Recent Progress in MOF-Based Electrochemical Sensors for Non-Enzymatic Glucose Detection
Abstract
1. Introduction
2. Pristine MOF and MOF Conjugates as Non-Enzymatic Glucose Sensors
2.1. Enzyme-Free Glucose Biosensor Based on Monometallic MOF
2.2. Enzyme-Free Glucose Sensor Based on Multi-Metal MOF Combination
3. Enzyme-Free Glucose Biosensors Based on MOF Complexes and Derivatives
3.1. Enzyme-Free Glucose Sensor Based on MOF with Carbon or Metal
3.2. Enzyme-Free Glucose Sensor Based on MOF Derivatives
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Wadman, M. Genital defects seen in sons of men taking major diabetes drug. Science 2022, 376, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Brusko, T.M.; Russ, H.A.; Stabler, C.L. Strategies for durable beta cell replacement in type 1 diabetes. Science 2021, 373, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. Mass screening weighed for type 1 diabetes risk. Science 2020, 368, 353. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Rauch, S.; Iurlaro, E.; Gunier, R.B.; Rego, A.; Gravett, M.G.; Cavoretto, P.I.; Deruelle, P.; Garcia-May, P.K.; Mhatre, M.; et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: The INTERCOVID study. Am. J. Obstet. Gynecol. 2022, 227, 74.e1–74.e16. [Google Scholar] [CrossRef]
- Barry, E.; Roberts, S.; Oke, J.; Vijayaraghavan, S.; Normansell, R.; Greenhalgh, T. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: Systematic review and meta-analysis of screening tests and interventions. Br. Med. J. 2017, 356, i6538. [Google Scholar] [CrossRef]
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens. Bioelectron. 2020, 165, 112331. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Qusti, A.H.; Al-Youbi, A.O.; Sun, X. Ultrathin graphitic carbon nitride nanosheets: A novel peroxidase mimetic, Fe doping-mediated catalytic performance enhancement and application to rapid, highly sensitive optical detection of glucose. Nanoscale 2013, 5, 11604–11609. [Google Scholar] [CrossRef]
- Dong, Q.; Ryu, H.; Lei, Y. Metal oxide based non-enzymatic electrochemical sensors for glucose detection. Electrochim. Acta 2021, 370, 137744. [Google Scholar] [CrossRef]
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors—A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef]
- Simon, D.; Obst, F.; Haefner, S.; Heroldt, T.; Peiter, M.; Simon, F.; Richter, A.; Voit, B.; Appelhans, D. Hydrogel/enzyme dots as adaptable tool for non-compartmentalized multi-enzymatic reactions in microfluidic devices. React. Chem. Eng. 2019, 4, 67–77. [Google Scholar] [CrossRef]
- Zhou, B.; Qi, Z.; Yan, D. Highly Efficient and Direct Ultralong All-Phosphorescence from Metal-Organic Framework Photonic Glasses. Angew. Chem. Int. Ed. 2022, 61, e202208735. [Google Scholar] [CrossRef]
- Zheng, S.; Sun, Y.; Xue, H.; Braunstein, P.; Huang, W.; Pang, H. Dual-ligand and hard-soft-acid-base strategies to optimize metal-organic framework nanocrystals for stable electrochemical cycling performance. Natl. Sci. Rev. 2022, 9, nwab197. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Xin, R.; Earnshaw, J.; Tang, J.; Hill, J.P.; Ashok, A.; Nanjundan, A.K.; Kim, J.; Young, C.; Sugahara, Y.; et al. MOF-derived nanoporous carbons with diverse tunable nanoarchitectures. Nat. Protoc. 2022, 17, 2990–3027. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wei, J.; Zhang, Y.; Qian, B.; Jia, Q.; Liu, J.; Zhao, X.; Shao, G. Ultralight Magnetic and Dielectric Aerogels Achieved by Metal-Organic Framework Initiated Gelation of Graphene Oxide for Enhanced Microwave Absorption. Nano-Micro Lett. 2022, 14, 1–16. [Google Scholar] [CrossRef]
- He, C.; Liang, J.; Zou, Y.-H.; Yi, J.-D.; Huang, Y.-B.; Cao, R. Metal-organic frameworks bonded with metal N-heterocyclic carbenes for efficient catalysis. Natl. Sci. Rev. 2022, 9, nwab157. [Google Scholar] [CrossRef]
- Jian, X.; Xu, J.; Guo, J.; Zhao, J.; Shen, T.; Gao, Z.; Song, Y.-Y. Cascade-Gates Guarded Asymmetrical Nanochannel Membrane: An Interference-Free Device for Straightforward Detection of Trace Biomarker in Undiluted Serum. Small 2023, 19, 2205995. [Google Scholar] [CrossRef]
- Sakthivel, R.; Lin, L.-Y.; Duann, Y.-F.; Chen, H.-H.; Su, C.; Liu, X.; He, J.-H.; Chung, R.-J. MOF-Derived Cu-BTC Nanowire-Embedded 2D Leaf-like Structured ZIF Composite-Based Aptamer Sensors for Real-Time In Vivo Insulin Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 28639–28650. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martin, J.M.; Abdelkader-Fernandez, V.K.; Cunha-Silva, L.; Balula, S.S. One-Pot Conversion of Glucose into 5-Hydroxymethylfurfural using MOFs and Bronsted-Acid Tandem Catalysts. Adv. Sustain. Syst. 2022, 6, 2100444. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, X.; Wang, L.; Qian, X.; Zhang, Y.; Wu, J.; Shao, S. Subacute thyroiditis complicated with multiple organ failure A case report. Medicine 2022, 101, e28710. [Google Scholar] [CrossRef]
