Comparison of Homo-Polyimide Films Derived from Two Isomeric Bis-Benzimidazole Diamines
Abstract
1. Introduction
2. Results and Discussion
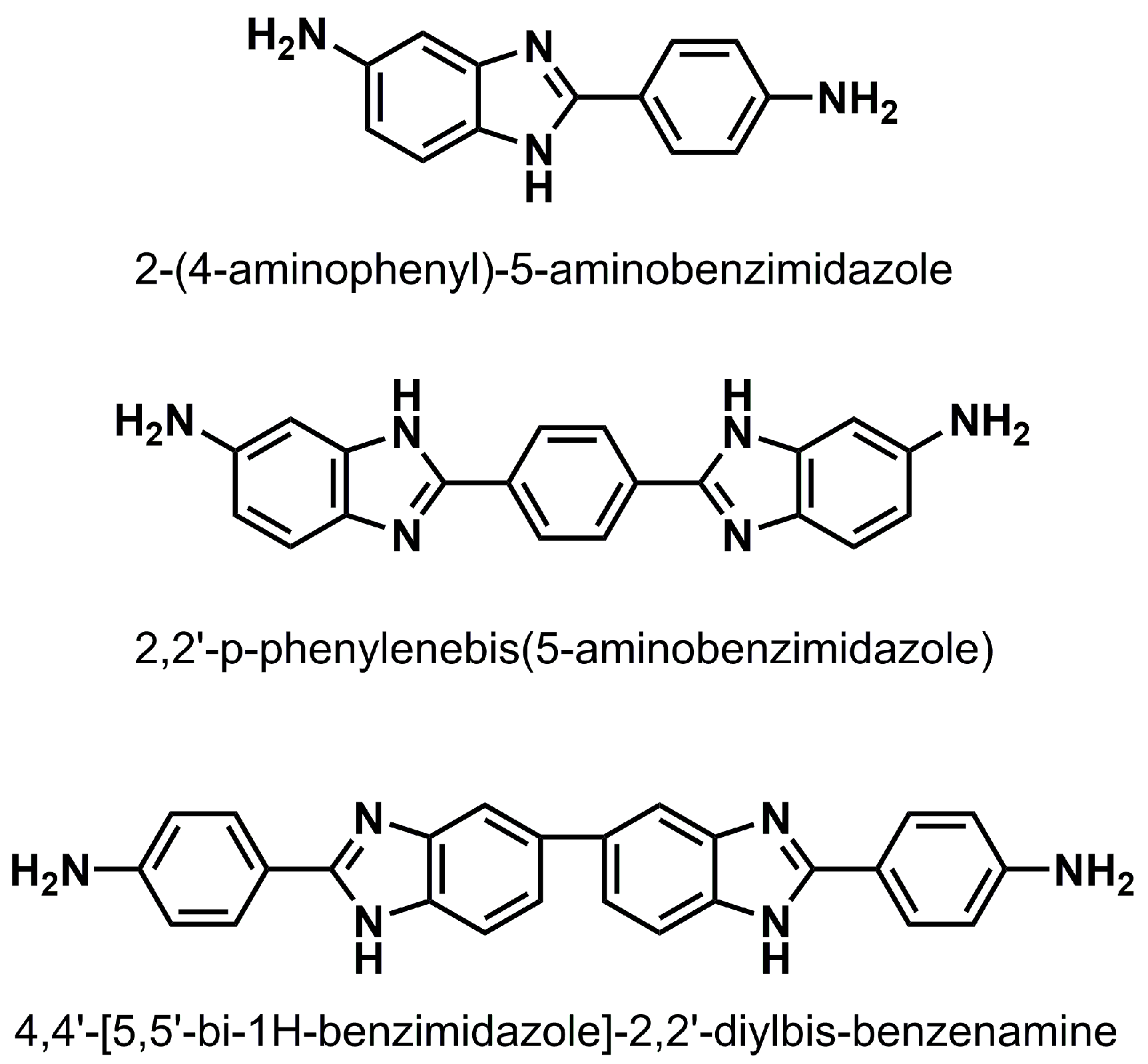
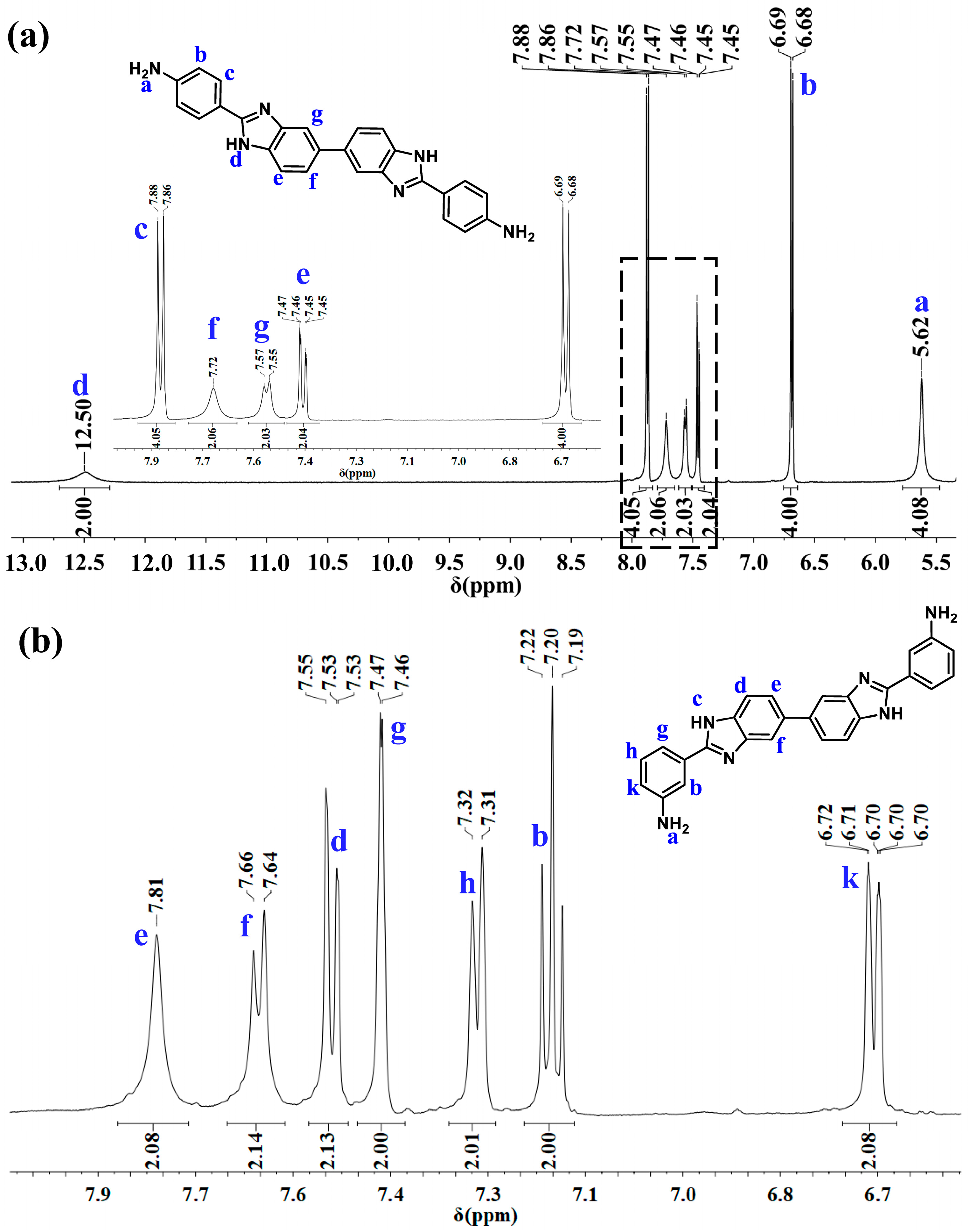
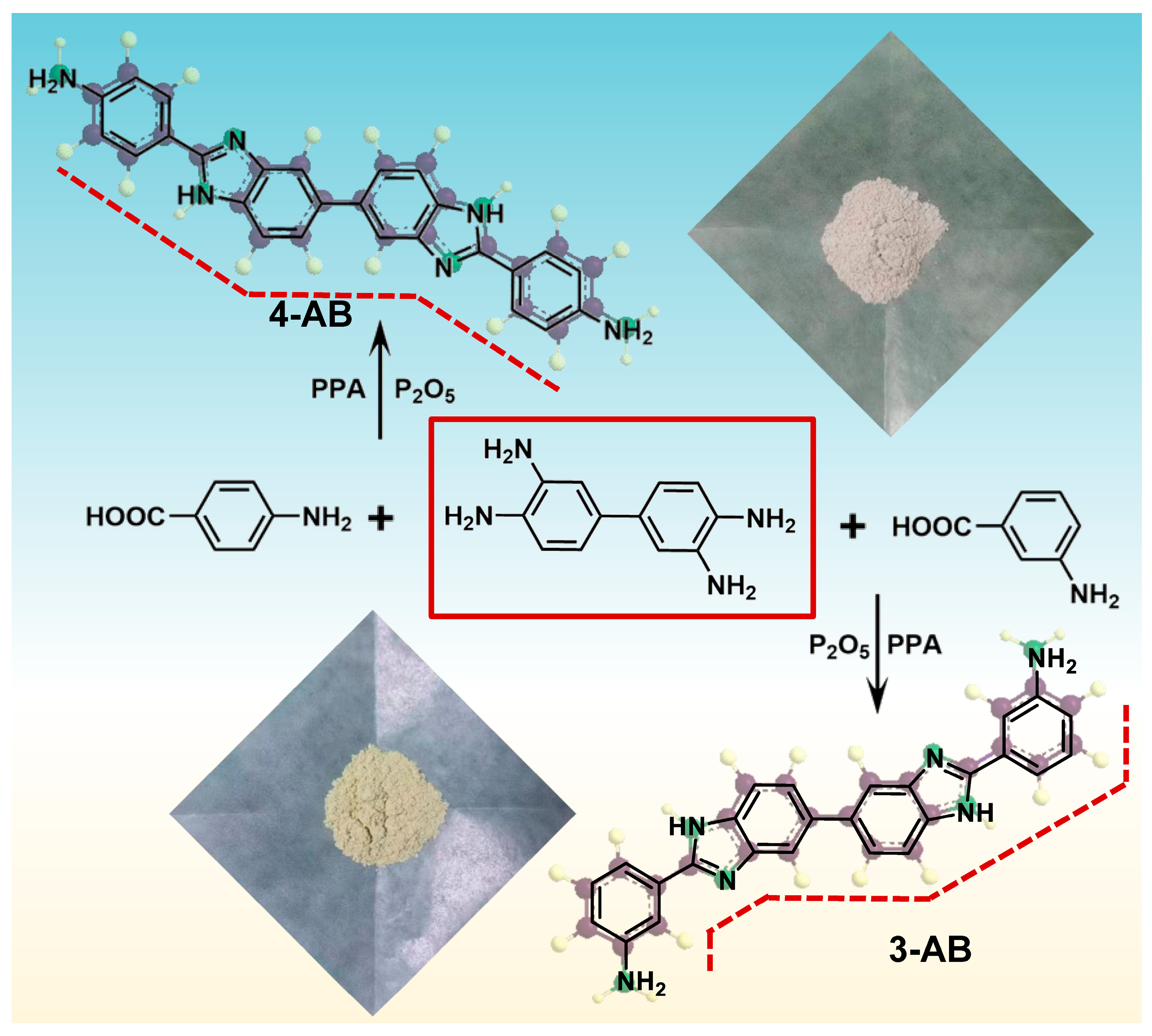
2.1. Monomer Characterization
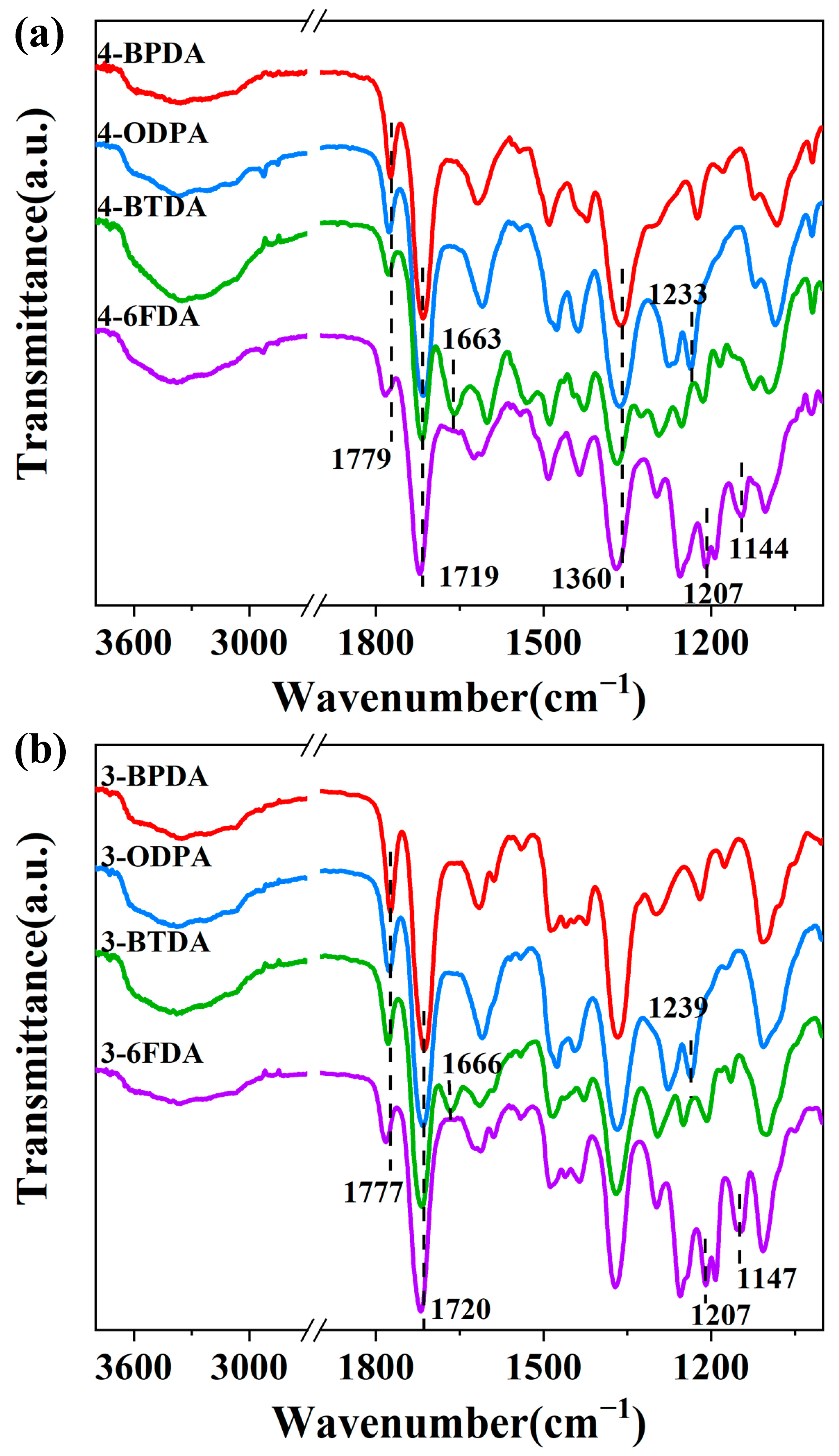
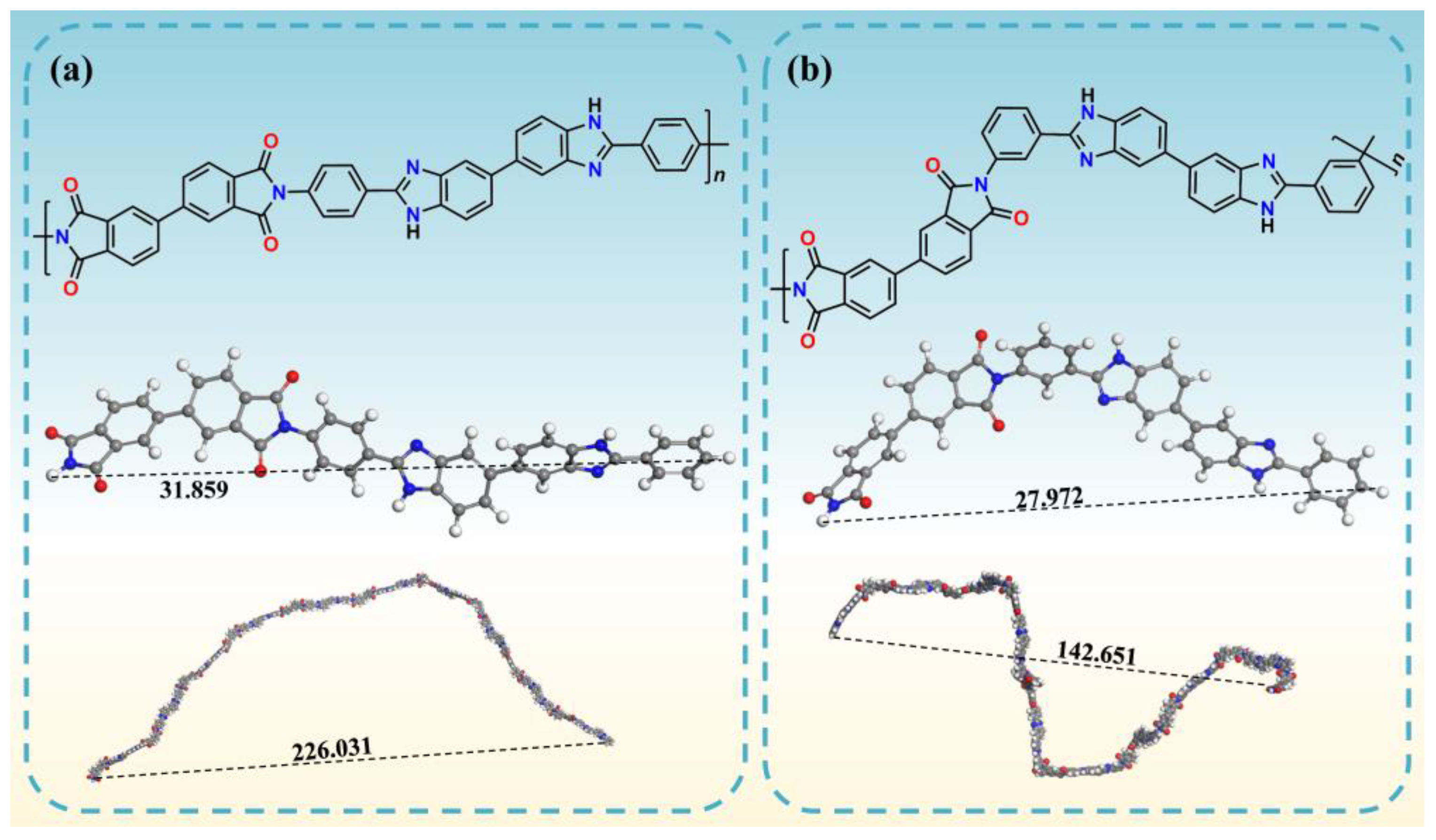
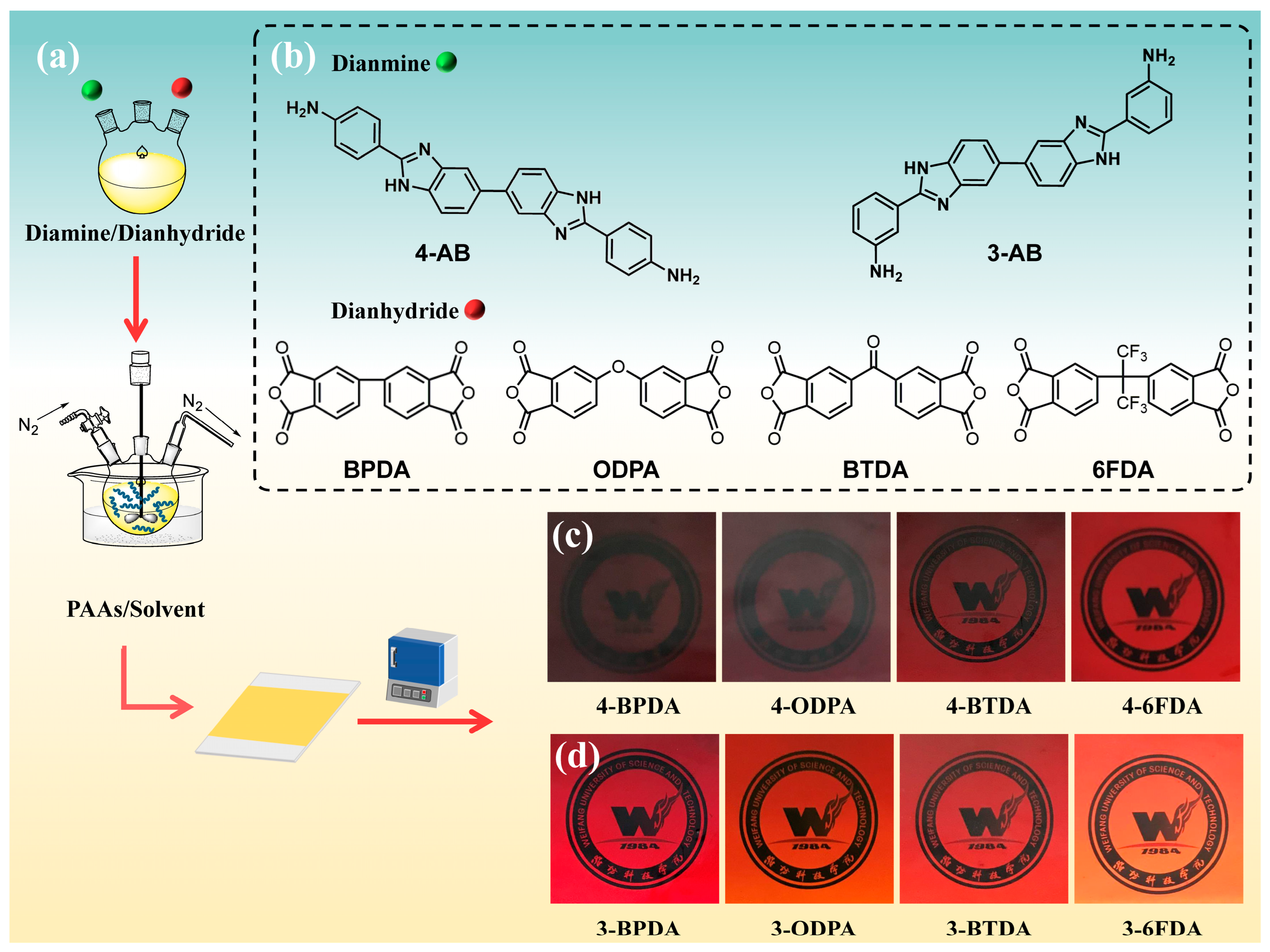
2.2. Polymer Characterization
2.3. Film Quality
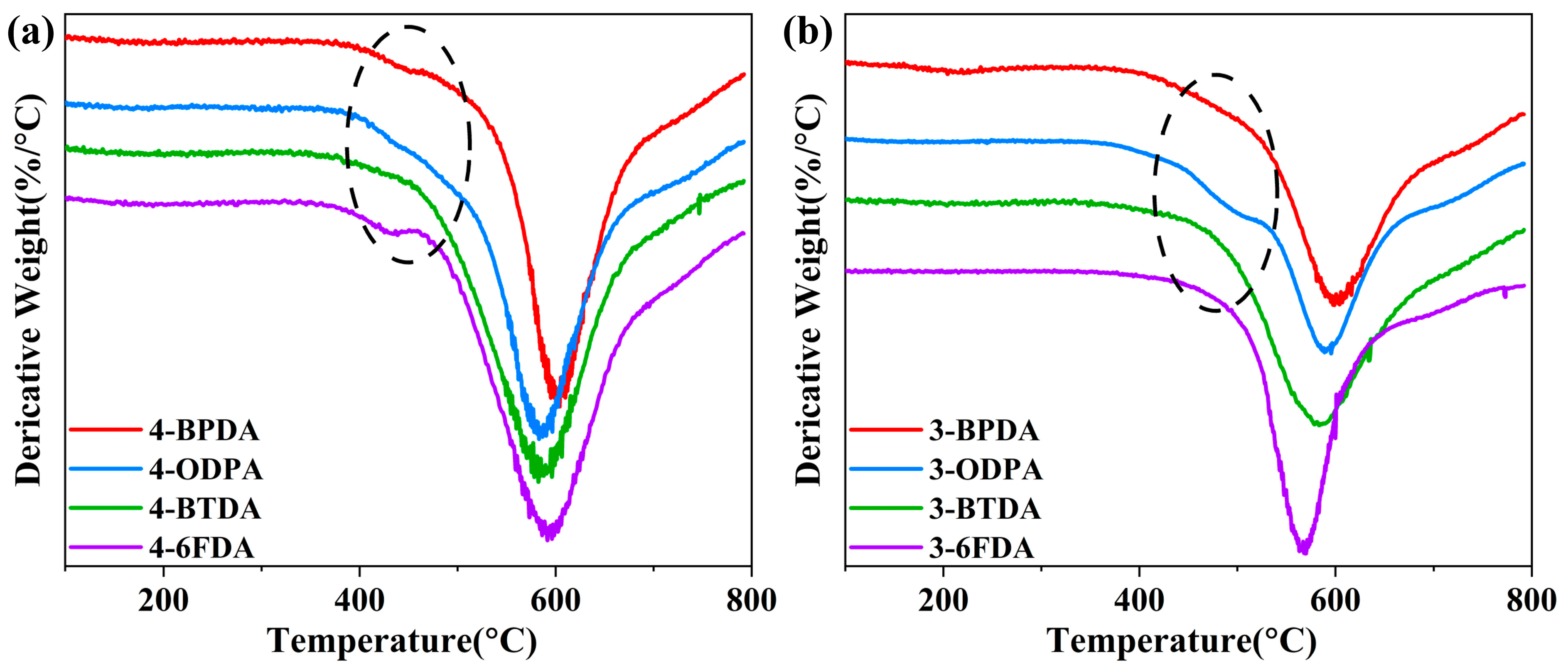
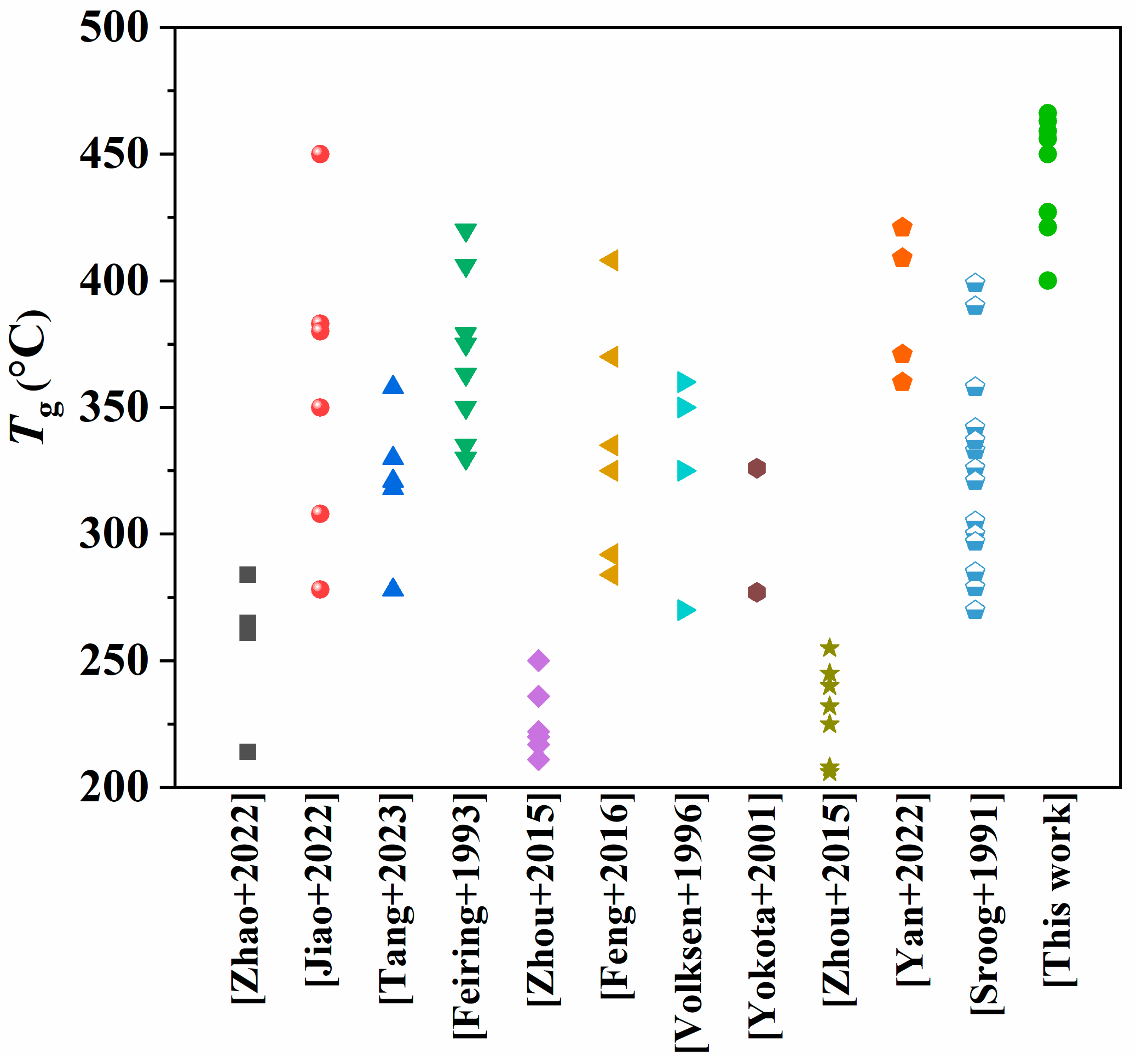
2.4. Thermal Properties
3. Experimental Section
3.1. Materials
3.2. Measurement
3.3. Monomer Synthesis
3.3.1. Synthesis of 4,4′-[5,5′-Bi-1H-benzimidazole]-2,2′-diylbis-benzenamine (4-AB)
3.3.2. Synthesis of 3,3′-[5,5′-Bi-1H-benzimidazole]-2,2′-diylbis-benzenamine (3-AB)
3.4. Polymer Synthesis
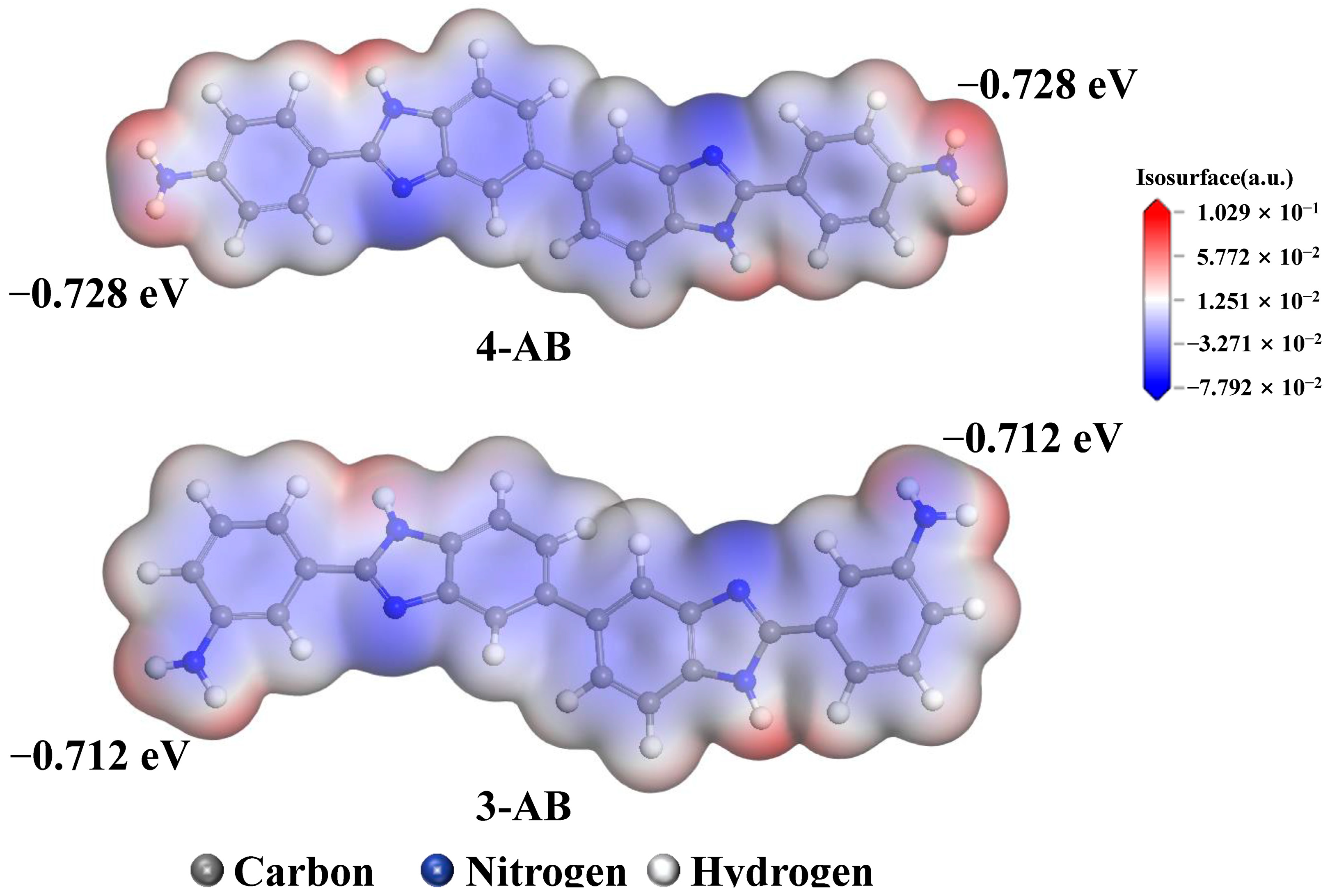
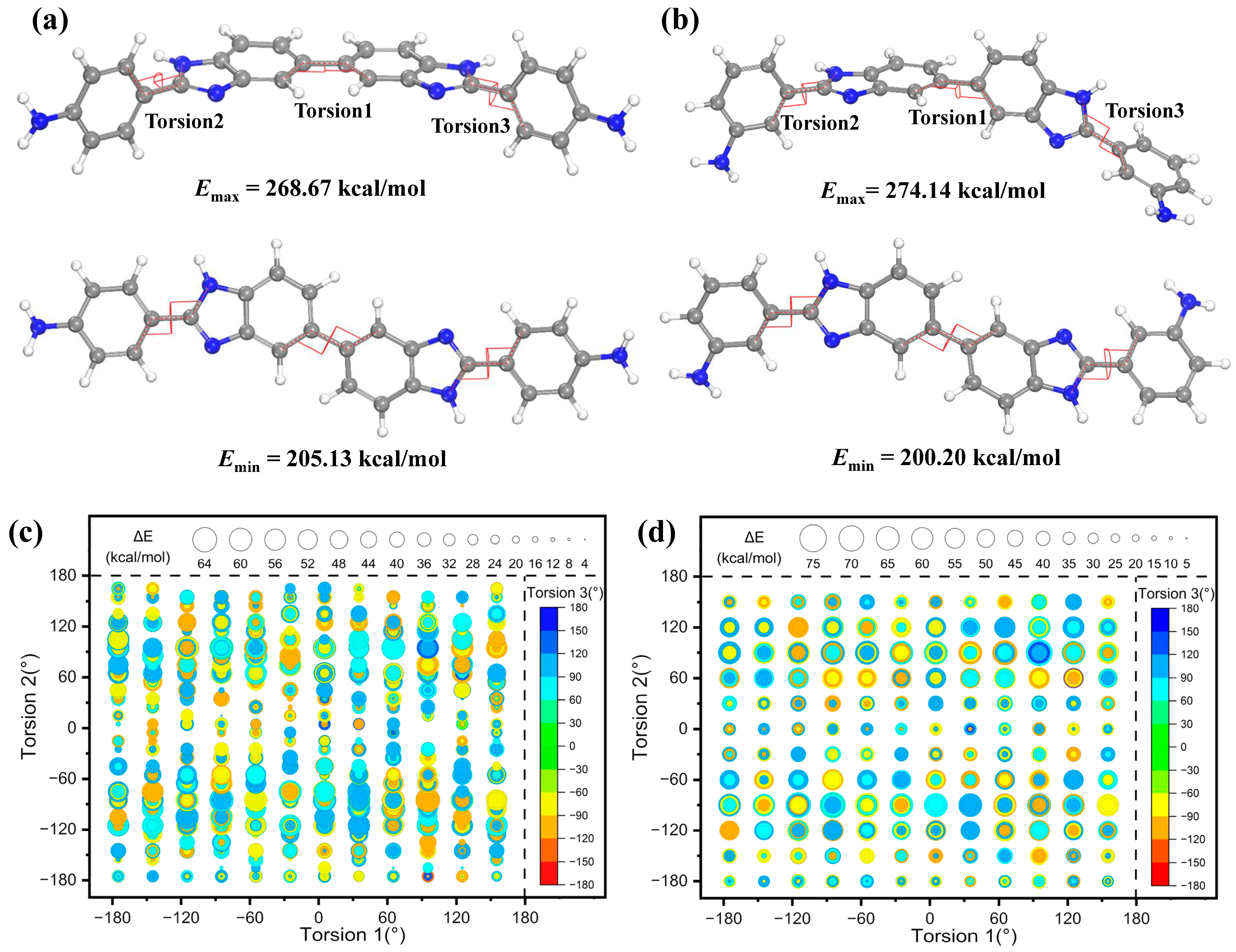
3.5. Molecular Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Kumar, A.; Priyam; Meena, H.; Prakash, J.; Wang, L.; Singh, G. Recent advances on semiconducting nanomaterials–ferroelectric liquid crystals nanocomposites. J. Phys. Condens. Matter 2022, 34, 013004. [Google Scholar] [CrossRef]
- Malik, P.; Supreet; Kumar, A.; Castagna, R.; Singh, G. Recent advances and future perspectives on nanoparticles-controlled alignment of liquid crystals for displays and other photonic devices. Crit. Rev. Solid State Mater. Sci. 2023, 48, 57–92. [Google Scholar] [CrossRef]
- Zuo, H.; Qian, G.; Li, H.-B.; Gan, F.; Fang, Y.; Li, X.; Dong, J.; Zhao, X.; Zhang, Q. Reduced coefficient of linear thermal expansion for colorless and transparent polyimide by introducing rigid-rod amide units: Synthesis and properties. Polym. Chem. 2022, 13, 2999–3008. