Tracking the Progression of the Simulated Bronze Disease—A Laboratory X-ray Microtomography Study
Abstract
1. Introduction
2. Experimental Methods
2.1. Corrosive Specimen and Environment
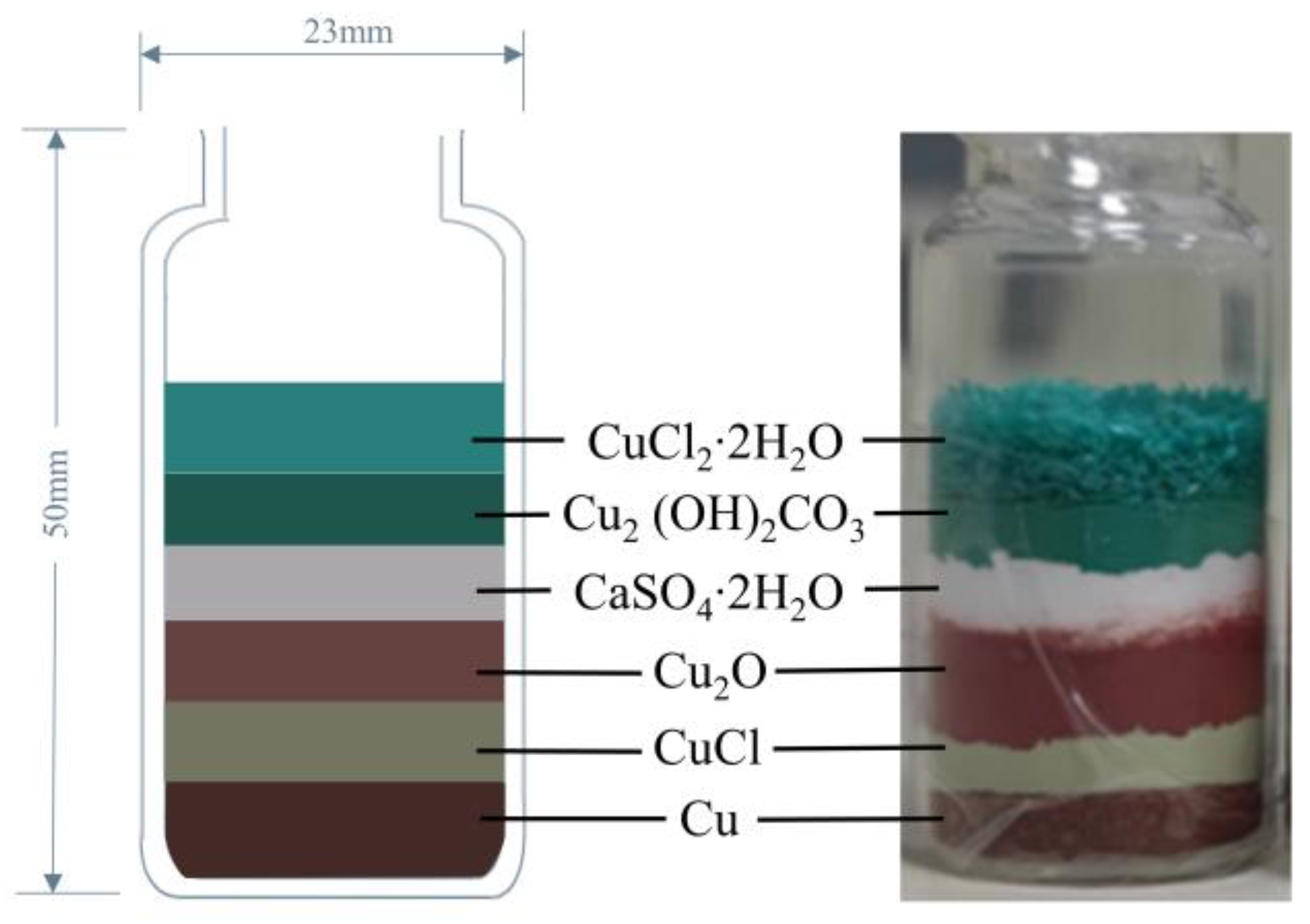
2.1.1. Corrosion Specimen Preparation
2.1.2. Corrosive Environment Setup
2.1.3. Experimental Principle and Expected Phenomenon
2.2. Experimental Process Detection and Product Analysis
2.2.1. X-ray Microtomography Experiment
2.2.2. Detection of Corrosion Products by XRD Analysis and OM Observation
3. Results and Discussion
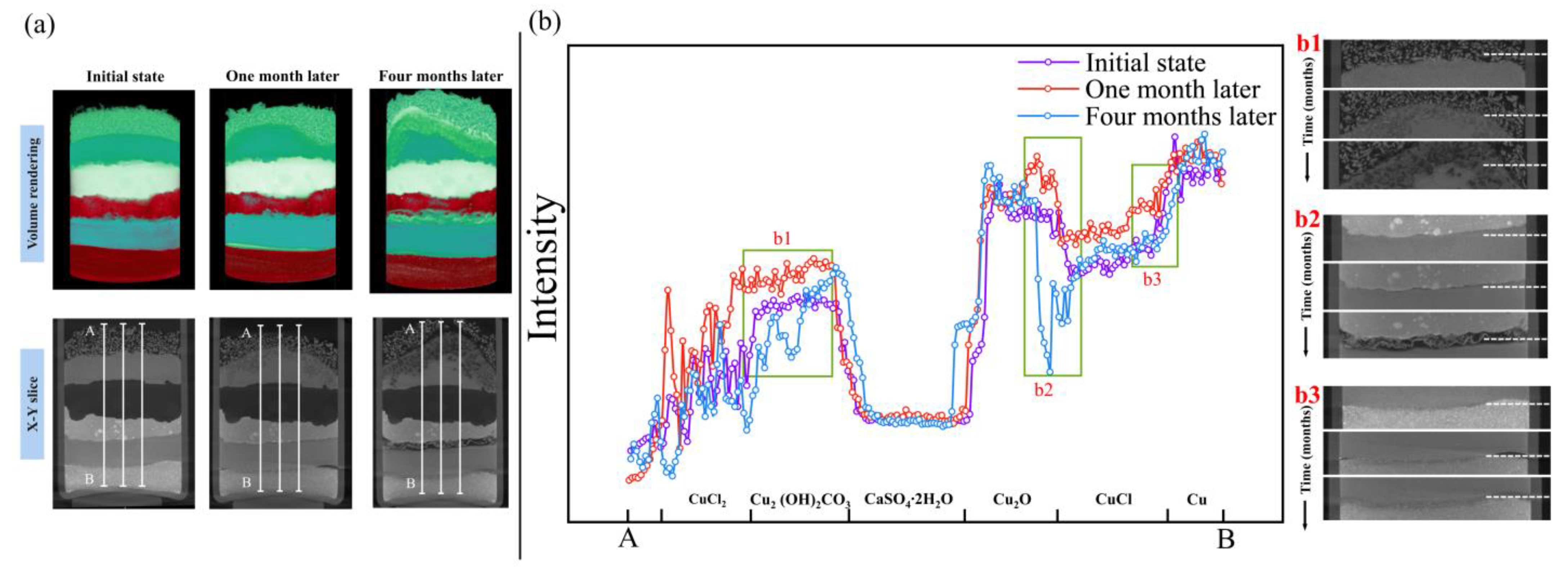
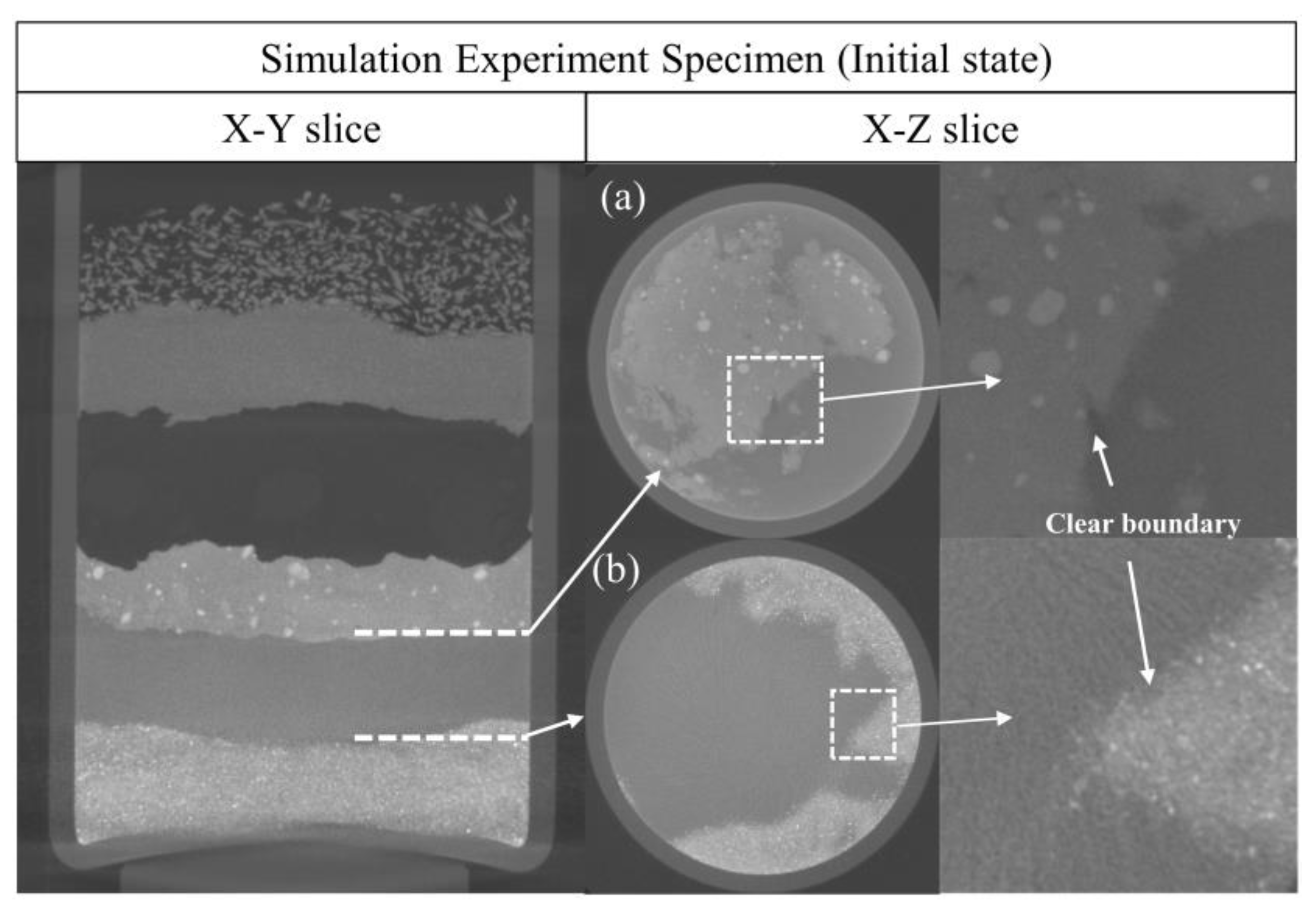
3.1. Corrosion of the Specimen by Using Micro-CT
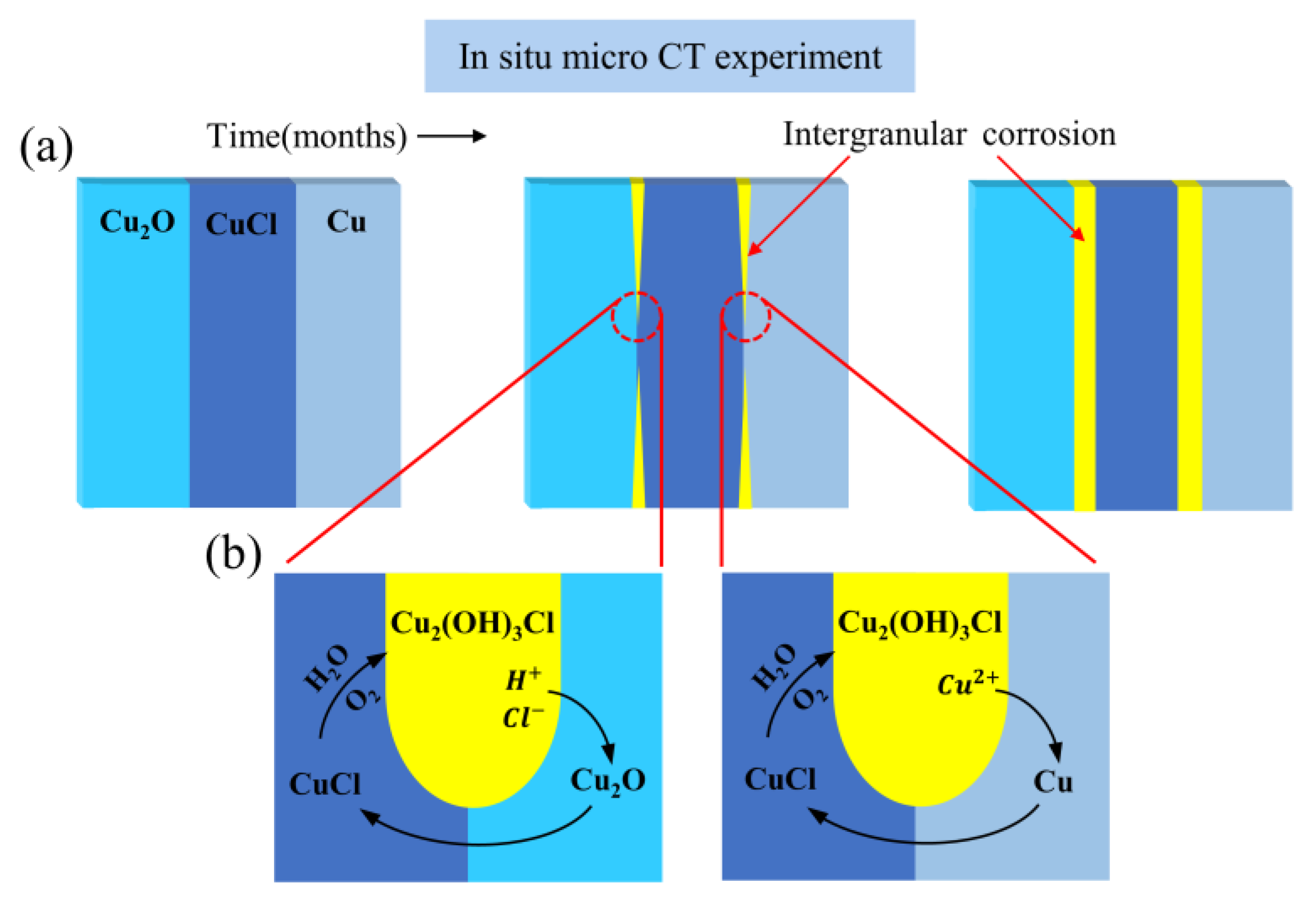
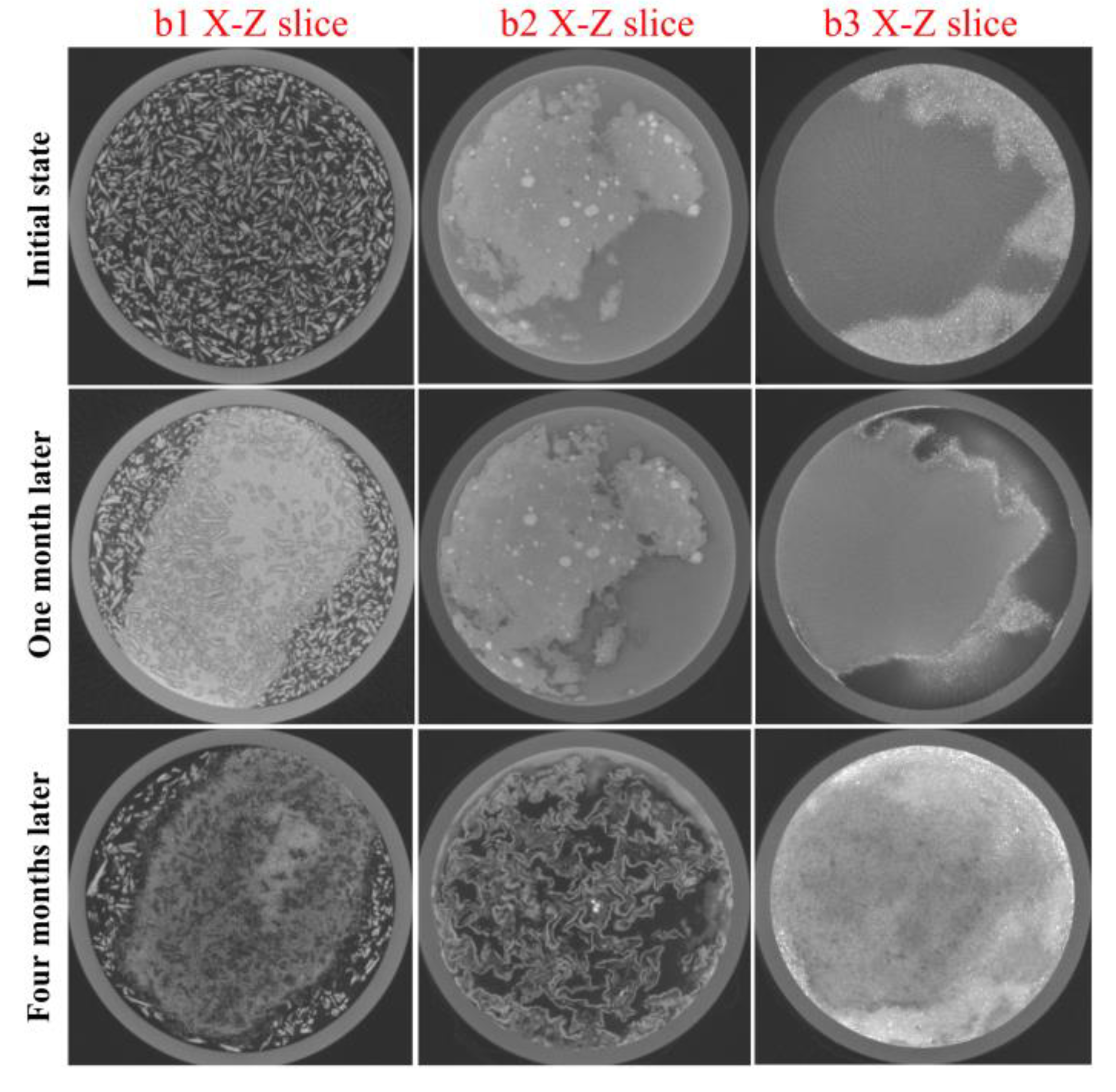
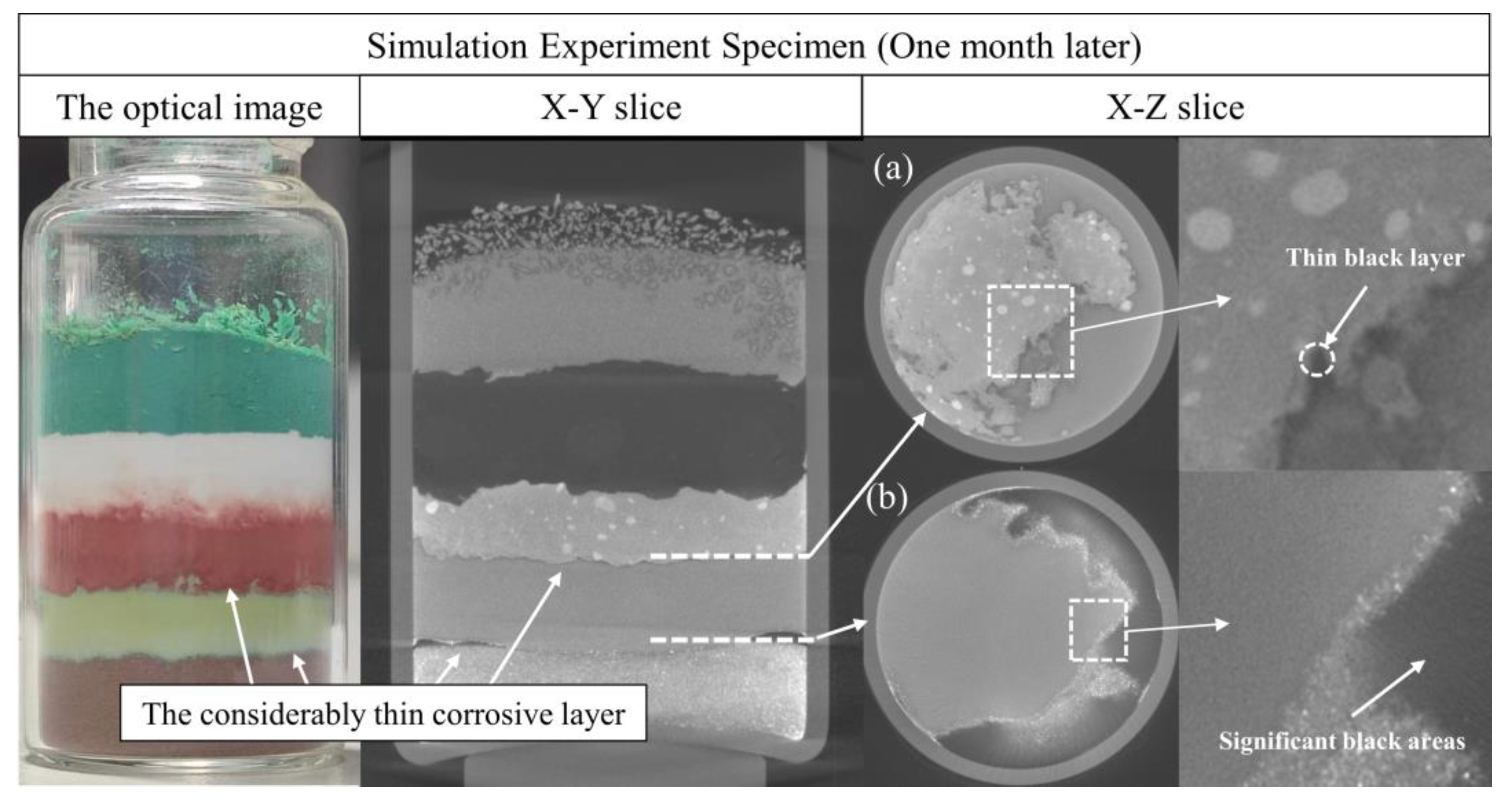
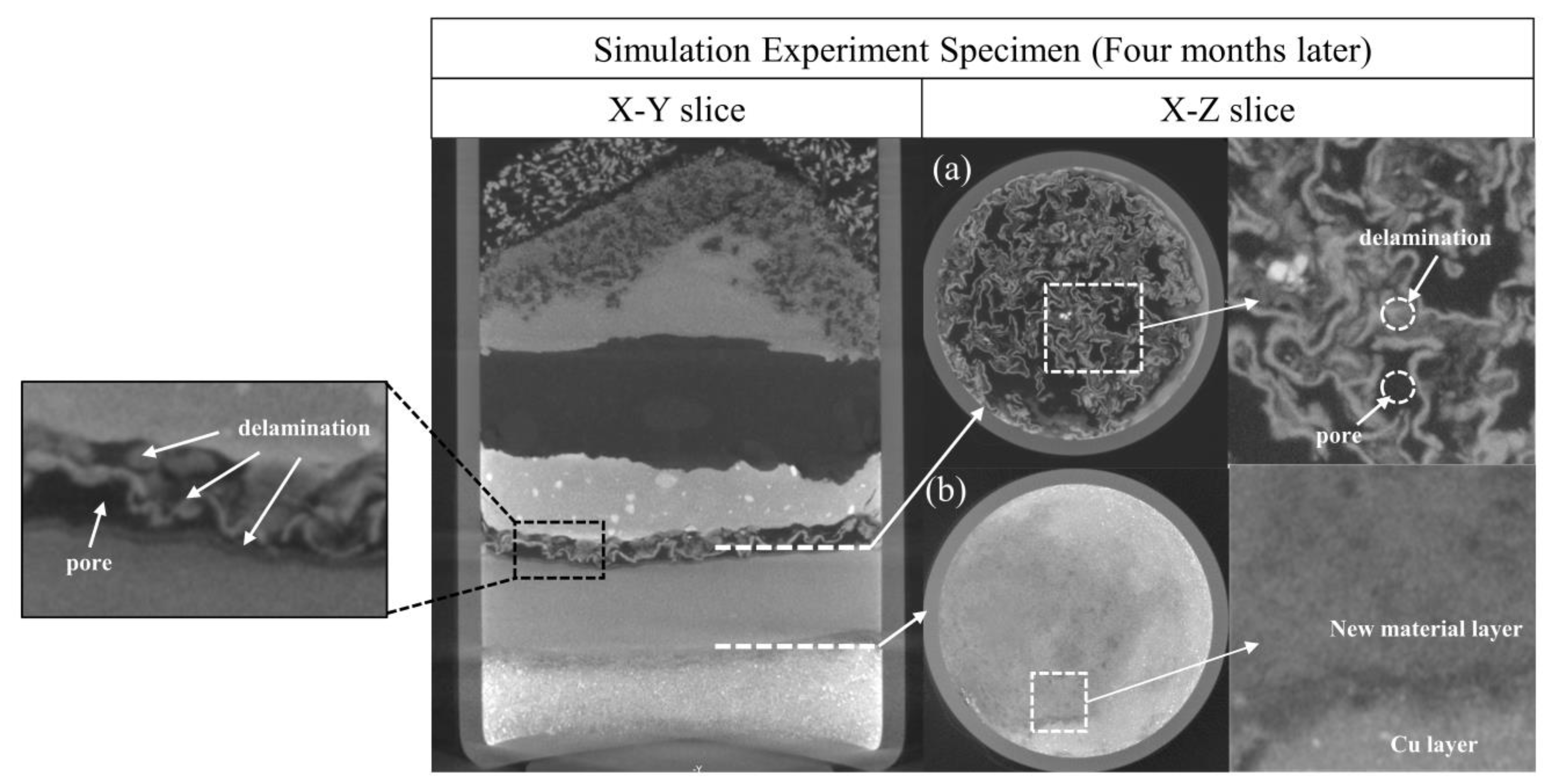
3.1.1. Evolution of the Reaction among Copper(I) Chloride, Copper and Copper(I) Oxide
3.1.2. Evolution of the Reaction between Copper(II) Chloride Dihydrate and Copper(II) Carbonate Hydroxide
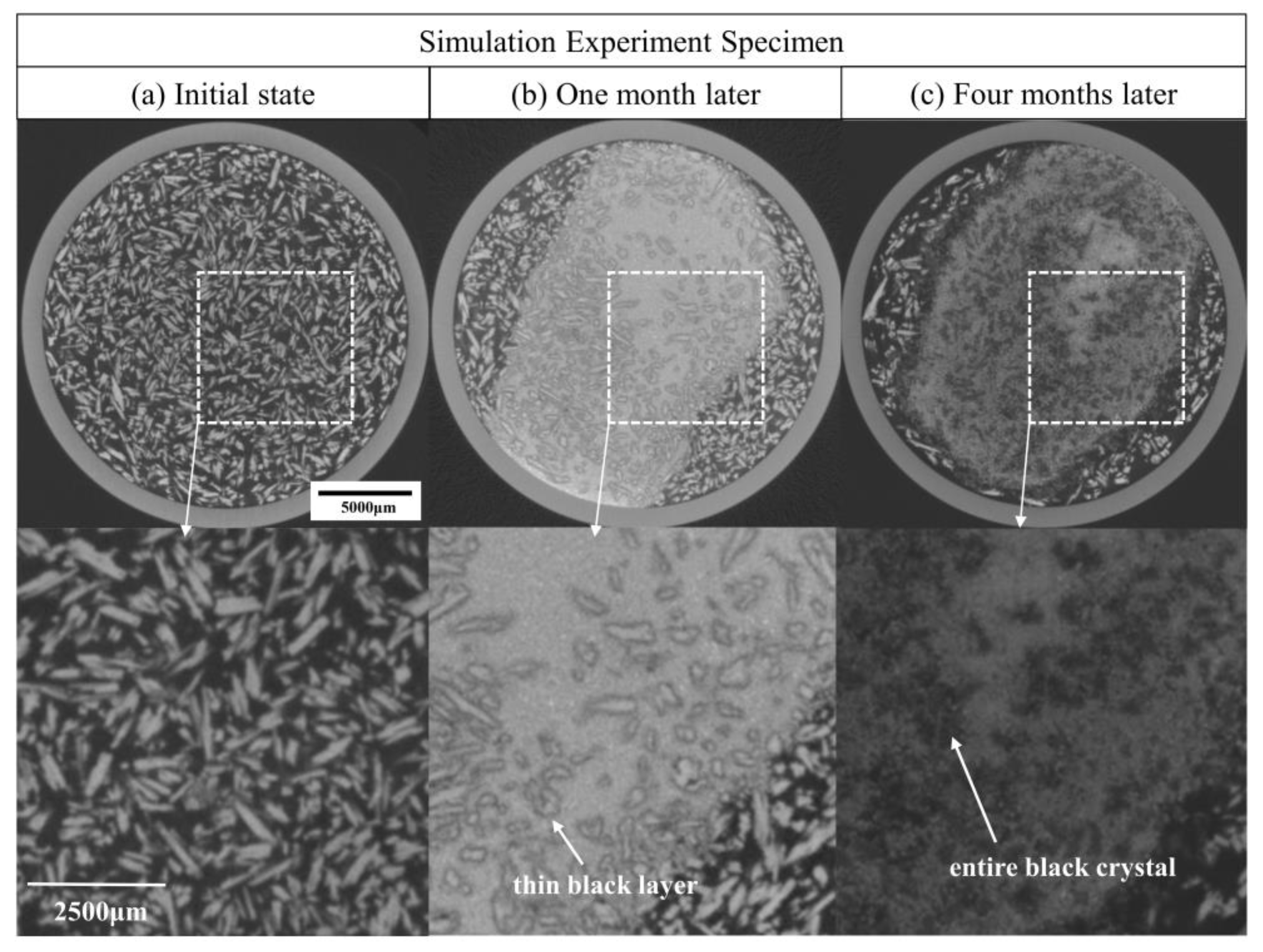
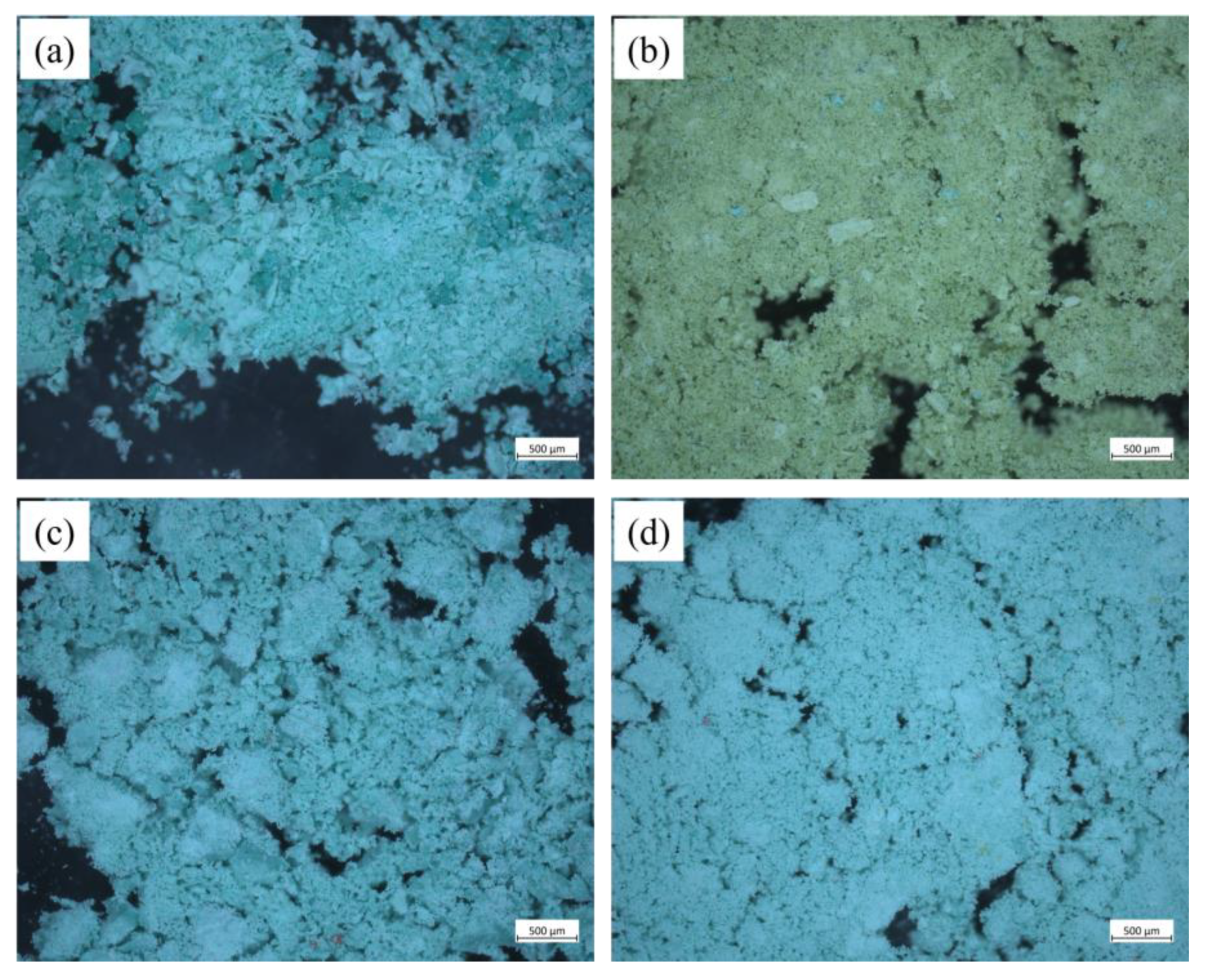
3.2. Analysis of the Corrosion Layer Using XRD and OM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Scott, D.A. Copper and Bronze in Art: Corrosion, Colorants, Conservation; Getty Conservation Institute: Los Angeles, CA, USA, 2002. [Google Scholar]
- Doménech-Carbó, A.; Doménech-Carbó, M.T.; Costa, V. Electrochemical Methods in Archaeometry, Conservation and Restoration; Springer: Berlin/Heidelberg, Germany, 2009; pp. 123–134. ISBN 978-3-540-92867-6. [Google Scholar]
