1,2-Diphenyl-o-carborane and Its Chromium Derivatives: Synthesis, Characterization, X-ray Structural Studies, and Biological Evaluations
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of 1,2-Diphenyl-o-carborane and Corresponding Chromium Metal Complexes
2.2. IR and NMR Spectroscopy
2.3. X-ray Structural Studies of 1,2-Diphenyl-o-carborane and Corresponding Chromium Complexes
2.4. Determination of IC50 and Incorporation of Boron into B16 and CT26 Cells
3. Materials and Methods
3.1. General Procedure
3.2. Crystal Structure Determination
3.3. Cell Viability Assay (MTT Assay)
3.4. In Vitro Boron Incorporation into B16 and CT26 Cancer Cells
3.5. Synthesis of 1,2-Diphenyl-o-carborane (1)
3.6. Synthesis of 1-(Phenyl-η6-chromium(0) tricarbonyl)-2-phenyl-o-carborane (2)
3.7. Synthesis of 1,2-bis(phenyl-η6-chromium(0) tricarbonyl)-o-carborane (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Yamamoto, T.; Nakai, K.; Matsumura, A. Boron neutron capture therapy for glioblastoma. Cancer Lett. 2008, 262, 143–152. [Google Scholar] [CrossRef]
- Pisarev, M.A.; Dagrosa, M.A.; Juvenal, G.J. Boron neutron capture therapy in cancer: Past, present and future. Arq Bras Endocrinol Metab. 2007, 51, 852–856. [Google Scholar] [CrossRef]
- Lesnikowski, Z.J. Boron units as pharmacophores—New applications and opportunities of boron cluster chemistry. Collect. Czech. Chem. Commun. 2007, 72, 1646–1658. [Google Scholar] [CrossRef]
- Tjarks, W.; Tiwari, R.; Byun, Y.; Narayanasamy, S.; Barth, R.F. Carboranyl thymidine analogues for neutron capture therapy. Chem. Commun. 2007, 4978–4991. [Google Scholar] [CrossRef] [PubMed]
- Bregadze, V.I.; Sivaev, I.B. Polyhedral Boron Compounds for BNCT in Boron Science: New Technologies and Applications; CRC Press: Boca Raton, FL, USA, 2011; Chapter 9; pp. 187–207. [Google Scholar]
- Armstrong, A.F.; Valliant, J.F. The bioinorganic and medicinal chemistry of carboranes: From new drug discovery to molecular imaging and therapy. Dalton Trans. 2007, 38, 4240–4251. [Google Scholar] [CrossRef] [PubMed]
- Korbe, S.; Schreiber, P.J.; Michl, J. Chemistry of the Carba-closo-dodecaborate(−) Anion, CB11H12−. Chem. Rev. 2006, 106, 5208–5249. [Google Scholar] [CrossRef]
- Jin, G.F.; Hwang, J.-H.; Lee, J.-D.; Wee, K.-R.; Suh, I.-H.; Kang, S.O. A three-dimensional π-electron acceptor, tri-phenyl-o-carborane, bearing a rigid conformation with end-on phenyl units. Chem. Commun. 2013, 49, 9398–9400. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Ma, S.-Y.; Kang, S.O.; Lee, J.-D. B-phenylated o-carboranes and its chromium derivatives: Synthesis, electrochemical properties, and X-ray structural studies. J. Organomet. Chem. 2018, 865, 100–108. [Google Scholar] [CrossRef]
- Semmelhack, M.F. Transition Metal Arene Complexes: Nucleophilic Addition, Comprehensive Organometallic Chemistry II; Abel, E.W., Stone, F.G.A., Wilkinson, G., Eds.; Pergamon Press: Oxford, UK, 1995; Volume 12, pp. 979–1013. [Google Scholar]
- Rose-Munch, F.; Rose, E. Arenetricarbonylchromium Complexes: Ipso, Cine, Tele Nucleophilic Aromatic Substitutions. In Modern Arene Chemistry; Astruc, D., Ed.; Wiley: Hoboken, NJ, USA, 2002; Chapter 11; pp. 368–397. [Google Scholar]
