The Promotion of Research Progress of Zinc Manganate Cathode Materials for Zinc-Ion Batteries by Characterization and Analysis Technology
Abstract
1. Introduction
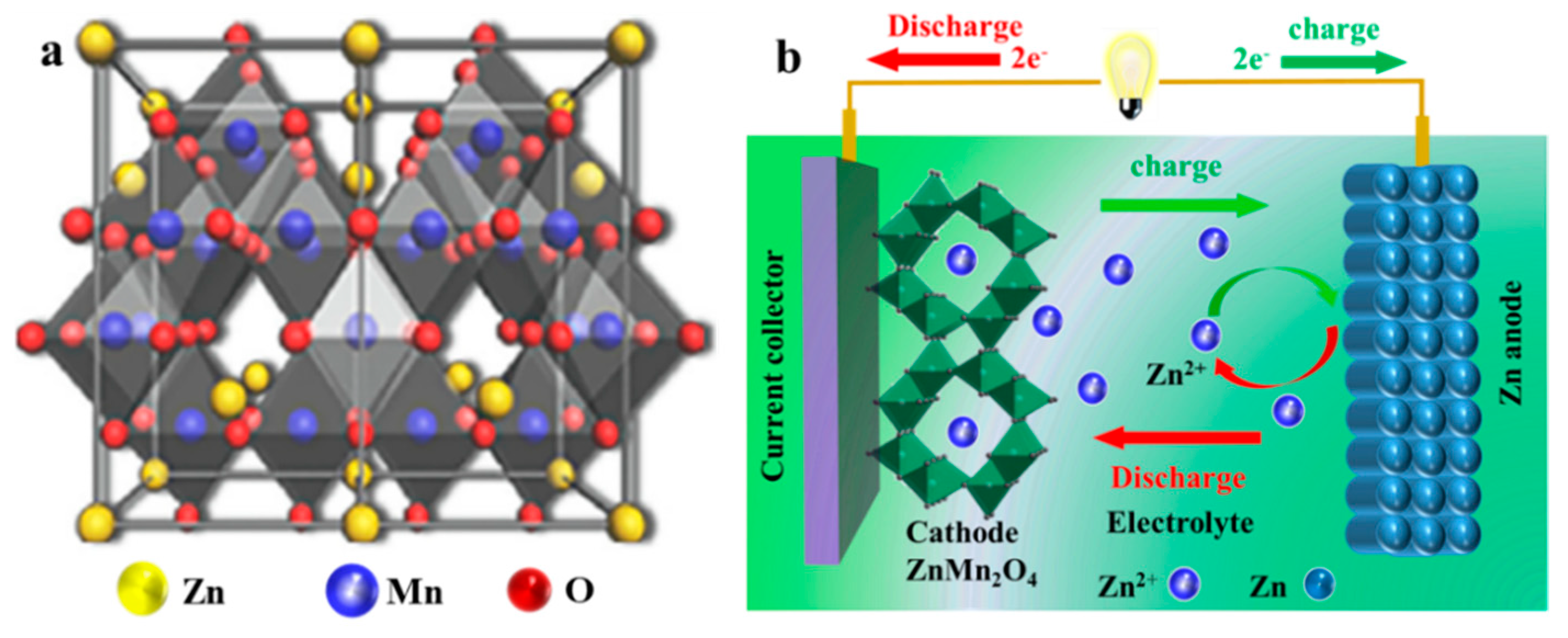
2. Zinc Storage Mechanism of ZMO
3. ZMO Modification Strategies by Characterization and Analysis Technology
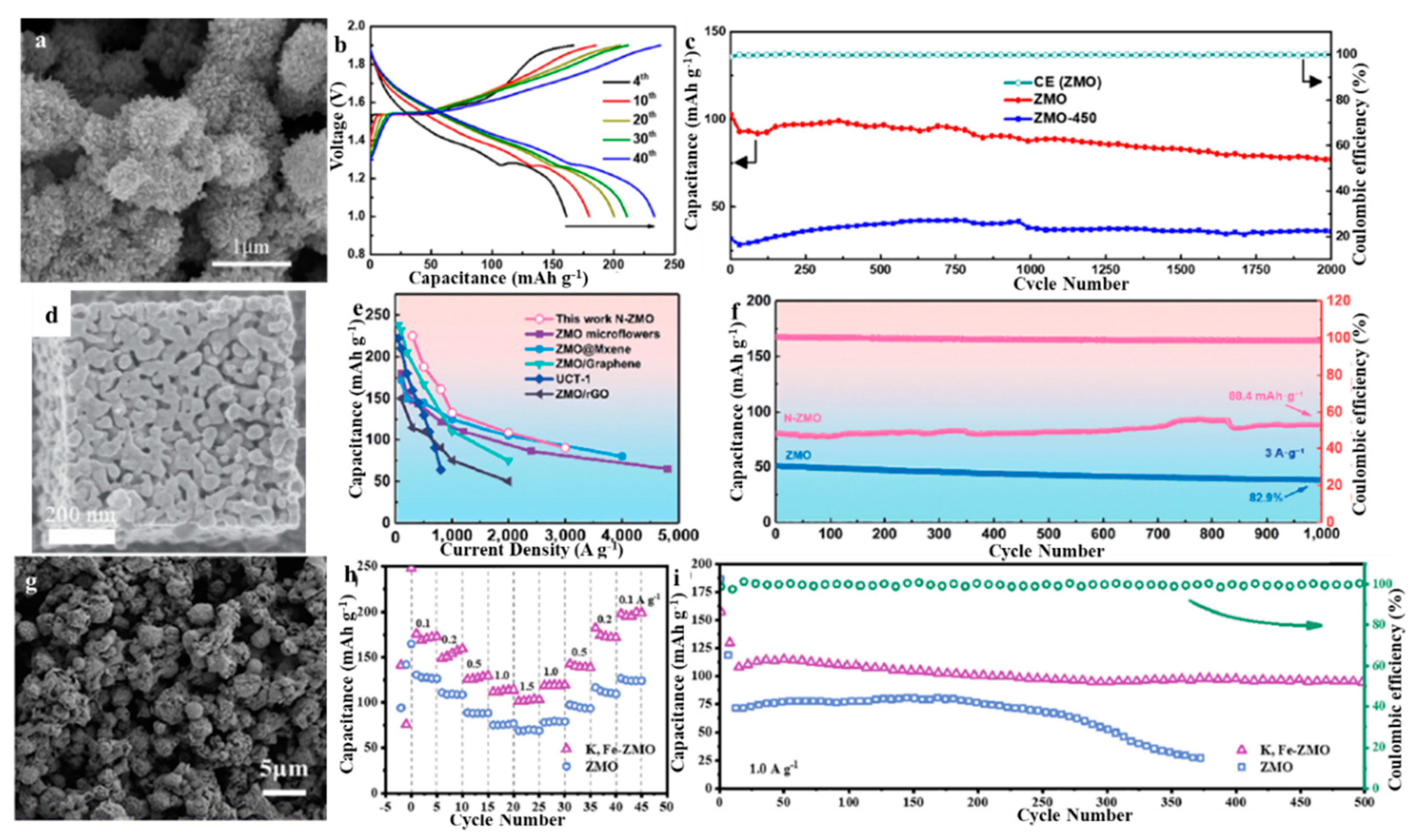
3.1. Design of Different Morphologies
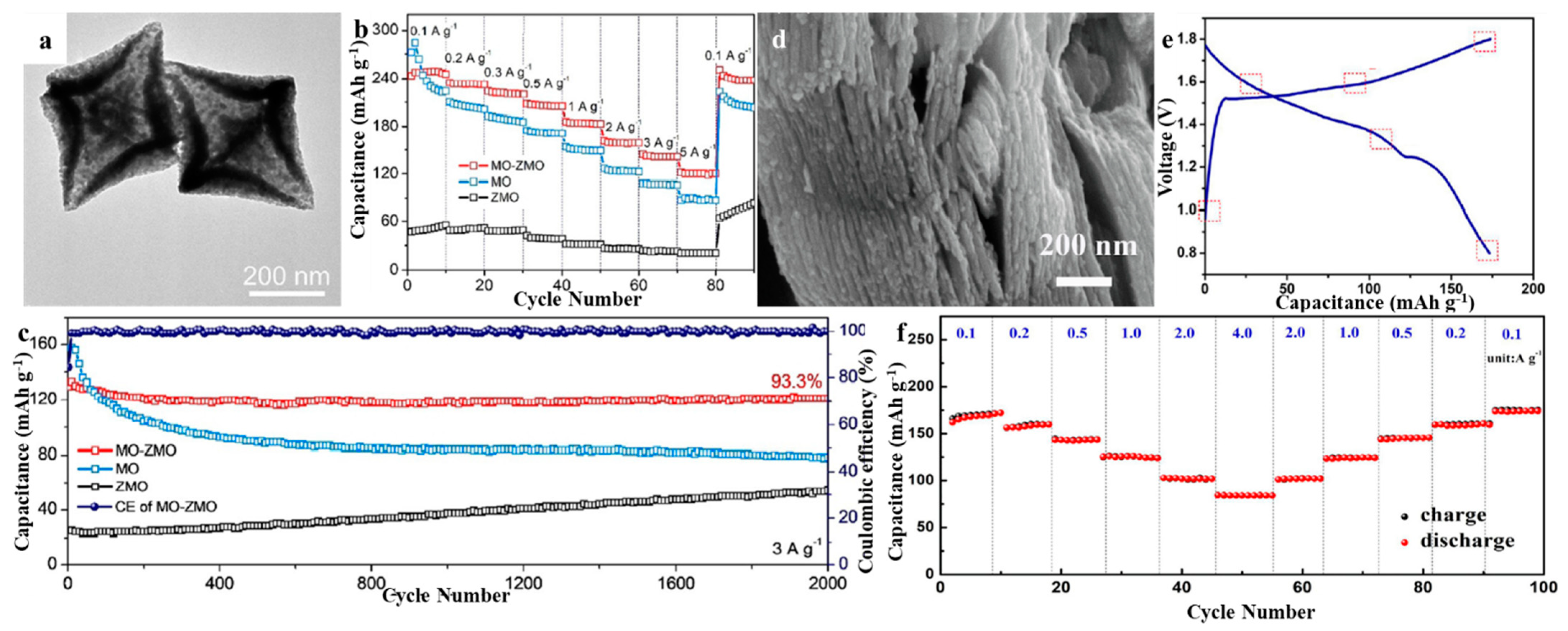
3.2. Construction of ZMO-Based Composites
3.2.1. Composite with Carbon Materials

3.2.2. Composite with Metal Oxides
3.3. Enlarging the Interlayer Spacing of ZMO
3.4. Introducing Deficiencies into ZMO
3.5. Doping Metal Ions of ZMO
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Obama, B. The irreversible momentum of clean energy. Science 2017, 355, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Carley, S.; Konisky, D.M. The justice and equity implications of the clean energy transition. Nat. Energy 2020, 5, 569–577. [Google Scholar] [CrossRef]
- Schill, W.P. Electricity storage and the renewable energy transition. Joule 2020, 4, 2059–2064. [Google Scholar] [CrossRef]
- Cao, M.; Leng, M.; Pan, W.; Wang, Y.; Tan, S.; Jiao, Y.; Yu, S.; Fan, S.; Xu, T.; Liu, T.; et al. 3D wearable piezoresistive sensor with waterproof and antibacterial activity for multimodal smart sensing. Nano Energy 2023, 112, 108492. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, Z.; Ming, F.; Zeng, Y.; Wei, B.; Jiang, Q.; Qi, Z.; Wang, Z.; Liang, H. Surface and Interface Engineering of Zn Anodes in Aqueous Rechargeable Zn-Ion Batteries. Small 2022, 18, 2200006. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Zhang, X.; Zeng, Z.; Qin, J.; Huang, Y. Strategies of regulating Zn2+ solvation structures for dendrite-free and side reaction-suppressed zinc-ion batteries. Energy Environ. Sci. 2022, 15, 499–528. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Zhang, W.; Cao, M.; Qiu, H.; Ji, X. Research progress on electrolytes for fast-charging lithium-ion batteries. Chin. Chem. Lett. 2022, 34, 107122. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, C.; Ou, T.; Zhang, S.; Li, L.; Ji, X. Constructing Advanced Electrode Materials for Low-temperature Lithium-ion batteries. Energy Rep. 2022, 8, 4525–4534. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, C.; Zhang, W.; Pan, W.; Wang, Q.; Li, L. Expanded-graphite-based materials for supercapacitors: A review. Molecules 2022, 27, 716. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J.; Cui, H.; Zhao, Y.; Cao, M. Carbon-based materials for fast charging lithium-ion batteries. Carbon 2021, 183, 721–734. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Zhang, W.; Cao, M.; Qiu, H.; Ji, X. Synthesis of expanded graphite-based materials for application in lithium-based batteries. J. Energy Storage 2023, 60, 106678. [Google Scholar] [CrossRef]