- Scott, D.A. New insights on the corrosion of ancient bronzes using X-ray powder diffraction: The importance of paratacamite, sampleite, and connellite. Stud. Conserv. 2017, 62, 410–418. [Google Scholar] [CrossRef]
- Oudbashi, O.; Emami, S.M.; Ahmadi, H.; Davami, P. Micro-stratigraphical investigation on corrosion layers in ancient Bronze artefacts by scanning electron microscopy energy dispersive spectrometry and optical microscopy. Herit. Sci. 2013, 1, 21. [Google Scholar] [CrossRef]
- Grayburn, R.; Dowsett, M.G.; Hand, M.; Sabbe, P.-J.; Thompson, P.; Adriaens, A. Tracking the progression of bronze disease—A synchrotron X-ray diffraction study of nantokite hydrolysis. Corros. Sci. 2015, 91, 220–223. [Google Scholar] [CrossRef]
- Oudbashi, O.; Hasanpour, A.; Davami, P. Investigation on corrosion stratigraphy and morphology in some Iron Age bronze alloys vessels by OM, XRD and SEM–EDS methods. Appl. Phys. A 2016, 122, 262. [Google Scholar] [CrossRef]
- Coccato, A.; Bersani, D.; Coudray, A.; Sanyova, J.; Moens, L.; Vandenabeele, P. Raman spectroscopy of green minerals and reaction products with an application in Cultural Heritage research. J. Raman Spectrosc. 2016, 47, 1429–1443. [Google Scholar] [CrossRef]
- Bradley, R.S.; Liu, Y.; Burnett, T.L.; Zhou, X.; Lyon, S.; Withers, P.; Gholinia, A.; Hashimoto, T.; Graham, D.; Gibbon, S.; et al. Time-lapse lab-based X-ray nano-CT study of corrosion damage. J. Microsc. 2017, 267, 98–106. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Wu, N.; Liu, S.; Chen, J. A new application of Fiber optics reflection spectroscopy (FORS): Identification of “bronze disease” induced corrosion products on ancient bronzes. J. Cult. Herit. 2021, 49, 19–27. [Google Scholar] [CrossRef]
