N,N-Bis(2,4-Dibenzhydryl-6-cycloalkylphenyl)butane-2,3-diimine–Nickel Complexes as Tunable and Effective Catalysts for High-Molecular-Weight PE Elastomers †
Abstract
1. Introduction
2. Results
2.1. Synthesis and Characterization
2.2. Ethylene Polymerization Studies
2.2.1. Selection of Aluminum Activator
2.2.2. Ethylene Polymerization Achieved with Activation of Ni1–Ni4 with EtAlCl2
2.2.3. Ethylene Polymerization Achieved with Activation of Ni1–Ni4 with Et2AlCl
2.2.4. Ethylene Polymerization Achieved with Activation of Ni1–Ni4 with MAO
2.3. Properties of Polyethylene
2.3.1. Analysis of Polyethylene Branching with 13C NMR Spectroscopy
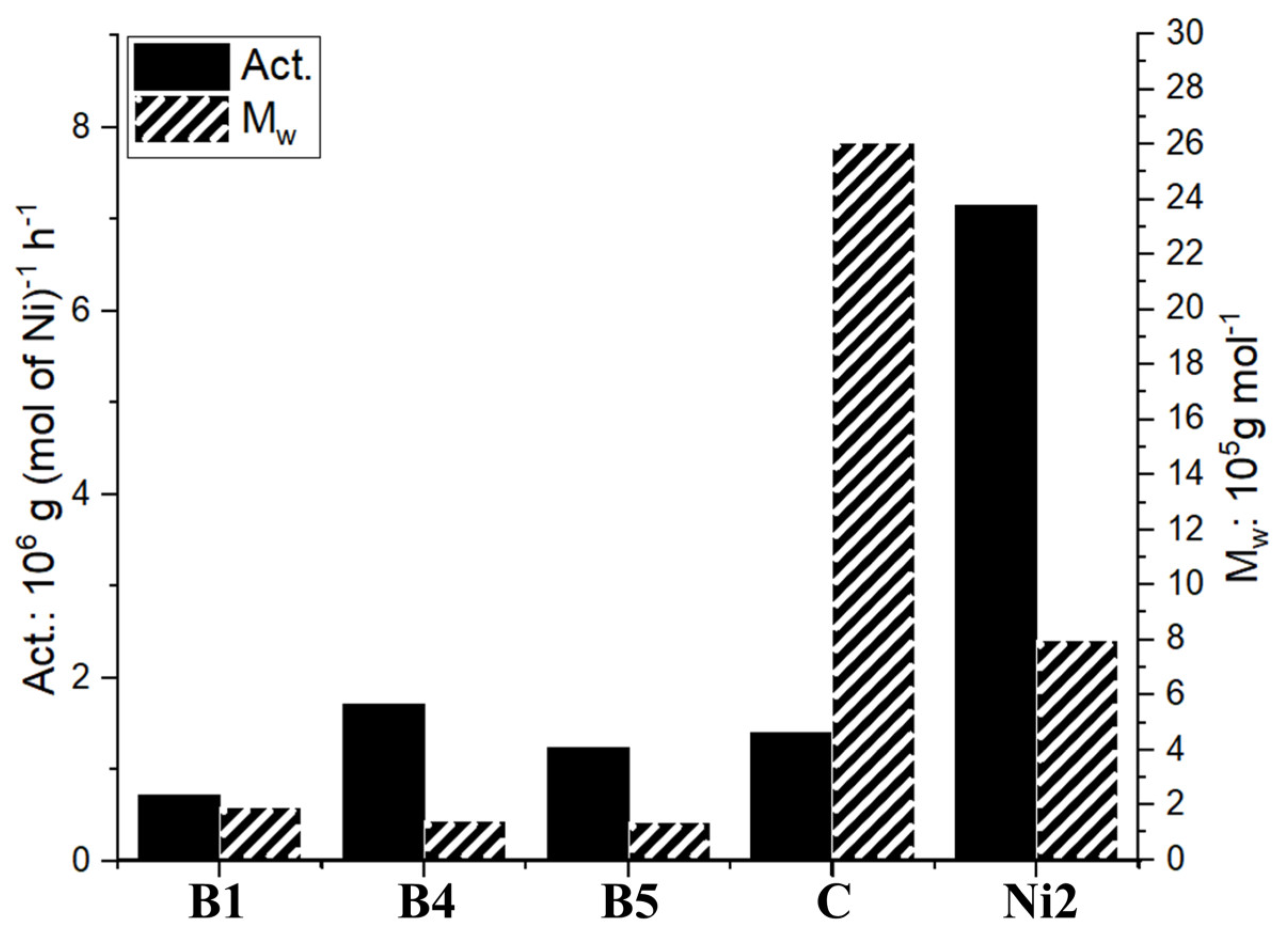
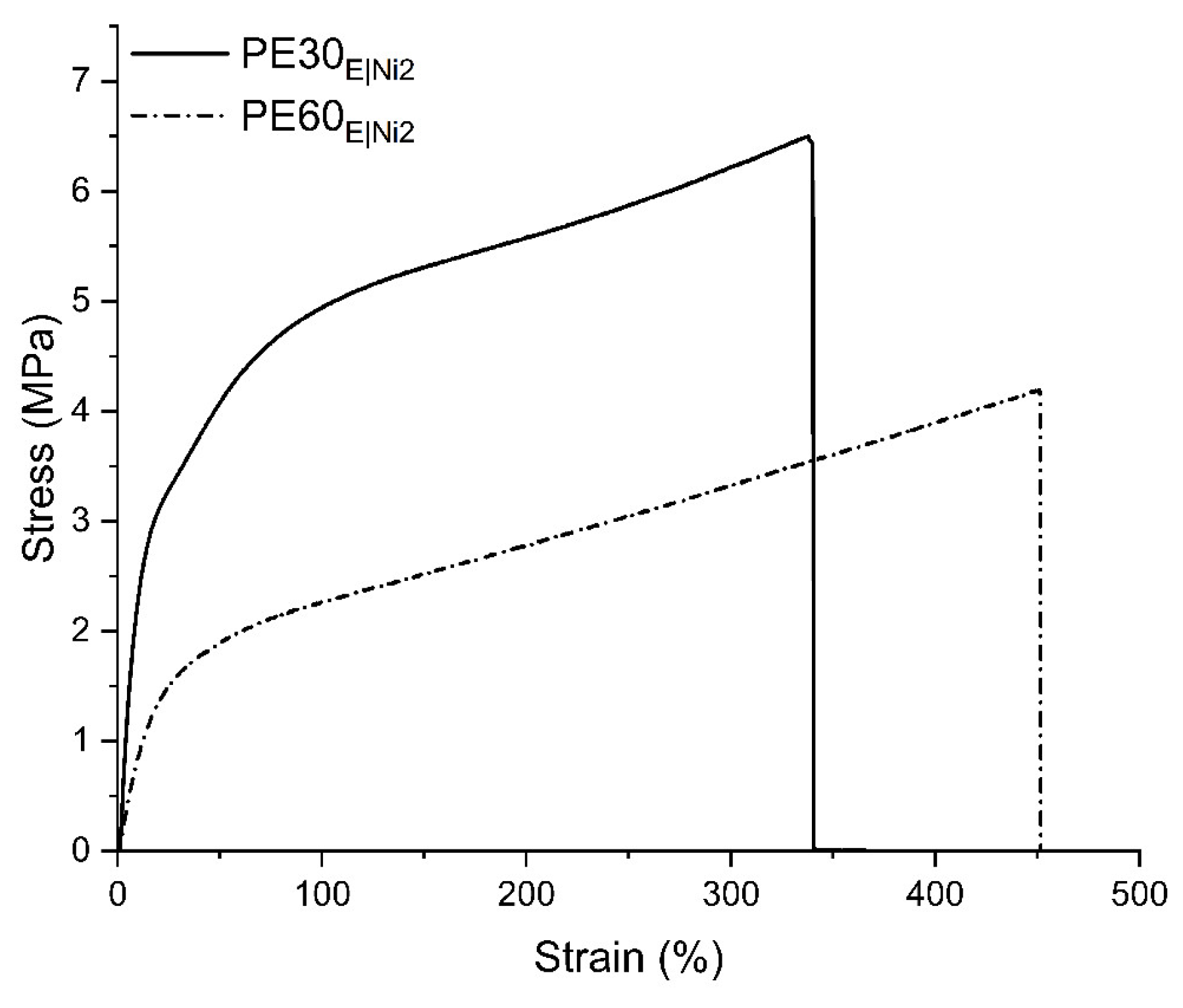
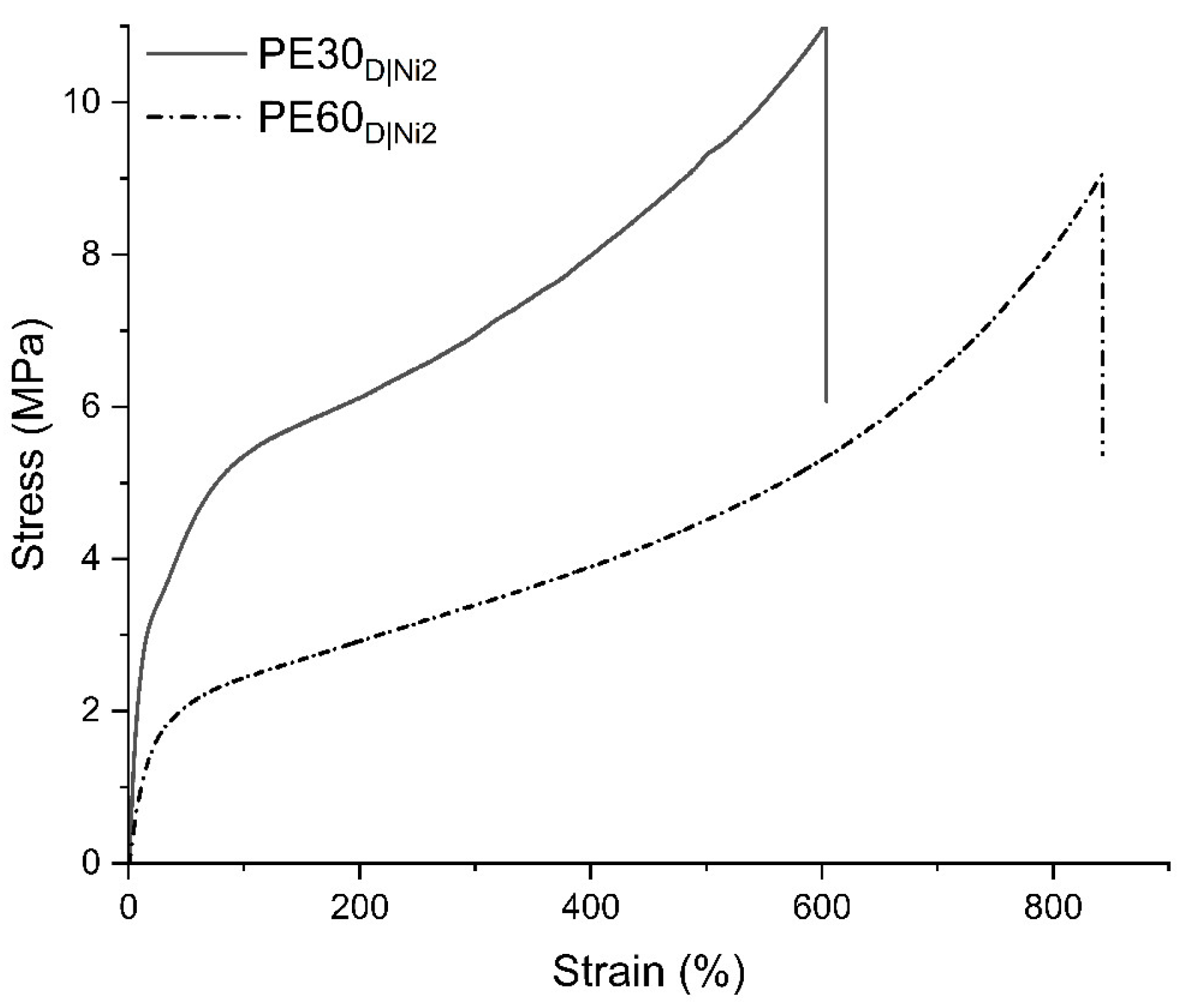
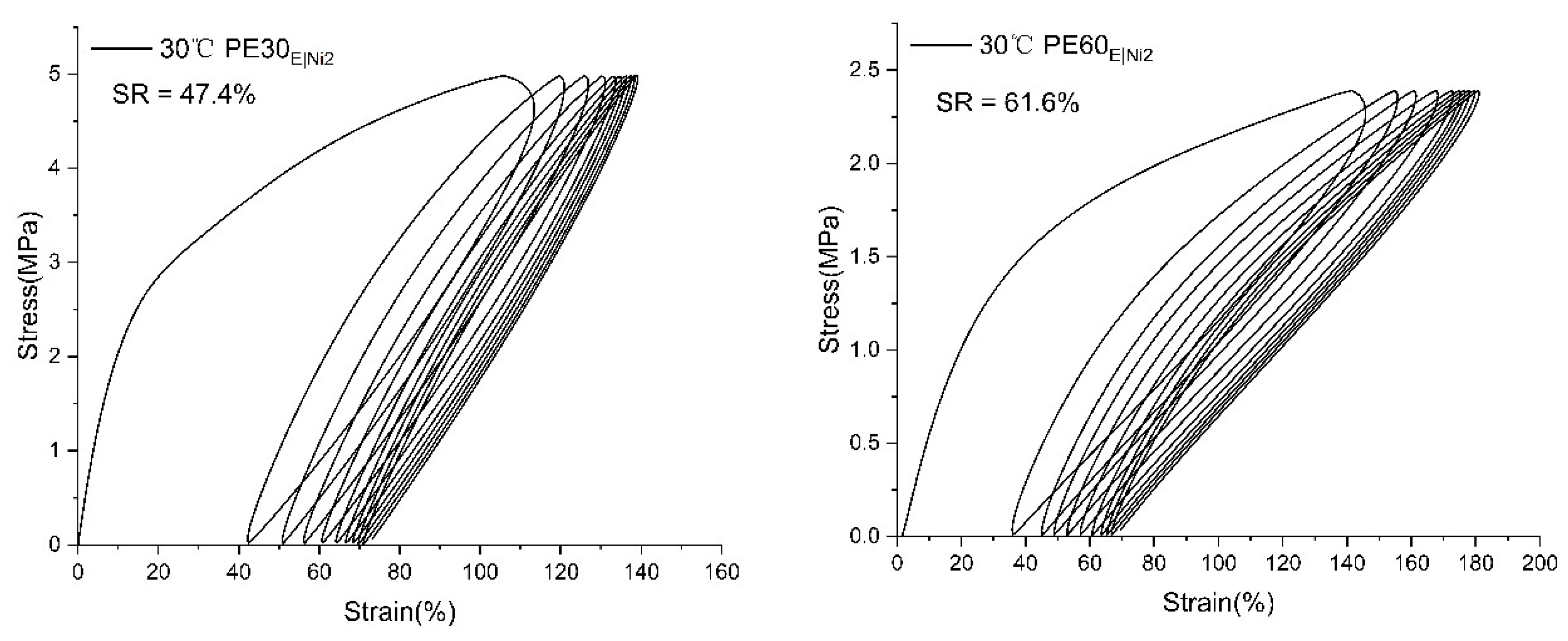