- Gumilar, G.; Henzie, J.; Yuliarto, B.; Patah, A.; Nugraha, N.; Iqbal, M.; Amin, M.A.; Hossain, M.S.A.; Yamauchi, Y.; Kaneti, Y.V. Performance enhancement strategies for surface plasmon resonance sensors in direct glucose detection using pristine and modified UiO-66: Effects of morphology, immobilization technique, and signal amplification. J. Mater. Chem. A 2022, 10, 6662–6678. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, P.; Li, J.; Sun, Z.; Huang, W. Construction of a novel Co-based coordination polymer and its study of non-enzymatic glucose sensors. J. Solid State Chem. 2022, 311, 123115. [Google Scholar] [CrossRef]
- Stefani, A.; Capello, K.; Carminato, A.; Wurzburger, W.; Furlanello, T.; Bertazzo, V.; Marsilio, E.; Albertin, E.; La Pietra, G.; Bozzato, E.; et al. Effects of leukoreduction on storage lesions in whole blood and blood components of dogs. J. Vet. Intern. Med. 2021, 35, 936–945. [Google Scholar] [CrossRef]
- Ronaghi, N.; Shade, D.; Moon, H.J.; Najmi, S.; Cleveland, J.W.; Walton, K.S.; France, S.; Jones, C.W. Modulation and Tuning of UiO-66 for Lewis Acid Catalyzed Carbohydrate Conversion: Conversion of Unprotected Aldose Sugars to Polyhydroxyalkyl and C-Glycosyl Furans. ACS Sustain. Chem. Eng. 2021, 9, 11581–11595. [Google Scholar] [CrossRef]
- Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martin, J.M.; Abdelkader-Fernandez, V.K.; Cunha-Silva, L.; Balula, S.S. Isomerization of glucose to fructose catalyzed by metal-organic frameworks. Sustain. Energy Fuels 2021, 5, 3847–3857. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Osman, D.I.; Ali, O.I.; Shousha, W.G.; Shoeib, M.A.; Shawky, S.M.; Sheta, S.M. A novel Ag/Zn bimetallic MOF as a superior sensitive biosensing platform for HCV-RNA electrochemical detection. Appl. Surf. Sci. 2021, 562, 150202. [Google Scholar] [CrossRef]
- Banga, I.; Paul, A.; Muthukumar, S.; Prasad, S. ZENose (ZIF-Based Electrochemical Nose) Platform for Noninvasive Ammonia Detection. ACS Appl. Mater. Interfaces 2021, 13, 16155–16165. [Google Scholar] [CrossRef]
- Luan, X.; Pan, Y.; Zhou, D.; He, B.; Liu, X.; Gao, Y.; Yang, J.; Song, Y. Cerium metal organic framework mediated molecular threading for point-of-care colorimetric assays. Biosens. Bioelectron. 2020, 165, 112406. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Su, X.; Yang, J.; Ji, P.; Sun, F.; Tian, Q.; Zhao, Z. Facile synthesis of high-entropy zirconate nanopowders and their sintering behaviors. J. Adv. Ceram. 2023, 12, 498–509. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, Y.; Fu, S.; He, P.; Sun, C.; Duan, X.; Jia, D.; Colombo, P.; Zhou, Y. 3D-printed Lunar regolith simulant-based geopolymer composites with bio-inspired sandwich architectures. J. Adv. Ceram. 2023, 12, 510–525. [Google Scholar] [CrossRef]
- Lai, L.; Zhao, Z.; Tian, S.; Ou, B.; Liang, G.; Li, B.; Dai, Y. Ultrahigh electrostrain with excellent fatigue resistance in textured Nb5+-doped (Bi0.5Na0.5)TiO3-based piezoceramics. J. Adv. Ceram. 2023, 12, 487–497. [Google Scholar] [CrossRef]
- Liu, Q.; He, Z.; Wang, H.; Feng, X.; Han, P. Magnetically controlled colorimetric aptasensor for chlorpyrifos based on copper-based metal-organic framework nanoparticles with peroxidase mimetic property. Microchim. Acta 2020, 187, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al Lawati, H.A.J.; Hassanzadeh, J. Dual-function 2D cobalt metal-organic framework embedded on paper as a point-of-care diagnostic device: Application for the quantification of glucose. Anal. Chim. Acta 2020, 1139, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Yang, X.; Liu, H.; Chai, X.; Wang, Y.; Fan, W.; Sun, D. Fe-MOF with U-Shaped Channels for C2H2/CO2 and C2H2/C2H4 Separation. Inorg. Chem. 2023, 62, 3722–3726. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wu, B.; Li, Z.; Bai, Y.; Kan, L.; Wang, M.; He, L.; Du, M. Hierarchical CoCoPBA@PCN-221 nanostructure for the highly sensitive detection of deoxynivalenol in foodstuffs. Food Chem. 2023, 403, 134370. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Gao, X.; DelaCruz, S.; Chen, C.; Tang, Z.; Shi, T.; Carraro, C.; Maboudian, R. In situ formation of metal-organic framework derived CuO polyhedrons on carbon cloth for highly sensitive non-enzymatic glucose sensing. J. Mater. Chem. B 2019, 7, 4990–4996. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Y.; Zhu, P.; Du, L.; Chen, W.; Wu, C. Electrochemically Activated Conductive Ni-Based MOFs for Non-enzymatic Sensors Toward Long-Term Glucose Monitoring. Front. Chem. 2020, 8, 602752. [Google Scholar] [CrossRef]