- Jonson, T.R.; Mann, B.E.; Clark, J.E.; Foresti, R.; Green, C.J.; Motterlini, R. Metal Carbonyls: A New Class of Pharmaceuticals? Angew. Chem. Int. Ed. 2003, 42, 3722–3729. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessières, A.; Butler, I. Bioorganometallic Chemistry: A Future Direction for Transition Metal Organometallic Chemistry? Acc. Chem. Res. 1993, 26, 361–369. [Google Scholar] [CrossRef]
- Hess, A.; Metzler-Nolte, N. Transition metal labels on peptide nucleic acid (PNA) monomers. Chem. Commun. 1999, 885–886. [Google Scholar] [CrossRef]
- Baldoli, C.; Maiorana, S.; Licandro, E.; Zinzalla, G.; Perdicchia, D. Synthesis of Chiral Chromium Tricarbonyl Labeled Thymine PNA Monomers via the Ugi Reaction. Org. Lett. 2002, 4, 4341–4344. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Zhigareva, G.G. Synthesis of o- and m-carboranylbenzenechromotricarbonyls by the reaction of lithium o- and m-carboranes with chlorobenzenechromotricarbonyl. Zhurnal Obshchei Khimii 1983, 53, 953–954. [Google Scholar]
- Vasyukova, N.I.; Nekrasov, Y.S.; Sukharev, Y.N.; Magomedov, G.K.; Frenkel, A.S. Mass spectrometry of π-complexes of transition metals. 33. Carboranyl derivatives of benzenechromium tricarbonyl. Izvestiya Akademii Nauk SSSR Seriya Khimicheskaya 1985, 1548–1549. [Google Scholar] [CrossRef]
- Magomedov, G.K.; Frenkel, A.S.; Kalinin, V.N.; Zakharkin, L.I. Synthesis of arylcarboranechromiumtricarbonyls. Izvestiya Akademii Nauk SSSR Seriya Khimicheskaya 1977, 949–952. [Google Scholar]
- Ohta, K.; Goto, T.; Endo, Y. 1,2-Dicarba-closo-dodecaboran-1-yl Naphthalene Derivatives. Inorg. Chem. 2005, 44, 8569–8573. [Google Scholar] [CrossRef]
- Kokado, K.; Nagai, A.; Chujo, Y. Poly(γ-glutamic acid) Hydrogels with Water-Sensitive Luminescence Derived from Aggregation-Induced Emission of o-Carborane. Macromolecules 2010, 43, 6463–6468. [Google Scholar] [CrossRef]
- Henly, T.J.; Knobler, C.B.; Hawthorne, M.F. Reactions of Anionic Carborane Nucleophiles with Chromium-Coordinated Haloarenes. Organometallics 1992, 11, 2313–2316. [Google Scholar] [CrossRef]
- Mahaffy, C.A.L.; Pauson, P.L. (η6-Arene)tricarbonylchromium Complexes. Inorg. Synth. 1979, 19, 154–158. [Google Scholar]
- Fischer, R.D. IR-spektroskopische Untersuchungen der ν-CO-Banden an Metallcarbonylkomplexen rnit zentrisch-x-gebundenen organischen Ringsystemen. Chem. Ber. 1960, 93, 165–175. [Google Scholar] [CrossRef]
- Brown, D.A.; Sloan, H. Molecular-orbital Theory of Organometallic Compounds. Part I V.l Substitution Reactions of Tricarbonylbenzenechromium. J. Chem. Soc. 1963, 4389–4394. [Google Scholar] [CrossRef]
- Brown, D.A.; Raju, J.R. Infrared and Proton Magnetic Resonance Spectra of π-Complexes of Substituted Condensed Hydrocarbons. J. Chem. Soc. A. 1966, 1617–1620. [Google Scholar] [CrossRef]
- Adams, D.M.; Squire, A. Vibrational Spectra of Some Monosubstituted-π-arene Tricarbonylchromium Complexes and of Methyl Benzoate. J. Chem. Soc. Dalton Trans. 1974, 6, 558–565. [Google Scholar] [CrossRef]