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Wang, Y.; Chu, W.; Liu, F.; Wang, B. Cross-linked and rigid polyimide composite foams with prominent fire resistant, thermal insulating, and wave-transparent properties. ACS Appl. Polym. Mater. 2022, 4, 6994–7003. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Shi, Y.; Zhu, S.; Zhang, Y.; Jin, J. Synergistic design of enhanced π–π interaction and decarboxylation cross-linking of polyimide membranes for natural gas separation. Macromolecules 2022, 55, 2970–2982. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, Q.; Xie, C.; Wang, Y.; Zhao, C.; Xiao, C.; Wei, Y.; Li, W. Mechanical-robust and recyclable polyimide substrates coordinated with cyclic Ti-oxo cluster for flexible organic solar cells. NPJ Flex. Electron. 2022, 6, 37. [Google Scholar] [CrossRef]
- Chung, I.S.; Kim, S.Y. Synthesis and characterization of poly(amide imides) containing benzimidazole-rings. Polym. Bull. 1997, 38, 627–634. [Google Scholar] [CrossRef]
- Yin, C.; Dong, J.; Tan, W.; Lin, J.; Chen, D.; Zhang, Q. Strain-induced crystallization of polyimide fibers containing 2-(4-aminophenyl)-5-aminobenzimidazole moiety. Polymer 2015, 75, 178–186. [Google Scholar] [CrossRef]
- Ma, X.; Kang, C.; Chen, W.; Jin, R.; Guo, H.; Qiu, X.; Gao, L. Effect of multiple H-bonding on the properties of polyimides containing the rigid rod groups. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 570–581. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, J.; Huang, J.; Feng, Y.; Peng, C.; Wang, X.; Liu, X. The dominant factor for mechanical property of polyimide films containing heterocyclic moieties: In-plane orientation, crystallization, or hydrogen bonding. J. Appl. Polym. Sci. 2016, 133, 44000. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, J.; Yang, C.; Wu, T.; Zhao, X.; Zhang, Q. Synthesis of poly(benzobisoxazole-co-imide) and fabrication of high-performance fibers. Polymer 2017, 133, 50–59. [Google Scholar] [CrossRef]
