Chemical and Sensory Characterization of Xinomavro Red Wine Using Grapes from Protected Designations of Northern Greece
Abstract
1. Introduction
2. Results and Discussion
2.1. Classical Analyses
2.2. Volatile Composition of Xinomavro Wines
2.3. Sensory Descriptive Analysis of the Wine
2.4. Odor Activity Values
2.5. Linking Chemical and Sensory Data of Xinomavro Wines
2.6. Olfactometric Data of Xinomavro Wines
3. Materials and Methods
3.1. Wine Samples
3.1.1. Winemaking Protocol
3.1.2. Basic Wine Composition
3.2. Quantification of Wine Volatile Compounds
3.2.1. Isolation of Volatiles for Liquid Injection
3.2.2. Gas Chromatography–Mass Spectrometry (GC-MS)
3.3. Sensory Evaluation
3.4. Isolation of Volatiles for GC-O
3.4.1. Solvent-Assisted Flavor Evaporation (SAFE) Extract
3.4.2. Gas Chromatography–Olfactometry
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kallithraka, S.; Tsoutsouras, E.; Tzourou, E.; Lanaridis, P. Principal phenolic compounds in Greek red wines. Food Chem. 2006, 99, 784–793. [Google Scholar] [CrossRef]
- Karimali, D.; Kosma, I.; Badeka, A. Varietal classifcation of red wine samples from four native Greek grape varieties based on volatile compound analysis, color parameters and phenolic composition. Eur. Food Res. Technol. 2019, 246, 41–53. [Google Scholar] [CrossRef]
- Koufos, G.C.; Mavromatis, T.; Koundouras, S.; Fyllas, N.M.; Theocharis, S.; Jones, G.V. Greek wine quality assessment and relationships with climate: Trends, future projections and uncertainties. Water 2022, 14, 573. [Google Scholar] [CrossRef]
- Souza Gonzaga, L.; Capone, D.L.; Bastian, S.E.P.; Jeffery, D.W. Defining wine typicity: Sensory characterisation and consumer perspectives. Aust. J. Grape Wine Res. 2021, 27, 246–256. [Google Scholar] [CrossRef]
- Koussissi, E.; Paterson, A.; Cristovam, E. Sensory discrimination of dry red wines from Greece. J. Wine Res. 2002, 13, 165–179. [Google Scholar] [CrossRef]
- Tzachristas, A.; Dasenaki, M.E.; Aalizadeh, R.; Thomaidis, N.S.; Proestos, C. Development of a wine metabolomics approach for the authenticity assessment of selected Greek red wines. Molecules 2021, 26, 2837. [Google Scholar] [CrossRef]
- Marinaki, M.; Sampsonidis, I.; Lioupi, A.; Arapitsas, P.; Thomaidis, N.; Zinoviadou, K.; Theodoridis, G. Development of two-level Design of Experiments for the optimization of a HS-SPME-GC-MS method to study Greek monovarietal PDO and PGI wines. Talanta 2023, 253, 123987. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Sykalia, D.; Mannu, A.; Badeka, A. Physico-chemical parameters complemented with aroma compounds fired up the varietal discrimination of wine using statistics. Eur. Food Res. Technol. 2020, 246, 2233–2248. [Google Scholar] [CrossRef]
- Mansfield, A.K.; Schirle-Keller, J.P.; Reineccius, G.A. Identification of odor-impact compounds in red table wines produced from Frontenac grapes. Am. J. Enol. Vitic. 2011, 62, 169–176. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef]
- Darici, M.; Cabaroglu, T.; Ferreira, V.; Lopez, R. Characterisation of the aroma of Çalkarası rosé. Aust. J. Grape Wine Res. 2014, 20, 340–346. [Google Scholar] [CrossRef]
- Darici, M.; Cabaroglu, T. Chemical and sensory characterization of Kalecik Karası wines produced from two different regions in Turkey using chemometrics. J. Food Proc. Preserv. 2022, 46, 16278. [Google Scholar] [CrossRef]
- Muñoz-González, C.; Rodríguez-Bencomo, J.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.A. Beyond the characterization of wine aroma compounds: Looking for analytical approaches in trying to understand aroma perception during wine consumption. Anal. Bioanal. Chem. 2011, 401, 1497–1512. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Palomo, E.; Delgado, J.A.; Ferrer, M.A.; González Viñas, M.A. The aroma of La Mancha Chelva wines: Chemical and sensory characterization. Food Res. Int. 2019, 119, 135–142. [Google Scholar] [CrossRef]
- Ling, M.; Chai, R.; Xiang, X.; Li, J.; Zhou, P.; Shi, Y.; Duan, C.; Lan, Y. Characterization of key odor-active compounds in Chinese Dornfelder wine and its regional variations by application of molecular sensory science approaches. Food Chem. 2023, 17, 100598. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- De-la-Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- Escudero, A.; Gogorza, B.; Melus, M.A.; Ortin, N.; Cacho, J.; Ferreira, V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. J. Agric. Food Chem. 2004, 52, 3516–3524. [Google Scholar] [CrossRef]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortin, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache rosé wines: aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Wang, X.; Capone, D.L.; Kang, W.; Roland, A.; Jeffery, D.W. Impact of accentuated cut edges (ACE) technique on volatile and sensory profiles of Shiraz wines. Food Chem. 2022, 372, 131–222. [Google Scholar] [CrossRef] [PubMed]
- Wimalasiri, P.M.; Olejar, K.J.; Harrison, R.; Hider, R.; Tian, B. Whole bunch fermentation and the use of grape stems: Effect on phenolic and volatile aroma composition of Vitis vinifera cv. Pinot Noir wine. Aust. J. Grape Wine Res. 2022, 28, 395–406. [Google Scholar] [CrossRef]
- Wines of Greece. Available online: https://winesofgreece.org/ (accessed on 7 May 2023).