- De Maeseneer, M.; Buls, N.; Cleeren, N.; Lenchik, L.; De Mey, J. An ancient Roman bowl embedded in a soil sample: Surface shaded three dimensional display using data from a multi-detector CT. JBR-BTR 2006, 89, 264–265. [Google Scholar]
- Li, J.; Guériau, P.; Bellato, M.; King, A.; Robbiola, L.; Thoury, M.; Baillon, M.; Fossé, C.; Cohen, S.X.; Moulhérat, C.; et al. Synchrotron-Based Phase Mapping in Corroded Metals: Insights from Early Copper-Base Artifacts. Anal. Chem. 2019, 91, 1815–1825. [Google Scholar] [CrossRef]
- Albertin, F.; Bettuzzi, M.; Brancaccio, R.; Morigi, M.P.; Casali, F. X-ray Computed Tomography In Situ: An Opportunity for Museums and Restoration Laboratories. Heritage 2019, 2, 2028–2038. [Google Scholar] [CrossRef]
- Bude, R.; Bigelow, E. Nano-CT Evaluation of Totally Corroded Coins: A Demonstration Study to Determine if Detail Might Still Be Discernible Despite the Lack of Internal, Non-Corroded, Metal. Archaeometry 2020, 62, 1195–1201. [Google Scholar] [CrossRef]
- Bettuzzi, M.; Casali, F.; Morigi, M.P.; Brancaccio, R.; Carson, D.; Chiari, G.; Maish, J. Computed tomography of a medium size Roman bronze statue of Cupid. Appl. Phys. A 2015, 118, 1161–1169. [Google Scholar] [CrossRef]
- Tian, H.; Zeng, X.; Guo, J.; Qu, L.; Chen, K. X-ray computed tomography reveals special casting techniques used with unusual bronze objects unearthed from the Sanxingdui site. Adv. Archaeomater. 2022, 3, 28–33. [Google Scholar] [CrossRef]
- Nguyen, H.Y.; Keating, S.; Bevan, G.; Gabov, A.; Daymond, M.; Schillinger, B.; Murray, A. Seeing through Corrosion: Using Micro-focus X-ray Computed Tomography and Neutron Computed Tomography to Digitally “Clean” Ancient Bronze Coins. MRS Online Proc. Libr. 2011, 1319, 305. [Google Scholar] [CrossRef]