- Shahriari, S.; Mollaamin, F.; Monajjemi, M. Increasing the Performance of {[(1-x-y) LiCo0.3Cu0.7] (Al and Mg doped)]O2}, xLi2MnO3, yLiCoO2 Composites as Cathode Material in Lithium-Ion Battery: Synthesis and Characterization. Micromachines 2023, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Min, X.; Monajjemi, M. A Novel Cathode Material Synthesis and Thermal Characterization of (1-x-y)LiCo1/3Ti1/3Fe1/3PO4, xLi2MnPO4, yLiFePO4 Composites for Lithium-Ion Batteries (LIBs). Molecules 2022, 27, 8486. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Quilty, C.D.; Wu, D.; Li, W.; Bock, D.C.; Wang, L.; Housel, L.M.; Abraham, A.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S. Electron and Ion Transport in Lithium and Lithium-Ion Battery Negative and Positive Composite Electrodes. Chem. Rev. 2023, 123, 1327–1363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, X.; Lai, F.; Liu, T.; Shearing, P.R.; Parkin, I.P.; He, G.; Brett, D.J.L. Rechargeable aqueous Zn-based energy storage devices. Joule 2021, 5, 2845–2903. [Google Scholar] [CrossRef]
- Zampardi, G.; Mantia, F.L. Lanthanum nitrate as aqueous electrolyte additive for favourable zinc metal electrodeposition. Nat. Commun. 2022, 13, 687. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cheng, Z.; Zhang, C. Improved strategies for separators in zinc ion batteries. ChemSusChem 2023, 16, e202202330. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cao, M.; Ji, Y.; Qiu, H.; Zhang, D. Research progress of “rocking chair” type zinc-ion batteries with zinc metal-free anodes. Chin. Chem. Lett. 2023, 108307. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cheng, Z.; Zhang, C. Improved strategies for ammonium vanadate-based zinc ion batteries. Nanoscale 2023. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cao, M.; Ji, Y.; Qiu, H.; Zhang, D. Research progress on transition metal sulfide-based materials as cathode materials for zinc-ion batteries. J. Energy Storage 2023, 67, 107614. [Google Scholar] [CrossRef]