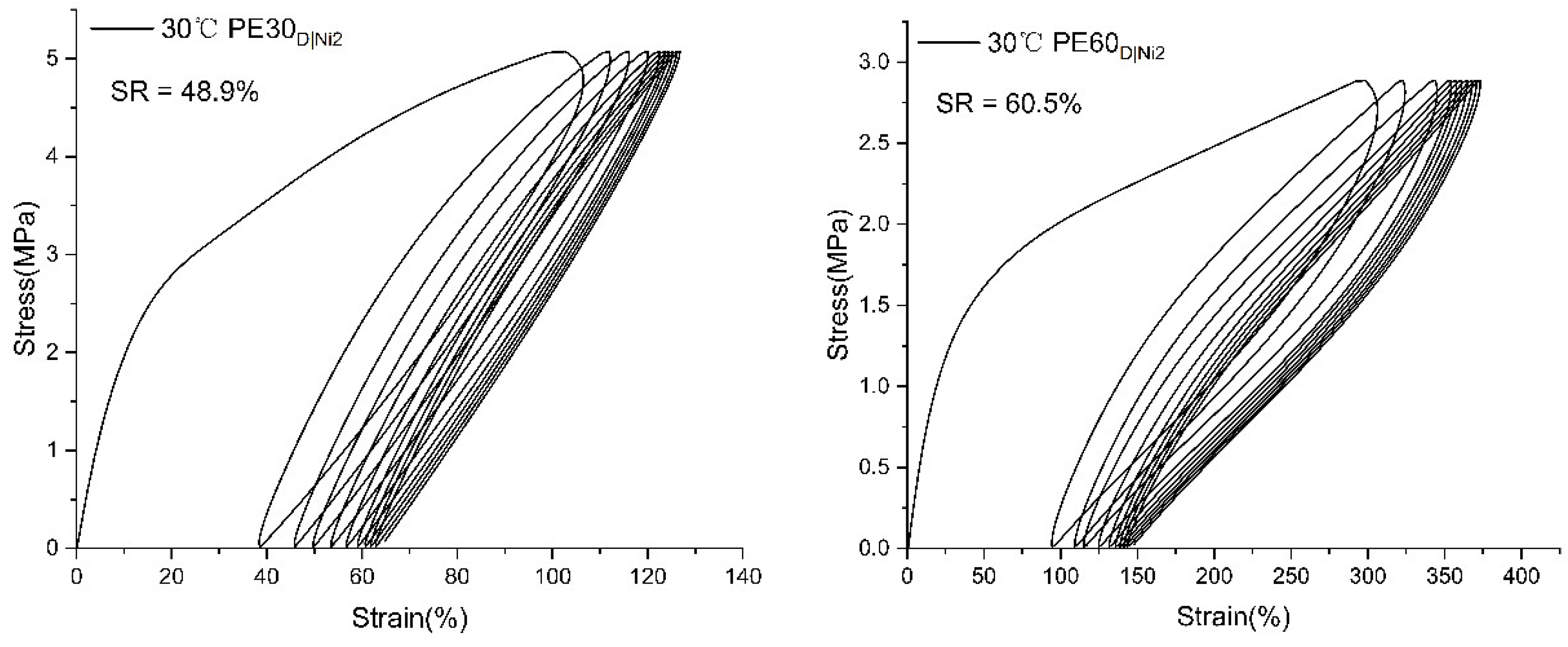
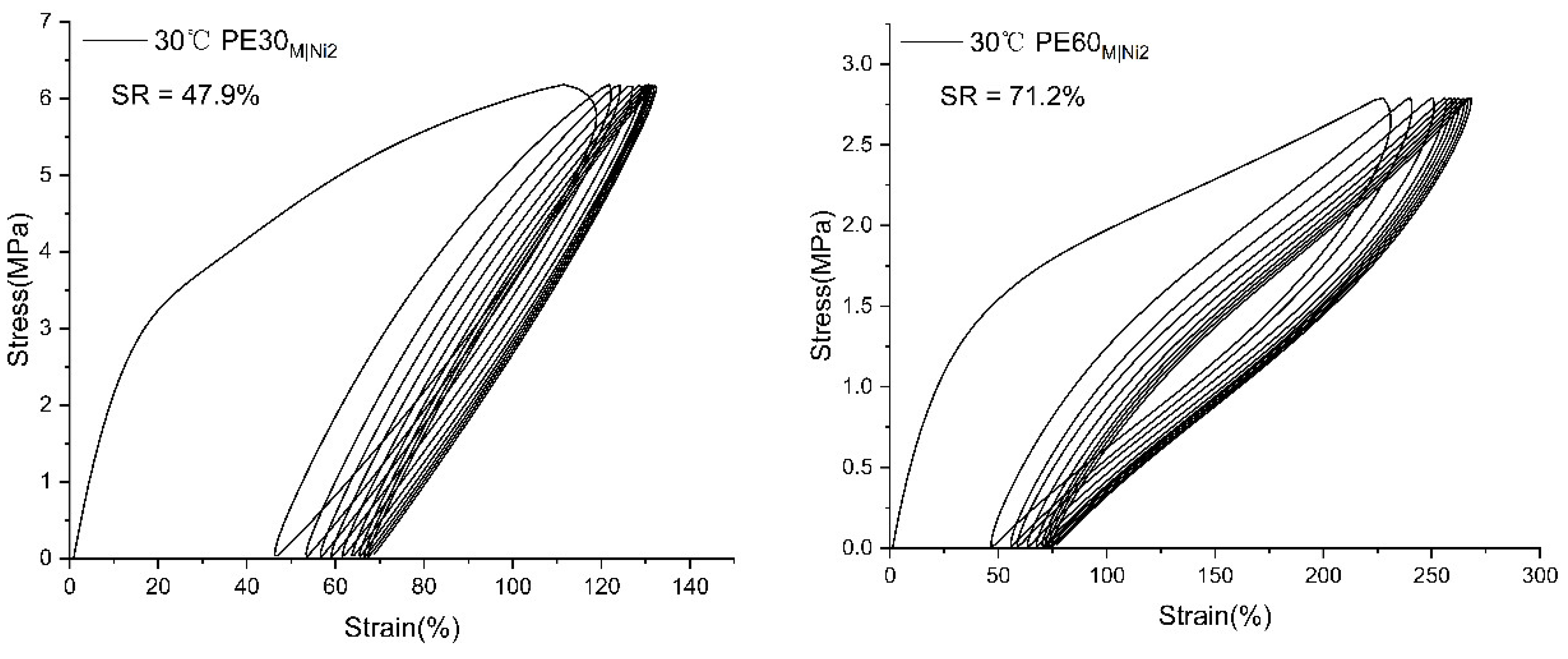
2.3.2. Mechanical Performance of Branched Polyethylenes
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of [ArN=C(Me)-C(Me)=NAr]NiBr2 (Ni1–Ni4)
- (a)
- Ar = 2-(C5H9)-4,6-(CHPh2)2C6H2 (Ni1). 2,3-Butanedione (0.009 g, 0.10 mmol), 2-cyclopentyl-4,6-dibenzhydrylaniline (0.198 g, 0.40 mmol) and (DME)NiBr2 (0.031 g, 0.10 mmol) were stirred and heated to reflux in glacial acetic acid (15 mL) for 6 h. After cooling to room temperature, excess anhydrous diethyl ether was added to induce precipitation. The precipitate was then filtered, washed with diethyl ether (4 × 20 mL) and dried under reduced pressure to give Ni1 (0.021 g, 17%) as brown powder. FT-IR (cm−1): 2954(w), 2869(w), 1644(w, vC=N), 1578(m), 1494(m), 1448(m), 1418(w), 1340(w), 