- Xia, A.; Guo, H.; Qiu, X.; Ding, M.; Gao, L. Syntheses and properties of polyimides derived from diamines containing 2,5-disubstituted pyridine group. J. Appl. Polym. Sci. 2006, 102, 1844–1851. [Google Scholar] [CrossRef]
- Gan, F.; Dong, J.; Tan, W.; Zhang, D.; Zhao, X.; Chen, X.; Zhang, Q. Fabrication and characterization of co-polyimide fibers containing pyrimidine units. J. Mater. Sci. 2017, 52, 9895–9906. [Google Scholar] [CrossRef]
- Gan, F.; Dong, J.; Zhang, D.; Tan, W.; Zhao, X.; Zhang, Q. High-performance polyimide fibers derived from wholly rigid-rod monomers. J. Mater. Sci. 2017, 53, 5477–5489. [Google Scholar] [CrossRef]
- Liu, T.Q.; Zheng, F.; Ding, T.M.; Zhang, S.Y.; Lu, Q. Design and synthesis of a novel quinoxaline diamine and its polyimides with high-Tg and red color. Polymer 2019, 179, 121612. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, H.; Dang, G.; Chen, C. Synthesis and characterization of thermally stable, high-modulus polyimides containing benzimidazole moieties. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2024–2031. [Google Scholar] [CrossRef]
- Liu, X.; Gao, G.; Dong, L.; Ye, G.; Gu, Y. Correlation between hydrogen-bonding interaction and mechanical properties of polyimide fibers. Polym. Adv. Technol. 2009, 20, 362–366. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Xia, Q.; Dong, J.; Xu, Q. Synthesis, characterization and properties of polyimides derived from a symmetrical diamine containing bis-benzimidazole rings. Polym. Degrad. Stab. 2012, 97, 987–994. [Google Scholar] [CrossRef]