- Song, X.; Ling, M.; Li, D.; Zhu, B.; Shi, Y.; Duan, C.; Lan, Y. Volatile profiles and sensory characteristics of Cabernet Sauvignon dry red wines in the sub-regions of the eastern foothills of Ningxia Helan Mountain in China. Molecules 2022, 27, 8817. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Guth, H. Quantification and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Etievant, P.X. Wine. In Volatile Compounds in Foods and Beverages, 1st ed.; Maarse, H., Ed.; Dekker: New York, NY, USA, 1991; pp. 483–546. [Google Scholar]
- Cacho, J.; Moncayo, L.; Palma, L.C.; Ferreira, V.; Culleré, L. Characterization of the aromatic profile of the Italia variety of Peruvian pisco by gas chromatography-olfactometry and gas chromatography coupled with flame ionization and mass spectrometry detection systems. Food Res. Int. 2012, 49, 117–125. [Google Scholar] [CrossRef]
- Flavornet and Human Odor Space. Available online: https://www.flavornet.org/ (accessed on 7 May 2023).
- Delgado, J.A.; Sánchez-Palomo, E.; Osorio Alises, M.; González Viñas, M.A. Chemical and sensory aroma typicity of La Mancha Petit Verdot wines. LWT 2022, 162, 113418. [Google Scholar] [CrossRef]
- Wang, J.; Capone, D.L.; Wilkinson, K.L.; Jeffery, D.W. Chemical and sensory profiles of rosé wines from Australia. Food Chem. 2016, 196, 682–693. [Google Scholar] [CrossRef]
- Liu, D.; Xing, R.R.; Li, Z.; Yang, D.M.; Pan, Q.H. Evolution of volatile compounds, aroma attributes, and sensory perception in bottle-aged red wines and their correlation. Eur. Food Res. Technol. 2016, 242, 1937–1948. [Google Scholar] [CrossRef]
- Nguyen, A.N.H.; Capone, D.L.; Johnson, T.E.; Jeffery, D.W.; Danner, L.; Bastian, S.E.P. Volatile Composition and Sensory Profiles of a Shiraz Wine Product Made with Pre- and Post-Fermentation Additions of Ganoderma lucidum Extract. Foods 2019, 8, 538. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Gao, X.; Shi, X.; Chen, S.; Xu, Y.; Tang, K. Comparison of the aroma-active compounds and sensory characteristics of different grades of light-flavor Baijiu. Foods 2023, 12, 1238. [Google Scholar] [CrossRef]
- González Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Relationships between Godello white wine sensory properties and its aromatic fingerprinting obtained by GC–MS. Food Chem. 2011, 129, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Kotseridis, Y.; Baumes, R. Identification of impact odorants in Bordeaux red grape juice, in the commercial yeast used for its fermentation, and in the produced wine. J. Agric. Food Chem. 2000, 48, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Jarauta, I.; López, R.; Cacho, J. Quantitative determination of sotolon, maltol and free furaneol in wine by solid-phase extraction and gas chromatography–ion-trap mass spectrometry. J. Chromatogr. A 2003, 1010, 95–103. [Google Scholar] [CrossRef]
- Kallio, H.P. Historical review on the identification of mesifurane, 2,5-dimethyl-4-methoxy-3(2H)-furanone, and its occurrence in berries and fruits. J. Agric. Food Chem. 2018, 66, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Falcao, L.D.; Lytra, G.; Darriet, P.; Barbe, J.-C. Identification of ethyl 2-hydroxy-4-methylpentanoate in red wines, a compound involved in blackberry aroma. Food Chem. 2012, 132, 230–236. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, A.R.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma precursors in grapes and wine: Flavor release during wine production and consumption. J. Agric. Food Chem. 2018, 66, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, M.; Bely, M.; Albertin, W.; Masneuf-Pomarède, I.; Colonna-Ceccaldi, B.; Marullo, P.; Barbe, J.C. Impact of grape maturity on ester composition and sensory properties of Merlot and Tempranillo wines. J. Agric. Food Chem. 2022, 70, 11520–11530. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Hu, L.; Liu, Y.; Cheng, C.F.; Chen, W.; Li, S.D.; He, F.; Duan, C.Q.; Wang, J. Manipulating the severe shoot topping delays the harvest date and modifies the flavor composition of Cabernet Sauvignon wines in a semi-arid climate. Food Chem. 2023, 405, 135008. [Google Scholar] [CrossRef] [PubMed]
- OIV. Oenological Practices: Wines. 2015. Available online: https://www.oiv.int (accessed on 7 May 2023).