- Fox, M.A.; Nervi, C.; Crivello, A.; Low, P.J. Carborane radical anions: Spectroscopic and electronic properties of a carborane radical anion with a 2n + 3 skeletal electron count. Chem. Commun. 2007, 23, 2372–2374. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.A.; Clegg, W.; Copley, R.C.B.; Davidson, M.G.; Fox, M.A.; Hibbert, T.G.; Howard, J.A.K.; Mackinnon, A.; Peace, R.J.; Wade, K. Exo-π-bonding to an ortho-carborane hypercarbon atom: Systematic icosahedral cage distortions reflected in the structures of the fluoro-, hydroxy- and amino-carboranes, 1-X-2-Ph-1,2-C2B10H10 (X = F, OH or NH2) and related anions. Dalton Trans. 2004, 17, 2786–2799. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A.; Peace, R.J.; Clegg, W.; Elsegood, M.R.J.; Wade, K. Trends in ortho-carboranes 1-X-2-R-1,2-C2B10H10 (R = Ph, Me) bearing an exo-CN-bonded substituent group (X = NO, N=NRʹ or NHRʺ). Polyhedron 2009, 28, 2359–2370. [Google Scholar] [CrossRef]
- Lewis, Z.G.; Welch, A.J. Structure of 1,2-Diphenylcarbaborane, 1,2-Ph2-1,2-closo-C2B10H10. Acta Crystallogr. Sect. C. 1993, 49, 705–710. [Google Scholar] [CrossRef]
- Calhorda, M.J.; Frazão, C.F.; Martinho-Simões, J.A. Metal-Carbon “Bond Strengths” in Cr(CO)6, Cr(η-C6H6)2, and Cr(CO)3(η-C6H6). J. Organomet. Chem. 1984, 262, 305–314. [Google Scholar] [CrossRef]
- Rees, B.; Coppens, P. Electronic Structure of Benzene Chromium Tricarbonyl by X-ray and Neutron Diffraction at 78 ºK. Acta Crystallogr. Sect. B 1973, 29, 2516–2528. [Google Scholar] [CrossRef]
- Bailey, M.F.; Dahl, L.F. Three-Dimensional Crystal Structure of Benzenechromium Tricarbonyl with Further Comments on the Dibenzenechromium Structure. Inorg. Chem. 1965, 4, 1314–1319. [Google Scholar] [CrossRef]
- Wang, Y.; Angermund, K.; Goddard, R.; Kruger, C. Redetermination of the Experimental Electron Deformation Density of Benzenetricarbonylchromium. J. Am. Chem. Soc. 1987, 109, 587–589. [Google Scholar] [CrossRef]
- Czerwinski, C.J.; Guzei, I.A.; Riggle, K.M.; Schroeder, J.R.; Spencer, L.C. Haptotropic rearrangement in tricarbonylchromium complexes of 2-aminobiphenyl and 4-aminobiphenyl. Dalton Trans. 2011, 40, 9439–9446. [Google Scholar] [CrossRef] [PubMed]
- Guzei, I.A.; Spencer, L.C.; Buechel, S.C.; Kaufmann, L.B.; Czerwinski, C.J. Intricacies of ligand coordination in tricarbonylchromium(0) complexes with ortho- and para-fluorobiphenyls. Acta Crystallogr. Sect. C 2017, 73, 638–644. [Google Scholar] [CrossRef]
- Davidson, M.G.; Hibbert, T.G.; Howard, J.A.K.; Mackinnon, A.; Wade, K. Definitive crystal structures of ortho-, meta- and para-carboranes: Supramolecular structures directed solely by C−H···O hydrogen bonding to hmpa (hmpa = hexamethylphosporamide). Chem. Commun. 1996, 2285–2286. [Google Scholar] [CrossRef]
- Llop, J.; Viñas, C.; Oliva, J.M.; Teixidor, F.; Flores, M.A.; Kivekäs, R.; Sillanpää, R. Modulation of the C−C distance in disubstituted 1,2-R2-o-carboranes. Crystal structure of closo 1,2-(SPh)2-1,2-C2B10H10. J. Organomet. Chem. 2002, 657, 232–238. [Google Scholar] [CrossRef]
- Oliva, J.M.; Allan, N.L.; Schleyer, P.v.R.; Viñas, C.; Teixidor, F. Strikingly Long C···C Distances in 1,2-Disubstituted ortho-Carboranes and Their Dianions. J. Am. Chem. Soc. 2005, 127, 13538–13547. [Google Scholar] [CrossRef]
- Hutton, B.W.; Maclntosh, F.; Ellis, D.; Herisse, F.; Macgregor, S.A.; McKay, D.; Petrie-Armstrong, V.; Rosair, G.M.; Perekalin, D.S.; Tricas, H.; et al. Unprecedented steric deformation of ortho-carborane. Chem. Commun. 2008, 42, 5345–5347. [Google Scholar] [CrossRef]
- Bruker Analytical X-ray Division, SMART and SAINT; Bruker: Madison, WI, USA, 2002.