- Maher, M. X-ray computed tomography of a late period falcon bronze coffin. Radiat. Phys. Chem. 2020, 166, 108475. [Google Scholar] [CrossRef]
- Schoell, R.; Xi, L.; Zhao, Y.; Wu, X.; Yu, Z.; Kenesei, P.; Almer, J.; Shayer, Z.; Kaoumi, D. In situ synchrotron X-ray tomography of 304 stainless steels undergoing chlorine-induced stress corrosion cracking. Corros. Sci. 2020, 170, 108687. [Google Scholar] [CrossRef]
- Hirayama, K.; Toda, H.; Fu, D.; Masunaga, R.; Su, H.; Shimizu, K.; Takeuchi, A.; Uesugi, M. Damage micromechanisms of stress corrosion cracking in Al-Mg alloy with high magnesium content. Corros. Sci. 2021, 184, 109343. [Google Scholar] [CrossRef]
- Gianni, L.; Cavallini, M.; Natali, S.; Adriaens, A. Wet and dry accelerated aging tests in a spray chamber to understand the effects of acid rain frequencies on bronze corrosion. Int. J. Electrochem. Sci. 2013, 8, 1822–1838. [Google Scholar]
- Mennucci, M.M.; Sanchez-Moreno, M.; Aoki, I.V.; Bernard, M.-C.; de Melo, H.G.; Joiret, S.; Vivier, V. Local electrochemical investigation of copper patina. J. Solid State Electrochem. 2012, 16, 109–116. [Google Scholar] [CrossRef]
- Šatović, D.; Žulj, L.V.; Desnica, V.; Fazinić, S.; Martinez, S. Corrosion evaluation and surface characterization of the corrosion product layer formed on Cu–6Sn bronze in aqueous Na2SO4 solution. Corros. Sci. 2009, 51, 1596–1603. [Google Scholar] [CrossRef]
- Fuente, D.D.; Simancas, J.; Morcillo, M. Morphological study of 16-year patinas formed on copper in a wide range of atmospheric exposures. Corros. Sci. 2008, 50, 268–285. [Google Scholar] [CrossRef]