- Ribéreau-Gayon, P.; Gloires, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments, 3rd ed.; John Wiley and Sons: New York, NY, USA, 2006. [Google Scholar]
- Ivanova, V.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Bíró, I.; Bufa, A.; Kilár, F. Validation of a method for analysis of aroma compounds in red wine using liquid–liquid extraction and GC–MS. Food Anal. Methods 2012, 5, 1427–1434. [Google Scholar] [CrossRef]
- Nanou, E.; Mavridou, E.; Milienos, F.S.; Papadopoulos, G.; Tempère, S.; Kotseridis, Y. Odor characterization of white wines produced from indigenous Greek grape varieties using the frequency of attribute citation method with trained assessors. Foods 2020, 9, 1396. [Google Scholar] [CrossRef]
- Wang, J.; Gambetta, J.M.; Jeffery, D.W. Comprehensive study of volatile compounds in two Australian rosé wines: Aroma extract dilution analysis (AEDA) of extracts prepared using solvent-assisted flavor evaporation (SAFE) or headspace solid-phase extraction (HS-SPE). J. Agric. Food Chem. 2016, 64, 3838–3848. [Google Scholar] [CrossRef]
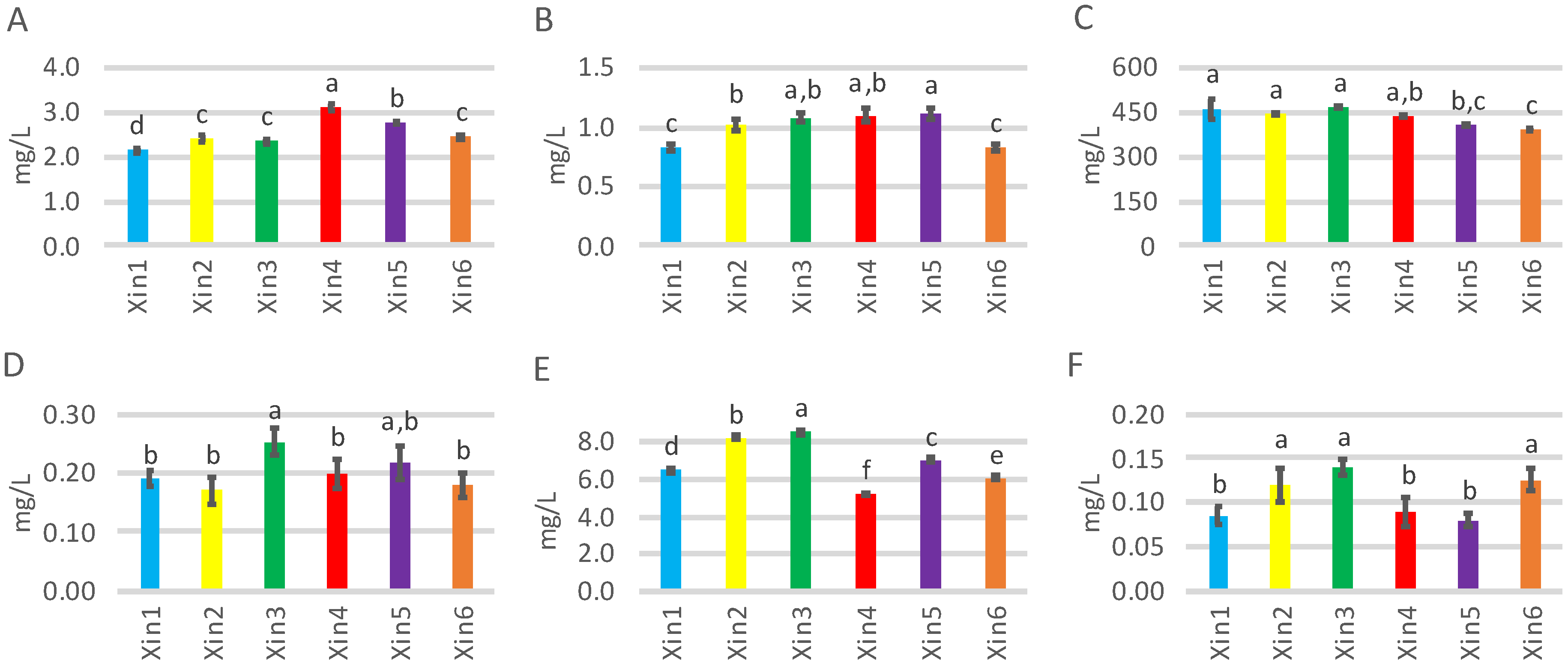
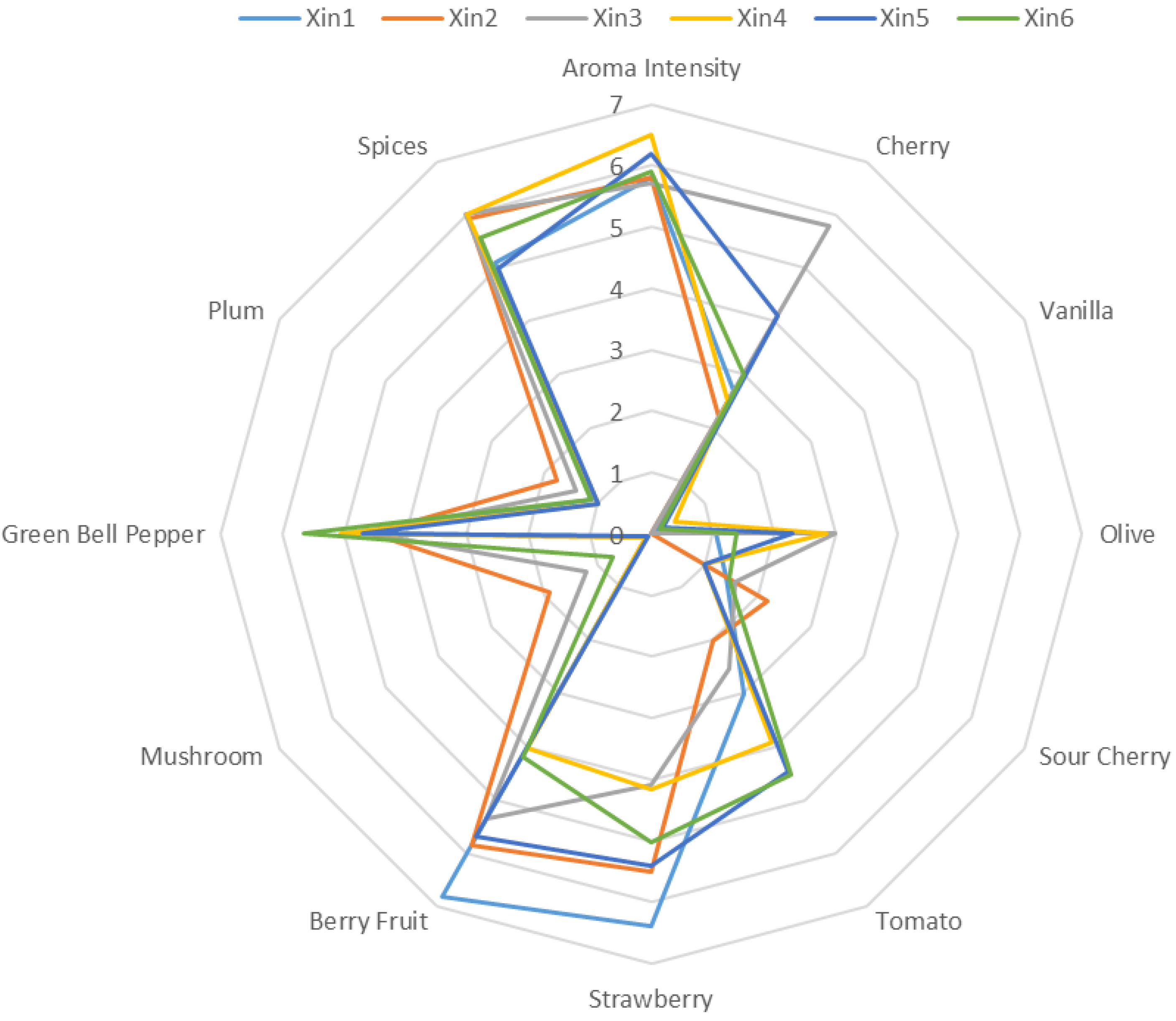
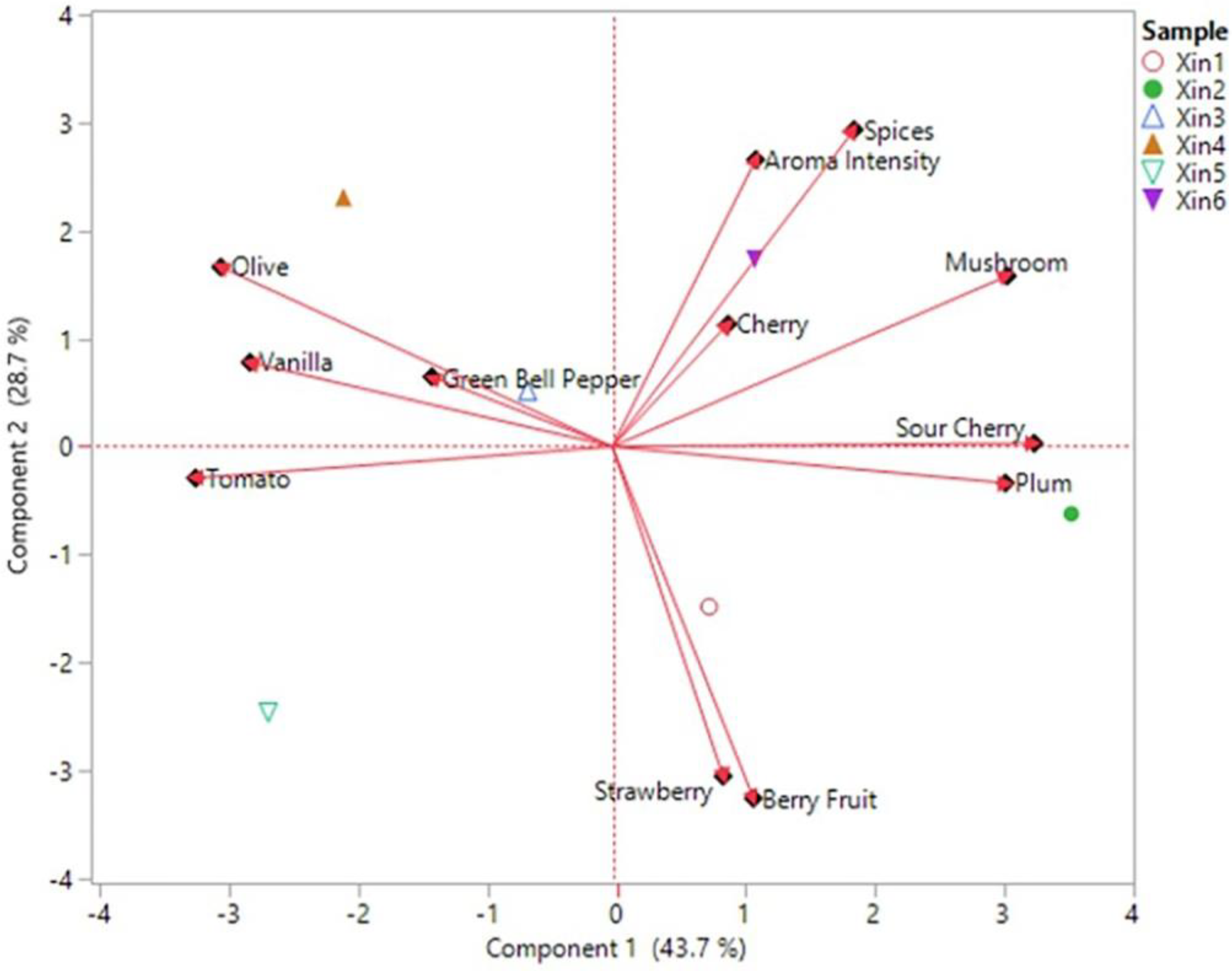
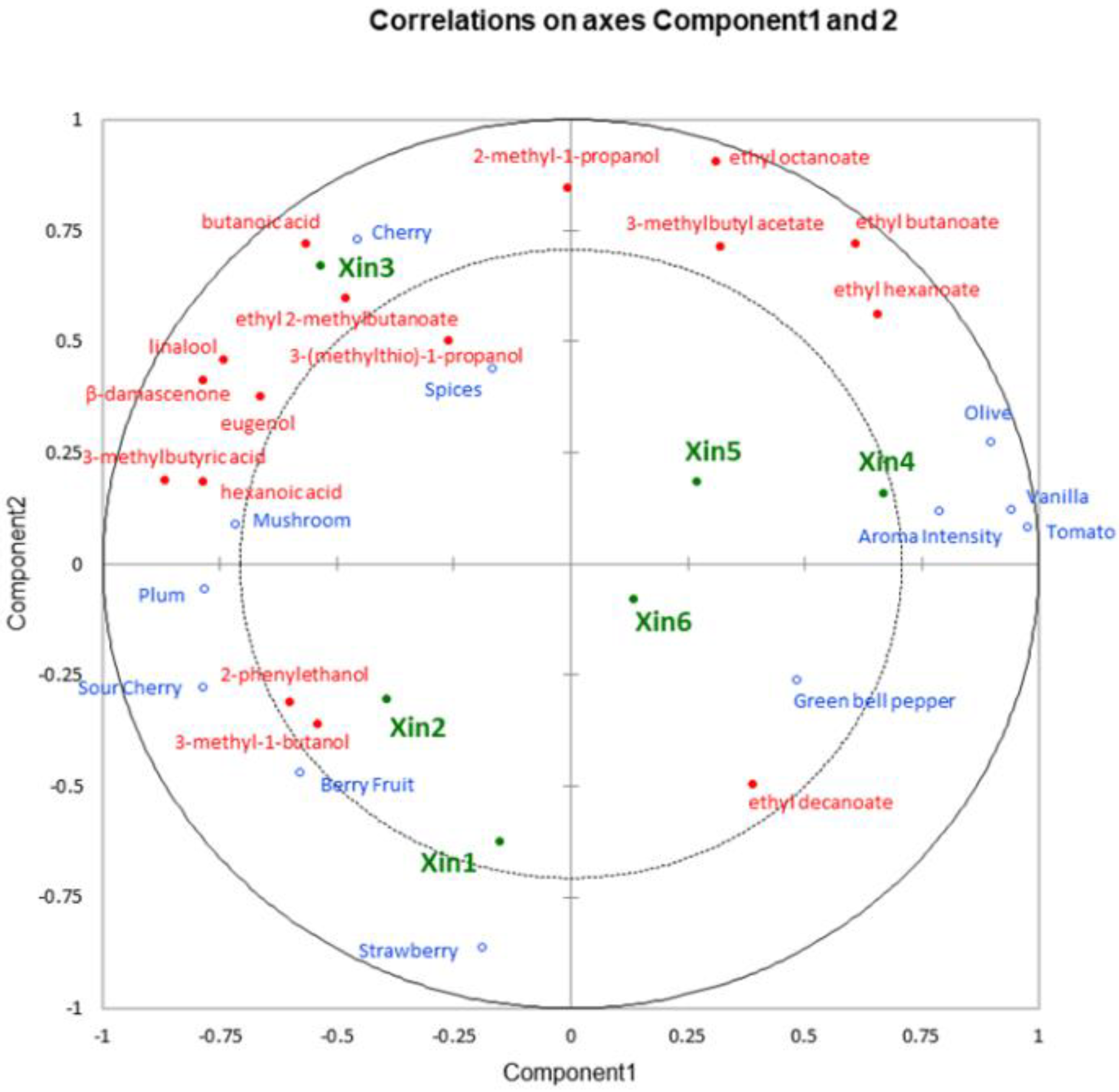
| Xin1 | Xin2 | Xin3 | Xin4 | Xin5 | Xin6 | p-Value | |
|---|---|---|---|---|---|---|---|
| Enological Parameters | |||||||
| Glucose and fructose (g/L) | 0.03 ± 0.01 ab | 0.02 ± 0.01 c | 0.02 ± 0.00 bc | 0.02 ± 0.00 bc | 0.02 ± 0.00 bc | 0.04 ± 0.00 a | 0.0004 |
| pH | 3.41 ± 0.04 b | 3.33 ± 0.01 c | 3.31 ± 0.01 c | 3.39 ± 0.01 b | 3.51 ± 0.00 a | 3.33 ± 0.00 c | <0.0001 |
| Titratable acidity (tartaric acid g/L) | 6.8 ± 0.1 c | 7.7 ± 0.0 b | 7.8 ± 0.0 ab | 7.9 ± 0.1 a | 6.6 ± 0.1 d | 7.8 ± 0.0 ab | <0.0001 |
| Volatile acidity (acetic acid g/L) | 0.38 ± 0.03 b | 0.40 ± 0.00 b | 0.42 ± 0.03 ab | 0.38 ± 0.02 b | 0.43 ± 0.05 ab | 0.49 ± 0.04 a | 0.0002 |
| Alcohol (% v/v) | 12.9 ± 0.1 d | 12.9 ± 0.1 d | 13.8 ± 0.1 ab | 13.9 ± 0.1 a | 13.1 ± 0.1 c | 13.7 ± 0.1 b | <0.0001 |
| Chromatic Characteristics | |||||||
| Color Intensity | 2.7 ± 0.1 cd | 3.3 ± 0.3 c | 4.5 ± 0.5 b | 5.9 ± 0.7 a | 2.3 ± 0.0 d | 3.7 ± 0.0 bc | <0.0001 |
| Hue | 0.6 ± 0.0 b | 0.6 ± 0.1 b | 0.6 ± 0.0 b | 0.5 ± 0.1 b | 0.7 ± 0.0 a | 0.6 ± 0.0 b | 0.0013 |
| Xin1 | Xin2 | Xin3 | Xin4 | Xin5 | Xin6 | p-Value | |