- Sheldrick, G.M. Bruker Analytical X-Ray Division, SHELXTL-PLUS Software Package; Bruker: Madison, WI, USA, 2002. [Google Scholar]



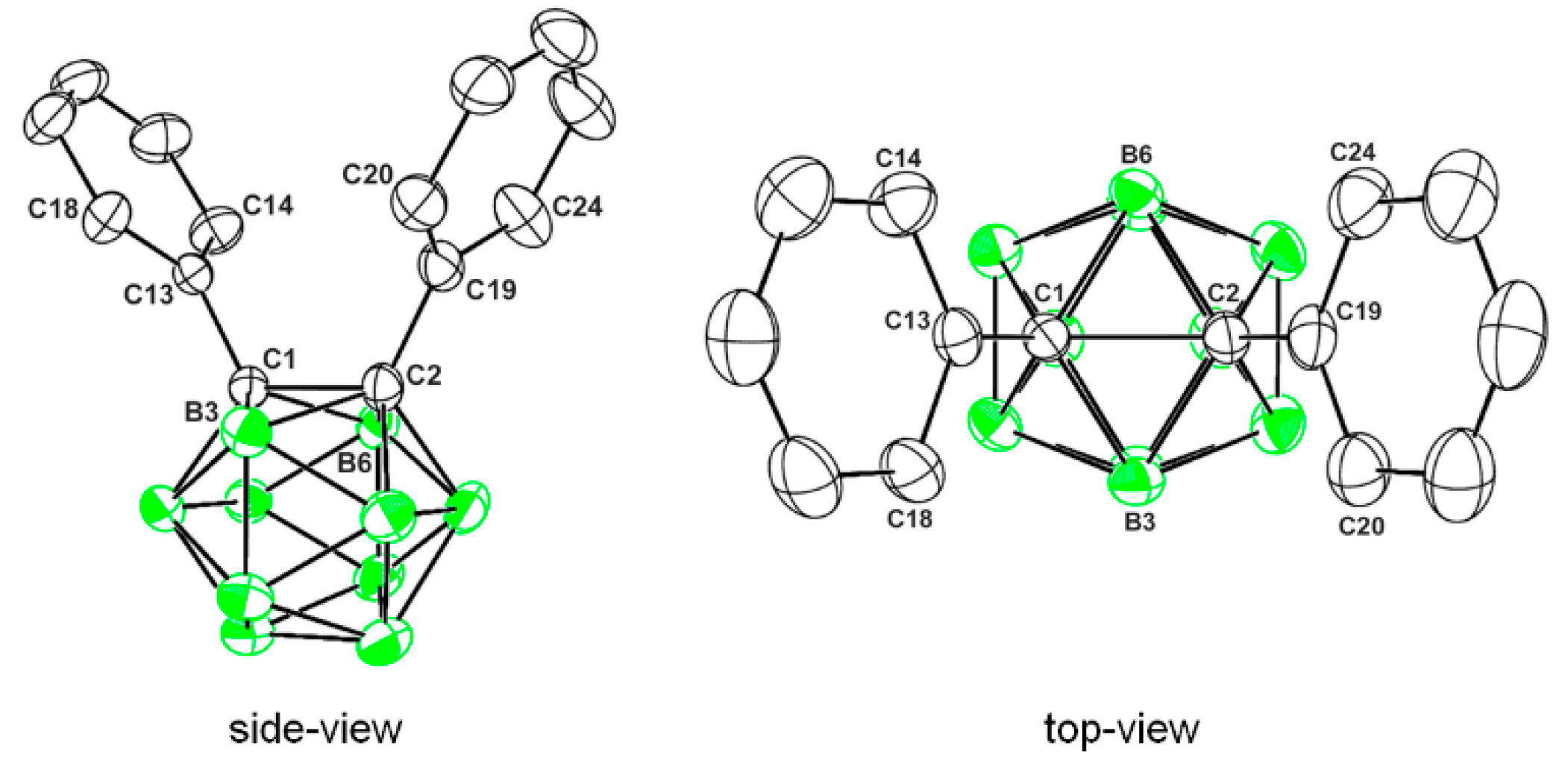

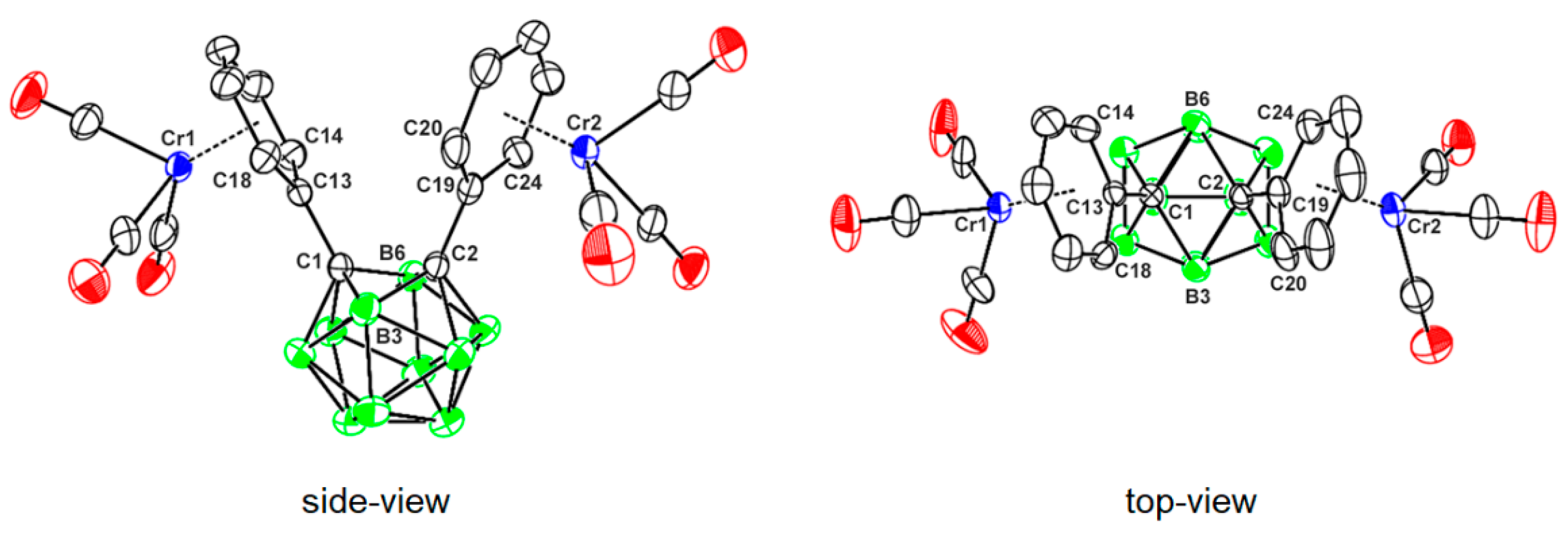
| 1 | 2 | 3 | |
|---|---|---|---|
| Identification code | K120504 | K130805 | K131105 |
| Empirical formula | C14H20B10 | C17H20B10Cr1O3 | C20H20B10Cr2O6 |
| Formula weight | 296.40 | 432.43 | 568.46 |
| Temperature | 293(2) K | 293(2) K | 293(2) K |
| Wavelength | 0.71073 Å | 0.71073 Å | 0.71073 Å |
| Crystal system, space group | Monoclinic, P21/n | Monoclinic, P21/n | Triclinic, Pī |
| Unit cell dimensions | a = 10.859(1) Å | a = 10.621(3) Å | a = 17.540(2) Å, α = 105.746(2)° |
| b = 24.953(3) Å, β = 111.854(2)° | b = 17.056(5) Å, β = 106.622(5)° | b = 18.060(2) Å, β = 110.226(2)° | |
| c = 13.938(2) Å | c = 12.174(4) Å | c = 19.484(3) Å, γ = 91.256(2)° | |
| Volume | 3505.3(8) | 2113.2(1) | 5528.0(1) |
| Z, Dcalc | 8, 1.123 | 4, 1.359 | 2, 0.342 |
| F(000) | 1232.0 | 880.0 | 572 |
| Crystal size | 0.15, 0.13, 0.12 | 0.17, 0.15, 0.13 | 0.20, 0.20, 0.15 |
| θ range for data collection | 1.63 to 28.37 | 2.12 to 28.46 | 1.17 to 28.38 |
| Limiting indices | −14 ≤ h ≤ 14, −33 ≤ k ≤ 32, −18 ≤ l ≤ 18 | −14 ≤ h ≤ 14, −22 ≤ k ≤ 22, −16 ≤ l ≤ 16 | −23 ≤ h ≤ 23, −24 ≤ k ≤ 24, −26 ≤ l ≤ 25 |
| Reflections collected/unique | 35826/8726 [R(int) = 0.0421] | 28490/5305 [R(int) = 0.0238] | 44259/18014 [R(int) = 0.0398] |