- Tommesani, L.; Brunoro, G.; Garagnani, G.L. Corrosion behaviour of modern bronzes simulating historical alloys. ArchéoSci. Rev. D’archéométrie 1997, 21, 131–139. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Lv, G. Formation processes of CuCl and regenerated Cu crystals on bronze surfaces in neutral and acidic media. Appl. Surf. Sci. 2006, 252, 6294–6303. [Google Scholar] [CrossRef]
- Casaletto, M.P. Sustainable Conservation of Bronze Artworks: Advanced Research in Materials Science; The J. Paul Getty Museum and Getty Conservation Institute: Los Angeles, CA, USA, 2017; pp. 280–288. [Google Scholar]
- MacLeod, I.D. Bronze Disease: An Electrochemical Explanation. ICCM Bull. 1981, 7, 16–26. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Guida, G.; Pascucci, M.; Giuliani, C.; Messina, E.; Fierro, G.; Di Carlo, G. Micro-chemical investigation of corrosion products naturally grown on archaeological Cu-based artefacts retrieved from the Mediterranean sea. Appl. Surf. Sci. 2019, 470, 695–706. [Google Scholar] [CrossRef]
- Robbiola, L.; Blengino, J.M.; Fiaud, C. Morphology and mechanisms of formation of natural patinas on archaeological Cu–Sn alloys. Corros. Sci. 1998, 40, 2083–2111. [Google Scholar] [CrossRef]
- Salzano de Luna, M.; Buonocore, G.G.; Giuliani, C.; Messina, E.; Di Carlo, G.; Lavorgna, M.; Ambrosio, L.; Ingo, G.M. Long-Lasting Efficacy of Coatings for Bronze Artwork Conservation: The Key Role of Layered Double Hydroxide Nanocarriers in Protecting Corrosion Inhibitors from Photodegradation. Angew. Chem. (Int. Ed. Engl.) 2018, 57, 7380–7384. [Google Scholar] [CrossRef]