- Gumilar, G.; Kaneti, Y.V.; Henzie, J.; Chatterjee, S.; Na, J.; Yuliarto, B.; Nugraha, N.; Patah, A.; Bhaumik, A.; Yamauchi, Y. General synthesis of hierarchical sheet/plate-like M-BDC (M = Cu, Mn, Ni, and Zr) metal-organic frameworks for electrochemical non-enzymatic glucose sensing. Chem. Sci. 2020, 11, 3644–3655. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, Q.; Lu, S.; Chen, G.; Gao, S.; Lu, W.; Sun, X. High-performance non-enzymatic glucose detection: Using a conductive Ni-MOF as an electrocatalyst. J. Mater. Chem. B 2020, 8, 5411–5415. [Google Scholar] [CrossRef]
- Xuan, X.; Qian, M.; Pan, L.; Lu, T.; Han, L.; Yu, H.; Wan, L.; Niu, Y.; Gong, S. A longitudinally expanded Ni-based metal-organic framework with enhanced double nickel cation catalysis reaction channels for a non-enzymatic sweat glucose biosensor. J. Mater. Chem. B 2020, 8, 9094–9109. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Wang, N.; Xu, Q.Q.; Xu, L.; Lin, M. Copper-based Metal-organic Framework for Non-enzymatic Electrochemical Detection of Glucose. Electroanalysis 2018, 30, 474–478. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.; Lee, H.N.; Park, Y.M.; Bae, Y.-S.; Kim, H.-J. Electrochemically derived CuO nanorod from copper-based metal-organic framework for non-enzymatic detection of glucose. Appl. Surf. Sci. 2019, 479, 720–726. [Google Scholar] [CrossRef]
- Hu, Q.; Qin, J.; Wang, X.-F.; Ran, G.-Y.; Wang, Q.; Liu, G.-X.; Ma, J.-P.; Ge, J.-Y.; Wang, H.-Y. Cu-Based Conductive MOF Grown in situ on Cu Foam as a Highly Selective and Stable Non-Enzymatic Glucose Sensor. Front. Chem. 2021, 9, 786970. [Google Scholar] [CrossRef]
- Zeraati, M.; Alizadeh, V.; Kazemzadeh, P.; Safinejad, M.; Kazemian, H.; Sargazi, G. A new nickel metal organic framework (Ni-MOF) porous nanostructure as a potential novel electrochemical sensor for detecting glucose. J. Porous Mater. 2022, 29, 257–267. [Google Scholar] [CrossRef]
- Sangeetha, S.; Jayasree, A.C.; Raj, K.; Prasad, N.L.; Krishnamurthy, G.; Nagashree, K.L. Cobalt metal-organic framework for low concentration detection of glucose. Inorg. Nano-Met. Chem. 2021, 51, 1–6. [Google Scholar] [CrossRef]
- Ma, Z.-Z.; Ma, Y.; Liu, B.; Xu, L.; Jiao, H. A high-performance Co-MOF non-enzymatic electrochemical sensor for glucose detection. N. J. Chem. 2021, 45, 21350–21358. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, N.; Cao, P.; Lin, M.; Xu, L.; Ma, H. Electrochemical non-enzymatic glucose sensor using ionic liquid incorporated cobalt-based metal-organic framework. Microchem. J. 2020, 159, 105343. [Google Scholar] [CrossRef]
- Koyappayil, A.; Yeon, S.-H.; Chavan, S.G.; Jin, L.; Go, A.; Lee, M.-H. Efficient and rapid synthesis of ultrathin nickel-metal organic framework nanosheets for the sensitive determination of glucose. Microchem. J. 2022, 179, 107462. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Ai, L.; Jiang, J. Synthesis of the crystalline porous copper oxide architectures derived from metal-organic framework for electrocatalytic oxidation and sensitive detection of glucose. J. Ind. Eng. Chem. 2019, 70, 330–337. [Google Scholar] [CrossRef]
- Arul, P.; John, S.A. Electrodeposition of CuO from Cu-MOF on glassy carbon electrode: A non enzymatic sensor for glucose. J. Electroanal. Chem. 2017, 799, 61–69. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.-W.; Ma, Y.; Zhang, J.-J.; Mo, G.-Q.; Du, H.-J.; Ye, J.-S. Cu(II) Metal-Organic Framework Encapsulated in Carbon Paste Electrode for High-Performance Non-Enzymatic Glucose Sensing. Chin. J. Anal. Chem. 2020, 48, E20038–E20046. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Liu, H.; Zhai, Y.; Li, L.; Wen, H. 2D MOF with electrochemical exfoliated graphene for nonenzymatic glucose sensing: Central metal sites and oxidation potentials. Anal. Chim. Acta 2020, 1122, 9–19. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Bu, Y.; Zhang, J.; Zhang, R. Copper Cobalt Sulfide Structures Derived from MOF Precursors with Enhanced Electrochemical Glucose Sensing Properties. Nanomaterials 2022, 12, 1394. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Liao, Y.; Shi, N.; Sun, S.; Liao, X.; Yin, G.; Huang, Z.; Pu, X.; Wang, J. Facile synthesis of bimetallic metal-organic frameworks on nickel foam for a high performance non-enzymatic glucose sensor. J. Electroanal. Chem. 2022, 904, 115887. [Google Scholar] [CrossRef]