- Lian, M.; Lu, X.; Lu, Q. Synthesis of superheat-resistant polyimides with high Tg and low coefficient of thermal expansion by introduction of strong intermolecular interaction. Macromolecules 2018, 51, 10127–10135. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q. Synthesis, characterization and properties of aromatic copolyimides containing bi-benzimidazole moiety. J. Polym. Res. 2015, 22, 78. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q. Synthesis and properties of polyimides derived from diamine monomer containing bi-benzimidazole unit. J. Polym. Res. 2014, 21, 424. [Google Scholar] [CrossRef]
- Lin, C.L.; Lin, W.L.; Rwei, S.P. Synthesis and characterization of poly(urethane-imide) derived from structural effect of diisocyanates. J. Polym. Res. 2023, 30, 54. [Google Scholar] [CrossRef]
- Lian, M.; Zheng, F.; Lu, X.; Lu, Q. Tuning the heat resistance properties of polyimides by intermolecular interaction strengthening for flexible substrate application. Polymer 2019, 173, 205–214. [Google Scholar] [CrossRef]
- Yan, X.; Dai, F.; Ke, Z.; Yan, K.; Chen, C.; Qian, G.; Li, H. Synthesis of colorless polyimides with high Tg from asymmetric twisted benzimidazole diamines. Eur. Polym. J. 2022, 164, 110975. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, X.; Zheng, F.; Lu, X.; Lu, Q. Synthesis of highly transparent and heat-resistant polyimides containing bulky pendant moieties. Polym. Int. 2019, 68, 1186–1193. [Google Scholar] [CrossRef]
- Yang, C.P.; Chen, R.S.; Chen, K.H. Effects of diamines and their fluorinated groups on the color lightness and preparation of organosoluble aromatic polyimides from 2,2-bis 4-(4-amino-2-trifluoromethylphenoxy) phenyl-hexafluoropropane. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 922–938. [Google Scholar] [CrossRef]