- Liang, Z.; Jiang, K.; Zhang, T.-a. Corrosion behaviour of lead bronze from the Western Zhou Dynasty in an archaeological-soil medium. Corros. Sci. 2021, 191, 109721. [Google Scholar] [CrossRef]
- Quaranta, M.; Catelli, E.; Prati, S.; Sciutto, G.; Mazzeo, R. Chinese archaeological artefacts: Microstructure and corrosion behaviour of high-leaded bronzes. J. Cult. Herit. 2014, 15, 283–291. [Google Scholar] [CrossRef]
- Sandu, I.; Ursulescu, N.; Bounegru, O.; Alexandru, A. Pedological stratification effect of corrosion and contamination products on Byzantine bronze artefacts. Corros. Eng. Sci. Technol. 2008, 43, 256–266. [Google Scholar] [CrossRef]
- Angelucci, S.; Fiorentino, P.; Kosinkova, J.; Marabelli, M. Pitting Corrosion in Copper and Copper Alloys: Comparative Treatment Tests. Stud. Conserv. 1978, 23, 147–156. [Google Scholar] [CrossRef]
- Scott, D.A. A Review of Copper Chlorides and Related Salts in Bronze Corrosion and as Painting Pigments. Stud. Conserv. 2000, 45, 39–53. [Google Scholar] [CrossRef]
- Hashemi, T.; Hogarth, C.A. The mechanism of corrosion inhibition of copper in NaCl solution by benzotriazole studied by electron spectroscopy. Electrochim. Acta 1988, 33, 1123–1127. [Google Scholar] [CrossRef]
- Pollard, A.M. The Copper Chloride System and Corrosion: A Complex Interplay of Kinetic and Thermodynamic Factors; National Association of Corrosion Engineers: Houston, TX, USA, 1992; pp. 123–133. ISBN 978-18-7791-438-6. [Google Scholar]
- White, R.; Kelly, S. Correlative, Multi-scale, Lab-based X-ray Tomography: From Millimeters to Nanometers. Microsc. Microanal. 2020, 26, 1002–1003. [Google Scholar] [CrossRef]