|---|---|---|---|---|---|---|---|
| Higher alcohols (mg/L) | |||||||
| 2-Methyl-1-propanol | 62.4 ± 1.3 e | 83.9 ± 1.2 c | 93.7 ± 3.3 a | 88.6 ± 0.9 b | 85.1 ± 2.6 bc | 71.8 ± 0.6 d | <0.0001 |
| 3-Methyl-1-butanol | 340 ± 33 a | 313 ± 3 abc | 318 ± 5 ab | 301 ± 5 bc | 285 ± 2 c | 282 ± 6 c | 0.0002 |
| 1-Hexanol | 2.33 ± 0.29 b | 2.41 ± 0.16 b | 3.58 ± 0.28 a | 2.32 ± 0.23 b | 2.40 ± 0.27 b | 2.30 ± 0.16 b | <0.0001 |
| (Z)-3-Hexenol | 0.17 ± 0.02 b | 0.15 ± 0.01 b | 0.21 ± 0.01 a | 0.11 ± 0.02 c | 0.14 ± 0.02 bc | 0.11 ± 0.01 c | <0.0001 |
| 3-(Methylthio)-1-propanol | 1.34 ± 0.03 cd | 2.12 ± 0.08 a | 2.22 ± 0.19 a | 1.17 ± 0.08 d | 1.79 ± 0.07 b | 1.50 ± 0.06 c | <0.0001 |
| 2-Phenylethanol | 53.3 ± 1.5 a | 47.4 ± 2.0 b | 48.2 ± 0.6 b | 41.9 ± 1.2 c | 37.3 ± 1.0 d | 35.1 ± 1.0 d | <0.0001 |
| Esters (μg/L) | |||||||
| 3-Methylbutyl acetate | 325 ± 26 d | 436 ± 12 c | 529 ± 32 b | 549 ± 40 b | 640 ± 36 a | 357 ± 32 d | <0.0001 |
| 2-Methylpropyl acetate | 138 ± 17 a | 141 ± 18 a | 113 ± 6 a | 70.9 ± 23 b | 63.4 ± 7.8 b | 48.1 ± 2.4 b | <0.0001 |
| Hexyl acetate | 206 ± 17 c | 246 ± 31 bc | 256 ± 2.8 b | 317 ± 14 a | 237 ± 15 bc | 247 ± 33 bc | <0.0001 |
| 2-Phenylethyl acetate | 166 ± 6.4 b | 197 ± 19 a | 181 ± 4.8 ab | 166 ± 17 b | 179 ± 6.4 ab | 178 ± 12 ab | 0.0184 |
| Ethyl octanoate | 1200 ± 25 e | 1450 ± 49 c | 1350 ± 20 b | 1880 ± 48 a | 1720 ± 28 b | 1490 ± 21 c | <0.0001 |
| Ethyl 2-methylbutanoate | 51.7 ± 1.8 ab | 34.9 ± 6.2 b | 61.0 ± 4.2 a | 45.5 ± 17 ab | 45.1 ± 5.0 ab | 46.4 ± 2.3 ab | 0.0069 |
| Ethyl 3-methylbutanoate | 45.4 ± 3.1 b | 61.4 ± 8.2 a | 61.5 ± 4.7 a | 56.5 ± 2.6 a | 52.5 ± 2.9 ab | 60.3 ± 4.3 a | 0.0009 |
| Ethyl hexanoate | 128 ± 11 c | 125 ± 9.4 c | 275 ± 18 a | 245 ± 4.1 b | 235 ± 16 b | 231 ± 12 b | <0.0001 |
| Ethyl decanoate | 687 ± 28 bc | 734 ± 16 ab | 521 ± 19 d | 764 ± 21 a | 635 ± 35 c | 540 ± 11 d | <0.0001 |
| Ethyl butanoate | 37.4 ± 7.9 c | 36.0 ± 11 c | 89.5 ± 20 b | 120 ± 6.3 a | 74.5 ± 9.9 b | 81.3 ± 15 b | <0.0001 |
| Fatty acids (mg/L) | |||||||
| Hexanoic Acid | 2.15 ± 0.15 b | 2.11 ± 0.08 b | 2.45 ± 0.13 a | 1.81 ± 0.08 c | 1.76 ± 0.03 c | 1.82 ± 0.14 c | <0.0001 |
| 2-Methylpropanoic acid | 2.08 ± 0.01 d | 2.76 ± 0.04 a | 2.61 ± 0.04 b | 1.46 ± 0.02 f | 2.35 ± 0.04 c | 1.54 ± 0.03 e | <0.0001 |