- Archana, V.; Xia, Y.; Fang, R.; Kumar, G.G. Hierarchical CuO/NiO-Carbon Nanocomposite Derived from Metal Organic Framework on Cello Tape for the Flexible and High Performance Nonenzymatic Electrochemical Glucose Sensors. ACS Sustain. Chem. Eng. 2019, 7, 6707–6719. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Hui, Z.; Zhao, S.; Zhao, Y.; Wang, L. Carbon cloth-supported nanorod-like conductive Ni/Co bimetal MOF: A stable and high-performance enzyme-free electrochemical sensor for determination of glucose in serum and beverage. Food Chem. 2021, 349, 129202. [Google Scholar] [CrossRef]
- Zou, H.; Tian, D.; Lv, C.; Wu, S.; Lu, G.; Guo, Y.; Liu, Y.; Yu, Y.; Ding, K. The synergistic effect of Co/Ni in ultrathin metal-organic framework nanosheets for the prominent optimization of non-enzymatic electrochemical glucose detection. J. Mater. Chem. B 2020, 8, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Jia, L.; Zhu, R.-R.; Du, L.; Zhao, Q.-H. High-performance non-enzymatic glucose electrochemical sensor constructed by transition nickel modified Ni@Cu-MOF. J. Electroanal. Chem. 2020, 858, 113783. [Google Scholar] [CrossRef]
- Chen, C.; Zhong, Y.; Cheng, S.; Huanga, Y.; Li, T.; Shi, T.; Liao, G.; Tang, Z. In Situ Fabrication of Porous Nanostructures Derived from Bimetal-Organic Frameworks for Highly Sensitive Non-Enzymatic Glucose Sensors. J. Electrochem. Soc. 2020, 167, 027531. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, L.; Wang, X.; Chai, L.; Wang, Y.; Jiang, M.; Li, T.-T.; Hu, Y.; Qian, J.; Huang, S. General approach to MOF-derived core-shell bimetallic oxide nanowires for fast response to glucose oxidation. Sens. Actuators B-Chem. 2020, 306, 127551. [Google Scholar] [CrossRef]
- Lu, M.; Deng, Y.; Li, Y.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Core-shell MOF@MOF composites for sensitive nonenzymatic glucose sensing in human serum. Anal. Chim. Acta 2020, 1110, 35–43. [Google Scholar] [CrossRef]
- Pan, W.; Zheng, Z.; Wu, X.; Gao, J.; Liu, Y.; Yuan, Q.; Gan, W. Facile synthesis of 2D/3D hierarchical NiCu bimetallic MOF for non-enzymatic glucose sensor. Microchem. J. 2021, 170, 106652. [Google Scholar] [CrossRef]
- Kim, S.E.; Muthurasu, A. Metal-organic framework-assisted bimetallic Ni@Cu microsphere for enzyme-free electrochemical sensing of glucose. J. Electroanal. Chem. 2020, 873, 114356. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Ezzati, M.; Hosseini, H. Fabrication of a sensitive and fast response electrochemical glucose sensing platform based on co-based metal-organic frameworks obtained from rapid in situ conversion of electrodeposited cobalt hydroxide intermediates. Talanta 2020, 210, 120696. [Google Scholar] [CrossRef]
- Li, X.; Dong, H.; Fan, Q.; Chen, K.; Sun, D.; Hu, T.; Ni, Z. One-pot, rapid microwave-assisted synthesis of bimetallic metal-organic framework for efficient enzyme-free glucose detection. Microchem. J. 2022, 179, 107468. [Google Scholar] [CrossRef]
- Sun, S.; Du, Q.; Shi, N.; Liao, X.; Yin, G. Facile synthesis of Cu/Co-ZIF nanoarrays for non-enzymatic glucose detection. Nanotechnology 2021, 32, 475508. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.; Bae, Y.-S. Zn-Co Bimetallic Zeolitic Imidazolate Frameworks as Nonenzymatic Electrochemical Glucose Sensors with Enhanced Sensitivity and Chemical Stability. ACS Sustain. Chem. Eng. 2022, 10, 11702–11709. [Google Scholar] [CrossRef]
- Kim, S.E.; Muthurasu, A. Highly Oriented Nitrogen-doped Carbon Nanotube Integrated Bimetallic Cobalt Copper Organic Framework for Non-enzymatic Electrochemical Glucose and Hydrogen Peroxide Sensor. Electroanalysis 2021, 33, 1333–1345. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhao, Y.; He, H.; Zheng, L.; Huang, Z.; Zhao, X.; Xu, J.; Wang, B.; Yin, Z. Engineering of a self-supported carbon electrode with 2D ultrathin heterostructures of NiCo LDH/NiCoS via a MOF-template for sensitive detection of glucose and H2O2. Mater. Adv. 2022, 3, 6028–6036. [Google Scholar] [CrossRef]
- Li, S.; Zhan, X.; Bai, W.; Zheng, J. Controllable Synthesis of Ni/Co-TCPP MOFs with Different Morphologies and Their Application in Electrochemical Detection of Glucose. J. Electrochem. Soc. 2020, 167, 127506. [Google Scholar] [CrossRef]
- Feng, Y.; Xiang, D.; Qiu, Y.; Li, L.; Li, Y.; Wu, K.; Zhu, L. MOF-Derived Spinel NiCo2O4 Hollow Nanocages for the Construction of Non-enzymatic Electrochemical Glucose Sensor. Electroanalysis 2020, 32, 571–580. [Google Scholar] [CrossRef]
- Li, S.; Bai, W.; Zhang, X.; Zheng, J. NiO/Cu-TCPP Hybrid Nanosheets as an Efficient Substrate for Supercapacitor and Sensing Applications. J. Electrochem. Soc. 2020, 167, 027534. [Google Scholar] [CrossRef]
- Chen, J.; Yin, H.; Zhou, J.; Wang, L.; Ji, Z.; Zheng, Y.; Nie, Q. Hybrid Ni3N-nitrogen-doped carbon microspheres (Ni3N@C) in situ derived from Ni-MOFs as sensitive non-enzymatic glucose sensors. Mater. Technol. 2021, 36, 286–295. [Google Scholar] [CrossRef]