| Completeness to θ = 28.38 | 99.3% | 99.2% | 65.0% |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 8726/0/581 | 5305/0/360 | 18014/0/1409 |
| Goodness-of-fit on F2 | 1.008 | 1.053 | 0.902 |
| Final R indices [I > 2θ(I)] | a R1 = 0.0616, b wR2 = 0.1545 | a R1 = 0.0332, b wR2 = 0.0958 | a R1 = 0.0563, b wR2 = 0.1505 |
| R indices (all data) | a R1 = 0.1037, b wR2 = 0.1897 | a R1 = 0.0388, b wR2 = 0.1018 | a R1 = 0.0877, b wR2 = 0.1612 |
| Largest diff. peak and hole | 0.220 and −0.355 e.Å−3 | 0.364 and −0.242 e.Å−3 | 0.687 and −0.375 e.Å−3 |
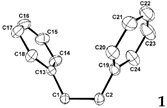 |  | 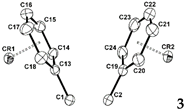 | |
|---|---|---|---|
| PhC−C (av) | 1.376 | 1.400 | 1.406 |
| PhC−C (av)−Cr | 2.210 | 2.212 | |
| CabC−C | 1.726(2) | 1.740(2) | 1.724(4) |
| Cent−Cr | 1.702 | 1.696(av) | |
| Cr–CO | 1.856(av) | 1.851(av) | |
| C1–C13 | 1.507(2) | 1.499(2) | 1.502(4) |
| C2–C19 | 1.501(2) | 1.500(2) | 1.510(4) |
| C13–C1–C2 | 118.3(1) | 116.6(1) | 116.4(2) |
| C19–C2–C1 | 119.0(1) | 119.6(1) | 116.1(2) |
| C1-C2-C19-C20 | 84.1(2) | 86.1(2) | 105.8(3) |
| C2-C1-C13-C14 | 81.7(2) | 102.3(1) | 112.4(3) |
| Compounds | B16 | CT26 | ||
|---|---|---|---|---|
| Cytotoxicity IC50 (M) a | Boron Accumulation (ppm) b | Cytotoxicity IC50 (M) a | Boron Accumulation (ppm) b | |
| 2 | 0.736 × 10−6 (±0.01) | 0.825 ± 0.003 | 0.833 × 10−6 (±0.03) | 0.755 ± 0.009 |