- Casali, F. X-ray and Neutron Digital Radiography and Computed Tomography for Cultural Heritage; Elsevier: Amsterdam, The Netherlands, 2006; pp. 41–123. ISBN 978-12-8064-174-9. [Google Scholar]
- Buzug, T.M. Computed Tomography; Springer: Berlin/Heidelberg, Germany, 2011; pp. 311–342. ISBN 978-3-540-74657-7. [Google Scholar]
- Bertrand, L.; Schoeder, S.; Anglos, D.; Breese, M.B.; Janssens, K.; Moini, M.; Simon, A. Mitigation strategies for radiation damage in the analysis of ancient materials. Trends Anal. Chem. 2015, 66, 128–145. [Google Scholar] [CrossRef]
- Swinehart, D.F. The Beer-Lambert Law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Fleet, M.E. The crystal structure of paratacamite, Cu2(OH)3Cl. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 183–187. [Google Scholar] [CrossRef]
- Quast, K.B. Leaching of atacamite (Cu2(OH)3Cl) using dilute sulphuric acid. Miner. Eng. 2000, 13, 1647–1652. [Google Scholar] [CrossRef]
- Giangrande, C. Identification of Bronze Corrosion Products by Infrared Absorption Spectroscopy; Jubilee Conservation Conference: London, UK, 1987; pp. 135–148. ISBN 978-09-5124-290-2. [Google Scholar]
- Hu, Y.; Wei, Y.; Li, L.; Zhang, J.; Chen, J. Same situ, different corrosion phenomena caused by chloride: The effect of the archaeological context on bronzes from Sujialong Cemetery, China. J. Cult. Herit. 2021, 52, 23–30. [Google Scholar] [CrossRef]
- Lewin, S.Z. A New Approach to Establishing the Authenticity of Patinas on Copper-Base Artifacts; Museum of Fine Arts: Boston, MA, USA, 1973; pp. 62–66. ISBN 978-08-7846-071-7. [Google Scholar]


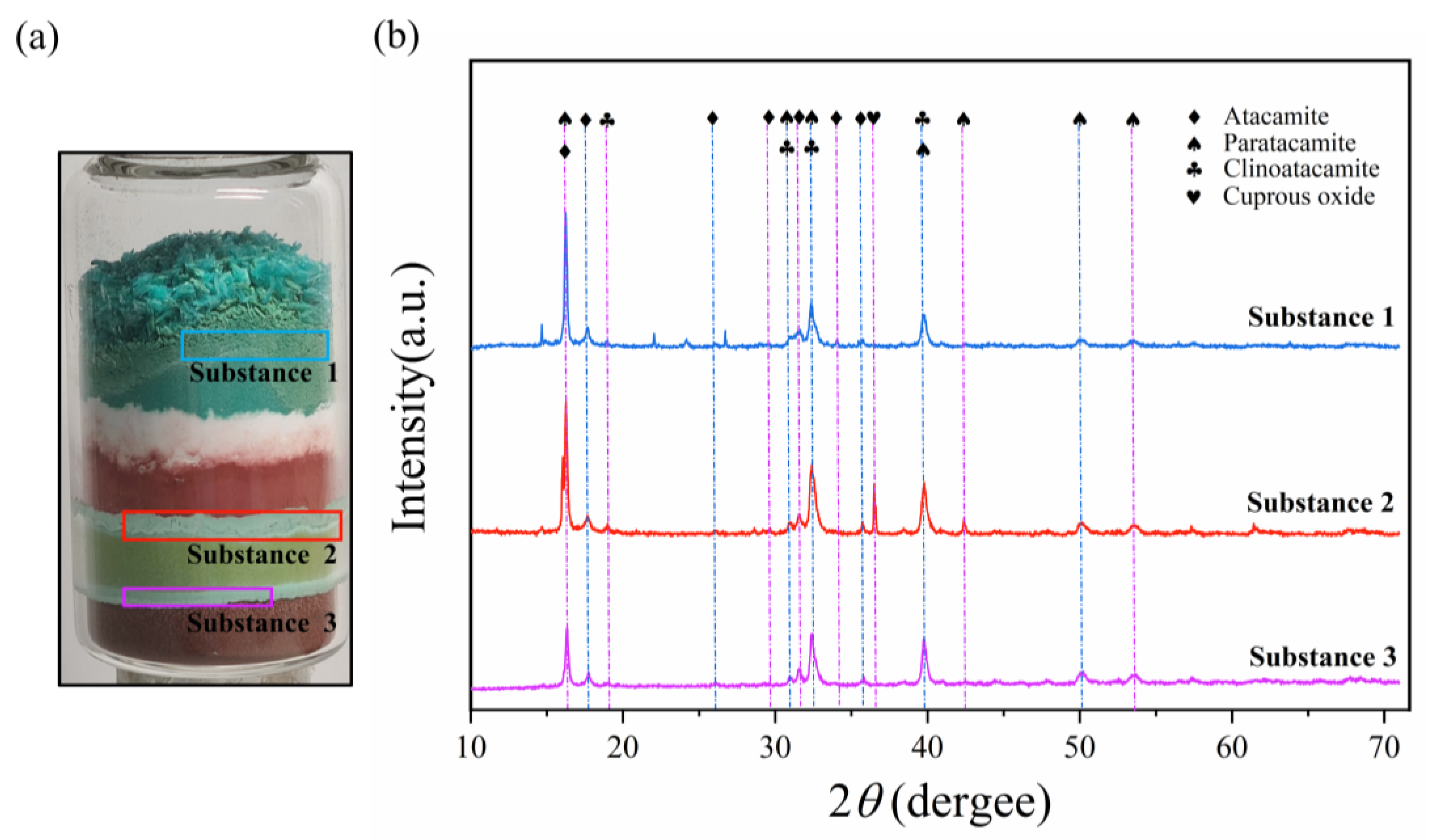
| Corrosion Product | Formula | Crystal System | Color |
|---|---|---|---|