- Ma, X.; Tang, K.-l.; Yang, M.; Shi, W.; Zhao, W. Metal-organic framework-derived yolk-shell hollow Ni/NiO@C microspheres for bifunctional non-enzymatic glucose and hydrogen peroxide biosensors. J. Mater. Sci. 2021, 56, 442–456. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Teng, J.; Wong, W.-L.; Qiu, B. In situ deposition of MOF-74(Cu) nanosheet arrays onto carbon cloth to fabricate a sensitive and selective electrocatalytic biosensor and its application for the determination of glucose in human serum. Microchim. Acta 2020, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, G.; Gong, C.; Chen, D.; Chen, X.; Zhang, Q.; Dong, H.; Zhang, Y.; Li, C.; Hu, J.; et al. Hydrogen-assisted synthesis of Ni-ZIF-derived nickel nanoparticle chains coated with nitrogen-doped graphitic carbon layers as efficient electrocatalysts for non-enzymatic glucose detection. Microchim. Acta 2022, 189, 1–12. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, S.; Li, T.; Wang, Z. Ni/NiO multivalent system encapsulated in nitrogen-doped graphene realizing efficient activation for non-enzymatic glucose sensing. Ceram. Int. 2021, 47, 22869–22880. [Google Scholar] [CrossRef]
- Yin, H.; Zhan, T.; Chen, J.; Wang, L.; Gong, J.; Zhao, S.; Ji, Z.; Nie, Q. Polyhedral NiO/C porous composites derived by controlled pyrolysis of Ni-MOF for highly efficient non-enzymatic glucose detection. J. Mater. Sci.-Mater. Electron. 2020, 31, 4323–4335. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, R.; He, F.; Hu, W.; Cao, X.; Jia, J.; Lu, W.; Sun, X. A comparative study of electrocatalytic oxidation of glucose on conductive Ni-MOF nanosheet arrays with different ligands. New J. Chem. 2020, 44, 17849–17853. [Google Scholar] [CrossRef]
- Tao, B.; Li, J.; Miao, F.; Zang, Y. Carbon Cloth Loaded NiCo2O4 Nano-Arrays to Construct Co-MOF@GO Nanocubes: A High-Performance Electrochemical Sensor for Non-Enzymatic Glucose. IEEE Sens. J. 2022, 22, 13898–13907. [Google Scholar] [CrossRef]
- Zang, G.; Hao, W.; Li, X.; Huang, S.; Gan, J.; Luo, Z.; Zhang, Y. Copper nanowires-MOFs-graphene oxide hybrid nanocomposite targeting glucose electro-oxidation in neutral medium. Electrochim. Acta 2018, 277, 176–184. [Google Scholar] [CrossRef]
- Pu, F.; Miao, H.; Lu, W.; Zhang, X.; Yang, Z.; Kong, C. High-performance non-enzymatic glucose sensor based on flower-like Cu2O-Cu-Au ternary nanocomposites. Appl. Surf. Sci. 2022, 581, 152389. [Google Scholar] [CrossRef]
- Zhai, X.; Cao, Y.; Sun, W.; Cao, S.; Wang, Y.; He, L.; Yao, N.; Zhao, D. Core-shell composite N-doped-Co-MOF@polydopamine decorated with Ag nanoparticles for nonenzymatic glucose sensors. J. Electroanal. Chem. 2022, 918, 116491. [Google Scholar] [CrossRef]
- Mo, G.; Zheng, X.; Ye, N.; Ruan, Z. Nitrogen-doped carbon dodecahedron embedded with cobalt nanoparticles for the direct electro-oxidation of glucose and efficient nonenzymatic glucose sensing. Talanta 2021, 225, 121954. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yin, H.; Zhou, J.; Wang, L.; Gong, J.; Ji, Z.; Nie, Q. Efficient Nonenzymatic Sensors Based on Ni-MOF Microspheres Decorated with Au Nanoparticles for Glucose Detection. J. Electron. Mater. 2020, 49, 4754–4763. [Google Scholar] [CrossRef]
- Arif, D.; Hussain, Z.; Abbasi, A.D.; Sohail, M. Ag Functionalized In2O3 Derived From MIL-68(In) as an Efficient Electrochemical Glucose Sensor. Front. Chem. 2022, 10, 507. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Li, J.; Hu, C.; Zhang, X.; Jiang, B.; Cao, Z.; Zhang, J.; Zhang, R. MOF-derived ZnCo2O4 porous micro-rice with enhanced electro-catalytic activity for the oxygen evolution reaction and glucose oxidation. RSC Adv. 2020, 10, 9063–9069. [Google Scholar] [CrossRef]
- Wang, L.; Miao, X.; Qu, Y.; Duan, C.; Wang, B.; Yu, Q.; Gao, J.; Song, D.; Li, Y.; Yin, Z. Rattle-type Au@NiCo LDH hollow core-shell nanostructures for nonenzymatic glucose sensing. J. Electroanal. Chem. 2020, 858, 113810. [Google Scholar] [CrossRef]
- Kachouei, M.A.; Shahrokhian, S.; Ezzati, M. Bimetallic CoZn-MOFs easily derived from CoZn-LDHs, as a suitable platform in fabrication of a non-enzymatic electrochemical sensor for detecting glucose in human fluids. Sens. Actuators B-Chem. 2021, 344, 130254. [Google Scholar] [CrossRef]
- Shakiba, M.; Afsharpour, M. Novel graphenic-SiC nanotubes (g-SiCNT) and Cu-doped g-SiCNT/CuO composite as the effective nonenzymatic glucose sensors. Appl. Surf. Sci. 2022, 602, 154405. [Google Scholar] [CrossRef]
- Ji, W.; Shen, C.; Xi, X.; Tang, W.; Wu, D.; Su, Y.; Liu, R. Batch-producible fibrous microelectrodes for enzyme-free electrochemical detection of glucose. J. Mater. Sci. Mater. Electron. 2022, 33, 11511–11522. [Google Scholar] [CrossRef]