- Sidra, L.R.; Chen, G.; Li, C.; Mushtaq, N.; Ma, K.; Fang, X. Processable, high Tg Polyimides from unsymmetrical diamines containing 4-phenoxy aniline and benzimidazole moieties. Polymer 2018, 148, 228–238. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride with controlled steric structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Morozova, S.M.; Lozinskaya, E.I.; Vlasov, P.S.; Gouveia, A.S.L.; Tome, L.C.; Marrucho, I.M.; Vygodskii, Y.S. Turning into poly(ionic liquid)s as a tool for polyimide modification: Synthesis, characterization and CO2 separation properties. Polym. Chem. 2016, 7, 580–591. [Google Scholar] [CrossRef]
- Tao, L.; Yang, H.; Liu, J.; Fan, L.; Yang, S. Synthesis and characterization of highly optical transparent and low dielectric constant fluorinated polyimides. Polymer 2009, 50, 6009–6018. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, F.; van Sittert, C.; Lu, Q. Role of intrinsic factors of polyimides in glass transition temperature: An atomistic investigation. J. Phys. Chem. B 2019, 123, 8569–8579. [Google Scholar] [CrossRef]
- Dinic, J.; Sharma, V. Flexibility, extensibility, and ratio of Kuhn length to packing length govern the pinching dynamics, coil-stretch transition, and rheology of polymer solutions. Macromolecules 2020, 53, 4821–4835. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hoshino, Y.; Katsura, N.; Ishii, J. Superheat-resistant polymers with low coefficients of thermal expansion. Polymer 2017, 111, 91–102. [Google Scholar] [CrossRef]
- Jiao, L.; Du, Z.; Dai, X.; Wang, H.; Yao, H.; Qiu, X. Multifunctional polyimide films with superheat-resistance, low coefficient of thermal expansion and fluorescence performance. Polymer 2022, 247, 124792. [Google Scholar] [CrossRef]