1265(w), 1215(w), 1186(w), 1163(w), 1077(w), 1031(w), 745(m), 699(s). Anal. Calcd for C78H72Br2N2Ni (1255.95): C, 74.59; H, 5.78; N, 2.23. Found: C, 74.23; H, 6.01; N, 2.04.
- (b)
- Ar = 2-(C6H11)-4,6-(CHPh2)2C6H2 (Ni2). Using a synthetic procedure similar to that described for Ni1 but using 2-cyclohexyl-4,6-dibenzhydrylaniline as the arylamine, Ni2 was isolated as brown powder (0.327 g, 64%). FT-IR (cm−1): 2921(w), 2850(w), 1637(w, vC=N), 1600(m), 1495(m), 1446(m), 1368(m), 1260(w), 1239(w), 1215(w), 1162(w), 1077(w), 1032(w), 776(w), 748(m), 699(s). Anal. Calcd for C80H76Br2N2Ni (1284.00): C, 74.83; H, 5.97; N, 2.18. Found: C, 74.67; H, 6.22; N, 1.94.
- (c)
- Ar = 2-(C8H15)-4,6-(CHPh2)2C6H2 (Ni3). Using a synthetic procedure similar to that described for Ni1 but using 2-cyclooctyl-4,6-dibenzhydrylaniline