- Guo, Q.; Zeng, W.; Liu, S.; Li, Y. In situ formation of Co3O4 hollow nanocubes on carbon cloth-supported NiCo2O4 nanowires and their enhanced performance in non-enzymatic glucose sensing. Nanotechnology 2020, 31, 265501. [Google Scholar] [CrossRef] [PubMed]
- Arul, P.; Gowthaman, N.S.K.; John, S.A.; Tominaga, M. Tunable electrochemical synthesis of 3D nucleated microparticles like Cu-BTC MOF-carbon nanotubes composite: Enzyme free ultrasensitive determination of glucose in a complex biological fluid. Electrochim. Acta 2020, 354, 136673. [Google Scholar] [CrossRef]
- Cao, J.; Yun, J.; Zhang, N.; Wei, Y.; Yang, H.; Xu, Z. Bifunctional Ag@Ni-MOF for high performance supercapacitor and glucose sensor. Synth. Met. 2021, 282, 116931. [Google Scholar] [CrossRef]
- Yang, N.; Guo, K.; Zhang, Y.; Xu, C. Engineering the valence state of ZIF-67 by Cu2O for efficient nonenzymatic glucose detection. J. Mater. Chem. B 2020, 8, 2856–2861. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Su, T.; Lu, Q.; Shang, Z.; Xu, Q.; Hu, X. Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection. Anal. Chem. 2021, 93, 16222–16230. [Google Scholar] [CrossRef]
- Chen, C.; Xiong, D.; Gu, M.; Lu, C.; Yi, F.-Y.; Ma, X. MOF-Derived Bimetallic CoFe-PBA Composites as Highly Selective and Sensitive Electrochemical Sensors for Hydrogen Peroxide and Nonenzymatic Glucose in Human Serum. ACS Appl. Mater. Interfaces 2020, 12, 35365–35374. [Google Scholar] [CrossRef]
- Abrori, S.A.; Septiani, N.L.W.; Nugraha; Anshori, I.; Suyatman; Suendo, V.; Yuliarto, B. Metal-Organic-Framework FeBDC-Derived Fe(3)O(4)for Non-Enzymatic Electrochemical Detection of Glucose. Sensors 2020, 20, 4891. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q.; Li, J.; Xu, F.; Sun, L.; Bu, Y.; Zou, Y.; Kraatz, H.-B.; Rosei, F. Tunable hierarchical surfaces of CuO derived from metal-organic frameworks for non-enzymatic glucose sensing. Inorg. Chem. Front. 2020, 7, 1512–1525. [Google Scholar] [CrossRef]
- Wei, C.; Li, X.; Xiang, W.; Yu, Z.; Liu, Q. MOF derived seaweed-like CoCu oxides nanorod arrays for electrochemical non-enzymatic glucose sensing with ultrahigh sensitivity. Sens. Actuators B-Chem. 2020, 324, 128773. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Guo, W.; Wan, K.; Zhang, T.; Arbiol, J.; Zhao, Y.-Q.; Xu, C.-L.; Xu, M.; Fransaer, J. A yolk-albumen-shell structure of mixed Ni-Co oxide with an ultrathin carbon shell for high-sensitivity glucose sensors. Mater. Adv. 2020, 1, 908–917. [Google Scholar] [CrossRef]
- Zhu, Q.; Hu, S.; Zhang, L.; Li, Y.; Carraro, C.; Maboudian, R.; Wei, W.; Liu, A.; Zhang, Y.; Liu, S. Reconstructing hydrophobic ZIF-8 crystal into hydrophilic hierarchically-porous nanoflowers as catalyst carrier for nonenzymatic glucose sensing. Sens. Actuators B-Chem. 2020, 313, 128031. [Google Scholar] [CrossRef]
- Lo, N.-C.; Hsu, W.-S.; Chen, Y.-T.; Sun, I.W.; Chen, P.-Y. Facile Nonenzymatic Glucose Electrode Composed of Commercial CuO Powder and Ionic Liquid Binder. Electroanalysis 2021, 33, 909–915. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Wang, B.; Duan, C.; Li, J.; Zheng, L.; Li, J.; Yin, Z. Bifunctional three-dimensional self-supporting multistage structure CC@MOF-74(NiO)@NiCo LDH electrode for supercapacitors and non-enzymatic glucose sensors. J. Alloys Compd. 2021, 885, 160899. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Y.; Liu, M.; Wang, H.; Ren, J.; Tian, Y.; Liu, X.; Zhou, Y.; Wang, J.; Zhu, W.; et al. In-situ two-step electrodeposition of alpha-CD-rGO/Ni-MOF composite film for superior glucose sensing. J. Alloys Compd. 2022, 923, 166418. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Gao, P.; Yin, W.; Yin, M.; Pu, H.; Sun, Q.; Liang, X.; Fa, H.-b. Bimetal-organic frameworks MnCo-MOF-74 derived Co/MnO@HC for the construction of a novel enzyme-free glucose sensor. Microchem. J. 2022, 175, 107097. [Google Scholar] [CrossRef]
- Arif, D.; Hussain, Z.; Sohail, M.; Liaqat, M.A.; Khan, M.A.; Noor, T. A Non-enzymatic Electrochemical Sensor for Glucose Detection Based on Ag@TiO2@ Metal-Organic Framework (ZIF-67) Nanocomposite. Front. Chem. 2020, 8, 573510. [Google Scholar] [CrossRef]
- Han, X.; Cao, K.; Yao, Y.; Zhao, J.; Chai, C.; Dai, P. A novel electrochemical sensor for glucose detection based on a Ti3C2Tx/ZIF-67 nanocomposite. RSC Adv. 2022, 12, 20138–20146. [Google Scholar] [CrossRef]
- Chen, Q.; Chu, D.; Yan, L.; Lai, H.; Chu, X.-Q.; Ge, D.; Chen, X. Enhanced non-enzymatic glucose sensing based on porous ZIF-67 hollow nanoprisms. New J. Chem. 2021, 45, 10031–10039. [Google Scholar] [CrossRef]
- Zhou, H.; Zheng, M.; Tang, H.; Xu, B.; Tang, Y.; Pang, H. Amorphous Intermediate Derivative from ZIF-67 and Its Outstanding Electrocatalytic Activity. Small 2020, 16, e1904252. [Google Scholar] [CrossRef]