| Butanoic acid | 0.94 ± 0.03 d | 1.16 ± 0.04 bc | 1.42 ± 0.05 a | 0.95 ± 0.02 d | 1.25 ± 0.03 ab | 1.01 ± 0.17 cd | <0.0001 |
| 3-Methylbutyric acid | 1.34 ± 0.02 d | 2.21 ± 0.02 a | 2.02 ± 0.08 b | 1.02 ± 0.06 e | 1.70 ± 0.03 c | 1.73 ± 0.02 c | <0.0001 |
| Terpenes (μg/L) | |||||||
| Linalool | 88.5 ± 13 b | 62.5 ± 14 c | 132.0 ± 17 a | 78.4 ± 10 b,c | 70.9 ± 8.9 bc | 67.8 ± 7.9 bc | <0.0001 |
| Nerol | 64.8 ± 9.5 b | 69.8 ± 12 b | 75.3 ± 20 b | 82.7 ± 16 ab | 110 ± 16 a | 72.4 ± 11 b | 0.0054 |
| Geraniol | 33.1 ± 3.8 ab | 29.4 ± 2.3 b | 36.1 ± 2.1 a | 32.3 ± 3.3 b | 31.5 ± 1.5 b | 32.4 ± 1.5 b | 0.0009 |
| β-Damascenone | 4.50 ± 0.70 bc | 5.90 ± 0.80 ab | 7.30 ± 1.10 a | 3.40 ± 0.35 c | 5.70 ± 0.80 ab | 6.10 ± 0.90 ab | <0.0001 |
| Volatile phenols (μg/L) | |||||||
| Eugenol | 85.9 ± 10 b | 122 ± 18 a | 138 ± 8.2 a | 89.2 ± 16.3 b | 81.1 ± 8.20 b | 125 ± 12.9 a | <0.0001 |
| Odor Threshold (mg/L) a | Sensory Descriptor b | OAV c | ||||||
|---|---|---|---|---|---|---|---|---|
| Xin1 | Xin2 | Xin3 | Xin4 | Xin5 | Xin6 | |||
| Higher alcohols | ||||||||
| 2-Methyl-1-propanol | 40 [27] | wine, solvent, bitter | 1.6 | 2.1 | 2.3 | 2.2 | 2.1 | 1.8 |
| 3-Methyl-1-butanol | 30 [28] | whiskey, malt, burnt | 11 | 10 | 10 | 10 | 9.5 | 9.3 |
| 1-Hexanol | 8 [27] | resin, flower, green | 0.3 | 0.3 | 0.5 | 0.3 | 0.3 | 0.3 |
| (Z)-3-Hexen-1-ol | 0.04 [27] | grass | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.5 |
| 3-(Methylthio)-1-propanol | 1 [27] | sweet, potato | 1.3 | 2.1 | 2.2 | 1.2 | 1.8 | 1.5 |
| 2-Phenylethanol | 14 [27] | honey, spice, rose, lilac | 3.8 | 3.4 | 3.5 | 3.0 | 2.7 | 2.5 |
| 2-Methyl-1-propanol | 40 [27] | wine, solvent, bitter | 1.6 | 2.1 | 2.3 | 2.2 | 2.1 | 1.8 |
| Esters | ||||||||
| 3-Methylbutyl acetate | 0.03 [27] | banana | 11 | 15 | 18 | 18 | 21 | 12 |
| 2-Methylpropyl acetate | 1.6 [22] | fruit, apple, banana | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| Hexyl acetate | 1.5 [29] | fruit, herb | 0.1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| 2-Phenylethyl acetate | 0.25 [28] | rose, honey, tobacco | 0.7 | 0.8 | 0.7 | 0.7 | 0.7 | 0.7 |
| Ethyl butanoate | 0.02 [28] | apple | 1.9 | 1.8 | 4.5 | 6.0 | 3.7 | 4.1 |
| Ethyl 2-methylbutanoate | 0.018 [27] | apple | 2.9 | 1.9 | 3.4 | 2.5 | 2.5 | 2.6 |
| Ethyl 3-methylbutanoate | 0.03 [27] | fruit | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Ethyl octanoate | 0.014 [27] | apple peel, fruit | 9.2 | 8.9 | 20 | 18 | 17 | 17 |