as the arylamine, Ni3 was isolated as brown powder (0.068 g, 20%). FT-IR (cm−1): 2919(w), 2857(w), 1644(w, vC=N), 1593(m), 1494(m), 1447(m), 1421(w), 1345(w), 1185(w), 1078(w), 1030(w), 914(w), 849(w), 744(m), 698(s). Anal. Calcd for C84H84Br2N2Ni (1340.11): C, 75.29; H, 6.32; N, 2.09. Found: C, 74.98; H, 6.44; N, 1.83.
- (d)
- Ar = 2-(C12H23)-4,6-(CHPh2)2C6H2 (Ni4). Using a similar synthetic procedure to that described for Ni1 but using 2-cyclododecyl-4,6-dibenzhydrylaniline as the arylamine, Ni4 was isolated as brown powder (0.072 g, 25%). FT-IR (cm−1): 2920(w), 2858(w), 1639(w, vC=N), 1594(m), 1494(m), 1445(m), 1373(w), 1258(w), 1212(w), 1161(w), 1075(w), 1031(w), 914(w), 847(w), 743(m), 697(s). Anal. Calcd for C92H100Br2N2Ni (1452.33): C, 76.09; H, 6.94; N, 1.93. Found: C, 75.83; H, 7.03; N, 1.77.