- Elizbit; Liaqat, U.; Hussain, Z.; Baig, M.M.; Khan, M.A.; Arif, D. Preparation of porous ZIF-67 network interconnected by MWCNTs and decorated with Ag nanoparticles for improved non-enzymatic electrochemical glucose sensing. J. Korean Ceram. Soc. 2021, 58, 598–605. [Google Scholar] [CrossRef]
- Almutairi, E.M.; Ghanem, M.A.; Al-Warthan, A.; Shaik, M.R.; Adil, S.F.; Almutairi, A.M. Chemical deposition and exfoliation from liquid crystal template: Nickel/nickel (II) hydroxide nanoflakes electrocatalyst for a non-enzymatic glucose oxidation reaction. Arab. J. Chem. 2022, 15, 103467. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Yuan, B.; Li, Y.; Yu, L.; Zhao, W. Three-dimensional hollow nickel phosphate microspheres with controllable hoya-like structure for high-performance enzymeless glucose detection and supercapacitor. Appl. Surf. Sci. 2022, 588, 152928. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, K.; Duan, J.; Zheng, S.; Jiang, J. The nickel phosphate rods derived from Ni-MOF with enhanced electrochemical activity for non-enzymatic glucose sensing. Talanta 2022, 247, 123587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.-Q.; Bai, Y.; Chu, W.; Sh, J. Confinement preparation of hierarchical NiO-N-doped carbon@reduced graphene oxide microspheres for high-performance non-enzymatic detection of glucose. Sens. Actuators B-Chem. 2020, 309, 127779. [Google Scholar] [CrossRef]
- Li, G.; Xie, G.; Chen, D.; Gong, C.; Chen, X.; Zhang, Q.; Pang, B.; Zhang, Y.; Li, C.; Hu, J.; et al. Facile synthesis of bamboo-like Ni3S2@NCNT as efficient and stable electrocatalysts for non-enzymatic glucose detection. Appl. Surf. Sci. 2022, 585, 152683. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zheng, X.; Li, Q.; Ye, N.; Mo, G. ZIFs derived polyhedron with cobalt oxide nanoparticles as novel nanozyme for the biomimetic catalytic oxidation of glucose and non-enzymatic sensor. Anal. Chim. Acta 2022, 1209, 339839. [Google Scholar] [CrossRef]
- Yan, L.; Chu, D.; Chu, X.-Q.; Ge, D.; Chen, X. Co/CoO nanoparticles armored by N-doped nanoporous carbon polyhedrons towards glucose oxidation in high-performance non-enzymatic sensors. New J. Chem. 2022, 46, 15071–15079. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Wang, D.; Qiao, Z.; Qu, L. Synthesis of nanoneedle-like copper oxide on N-doped reduced graphene oxide: A three-dimensional hybrid for nonenzymatic glucose sensor. Sens. Actuators B-Chem. 2017, 238, 588–595. [Google Scholar] [CrossRef]
- Gong, Q.; Sun, L.-P.; Wu, Z.; Huo, L.-H.; Zhao, H. Enhanced non-enzymatic glucose sensing of Cu-BTC-derived porous copper@carbon agglomerate. J. Mater. Sci. 2018, 53, 7305–7315. [Google Scholar] [CrossRef]
- He, J.; Lu, X.; Yu, J.; Wang, L.; Song, Y. Hierarchical Co(OH)(2) nanostructures/glassy carbon electrode derived from Co(BTC) metal-organic frameworks for glucose sensing. J. Nanoparticle Res. 2016, 18, 1–11. [Google Scholar] [CrossRef]
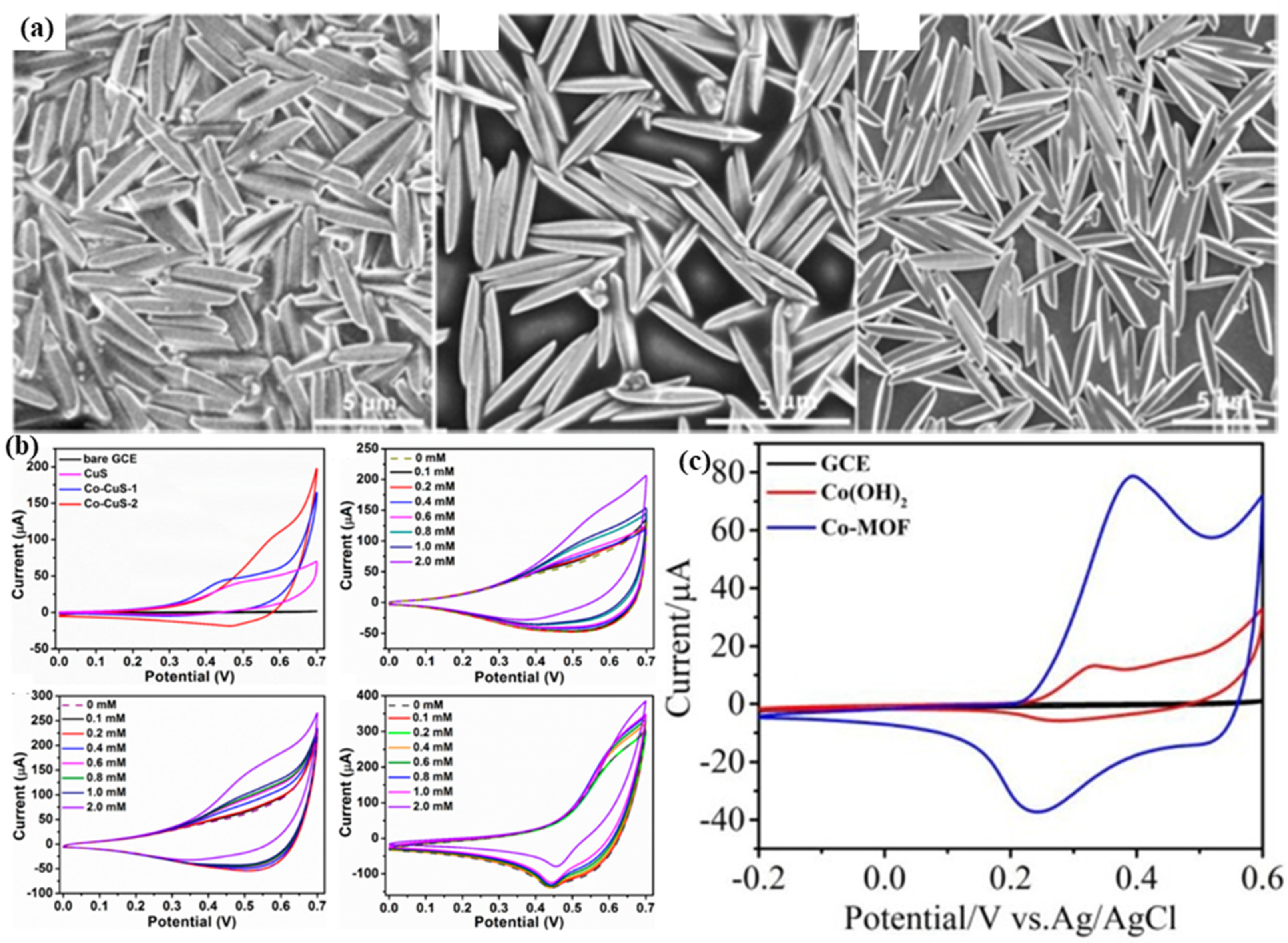
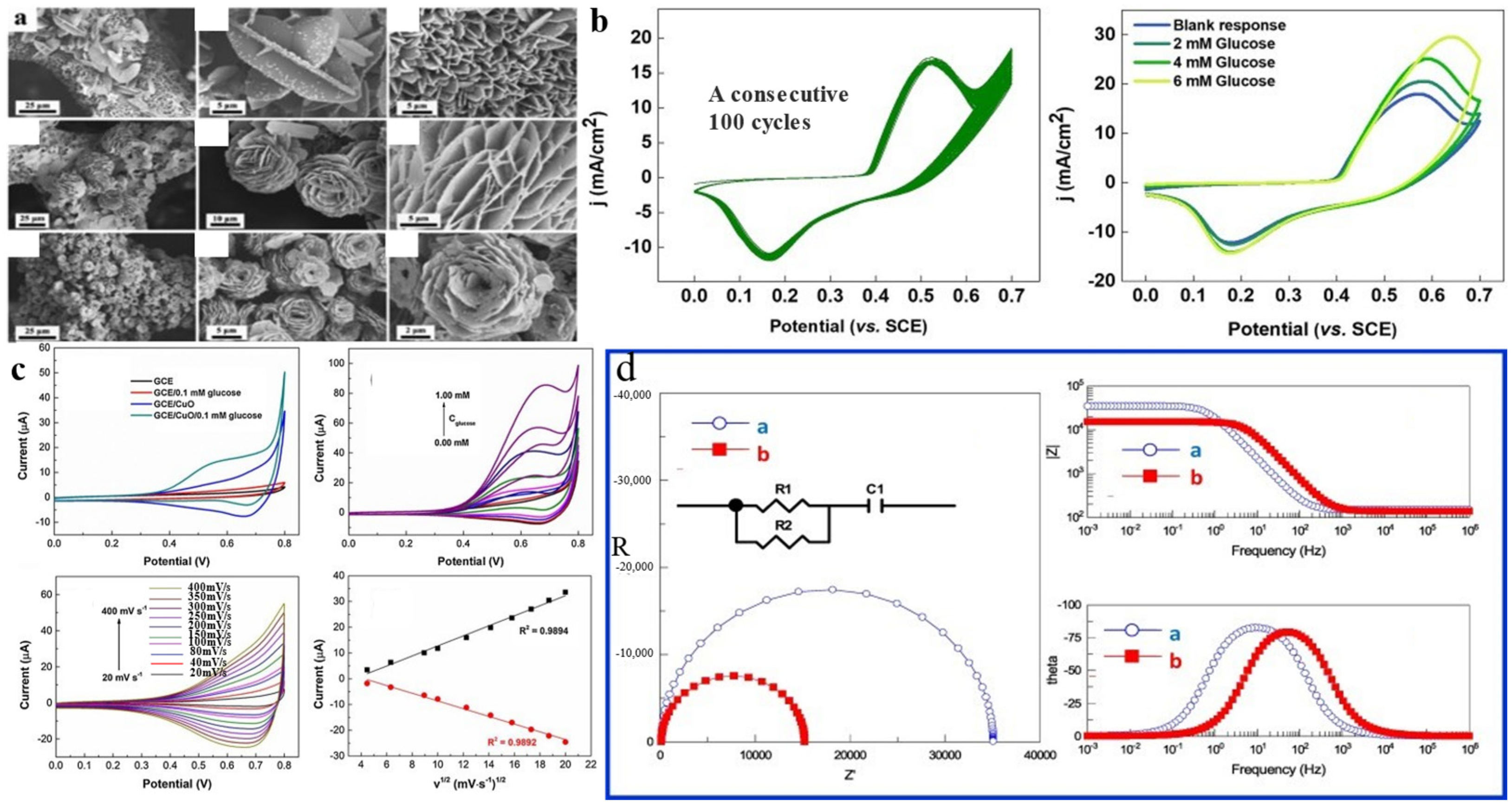
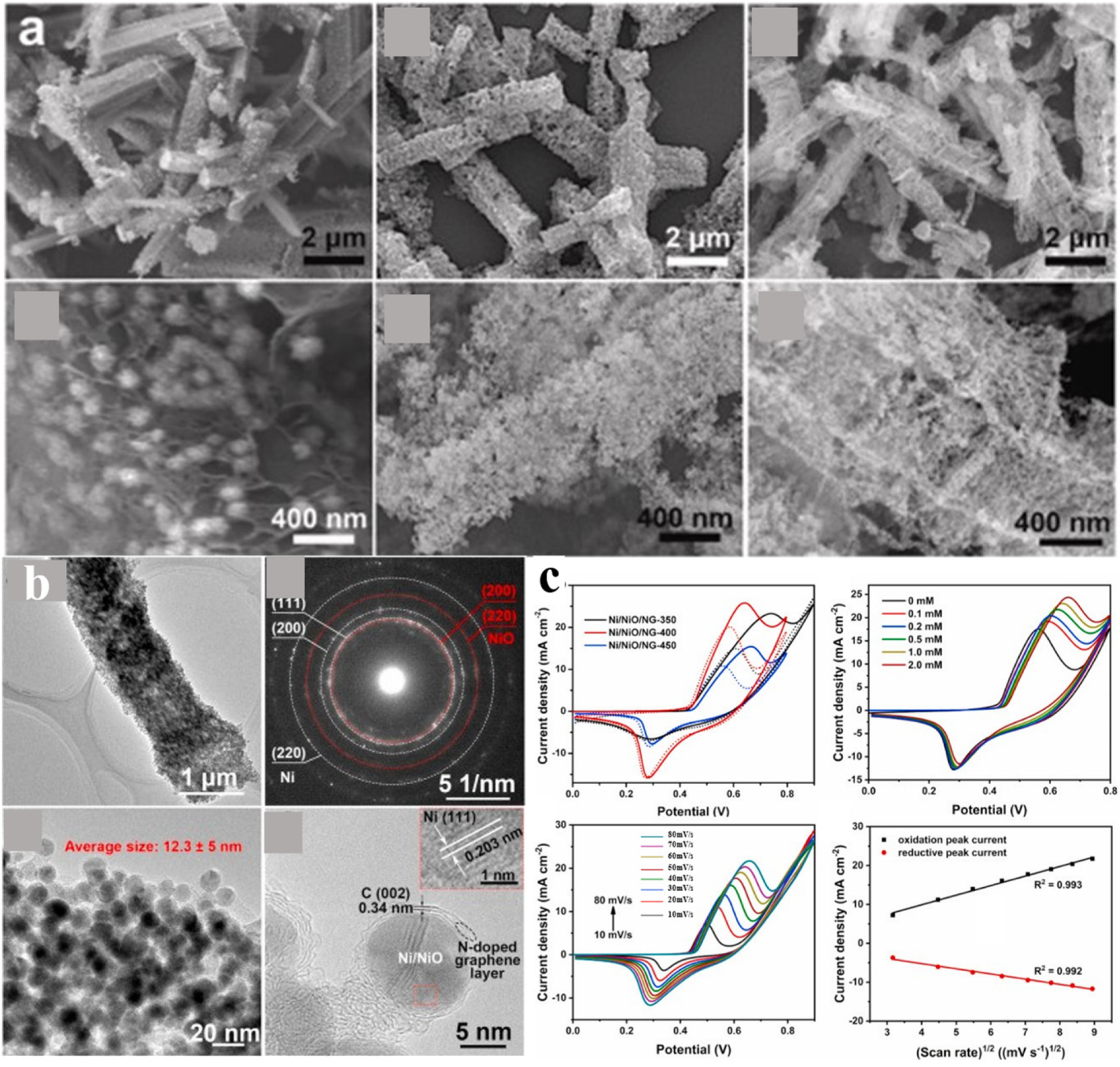
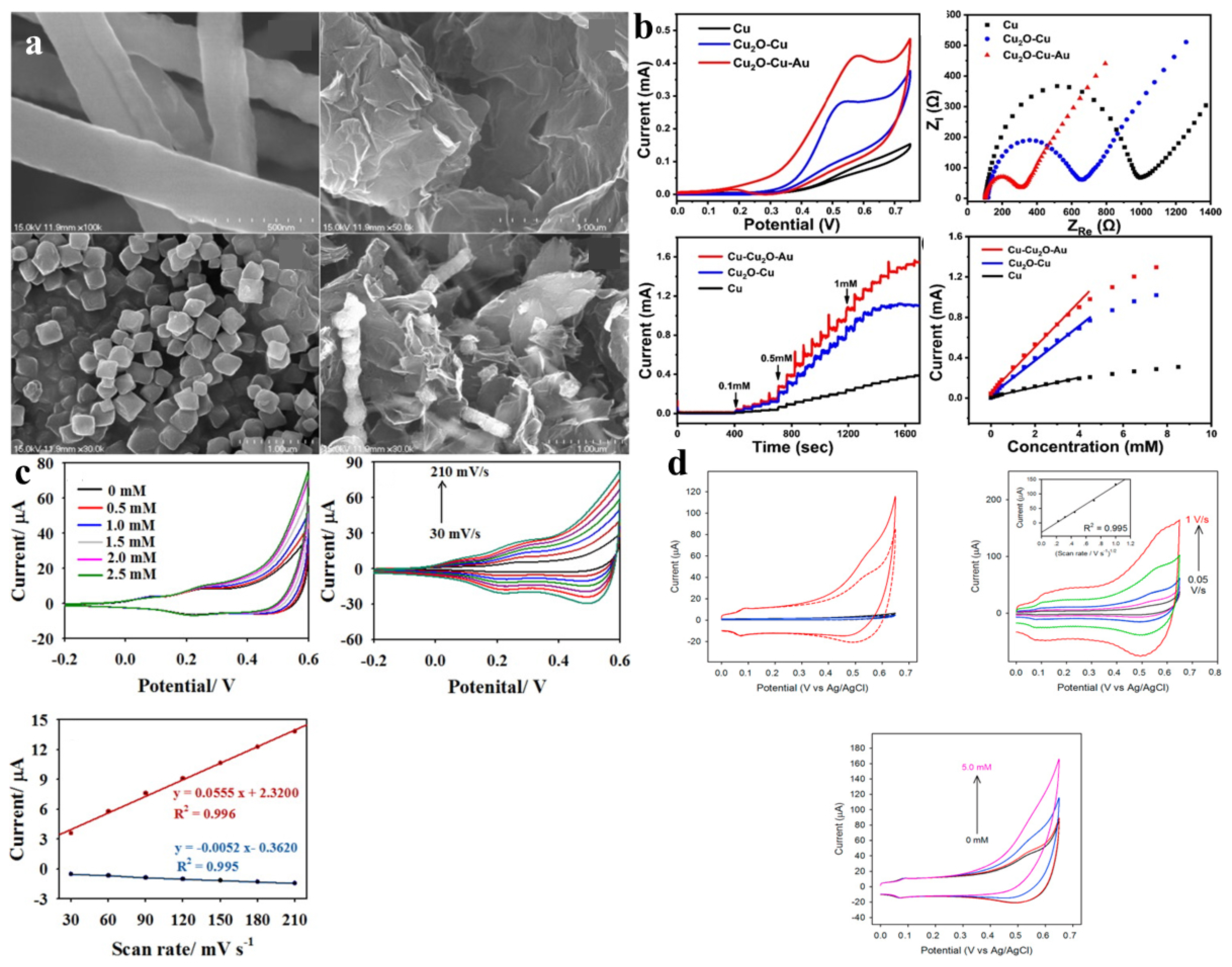
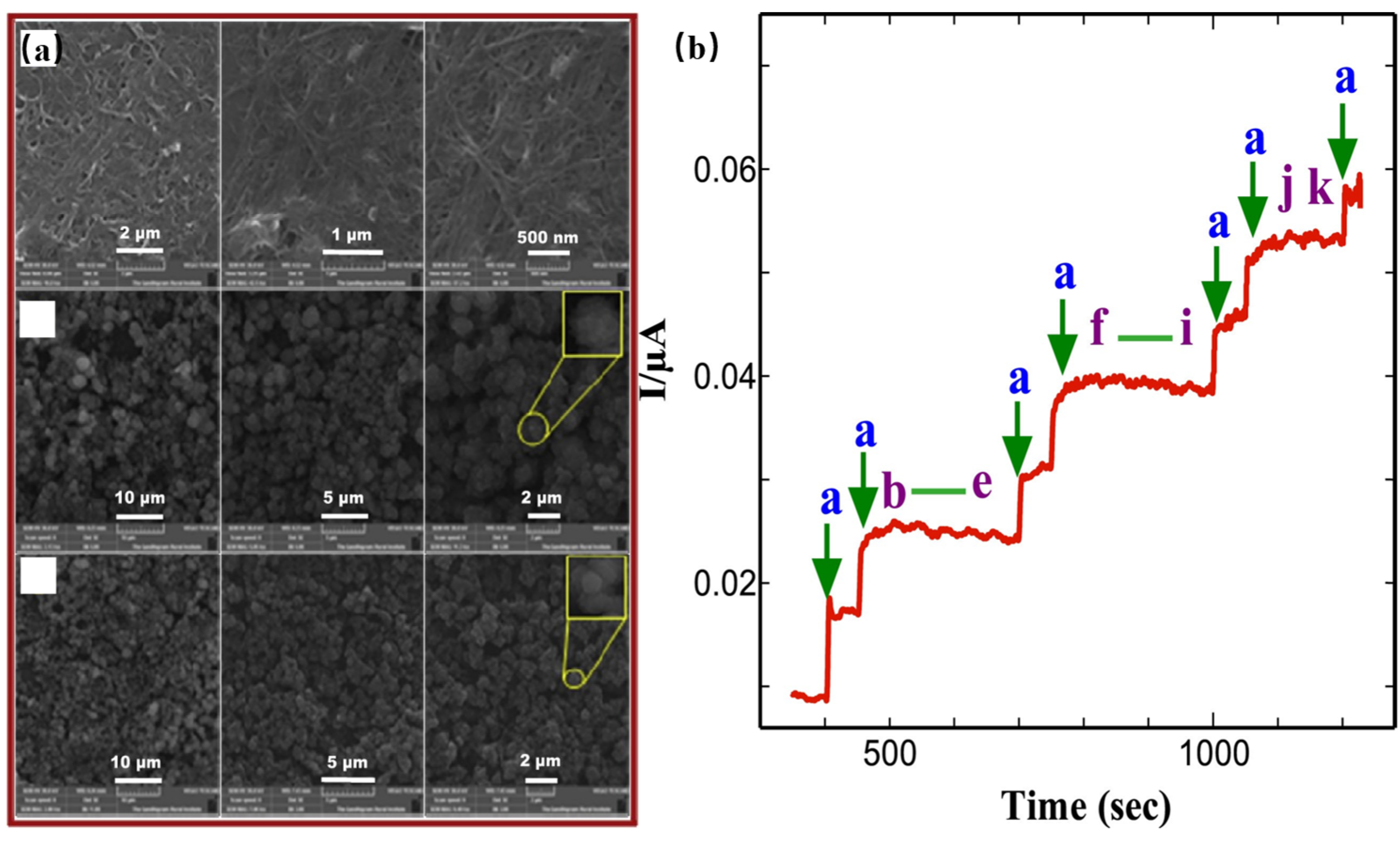
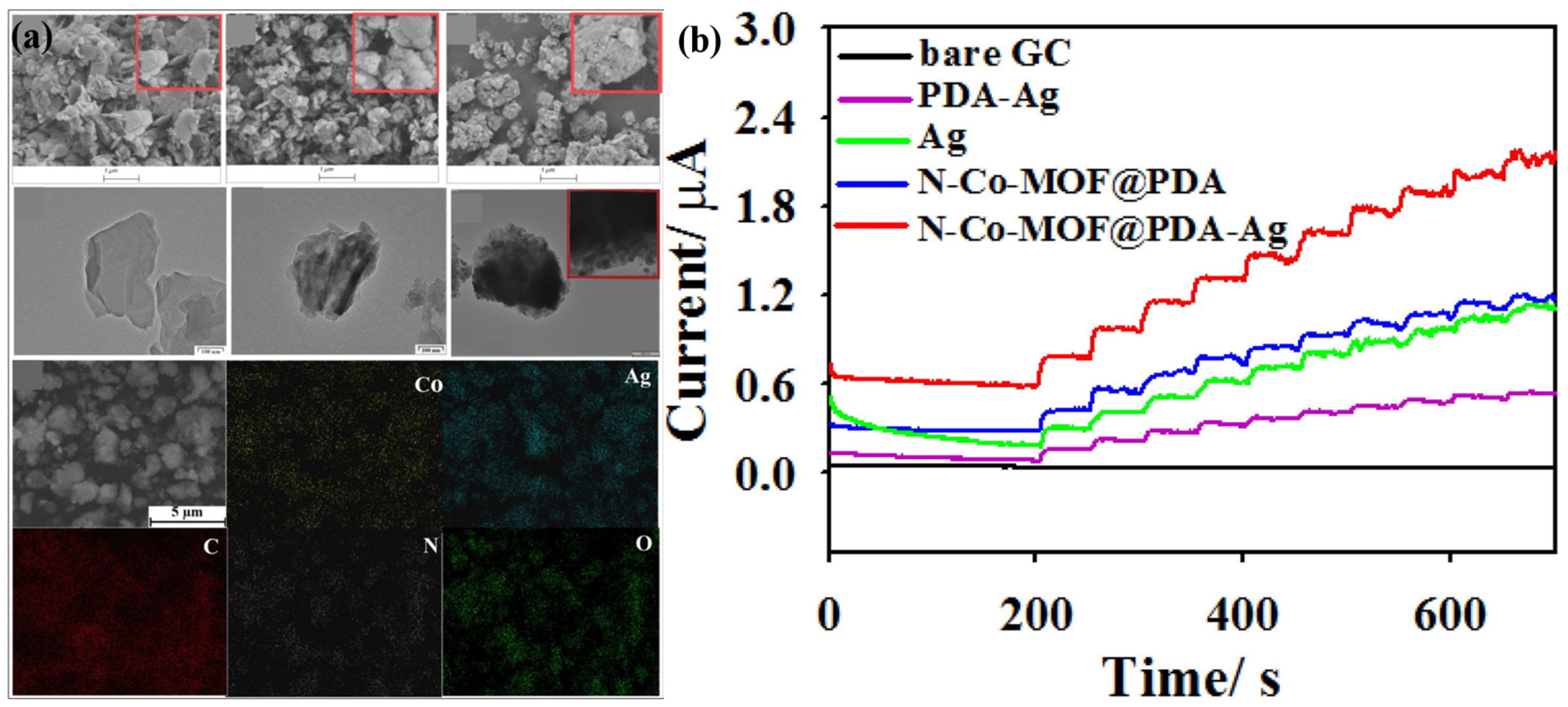


| Sensor Materials | Real Samples | Linear Range (mM) | LOD (μM) | Sensitivity (μA mM−1 cm−2) | Ref |
|---|---|---|---|---|---|
| Ni-MOF | - | - | 0.66 | 21,744 | [39] |
| Ni-MOF | sweat glucose | 0.001–0.4 | 0.89 | 3297.1 | [40] |
| Cu-MOF | urine | 0.06–5000 | 0.01 | - | [41] |
| CuO nanowires | - | - | 0.002 | 648.2 | [42] |
| Cu-MOF | human serum | 0.001–0.95 | 0.076 | 30,030 | [43] |
| Ni-MOF | 0.001–1.6 | 0.46 | 2859.95 | [44] | |
| Co-MOF | - | - | 0.3 | - | [45] |
| Co-MOFs | - | 10–1200 | 3.2 | 160.75 | [46] |
| Co-MOF | - | 0.005–0.9 | 1.6 | 169 | [47] |
| Ni-MOF | human serum | 0.025–3.15 | 0.6 | 402.3 | [48] |
| CuO architectures | 2.8 | 0.1 | 934.2 | [49] | |
| CuO | human serum | 0.0005–5.0 | - | - | [50] |
| Cu-MOF/CPE | human serum | 0.005–10.95 | 0.11 | - | [51] |
| Co-MOF/EG | - | 1–3330 | 0.58 | 23,330 | [52] |
| Sensor Materials | Real Samples | Linear Range (mM) | LOD (μM) | Sensitivity (μA mM−1 cm−2) | Ref |
|---|---|---|---|---|---|
| Ni/Co-MOF | human serum | 0.3–2.312 | 0.1 | 3250 | [56] |
| Ni/Co-MOF | human serum | 0.1–1400 | 0.047 | 2086.7 | [57] |
| Ni@Cu-MOF | human serum | 0.005–2.5 | 1.67 | 1703.33 | [58] |
| Cu-Co ZIF derivative | human serum | 0.02–0.8 | - | 18,680 | [59] |
| CuOx@Co3O4 | human serum | 0.0001–1.3 | - | 27,778 | [60] |
| UiO-67@Ni-MOF | human serum | 5–3900 | 0.98 | - | [61] |
| NiCu-MOF-6 | human serum | 0.02–4.93 | 15 | 1832 | [62] |
| Cu@ Ni MOF | human blood serum | 0–5 | 0.0004 | 496 | [63] |
| Co3(BTC)2 MOFs | 1–330 | 0.33 | 1792 | [64] | |