| Nantokite | CuCl | Cubic | Pale green |
| Atacamite a | Cu2(OH)3Cl | Orthorhombic | Pale green |
| Clinoatacamite a | Cu2(OH)3Cl | Rhombohedral | Pale green |
| Experiment Stages | CuCl2·2H2O | Cu2(OH)2CO3 | CaSO4·2H2O | Cu2O | CuCl | Cu |
|---|---|---|---|---|---|---|
| Initial state | −369.4 | −416.5 | −712.1 | −165.8 | −274.8 | 0 |
| One month later | −302.7 | −327.3 | −690.9 | −152.1 | −231.1 | 0 |
| Four months later | −212.1 | −258.1 | −644.8 | −101.3 | −211.9 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xi, X.; Li, L.; Zhang, Z.; Han, Y.; Wang, X.; Sun, Z.; Zhao, H.; Yuan, N.; Li, H.; et al. Tracking the Progression of the Simulated Bronze Disease—A Laboratory X-ray Microtomography Study. Molecules 2023, 28, 4933. https://doi.org/10.3390/molecules28134933
Wang Z, Xi X, Li L, Zhang Z, Han Y, Wang X, Sun Z, Zhao H, Yuan N, Li H, et al. Tracking the Progression of the Simulated Bronze Disease—A Laboratory X-ray Microtomography Study. Molecules. 2023; 28(13):4933. https://doi.org/10.3390/molecules28134933
Chicago/Turabian StyleWang, Zedong, Xiaoqi Xi, Lei Li, Zhicun Zhang, Yu Han, Xinguang Wang, Zhaoying Sun, Hongfeng Zhao, Ning Yuan, Huimin Li, and et al. 2023. "Tracking the Progression of the Simulated Bronze Disease—A Laboratory X-ray Microtomography Study" Molecules 28, no. 13: 4933. https://doi.org/10.3390/molecules28134933
APA StyleWang, Z., Xi, X., Li, L., Zhang, Z., Han, Y., Wang, X., Sun, Z., Zhao, H., Yuan, N., Li, H., Yan, B., & Chen, J. (2023). Tracking the Progression of the Simulated Bronze Disease—A Laboratory X-ray Microtomography Study. Molecules, 28(13), 4933. https://doi.org/10.3390/molecules28134933