3.3. X-ray Diffraction Studies
3.4. Polymerization Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Knowles, W.S.; Sabacky, M.J. Catalytic asymmetric hydrogenation employing a soluble, optically active, rhodium complex. Chem. Commun. 1968, 22, 1445–1446. [Google Scholar] [CrossRef]
- Ostoja Starzewski, K.A.; Witte, J. Control of the molecular weight of polyethene in syntheses with bis(ylide)nickel catalysts. Angew. Chem. Int. Ed. 1987, 26, 63–64. [Google Scholar] [CrossRef]
- Mu, H.; Pan, L.; Song, D.; Li, Y. Neutral Nickel Catalysts for Olefin Homo- and Copolymerization: Relationships between Catalyst Structures and Catalytic Properties. Chem. Rev. 2015, 115, 12091–12137. [Google Scholar] [CrossRef] [PubMed]
- Chen, C. Designing catalysts for olefin polymerization and copolymerization: Beyond electronic and steric tuning. Nat. Rev. Chem. 2018, 2, 6–14. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)- and Ni(II)-Based Catalysts for Polymerization of Ethylene and alpha-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Walsh, D.J.; Hyatt, M.G.; Miller, S.A.; Guironnet, D. Recent Trends in Catalytic Polymerizations. ACS Catal. 2019, 9, 11153–11188. [Google Scholar] [CrossRef]
- Mecking, S. Olefin Polymerization by Late Transition Metal Complexes-A Root of Ziegler Catalysts Gains New Ground. Angew. Chem. Int. Ed. 2001, 40, 534–540. [Google Scholar] [CrossRef]
- Gansukh, B.; Zhang, R.; Guo, J.; Zhang, W.; Sun, W.-H. Paradox of Late Transition-Metal Catalysts in Ethylene Polymerization. Gen. Chem. 2020, 6, 190031. [Google Scholar] [CrossRef]
- Dai, S.; Li, S.; Xu, G.; Wu, C.; Liao, Y.; Guo, L. Flexible cycloalkyl substituents in insertion polymerization with α-diimine nickel and palladium species. Polym. Chem. 2020, 11, 1393–1400. [Google Scholar] [CrossRef]
- Ittel, S.D.; Johnson, L.K.; Brookhart, M. Late-Metal Catalysts for Ethylene Homo- and Copolymerization. Chem. Rev. 2000, 100, 1169–1203. [Google Scholar] [CrossRef]
- Guan, Z.; Cotts, P.M.; McCord, E.F.; McLain, S.J. Chain Walking: A New Strategy to Control Polymer Topology. Science 1999, 283, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- Anderson, W.C., Jr.; Rhinehart, L.; Tennyson, A.G.; Long, B.K. Redox-Active Ligands: An Advanced Tool to Modulate Polyethylene Microstructure. J. Am. Chem. Soc. 2016, 138, 774–777. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xin, L.; Hao, Z.Q.; Li, G.D.; Su, J.-H.; Zhou, L.J.; Mu, Y. The ligand redox behavior and role in 1,2-bis[(2,6-diisopropylphenyl)imino]-acenaphthene nickel-TMA(MAO) systems for ethylene Polymerization. Chem. Commun. 2015, 51, 7004–7007. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C. A continuing legend: The Brookhart-type α-diimine nickel and palladium catalysts. Polym. Chem. 2019, 10, 2354–2369. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Solan, G.A.; Sun, W.-H. Recent advances in Ni-mediated ethylene chain growth: Nimine-donor ligand effects on catalytic activity, thermal stability and oligo-/polymer structure. Coord. Chem. Rev. 2017, 350, 68–83. [Google Scholar] [CrossRef]
- Mahmood, Q.; Li, X.; Qin, L.; Wang, L.; Sun, W.-H. Structural evolution of iminopyridine support for nickel/palladium catalysts in ethylene (oligo)polymerization. Dalton Trans. 2022, 51, 14375–14407. [Google Scholar] [CrossRef]
- Gates, D.P.; Svejda, S.A.; Oňate, E.; Killian, C.M.; Johnson, L.K.; White, P.S.; Brookhart, M. Synthesis of Branched Polyethylene Using (α-Diimine)nickel(II) Catalysts: Influence of Temperature, Ethylene Pressure, and Ligand Structure on Polymer Properties. Macromolecules 2000, 33, 2320–2334. [Google Scholar] [CrossRef]