- Meng, X.; Wang, J.; Li, L. Layered-oxide cathode materials for fast-charging lithium-ion batteries: A review. Molecules 2023, 28, 4007. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active Materials for Aqueous Zinc Ion Batteries: Synthesis, Crystal Structure, Morphology, and Electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Yi, T.F.; Qiu, L.; Qu, J.P.; Liu, H.; Zhang, J.H.; Zhu, Y.R. Towards high-performance cathodes: Design and energy storage mechanism of vanadium oxides-based materials for aqueous Zn-ion batteries. Coordin. Chem. Rev. 2021, 446, 214124. [Google Scholar] [CrossRef]
- Liu, X.; Ni, W.; Wang, Y.; Liang, Y.; Wu, B.; Xu, G.; Wei, X.; Yang, L. Water-Processable and Multiscale-Designed Vanadium Oxide Cathodes with Predominant Zn2+ Intercalation Pseudocapacitance toward High Gravimetric/Areal/Volumetric Capacity. Small 2022, 18, 2105796. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, X. Strategies for constructing manganese-based oxide electrode materials for aqueous rechargeable zinc-ion batteries. Chin. Chem. Lett. 2022, 33, 1236. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, W.; Chen, D.; Liu, J.; Yuan, Z.; Lu, M.; Shen, L.; Shulga, V.; Han, W.; Chao, D. The origin of capacity fluctuation and rescue of dead Mn-based Zn-ion batteries: A Mn-based competitive capacity evolution protocol. Energy Environ. Sci. 2022, 15, 1106–1118. [Google Scholar] [CrossRef]
- Zampardi, G.; Mantia, F.L. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Li, Z.; Liu, T.; Meng, R.; Gao, L.; Zou, Y.; Peng, P.; Shao, Y.; Liang, X. Insights into the Structure Stability of Prussian Blue for Aqueous Zinc Ion Batteries. Energy Environ. Mater. 2021, 4, 111–116. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, M.; Ma, W.; Zhu, J.; Song, W. Application of Carbon Materials in Aqueous Zinc Ion Energy Storage Devices. Small 2021, 17, 2100219. [Google Scholar] [CrossRef]
- Wu, L.; Dong, Y. Recent progress of carbon nanomaterials for high-performance cathodes and anodes in aqueous zinc ion batteries. Energy Storage Mater. 2021, 41, 715–737. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Zhao, Y.; Hong, H.; Li, H.; Huang, Z.; Liang, G.; Yang, Q.; Zhi, C. Insight on Organic Molecules in Aqueous Zn-Ion Batteries with an Emphasis on the Zn Anode Regulation. Adv. Energy Mater. 2022, 12, 2102707. [Google Scholar] [CrossRef]
- He, G.; Liu, Y.; Gray, D.E.; Othon, J. Conductive polymer composites cathodes for rechargeable aqueous Zn-ion batteries: A mini-review. J. Compos. Commun. 2021, 27, 100882. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Xiong, T.; Wang, X.; Xue, J. Unraveling MoS2 and Transition Metal Dichalcogenides as Functional Zinc-Ion Battery Cathode: A Perspective. Small Methods 2021, 5, 2000815. [Google Scholar] [CrossRef] [PubMed]