| Ethyl hexanoate | 0.58 [28] | fruit, fat | 2.1 | 2.5 | 2.6 | 3.3 | 3.0 | 2.3 |
| Ethyl decanoate | 0.2 [27] | grape | 3.5 | 3.7 | 2.6 | 3.8 | 3.2 | 2.7 |
| Fatty acids | ||||||||
| Hexanoic acid | 0.42 [27] | sweat | 5.1 | 5.0 | 5.8 | 4.3 | 4.2 | 4.3 |
| 2-Methylpropanoic acid | 8.1 [27] | rancid, butter, cheese | 0.3 | 0.3 | 0.3 | 0.2 | 0.3 | 0.2 |
| Butanoic acid | 0.173 [27] | rancid, cheese, sweat | 5.4 | 6.7 | 8.2 | 5.5 | 7.2 | 5.8 |
| 3-Methylbutyric acid | 0.033 [27] | sweat, acid, rancid | 40 | 67 | 61 | 31 | 51 | 58 |
| Terpenes | ||||||||
| Linalool | 0.025 [27] | flower, lavender | 3.5 | 2.5 | 5.3 | 3.1 | 2.8 | 2.7 |
| Nerol | 0.5 [30] | sweet | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | 0.1 |
| Geraniol | 0.036 [20] | rose, geranium | 0.9 | 0.8 | 1 | 0.9 | 0.9 | 0.9 |
| β-Damascenone | 0.00005 [28] | apple, rose, honey | 9.0 | 12 | 15 | 6.9 | 11 | 12 |
| Phenols | ||||||||
| Eugenol | 0.006 [27] | clove, honey | 14 | 20 | 23 | 15 | 14 | 21 |
| No. | RI | Odorant Description a | Identity Determined by b | Compound | Group Code c | MF (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Xin1 | Xin4 | Xin5 | Xin6 | ||||||
| 1 | 997 | Fruity, sweet | MS, RI, O | 2-methylpropyl acetate | F | 58 | - d | - | - |
| 2 | 1020 | Red fruit | MS, RI, O | ethyl butanoate | F | 47 | 82 | 82 | 47 |
| 3 | 1049 | Apple | MS, RI, O | ethyl 2-methylbutanoate | F | 33 | 47 | 33 | 33 |
| 4 | 1064 | Fruity | MS, RI, O | ethyl 3-methylbutanoate | F | 47 | 33 | 47 | 33 |
| 5 | 1117 | Banana | MS, RI, O | 3-methylbutyl acetate | F,B | 67 | 33 | 33 | 58 |
| 6 | 1156 | Wine | RI, O | 1-butanol | - | 67 | 67 | - | |
| 7 | 1210 | Burnt, solvent | MS, RI, O | 3-methyl-1-butanol | 100 | 100 | 100 | 100 | |
| 8 | 1240 | Fruity | MS, RI, O | ethyl hexanoate | F,B | 47 | 33 | 67 | 82 |
| 9 | 1279 | Fruit, herb | MS, RI, O | hexyl acetate | F | 58 | 47 | 67 | 67 |
| 10 | 1309 | Burnt | MS, RI, O | 2-methyl-3-furanthiol | 47 | 47 | 33 | 67 | |
| 11 | 1356 | Resin, flower, grass | MS, RI, O | 1-hexanol | V | 67 | 82 | 82 | 82 |
| 12 | 1379 | Fat | MS, RI, O | nonanal | 47 | 67 | 33 | 33 | |
| 13 | 1391 | Grass | MS, RI, O | (Z)-3-hexen-1-ol | V | 47 | 82 | 82 | 67 |
| 14 | 1433 | Fruit | MS, RI, O | ethyl octanoate | F,B | 47 | 33 | 67 | 58 |