- Rhinehart, J.L.; Brown, L.A.; Long, B.K. A Robust Ni (II) α-Diimine catalyst for high temperature ethylene polymerization. J. Am. Chem. Soc. 2013, 135, 16316–16319. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, W.; Liu, W.; Wang, L.; Redshaw, C.; Sun, W.-H. Unsymmetrical α-diiminonickel bromide complexes: Synthesis, characterization and their catalytic behavior toward ethylene. Catal. Sci. Technol. 2013, 3, 2737–2745. [Google Scholar] [CrossRef]
- Fan, L.; Du, S.; Guo, C.-Y.; Hao, X.; Sun, W.-H. 1-(2,6-dibenzhydryl-4-fluorophenylimino)-2-aryliminoacenaphthylylnickel halides highly polymerizing ethylene for the polyethylenes with high branches and molecular weights. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1369–1378. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Mahmood, Q.; Yuan, S.; Li, X.; Qin, L.; Zou, S.; Liang, T.; Sun, W.-H. Enhancing the Thermal Stability of α-Diimine Ni(II) Catalysts for ethylene polymerization via Symmetrical axial shielding strategy. Eur. Polym. J. 2023, 194, 112112. [Google Scholar] [CrossRef]
- Gao, M.; Du, S.; Ban, Q.; Xing, Q.; Sun, W.-H. Ethylene polymerization by 2,3-diiminobutylnickel bromide pre-catalysts bearing remote benzhydryl substituents. J. Organomet. Chem. 2015, 798, 401–407. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Li, W.; Chen, M. Ligand steric effects on α-diimine nickel catalyzed ethylene and 1-hexene polymerization. RSC Adv. 2017, 7, 55051–55059. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, D.; Zhang, W.; Zada, M.; Solan. G. A. Remote dibenzocycloheptyl-substitution of an iminotrihydroquinoline-nickel catalyst as a route to narrowly dispersed branched polyethylene waxes with alkene functionality. Eur. Polym. J. 2018, 107, 315–328. [Google Scholar] [CrossRef]
- Lin, W.; Liu, M.; Xu, L.; Ma, Y.; Zhang, L.; Flisak, Z.; Hu, X.; Liang, T.; Sun. W.-H. Nickel (II) complexes with sterically hindered 5,6,7-trihydroquinoline derivatives selectively dimerizing ethylene to 1-butene. Appl. Organomet. Chem. 2022, 36, 6596. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, W.; Jia, D.; Hao, X.; Redshaw, C.; Sun. W.-H. 2-{2,6-Bis[bis(4-fluorophenyl)methyl]-4-chlorophenylimino}-3-aryliminobutylnickel(II) bromide complexes: Synthesis, characterization, and investigation of their catalytic behavior. Appl. Catal. A Gen. 2014, 475, 195–202. [Google Scholar] [CrossRef]
- Tempel, D.J.; Johnson, L.K.; Huff, R.L.; White, P.S.; Brookhart, M. Mechanistic studies of Pd(II)-α-diimine-catalyzed olefin polymerizations. J. Am. Chem. Soc. 2000, 122, 6686–6700. [Google Scholar] [CrossRef]
- Mahmood, Q.; Zeng, Y.; Wang, X.; Sun, Y.; Sun, W.-H. Advancing polyethylene properties by incorporating NO2 moiety in 1,2-bis(arylimino)acenaphthylnickel precatalysts: Synthesis, characterization and ethylene polymerization. Dalton Trans. 2017, 46, 6934–6947. [Google Scholar] [CrossRef]
- Wang, F.; Yuan, J.; Song, F.; Li, J.; Jia, Z.; Yuan. B. New chiral α-diimine nickel(II) complexes bearing ortho-sec-phenethyl groups for ethylene polymerization. Appl. Organomet. Chem. 2013, 27, 319–327. [Google Scholar] [CrossRef]
- Chen, Y.; Du, S.; Huang, C.; Solan, G.A.; Hao, X.; Sun, W.-H. Balancing high thermal stability with high activity in diaryliminoacenaphthene-nickel(II) catalysts for ethylene polymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1971–1983. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, W.; Yu, J.; Yang, W.; Hao, X.; Redshaw, C.; Chen, L.; Sun, L. Synthesis, characterization and ethylene polymerization behavior of nickel dihalide complexes bearing bulky unsymmetrical α-diimine ligands. Catal. Sci. Technol. 2012, 2, 415–422. [Google Scholar] [CrossRef]
- Xia, J.; Kou, S.; Zhang, Y.; Jian, Z. Strategies cooperation on designing nickel catalysts to access ultrahigh molecular weight polyethylenes. Polymer 2022, 240, 124478. [Google Scholar] [CrossRef]
- Yuan, S.; Fan, Z.; Zhang, Q.; Flisak, Z.; Ma, Y.; Sun, Y.; Sun, W.-H. Enhancing performance of α-diiminonickel precatalyst for ethylene polymerization by substitution with the 2,4-bis (4,4’-dimethoxybenzhydryl)-6-methylphenyl group. Appl. Organomet. Chem. 2020, 34, e5638. [Google Scholar] [CrossRef]