- Boruah, B.D.; Wen, B.; Volder, M.D. Molybdenum Disulfide—Zinc Oxide Photocathodes for Photo-Rechargeable Zinc-Ion Batteries. ACS Nano 2021, 15, 16616–16624. [Google Scholar] [CrossRef]
- Dong, Y.; Shi, H.; Wu, Z.S. Recent Advances and Promise of MXene-Based Nanostructures for High-Performance Metal Ion Batteries. Adv. Funct. Mater. 2020, 30, 2000706. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.; Liu, K. Rational modulation of emerging MXene materials for zinc-ion storage. Carbon Energy 2022, 4, 60–76. [Google Scholar] [CrossRef]
- Hao, J.; Yuan, L.; Chao, Y.; Chao, D.; Davey, K.; Guo, Z.; Qiao, S.Z. Boosting Zinc Electrode Reversibility in Aqueous Electrolytes by Using Low-Cost Antisolvents. Angew. Chem. Int. Ed. 2021, 60, 7366–7375. [Google Scholar] [CrossRef]
- Fenta, F.W.; Olbasa, B.W.; Tsai, M.C.; Weret, M.A.; Zegeye, T.A.; Huang, C.J.; Huang, W.H.; Zeleke, T.S.; Sahalie, N.A.; Pao, C.W.; et al. Electrochemical transformation reaction of Cu-MnO in aqueous rechargeable zinc-ion batteries for high performance and long cycle life. J. Mater. Chem. A 2020, 8, 17595–17607. [Google Scholar] [CrossRef]
- Wu, X.; Xiang, Y.; Peng, Q.; Wu, X.; Li, Y.; Tang, F.; Song, R.; Liu, Z.; He, Z.; Wu, X. Green-low-cost rechargeable aqueous zinc-ion batteries using hollow porous spinel ZnMn2O4 as the cathode material. J. Mater. Chem. A 2017, 5, 17990–17997. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, R. Zn-ion batteries: ZnMn2O4 as cathode material. Mater. Today Proc. 2020, 26, 3378–3385. [Google Scholar]
- Soundharrajan, V.; Sambandam, B.; Kim, S.; Islam, S.; Jo, J.; Kim, S.; Mathew, V.; Sun, Y.K.; Kim, J. The dominant role of Mn2+ additive on the electrochemical reaction in ZnMn2O4 cathode for aqueous zinc-ion batteries. Energy Storage Mater. 2020, 28, 407. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Deng, J.; Gou, Y.; Fang, J. Review—Preparation and Application of Graphene-Based Hybrid Materials through Electrochemical Exfoliation. J. Electrochem. Soc. 2020, 167, 086511. [Google Scholar]
- Chen, L.; Yang, Z.; Qin, H.; Zeng, X.; Meng, J. Advanced electrochemical performance of ZnMn2O4/N-doped graphene hybrid as cathode material for zinc ion battery. J. Power Sources 2019, 425, 162–169. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Qin, H.; Zeng, X.; Meng, J.; Chen, H. Graphene-wrapped hollow ZnMn2O4 microspheres for high-performance cathode materials of aqueous zinc ion batteries. Electrochim. Acta 2019, 317, 155. [Google Scholar] [CrossRef]