- Musto, P.; Karasz, F.E.; MacKnight, W.J. Fourier transform infra-red spectroscopy on the thermo-oxidative degradation of polybenzimidazole and of a polybenzimidazole/polyetherimide blend. Polymer 1993, 34, 2934–2945. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Zhao, H.; Song, L.; Fang, X. Synthesis and properties of transparent polyimides derived from trans-1,4-bis(2,3-dicarboxyphenoxy)cyclohexane dianhydride. RSC Adv. 2015, 5, 53926–53934. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Wang, W.; Wei, L.; Zhang, Q.; Song, L.; Fang, X. Synthesis and characterization of transparent polyimides derived from ester-containing dianhydrides with different electron affinities. RSC Adv. 2015, 5, 79207–79215. [Google Scholar] [CrossRef]
- Volksen, W.; Cha, H.J.; Sanchez, M.I.; Yoon, D.Y. Polyimides derived from nonaromatic monomers: Synthesis, characterization and potential applications. React. Funct. Polym. 1996, 30, 61–69. [Google Scholar] [CrossRef]
- Sroog, C.E. Polyimides. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.; Wang, X.; Yi, C.; Su, S. The influence of alkyl groups at ortho-position of amino group on the structure and properties of polyimides. Polym. Bull. 2022, 1–7. [Google Scholar] [CrossRef]
- Feiring, A.E.; Auman, B.C.; Wonchoba, E.R. Synthesis and properties of fluorinated polyimides from novel 2,2′-bis(fluoroalkoxy)benzidines. Macromolecules 1993, 26, 2779–2784. [Google Scholar] [CrossRef]
- Tang, Y.; Yao, H.; Xu, W.; Zhu, L.; Zhang, Y.; Jiang, Z. Side-chain-type high dielectric-constant dipolar polyimides with temperature resistance. Macromol. Rapid Commun. 2023, 44, 2200639. [Google Scholar] [CrossRef]