- Wang, Y.; Vignesh, A.; Qua, M.; Wang, Z.; Sun, Y.; Sun, W.-H. Access to polyethylene elastomers via ethylene homo-polymerization using N, N′-nickel (II) catalysts appended with electron withdrawing difluorobenzhydryl group. Eur. Polym. J. 2019, 117, 254–271. [Google Scholar] [CrossRef]
- Du, S.; Kong, S.; Shi, Q.; Mao, J.; Guo, C.; Yi, J.; Liang, T.; Sun, W.-H. Enhancing the Activity and Thermal Stability of Nickel complex precatalysts Using 1-[2,6-bis(bis(4-fluorophenyl)methyl)-4-methylphenylimino]-2-aryliminoacenaphthylene Derivatives. Organometallics 2015, 34, 582–590. [Google Scholar] [CrossRef]
- Zada, M.; Vignesh, A.; Guo, L.; Zhang, R.; Zhang, W.; Ma, Y.; Sun, Y.; Sun, W.-H. Sterically and Electronically Modified Aryliminopyridyl-Nickel Bromide Precatalysts for an Access to Branched Polyethylene with Vinyl/Vinylene End Groups. ACS Omega 2020, 5, 10610–10625. [Google Scholar] [CrossRef]
- Yuan, S.; Duan, T.; Zhang, R.; Solan, G.A.; Ma, Y.; Liang, T.; Sun, W.-H. Alkylaluminum activator effects on polyethylene branching using a N,N′-nickel precatalyst appended with bulky 4,4′-dimethoxybenzhydryl groups. Appl. Organomet. Chem. 2019, 33, e4785. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Ma, Y.; Solan, G.A.; Sun, Y.; Sun, W.-H. Plastomeric-like polyethylenes achievable using thermally robust N,N’-nickel catalysts appended with electron withdrawing difluorobenzhydryl and nitro groups. Dalton Trans. 2019, 48, 1878–1891. [Google Scholar] [CrossRef]
- Zada, M.; Guo, L.; Zhang, R.; Zhang, W.; Ma, Y.; Solan, G.A.; Sun, Y.; Sun, W.-H. Moderately branched ultra-high molecular weight polyethylene by using N,N′-nickel catalysts adorned with sterically hindered dibenzocycloheptyl groups. Appl. Organomet. Chem. 2019, 33, e4749. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, R.; Ma, Y.; Han, M.; Solan, G.A.; Yang, W.; Liang, T.; Sun, W.-H. Trifluoromethoxy-substituted nickel catalysts for producing highly branched polyethylenes: Impact of solvent, activator and N,N’-ligand on polymer properties. Polym. Chem. 2022, 13, 1040–1058. [Google Scholar] [CrossRef]
- Cotts, P.M.; Guan, Z.; McCord, E.; McLain, S. Novel Branching Topology in Polyethylenes As Revealed by Light Scattering and 13C NMR. Macromolecules 2000, 33, 6945–6952. [Google Scholar] [CrossRef]
- Jurkiewicz, A.; Eilerts, N.W.; Hsieh, E.T. 13C NMR Characterization of Short Chain Branches of Nickel Catalyzed Polyethylene. Macromolecules 1999, 32, 5471–5476. [Google Scholar] [CrossRef]
- Kunrath, F.A.; Mota, F.F.; Casagrande, O.L., Jr.; Mauler, R.S.; de Souza, R.F. Synthesis and Characterization of Hyperbranched Polyethylenes Made with Nickel-α-Diimine Catalysts. Macromol. Chem. Phys. 2002, 203, 2407–2411. [Google Scholar] [CrossRef]
- Giraudeau, P.; Baguet, E. Improvement of the inverse-gated decoupling sequence for a faster quantitative analysis of various samples by 13C NMR spectroscopy. J. Magn. Reson. 2006, 180, 110–117. [Google Scholar] [CrossRef]
- Galland, G.B.; Souza, R.F.; Mauler, R.S.; Nunes, F.F. 13C NMR Determination of the Composition of Linear Low-Density Polyethylene Obtained with [η3-Methallyl-nickel-diimine]PF6 Complex. Macromolecules 1999, 32, 1620–1625. [Google Scholar] [CrossRef]
- Leatherman, M.D.; Svejda, S.A.; Johnson, L.K.; Brookhart, M. Mechanistic Studies of Nickel(II) Alkyl Agostic Cations and Alkyl Ethylene Complexes: Investigations of Chain Propagation and Isomerization in (α-diimine)Ni(II)-Catalyzed Ethylene Polymerization. J. Am. Chem. Soc. 2003, 125, 3068–3081. [Google Scholar] [CrossRef]
- Michalak, A.; Ziegler, T. Polymerization of Ethylene Catalyzed by a Nickel(+2) Anilinotropone-Based Catalyst: DFT and Stochastic Studies on the Elementary Reactions and the Mechanism of Polyethylene Branching. Organometallics 2003, 22, 2069–2079. [Google Scholar] [CrossRef]