- Tao, Y.; Li, Z.; Tang, L.; Pu, X.; Cao, T.; Cheng, D.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Nickel and cobalt Co-substituted spinel ZnMn2O4@N-rGO for increased capacity and stability as a cathode material for rechargeable aqueous zinc-ion battery. Electrochim. Acta 2020, 331, 135296. [Google Scholar] [CrossRef]
- Qiu, W.; Lin, Z.; Xiao, H.; Zhang, G.; Gao, H.; Feng, H.; Lu, X. Construction of chemical self-charging zinc ion batteries based on defect coupled nitrogen modulation of zinc manganite vertical graphene arrays. Mater. Adv. 2021, 2, 6694–6702. [Google Scholar]
- Yao, Z.; Cai, D.; Cui, Z.; Wang, Q.; Zhan, H. Strongly coupled zinc manganate nanodots and graphene composite as an advanced cathode material for aqueous zinc ion batteries. Ceram. Int. 2020, 46, 11237–11245. [Google Scholar]
- Yang, C.; Han, M.; Yan, H.; Li, F.; Shi, M.; Zhao, L. In-situ probing phase evolution and electrochemical mechanism of ZnMn2O4 nanoparticles anchored on porous carbon polyhedrons in high-performance aqueous Zn-ion batteries. J. Power Sources 2020, 452, 227826. [Google Scholar]
- Wang, S.; Zhang, S.; Chen, X.; Yuan, G.; Wang, B.; Bai, J.; Wang, H.; Wang, G. Double-shell zinc manganate hollow microspheres embedded in carbon networks as cathode materials for high–performance aqueous zinc-ion batteries. J. Colloid Interf. Sci. 2020, 580, 528–539. [Google Scholar]
- Islam, S.; Alfaruqi, M.H.; Putro, D.Y.; Park, S.; Kim, S.; Lee, S.; Ahmed, M.S.; Mathew, V.; Sun, Y.K.; Hwang, J.Y.; et al. In Situ Oriented Mn Deficient ZnMn2O4@C Nanoarchitecture for Durable Rechargeable Aqueous Zinc-Ion Batteries. Adv. Sci. 2021, 8, 2002636. [Google Scholar] [CrossRef] [PubMed]
- Sassin, M.B.; Helms, M.E.; Parker, J.F.; Chervin, C.N.; DeBlock, R.H.; Ko, J.S.; Rolison, D.R.; Long, J.W. Elucidating zinc-ion battery mechanisms in freestanding carbon electrode architectures decorated with nanocrystalline ZnMn2O4. Mater. Adv. 2021, 2, 2730–2738. [Google Scholar] [CrossRef]
- Deng, S.; Tie, Z.; Yue, F.; Cao, H.; Yao, M.; Niu, Z. Rational Design of ZnMn2O4 Quantum Dots in a Carbon Framework for Durable Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2022, 134, e202115877. [Google Scholar] [CrossRef]
- Jia, H.; Li, Y.; Ali, U.; Li, Y.; Hao, Y.; Liu, B.; Wang, C.; Li, L.; Wang, H.G. In-situ formation of ultrafine ZnMn2O4-MnOOH composite nanoparticles embedded into porous carbon nanospheres for stable aqueous zinc-ion batteries. Appl. Surf. Sci. 2022, 592, 153279. [Google Scholar] [CrossRef]