| 15 | 1442 | Sour | MS, RI, O | acetic acid | 82 | 82 | 82 | 82 | |
| 16 | 1446 | Sweet, bread | MS, RI, O | furfural | 47 | 47 | 47 | - | |
| 17 | 1466 | Cooked potato | MS, RI, O | 3-(methylthio)propanal (methional) | 58 | 58 | 33 | 33 | |
| 18 | 1516 | Green pepper | RI, O | 3-isobutyl-2-methoxypyrazine | V | 33 | 33 | 82 | 67 |
| 19 | 1533 | Flower | MS, RI, O | linalool | 58 | 58 | 58 | 58 | |
| 20 | 1553 | Butter, cheese | MS, RI, O | 2-methylpropanoic acid | 47 | 82 | 67 | 58 | |
| 21 | 1584 | Cotton candy, strawberry | MS, RI, O | 2,5-dimethyl-4-methoxy-3(2H)-furanone (dimethylmethoxy furanone) | ST | 47 | 82 | 58 | 33 |
| 22 | 1607 | Rancid, cheese | MS, RI, O | butanoic acid | 58 | 58 | 58 | 58 | |
| 23 | 1640 | Grape, flower | MS, RI, O | ethyl decanoate | F | 58 | 82 | 58 | 47 |
| 24 | 1647 | Honey | MS, RI, O | γ-butyrolactone | - | 47 | - | 67 | |
| 25 | 1651 | Sweat, rancid | MS, RI, O | 3-methylbutyric acid | 82 | 100 | 58 | 58 | |
| 26 | 1718 | Potato, mushroom | MS, RI, O | 3-(methylthio)-1-propanol (methionol) | 82 | 58 | 100 | 82 | |
| 27 | 1781 | Fruit, sweet, rose | MS, RI, O | citronellyl butyrate | F | 47 | 58 | 33 | 33 |
| 28 | 1816 | Fresh-canned fruit | MS, RI, O | β-damascenone | S | 33 | 100 | 67 | 47 |
| 29 | 1833 | Sweat | MS, RI, O | hexanoic acid | 82 | 82 | 82 | 67 | |
| 30 | 1880 | Green, herb | MS, RI, O | 3-mercapto-1-hexanol | V | - | 33 | 47 | 33 |
| 31 | 1835 | Tobacco, honey | MS, RI, O | 2-phenylethyl acetate | 47 | 47 | 58 | 33 | |
| 32 | 1914 | Rose, lilac | MS, RI, O | 2-phenylethanol | 82 | 100 | 82 | 82 | |
| 33 | 1979 | Flower | MS, RI, O | β-ionone | 47 | 33 | 47 | 47 | |
| 34 | 1997 | Spice | RI, O | bulnesol | 58 | 47 | - | - | |
| 35 | 2038 | Caramel, candy | MS, RI, O | 4-hydroxy-2,5-dimethyl-3(2H)-furanone (furaneol) | ST | 82 | 67 | 67 | 47 |
| 36 | 2079 | Cotton candy | MS, RI, O | 2-ethyl-4-hydroxy-5-methyl-3(2H)-furanone (homofuraneol) | ST | 82 | 33 | 33 | 67 |
| 37 | 2084 | Sweat | MS, RI, O | octanoic acid | - | 47 | 47 | 33 | |
| 38 | 2154 | Clove, spice | MS, RI, O | eugenol | S | 67 | 47 | 33 | 33 |
| 39 | 2235 | Curry | MS, RI, O | 3-hydroxy-4,5-dimethylfuran-2(5H)-one (sotolon) | S | 67 | 47 | 58 | 82 |
| 40 | 2353 | Sweet, grape must | MS, RI, O | diethyl tartrate | 33 | 67 | - | - | |