| 3 | 0.681 × 10−6 (±0.04) | 0.620 ± 0.002 | 0.314 × 10−6 (±0.07) | 0.694 ± 0.002 |
| Ph3C2BCr2 | 0.411 × 10−6 (±0.06) | 0.384 ± 0.006 | 0.164 × 10−6 (±0.05) | 0.402 ± 0.002 |
| Ph3C2BCr3 | 0.091 × 10−6 (±0.03) | 0.221 ± 0.001 | 0.089 × 10−6 (±0.08) | 0.247 ± 0.001 |
| BPA | 4.871 × 10−5 (±0.03) | 0.103 ± 0.002 | 3.862 × 10−3 (±0.04) | 0.514 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, T.-J.; Im, D.-K.; Kim, S.-M.; Lee, J.-D. 1,2-Diphenyl-o-carborane and Its Chromium Derivatives: Synthesis, Characterization, X-ray Structural Studies, and Biological Evaluations. Molecules 2023, 28, 4942. https://doi.org/10.3390/molecules28134942
Ha T-J, Im D-K, Kim S-M, Lee J-D. 1,2-Diphenyl-o-carborane and Its Chromium Derivatives: Synthesis, Characterization, X-ray Structural Studies, and Biological Evaluations. Molecules. 2023; 28(13):4942. https://doi.org/10.3390/molecules28134942
Chicago/Turabian StyleHa, Tae-Jin, Dong-Kyung Im, Seung-Min Kim, and Jong-Dae Lee. 2023. "1,2-Diphenyl-o-carborane and Its Chromium Derivatives: Synthesis, Characterization, X-ray Structural Studies, and Biological Evaluations" Molecules 28, no. 13: 4942. https://doi.org/10.3390/molecules28134942
APA StyleHa, T.-J., Im, D.-K., Kim, S.-M., & Lee, J.-D. (2023). 1,2-Diphenyl-o-carborane and Its Chromium Derivatives: Synthesis, Characterization, X-ray Structural Studies, and Biological Evaluations. Molecules, 28(13), 4942. https://doi.org/10.3390/molecules28134942