- Gao, F.; Mei, B.; Xu, X.; Ren, J.; Zhao, D.; Zhang, Z.; Wang, Z.; Wu, Y.; Liu, X.; Zhang, Y. Rational design of ZnMn2O4 nanoparticles on carbon nanotubes for high-rate and durable aqueous zinc-ion batteries. Chem. Eng. J. 2022, 448, 137742. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, M.; Wu, X.; Wu, X.; Zeng, F.; Li, Y.; Duan, S.; Fan, D.; Yang, Y.; Wu, X. The excellent electrochemical performances of ZnMn2O4/Mn2O3: The composite cathode material for potential aqueous zinc ion batteries. J. Electroanal. Chem. 2019, 832, 69–74. [Google Scholar] [CrossRef]
- Ma, S.C.; Sun, M.; Wang, S.X.; Li, D.S.; Liu, W.; Liu, M.; Ren, M.; Kong, F.G.; Wang, S.J.; Xia, Y.M. Zinc manganate/manganic oxide bi-component nanorod as excellent cathode for zinc-ion battery. Scr. Mater. 2021, 194, 113707. [Google Scholar] [CrossRef]
- Qin, L.; Zhu, Q.; Li, L.; Fang, G.; Li, S.; Cheng, H.; Guo, W.; Gao, H. Improved electrochemical performance of ZnMn2O4/CuO composite as cathode materials for aqueous zinc-ion batteries. Ionics 2021, 27, 4783–4792. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Jin, Q.; Pei, Z.; Luan, D.; Zhang, X.; Lou, X. Rationally Designed Mn2O3–ZnMn2O4 Hollow Heterostructures from Metal-Organic Frameworks for Stable Zn-Ion Storage. Angew. Chem. 2021, 133, 25997. [Google Scholar] [CrossRef]
- Shi, M.; Wang, B.; Shen, Y.; Jiang, J.; Zhu, J.; Su, Y.; Narayanasamy, M.; Angaiah, S.; Yan, C.; Peng, Q. 3D assembly of MXene-stabilized spinel ZnMn2O4 for highly durable aqueous zinc-ion batteries. Chem. Eng. J. 2020, 399, 125627. [Google Scholar] [CrossRef]
- Wu, T.H.; Liang, W.Y. Reduced Intercalation Energy Barrier by Rich Structural Water in Spinel ZnMn2O4 for High-Rate Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 23822–23832. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Huang, C.C.; Cheng, S.L.; Lin, C.C. Expanded spinel ZnxMn2O4 induced by electrochemical activation of glucose-mediated manganese oxide for stable cycle performance in zinc−ion batteries. J. Colloid Interf. Sci. 2020, 617, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Q.; Zhang, D.; Shen, G. Coupling N-doping and rich oxygen vacancies in mesoporous ZnMn2O4 nanocages toward advanced aqueous zinc ion batteries. Nano Res. 2022, 15, 8118–8127. [Google Scholar] [CrossRef]