| Descriptor | Reference Standard | Amount a |
|---|---|---|
| Cherry | standard (Vioryl, Afidnes, Greece, https://www.vioryl.gr/el/) | 10 μL |
| Sour cherry | sour cherry syrup (Jiotis, Athens, Greece, https://www.jotis.gr/) | 5 g |
| Strawberry | standard (Vioryl, https://www.vioryl.gr/el/) | 13 μL |
| Berry fruit | standard (Vioryl, https://www.vioryl.gr/el/) | 20 μL |
| Plum | plum juice (Sunsweet, Yuba City, CA, USA, https://www.sunsweet.gr/) | 33 mL |
| Green bell pepper | green bell pepper (fresh) | 8 g 10 cm2 pieces |
| Tomato | tomato paste (Kyknos, Savalia, Greece, https://kyknoscanning.com/el/) | 7.5 g |
| Olive | olives (Edem, Peania, Greece, https://www.edem.com.gr/) | 4 g crushed |
| Mushroom | 1-octen-3-ol 1 g/L (Sigma-Aldrich, Saint Louis, MO, USA, https://www.sigmaaldrich.com/) | 800 μL |
| Spices | grated black pepper (Kagia) and grated clove (Kagia, Patra, Greece, https://www.kagiaspices.gr/) | 0.1 g and 0.01 g, respectively |
| Vanilla | vanillin powder (Captain’s, Athens, Greece, http://www.captainspices.gr/) | 0.3 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goulioti, E.; Jeffery, D.W.; Kanapitsas, A.; Lola, D.; Papadopoulos, G.; Bauer, A.; Kotseridis, Y. Chemical and Sensory Characterization of Xinomavro Red Wine Using Grapes from Protected Designations of Northern Greece. Molecules 2023, 28, 5016. https://doi.org/10.3390/molecules28135016
Goulioti E, Jeffery DW, Kanapitsas A, Lola D, Papadopoulos G, Bauer A, Kotseridis Y. Chemical and Sensory Characterization of Xinomavro Red Wine Using Grapes from Protected Designations of Northern Greece. Molecules. 2023; 28(13):5016. https://doi.org/10.3390/molecules28135016
Chicago/Turabian StyleGoulioti, Elli, David W. Jeffery, Alexandros Kanapitsas, Despina Lola, Georgios Papadopoulos, Andrea Bauer, and Yorgos Kotseridis. 2023. "Chemical and Sensory Characterization of Xinomavro Red Wine Using Grapes from Protected Designations of Northern Greece" Molecules 28, no. 13: 5016. https://doi.org/10.3390/molecules28135016
APA StyleGoulioti, E., Jeffery, D. W., Kanapitsas, A., Lola, D., Papadopoulos, G., Bauer, A., & Kotseridis, Y. (2023). Chemical and Sensory Characterization of Xinomavro Red Wine Using Grapes from Protected Designations of Northern Greece. Molecules, 28(13), 5016. https://doi.org/10.3390/molecules28135016