- Feng, Y.; Luo, L.B.; Huang, J.; Li, K.; Li, B.; Wang, H.; Liu, X. Effect of molecular rigidity and hydrogen bond interaction on mechanical properties of polyimide fibers. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Yokota, R.; Yamamoto, S.; Yano, S.; Sawaguchi, T.; Hasegawa, M.; Yamaguchi, H.; Ozawa, H.; Sato, R. Molecular design of heat resistant polyimides having excellent processability and high glass transition temperature. High Perform. Polym. 2001, 13, S61–S72. [Google Scholar] [CrossRef]
- Hayes, R.A. The relationship between glass temperature, molar cohesion, and polymer structure. J. Appl. Polym. Sci. 1961, 5, 318–321. [Google Scholar] [CrossRef]
- Jou, J.H.; Huang, P.T.; Chen, H.C.; Liao, C.N. Coating thickness effect on the orientation and thermal expansion coefficient of polyimide films. Polymer 1992, 33, 967–974. [Google Scholar] [CrossRef]
- Jou, J.H.; Huang, P.T. Effect of thermal curing on the structures and properties of aromatic polyimide films. Macromolecules 1991, 24, 3796–3803. [Google Scholar] [CrossRef]
- Hasegawa, M.; Matano, T.; Shindo, Y.; Sugimura, T. Spontaneous molecular orientation of polyimides induced by thermal imidization. 2. in-plane orientation. Macromolecules 1996, 29, 7897–7909. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B Condens. Matter 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.L. Chemical Microstructure of Polymer Chains; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Wang, X.-Y.; in’t Veld, P.J.; Lu, Y.; Freeman, B.D.; Sanchez, I.C. A molecular simulation study of cavity size distributions and diffusion in para and meta isomers. Polymer 2005, 46, 9155–9161. [Google Scholar] [CrossRef]
- Hofman, D.; Ulbrich, J.; Fritsch, D.; Paul, D. Molecular modelling simulation of gas transport in amorphous polyimide and poly(amide imide) membrane materials. Polymer 1996, 37, 4773–4785. [Google Scholar] [CrossRef]

| Mn a (×104 g/mol) | Mw b (×104 g/mol) | PDI c | D d (μm) | |
|---|---|---|---|---|
| 4/3-BPDA | 2.95/2.56 | 8.85/6.91 | 3.0/2.7 | 25/22 |