- Shao, T.; Zhang, Y.; Cao, T.; Yang, Y.; Li, Z.; Liu, H.; Wang, Y.; Xia, Y. Structural Regulation of ZnMn2O4 cathode material by K, Fe-Double doping to improve its rate and cycling stability for rechargeable aqueous zinc-based batteries. Chem. Eng. J. 2022, 431, 133735. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Liu, Q.; He, W.; Lai, Z.; Zhang, X.; Yu, M.; Tong, Y.; Lu, X. Extracting oxygen anions from ZnMn2O4: Robust cathode for flexible all-solid-state Zn-ion batteries. Energy Storage Mater. 2019, 21, 154–161. [Google Scholar] [CrossRef]
- Qiu, W.; Xiao, H.; Feng, H.; Lin, Z.; Gao, H.; He, W.; Lu, X. Defect modulation of ZnMn2O4 nanotube arrays as high-rate and durable cathode for flexible quasi-solid-state zinc ion battery. Chem. Eng. J. 2021, 422, 129890. [Google Scholar] [CrossRef]
- Mallick, S.; Choutipalli, V.S.K.; Bag, S.; Subramanian, V.; Raj, C.R. Defect Engineered Ternary Spinel: An Efficient Cathode for an Aqueous Rechargeable Zinc-Ion Battery of Long-Term Cyclability. ACS Appl. Mater. Interfaces 2022, 14, 37577–37586. [Google Scholar] [CrossRef]



| Devices (Anode//Cathode) | Voltage Window (V) | Electrolyte | Cycle Performance | Specific Capacitance | Ref |
|---|---|---|---|---|---|
| Zn foil//hollow porous ZMO | 0.8–1.9 | 1 M ZnSO4 + 0.05 M MnSO4 | 59.2%, 300 cycles, 0.1 A·g−1 | 137.4 mAh·g−1 | [40] |
| Zn foil//ZMO | 0.1–2.1 | 1 M ZnSO4 | 99.26%, 25 cycles, 0.5 A·g−1 | 50.2 F·g−1 | [41] |
| Zn foil//ZMO | 0.6–1.9 | 1 M ZnSO4 + 0.05 M MnSO4 | 79%, 1000 cycles, 2 A·g−1 | 172 mAh·g−1 | [42] |
| Zn foil//ZMO/NG | 0.8–1.8 | 1 M ZnSO4 + 0.05 M MnSO4 | 97.4%, 2500 cycles, 1 A·g−1 | 221 mAh·g−1 | [44] |
| Zn foil// rGO@HM-ZMO | 0.8–1.8 | 1 M ZnSO4 + 0.05 M MnSO4 | 94.1%, 650 cycles, 1 A·g−1 | 146.9 mAh·g−1 | [45] |
| Zn foil//ZnNixCoy Mn2−x−yO4@N-rGO | 0.7–1.7 | 2 M ZnSO4 + 0.2 M MnSO4 | 79%, 900 cycles, 1 A·g−1 | 200.5 mAh·g−1 | [46] |
| Zn foil// N-ZnMn2O4-x/VG | 0.8–1.8 | 4 M Zn(CF3SO3)2 | 92.6%, 3000 cycles, 1 A·g−1 | 222 mAh·g−1 | [47] |
| Zn foil//ZMO NDs/rGO | 1.0–1.8 | 1 M ZnSO4 + 0.1 M MnSO4 | 84.1%, 400 cycles, 1 A·g−1 | 207.6 mAh·g−1 | [48] |
| Zn foil//ZMO@PCPs | 0.8–1.8 | 1 M ZnSO4 + 0.5 M MnSO4 | 90.3%, 2000 cycles, 1 A·g−1 | 125.6 mAh·g−1 | [49] |
| Zn foil//ZMO@C | 1.0–1.8 | 2 M ZnSO4 + 0.1 M MnSO4 | - | 481 mAh·g−1 | [50] |
| Zn foil//Mn-d-ZMO@C | 0.8–1.9 | 2 M ZnSO4 + 0.2 M MnSO4 | 84%, 2000 cycles, 0.1 A·g−1 | 194 mAh·g−1 | [51] |
| Zn foil//ZMO@CNF | 0.9–1.8 | 1 M ZnSO4 | 50%, 400 cycles, 1 C | 139 mAh·g−1 | [52] |
| Zn foil//ZMO QD@C | 0.9–1.8 | 1 M ZnSO4 | 86.4%, 1500 cycles, 1 A·g−1 | 320.6 mAh·g−1 | [53] |
| Zn foil// ZMO-MOH/C NSs | 0.8–1.8 | 2 M ZnSO4 + 0.2 M MnSO4 | 79.1%, 1000 cycles, 1 A·g−1 | 336.7 mAh·g−1 | [54] |
