexplored Potential of Polyaniline Embedded Barium Chloride Nanocomposite in the Synthesis of Styrylquinoxalin-2(1H)-Ones. Polycyclic Aromat. Compd. 2023, 43, 2104–2122. [Google Scholar] [CrossRef]
- He, Y.; Wang, G.; Hu, W.; Wei, D.; Jia, J.; Li, H.; Yuan, B. Piezocatalyzed Decarboxylative Acylation of Quinoxalin-2(1H)-ones Using Ball Milling. ACS Sustain. Chem. Eng. 2023, 11, 910–920. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, C.; Wang, X.; Zhang, J.; Wang, X.; Hu, Y. Silver-catalyzed decarboxylative acylation of quinoxalin-2(1H)-ones with α-oxo-carboxylic acids. Org. Biomol. Chem. 2017, 15, 8929–8935. [Google Scholar] [CrossRef]
- Wang, R.-N.; Zeng, F.-L.; Chen, X.-L.; Zhu, H.-L.; Qu, L.-B.; Huang, X.-Q.; Tang, S.; Zhao, Y.-F.; Yu, B. Recyclable ZnIn2S4 Microspheres for Photocatalytic Azolation of N-Heterocycles. ACS Sustain. Chem. Eng. 2022, 10, 14212–14219. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).