| ZIF67/ZIF8 | - | - | 6.5 | 833.61 | [65] |
| Cu/Co-ZIF-20 | - | 0.05–6 | - | 0.03 | [66] |
| ZIF-Zn0.5Co0.5 | up to 1.25 | 9 | 1105.6 | [67] | |
| Cu1Co2-MOF/Ni foam | 0.05–0.5 | 8304.4 | [54] |
| Sensor Materials | Real Samples | Linear Range (mM) | LOD (μM) | Sensitivity (μA mM−1 cm−2) | Ref |
|---|---|---|---|---|---|
| CuO polyhedrons/CC | - | 0.5–800 μM | 0.46 | 13,575 | [36] |
| Co–Ni–C-MOF | - | 5–1000 | 0.75 | 1964 | [62] |
| Bimetallic NCNT MOF CoCu nanostructure | - | 0.05–5.5 | 0.00015 | 1027 | [68] |
| NiCo2O4 HNCs | human serum | 0.00018–5.1 | 0.027 | 1306 | [71] |
| Ni/NiO@C | blood serum | 0.01–2 | 0.116 | 1291 | [74] |
| NiO/C-MOF | - | 0.005–4.1 | 0.92 | 2918 | [78] |
| Ni-MOF NSAs/CC | human blood serum | 0.001–7 | 0.57 | 13,428.89 | [79] |
| Cu NWs-MOFs-GO | human serum | 26.6 | 0.007 | - | [81] |
| Cu2O-Cu-Au | human serum | - | 1.71 | 1 × 106 | [82] |
| N-Co-MOF@ PDA-Ag | human serum | 0.001–2 | 0.5 | 183.6 | [83] |
| Co@NCD | human serum | 0.0002–12.0 | 0.11 | 125 | [84] |
| Au@Ni-MOF | bovine serum | 0.0005–1 | 0.36 | 246.8 | [85] |
| Ag@In2O3/NF | - | 10 μM–2.16 mM | 0.49 | 3310 | [86] |
| ZnCo2O4 microrice | - | 0.55–2.65 | 5 | 215.1 | [87] |
| Au@NiCo LDH | - | 0.005–12 | 0.028 | 864.7 | [88] |
| CoZn-LDHs | - | 0.001–0.255 | 15.6 | 1218 | [89] |
| Cu/g-SiCNT/CuO | - | 0.001–4.48 | 0.8 | 2051 | [90] |
| ZIF-67/rGO/CF | - | 0.0001–1 | 0.2 | - | [91] |
| Sensor Materials | Real Samples | Linear Range(mM) | LOD (μM) | Sensitivity (μA mM−1 cm−2) | Ref |
|---|---|---|---|---|---|
| Ni3(HITP)2 MOFs | - | 0–10 | - | - | [37] |
| 3D M-BDC-MOF | - | 0.01–0.8 | 6.68 | 636 | [38] |
| NiCo LDH/NiCoS/CC | - | 0.001–3, 4–9 | 0.208 | 2167, 1417 | [69] |
| Ni/Co-TCPP-MOF | human serum | 0.001–3.8 | 0.3 | 2800 | [70] |
| NiO/Cu-TCPP | - | 0.00285-0.2885 | 4666 | [72] | |
| Ni3N@C | bovine serum | 1–3000 | 0.3 | 1511.59 | [73] |
| Ni/Ni(OH)2-NFs/CP | - | 0.2–60 | 800 | 1078 | [74] |
| MOF-74(Cu) NS-CC | - | 0.1–1 | 0.14 | 381 | [75] |
| Ni@NC7H | human serum | 0.001–1.805 | 0.34 | 1440 | [76] |
| Ni/NiO/NG-400 | Human serum | 0.001–3.568 | 0.032 | 3251.8 | [77] |
| ZIF-67@GO/NiCo2O4/CC | - | 0.0003–5.407 | 0.16 | 990.12 | [80] |
| Hierarchical Co3O4/NiCo2O4/CC | human serum | 0.001–1.127 | 0.64 | 12,835 | [92] |
| SWCNTs-MPsLCu-MOF | human saliva | 0.000020–0.08 | 1.72 | 573 | [93] |
| Ni–Co MOF/Ag/rGO/PU | sweat glucose | 10–660 | - | 425.9 | [96] |
| CoFe-PBA/Co-ZIF/NF | - | 1.4–1500 | 0.02 | 5270 | [97] |
| FeBDC-derived Fe3O4 | - | Up to 9.0 mM | 15.7 | 4670 | [98] |
| CuO-350-NA/GCE | - | 5–1165 | 0.63 | 1614.4 | [99] |
| NiCo2O4 nanowire arrays/Ni foam | human serum | 0.001−3.987 | - | 5916 | [100] |
| Co–Ni–C-MOF | - | 5–1000 | 0.75 | 1964 | [101] |
| Cu@HHNs | - | 5–3000 | 1.97 | 1594.2 | [102] |
| SPCE\15 %CuO−IL | - | 1–2800 | 1 | 820 | [103] |
| CC@MOF-74(NiO)@NiCo LDH | - | 10–1100 | 1699 | [104] | |
| α-CD-rGO/Ni-MOF/TM | - | 0.65–4.828 | 0.3 | 1395 | [105] |
| Nafion/Co/MnO@HC/GCE | - | Up to 6.9 | 1.31 | 233.8 | [106] |
| Ag@TiO2@ZIF-67 | - | - | 0.99 | 7880 | [107] |
| Ti3C2Tx/ZIF-67 | - | 0.005–7.5 | 3.81 | - | [108] |
| ZIF-67 HNPs | - | 0.005–3.3 | 0.96 | 445.7 | [109] |
| HS-ZIF-67 | - | - | 3.9 × 10–6 | 1074.22 | [110] |
| Ag@ZIF-67@MWCNT/NF | 0.033–0.44 | 0.49 | 13,014 | [111] | |
| Ni/Ni(OH)2-NFs/CP | - | 0.2–60 | 800 | 1078 | [112] |
| NiP-450 | - | 0.001–0.6 | 0.16 | 1373.7 | [113] |
| r-NiPO | human serum | 0.001–3 | 1 | 3169 | [114] |
| NiO-NC@rGO/GCE | - | 0.5–20 | 0.07 | 4254 | [115] |
| Ni3S2@NCNT | - | 0.46–3190 | 0.14 | 1447.64 | [116] |
| NHCN–Co3O4 | human serum | 0.001–32.0 | 0.2 | 12,900 | [117] |
| Co/CoO/NC | - | 0.01–2.75 | 0.8 | 143.9 | [118] |
| NN-CuO/N-rGO | - | 0.5–639 | 0.001 | 3400 | [119] |
| Porous copper@carbon agglomerate | - | 0.005–3.33 | 0.29 | 614.3 | [120] |
| Integrated Co(OH)2/GCE | human serum | 0.005–6.7 | 1.73 | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zeng, W.; Li, Y. Recent Progress in MOF-Based Electrochemical Sensors for Non-Enzymatic Glucose Detection. Molecules 2023, 28, 4891. https://doi.org/10.3390/molecules28134891
Li Z, Zeng W, Li Y. Recent Progress in MOF-Based Electrochemical Sensors for Non-Enzymatic Glucose Detection. Molecules. 2023; 28(13):4891. https://doi.org/10.3390/molecules28134891
Chicago/Turabian StyleLi, Ziteng, Wen Zeng, and Yanqiong Li. 2023. "Recent Progress in MOF-Based Electrochemical Sensors for Non-Enzymatic Glucose Detection" Molecules 28, no. 13: 4891. https://doi.org/10.3390/molecules28134891
APA StyleLi, Z., Zeng, W., & Li, Y. (2023). Recent Progress in MOF-Based Electrochemical Sensors for Non-Enzymatic Glucose Detection. Molecules, 28(13), 4891. https://doi.org/10.3390/molecules28134891