| Zn foil//ZMO/CNTs | 0.4–1.8 | 1 M ZnSO4 + 0.1 M MnSO4 | 97.1%, 2000 cycles, 3 A·g−1 | 220.3 F·g−1 | [55] |
| Zn foil//ZMO/Mn2O3 | 0.8–1.8 | 1 M ZnSO4 | 74%, 300 cycles, 0.5 A·g−1 | 151.9 mAh·g−1 | [56] |
| Zn foil//ZMO/Mn2O3 | 0.8–1.9 | 2 M ZnSO4 | 79%, 120 cycles, 0.1 A·g−1 | 243.6 mAh·g−1 | [57] |
| Zn foil//ZMO/CuO | 0.6–1.8 | 2 M ZnSO4 + 0.1 M MnSO4 | 96%, 1000 cycles, 2 A·g−1 | 150 mAh·g−1 | [58] |
| Zn//MO-ZMO HOs | 0.8–1.8 | 2 M ZnSO4 | 93.3%, 2000 cycles, 3 A·g−1 | 247.4 mAh·g−1 | [59] |
| Zn foil// ZMO@Ti3C2Tx | 0.8–1.8 | 2 M ZnSO4 + 0.1 M MnSO4 | 92.4%, 5000 cycles, 1 A·g−1 | 172.6 mAh·g−1 | [60] |
| Zn foil//ZMO | 1.0–1.9 | 1 M ZnSO4 | 75%, 2000 cycles, 4 A·g−1 | 230 mAh·g−1 | [61] |
| Zn foil//ZnxMn2O4 | 1.0–1.9 | 3 M ZnSO4 | 85%, 3000 cycles, 4 A·g−1 | 170 mAh·g−1 | [62] |
| Zn foil//ZMO/carbon | 0.8–2.0 | 3M Zn(CF3SO3)2 | 94%, 500 cycles, 0.5 A·g−1 | 150 mAh·g−1 | [65] |
| Zn foil//ZMO | 0.8–1.9 | 1 M ZnSO4 | 93.8%, 300 cycles, 0.5 A·g−1 | 221 mAh·g−1 | [66] |
| Zn foil//N-ZMO NTAs | 0.8–1.8 | 2 M ZnSO4 | 92.1%, 1500 cycles, 0.5 A·g−1 | 223 mAh·g−1 | [67] |
| Zn foil//N-ZMO | 0.8–1.8 | 2 M ZnSO4 + 0.2 M MnSO4 | 88.4%, 1000 cycles, 3 A·g−1 | 225.4 mAh·g−1 | [63] |
| Zn foil//Zn0.65Ni0.58 Mn1.75O4 | 0.8–2.0 | 3 M ZnSO4 + 0.1 M MnSO4 | 94%, 5000 cycles, 2 A·g−1 | 265 mAh·g−1 | [68] |
| Zn foil// K, Fe-ZMO | 0.7–1.7 | 2 M ZnSO4 + 0.2 M MnSO4 | 88.1%, 500 cycles, 1 A·g−1 | 221.2 mAh·g−1 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Cheng, Z.; Li, L. The Promotion of Research Progress of Zinc Manganate Cathode Materials for Zinc-Ion Batteries by Characterization and Analysis Technology. Molecules 2023, 28, 4459. https://doi.org/10.3390/molecules28114459
Meng X, Cheng Z, Li L. The Promotion of Research Progress of Zinc Manganate Cathode Materials for Zinc-Ion Batteries by Characterization and Analysis Technology. Molecules. 2023; 28(11):4459. https://doi.org/10.3390/molecules28114459
Chicago/Turabian StyleMeng, Xin, Ziyi Cheng, and Le Li. 2023. "The Promotion of Research Progress of Zinc Manganate Cathode Materials for Zinc-Ion Batteries by Characterization and Analysis Technology" Molecules 28, no. 11: 4459. https://doi.org/10.3390/molecules28114459
APA StyleMeng, X., Cheng, Z., & Li, L. (2023). The Promotion of Research Progress of Zinc Manganate Cathode Materials for Zinc-Ion Batteries by Characterization and Analysis Technology. Molecules, 28(11), 4459. https://doi.org/10.3390/molecules28114459