| 4/3-ODPA | 2.78/1.97 | 9.45/7.09 | 3.4/3.6 | 26/20 |
| 4/3-BTDA | 3.21/2.75 | 9.31/8.81 | 2.9/3.2 | 21/24 |
| 4/3-6FDA | 2.47/2.32 | 8.65/7.89 | 3.5/3.4 | 19/17 |
| l a (Å) | h b (Å) | b c (Å) | |
|---|---|---|---|
| 4-BPDA | 31.859 | 226.031 | 160.363 |
| 3-BPDA | 27.972 | 142.651 | 72.749 |
| Film Quality | σ b (MPa) | E c (GPa) | ε d (%) | ||||
|---|---|---|---|---|---|---|---|
| 4-AB | 3-AB | 4-AB | 3-AB | 4-AB | 3-AB | ||
| BPDA | F&T a | 117.4 ± 10.3 | 105.2 ± 11.3 | 4.8 ± 0.8 | 2.6 ± 0.4 | 2.6 ± 0.4 | 4.1 ± 0.3 |
| ODPA | F&T | 104.3 ± 12.6 | 91.9 ± 8.6 | 4.3 ± 0.6 | 3.9 ± 0.5 | 2.6 ± 0.5 | 2.3 ± 0.2 |
| BTDA | F&T | 91.2 ± 8.9 | 83.2 ± 7.9 | 3.5 ± 0.7 | 2.9 ± 0.5 | 2.5 ± 0.3 | 1.8 ± 0.4 |
| 6FDA | B&T | 102.4 ± 7.6 | 98.5 ± 9.7 | 4.3 ± 0.9 | 3.1 ± 0.4 | 2.2 ± 0.4 | 3.1 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, M.; Zheng, F.; Meng, L.; Zhao, F.; Liu, J.; Song, J.; Lu, Q. Comparison of Homo-Polyimide Films Derived from Two Isomeric Bis-Benzimidazole Diamines. Molecules 2023, 28, 4889. https://doi.org/10.3390/molecules28134889
Lian M, Zheng F, Meng L, Zhao F, Liu J, Song J, Lu Q. Comparison of Homo-Polyimide Films Derived from Two Isomeric Bis-Benzimidazole Diamines. Molecules. 2023; 28(13):4889. https://doi.org/10.3390/molecules28134889
Chicago/Turabian StyleLian, Meng, Feng Zheng, Lingbin Meng, Fei Zhao, Jun Liu, Jimei Song, and Qinghua Lu. 2023. "Comparison of Homo-Polyimide Films Derived from Two Isomeric Bis-Benzimidazole Diamines" Molecules 28, no. 13: 4889. https://doi.org/10.3390/molecules28134889
APA StyleLian, M., Zheng, F., Meng, L., Zhao, F., Liu, J., Song, J., & Lu, Q. (2023). Comparison of Homo-Polyimide Films Derived from Two Isomeric Bis-Benzimidazole Diamines. Molecules, 28(13), 4889. https://doi.org/10.3390/molecules28134889