- Falivene, L.; Wiedemann, T.; Göttker-Schnetmann, I.; Caporaso, L.; Cavallo, L.; Mecking, S. Control of Chain Walking by Weak Neighboring Group Interactions in Unsymmetrical Catalysts. J. Am. Chem. Soc. 2018, 140, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Liu, F.; Liao, H.; Gao, J.; Zhong, L.; Gao, H.; Wu, Q. Synthesis of Polyethylenes with Controlled Branching with α-Diimine Nickel Catalysts and Revisiting Formation of Long-Chain Branching. ACS Catal. 2018, 8, 1104–1113. [Google Scholar] [CrossRef]
- Camacho, D.H.; Salo, E.V.; Ziller, J.W.; Guan, Z. Cyclophane-Based Highly Active Late-Transition-Metal Catalysts for Ethylene Polymerization. Angew. Chem. Int. Ed. 2004, 43, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Meinhard, D.; Wegner, M.; Kipiani, G.; Hearley, A.; Reuter, P.; Fischer, S.; Marti, O.; Rieger, B. New Nickel(II) Diimine Complexes and the Control of Polyethylene Microstructure by Catalyst Design. J. Am. Chem. Soc. 2007, 129, 9182–9191. [Google Scholar] [CrossRef]
- Meiries, S.; Speck, K.; Cordes, D.B.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*OMe)(acac)Cl]: Tuning the N-Heterocyclic Carbene in Catalytic C-N Bond Formation. Organometallics 2013, 32, 330–339. [Google Scholar] [CrossRef]
- He, Z.; Liang, Y.; Yang, W.; Uchino, H.; Yu, J.; Sun, W.-H.; Han, C.C. Random hyperbranched linear polyethylene: One step production of thermoplastic elastomer. Polymer 2015, 56, 119–122. [Google Scholar] [CrossRef]
- Wu, R.; Wang, Y.; Zhang, R.; Guo, C.-Y.; Flisak, Z.; Sun, Y.; Sun, W.-H. Finely tuned nickel complexes as highly active catalysts affording branched polyethylene of high molecular weight: 1-(2,6-Dibenzhydryl-4-methoxyphenylimino)-2-(arylimino)acenaphthylenenickel halides. Polymer 2018, 153, 574–586. [Google Scholar] [CrossRef]
- Vignesh, A.; Zhang, Q.; Ma, Y.; Liang, T.; Sun, W.-H. Attaining highly branched polyethylene elastomers by employing modified α-diiminonickel(II) catalysts: Probing the effects of enhancing fluorine atom on the ligand framework towards mechanical properties of polyethylene. Polymer 2020, 187, 122089. [Google Scholar] [CrossRef]
- Suo, H.; Oleynik, I.V.; Huang, C.; Oleynik, I.I.; Solan, G.A.; Ma, Y.; Liang, T.; Sun, W.-H. ortho-Cycloalkyl substituted N,N′-diaryliminoacenaphthene-Ni(ii) catalysts for polyethylene elastomers; exploring ring size and temperature effects. Dalton Trans. 2017, 46, 15684–15697. [Google Scholar] [CrossRef] [PubMed]
- Oleinik, I.I.; Oleinik, I.V.; Abdrakhmanov, I.B.; Ivanchev, S.S.; Tolstikov, G.A. Design of Arylimine Postmetallocene Catalytic Systems for Olefin Polymerization: I. Synthesis of Substituted 2-Cycloalkyland 2,6-Dicycloalkylanilines. Rus. J. Gen. Chem. 2004, 74, 1423–1427. [Google Scholar] [CrossRef]
- Ivanchev, S.S.; Yakimansky, A.V.; Rogozin, D.G. Quantum-chemical calculations of the effect of cycloaliphatic groups in α-diimine and bis(imino)pyridine ethylene polymerization precatalysts on their stabilities with respect to deactivation reactions. Polymer 2004, 45, 6453–6459. [Google Scholar] [CrossRef]
- Chartoire, A.; Claver, C.; Corpet, M.; Krinsky, J.; Mayen, J.; Nelson, D.; Nolan, S.P.; Penafiel, I.; Woodward, R.; Meadows, R.E. Recyclable NHC Catalyst for the Development of a Generalized Approach to Continuous Buchwald-Hartwig Reaction and Workup. Org. Process Res. Dev. 2016, 20, 551–557. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, P.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. Sect. A Found. Adv. 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows-a version of ORTEPIII with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]

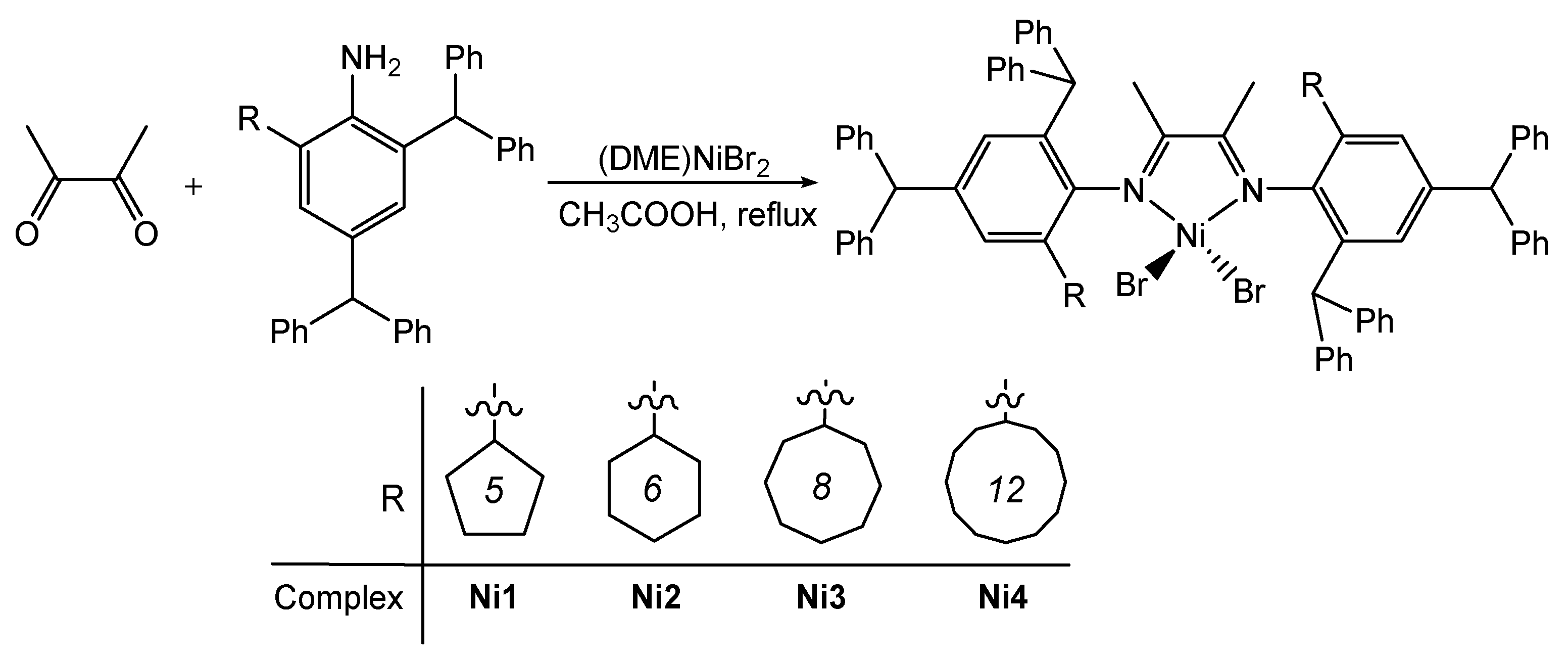
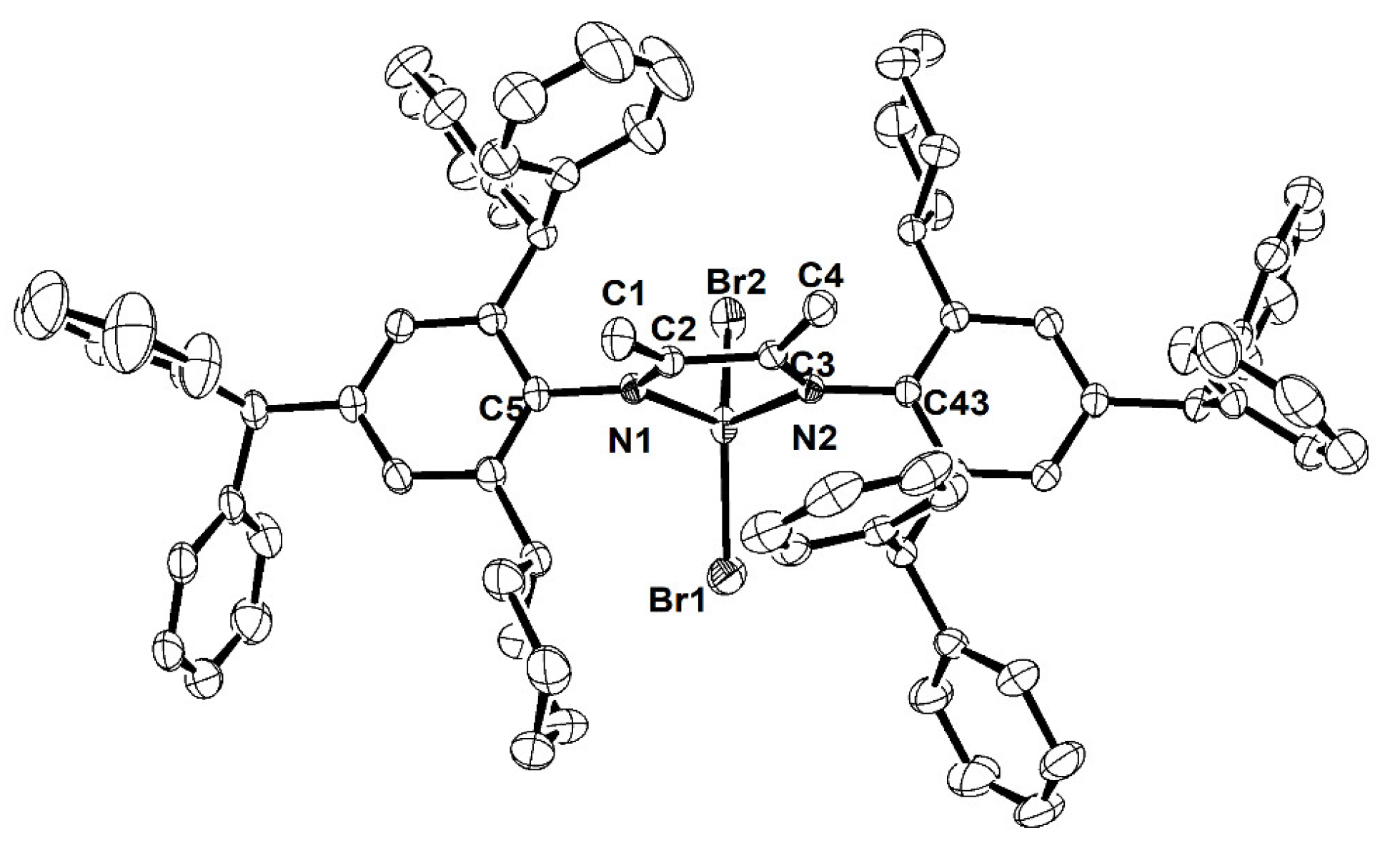
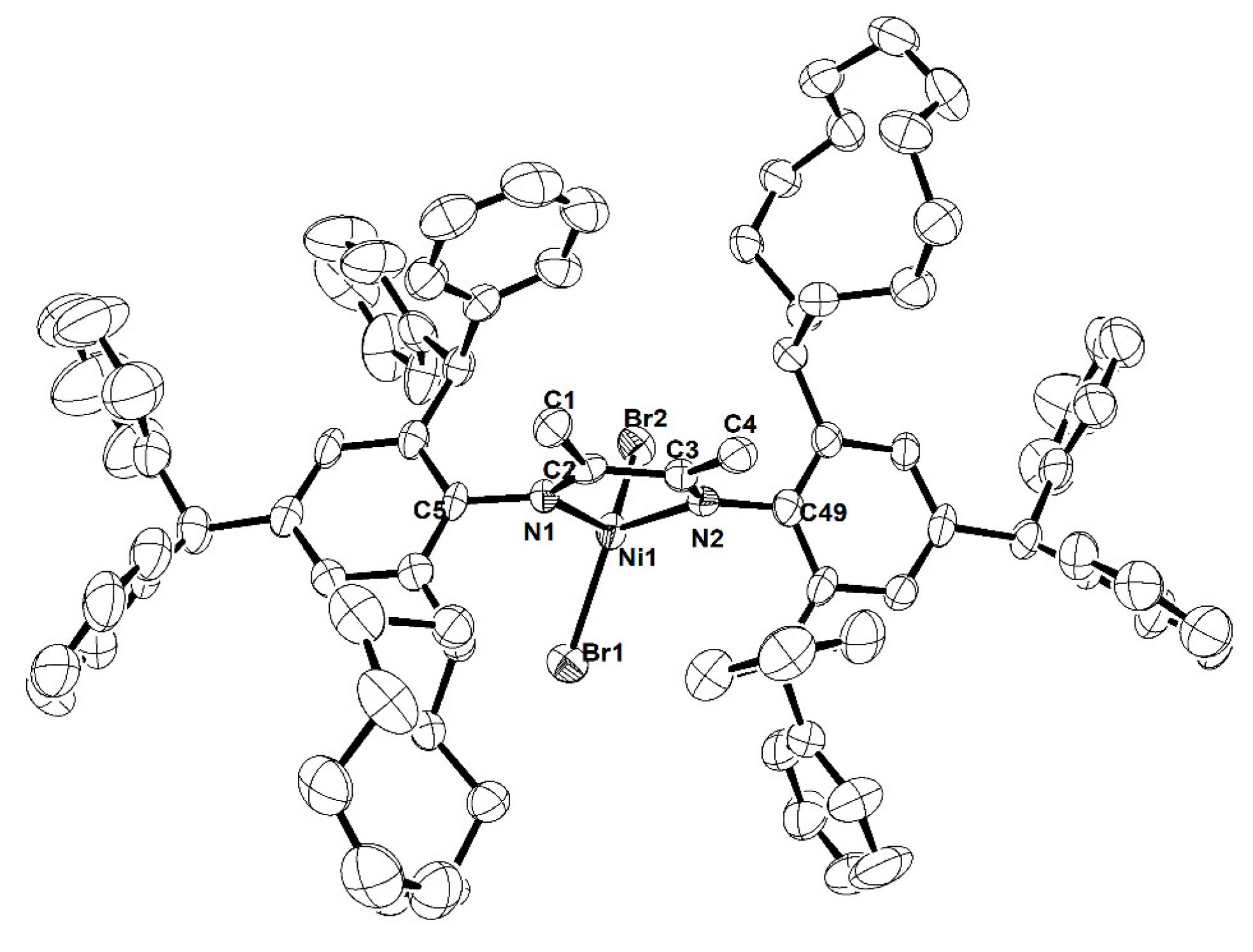
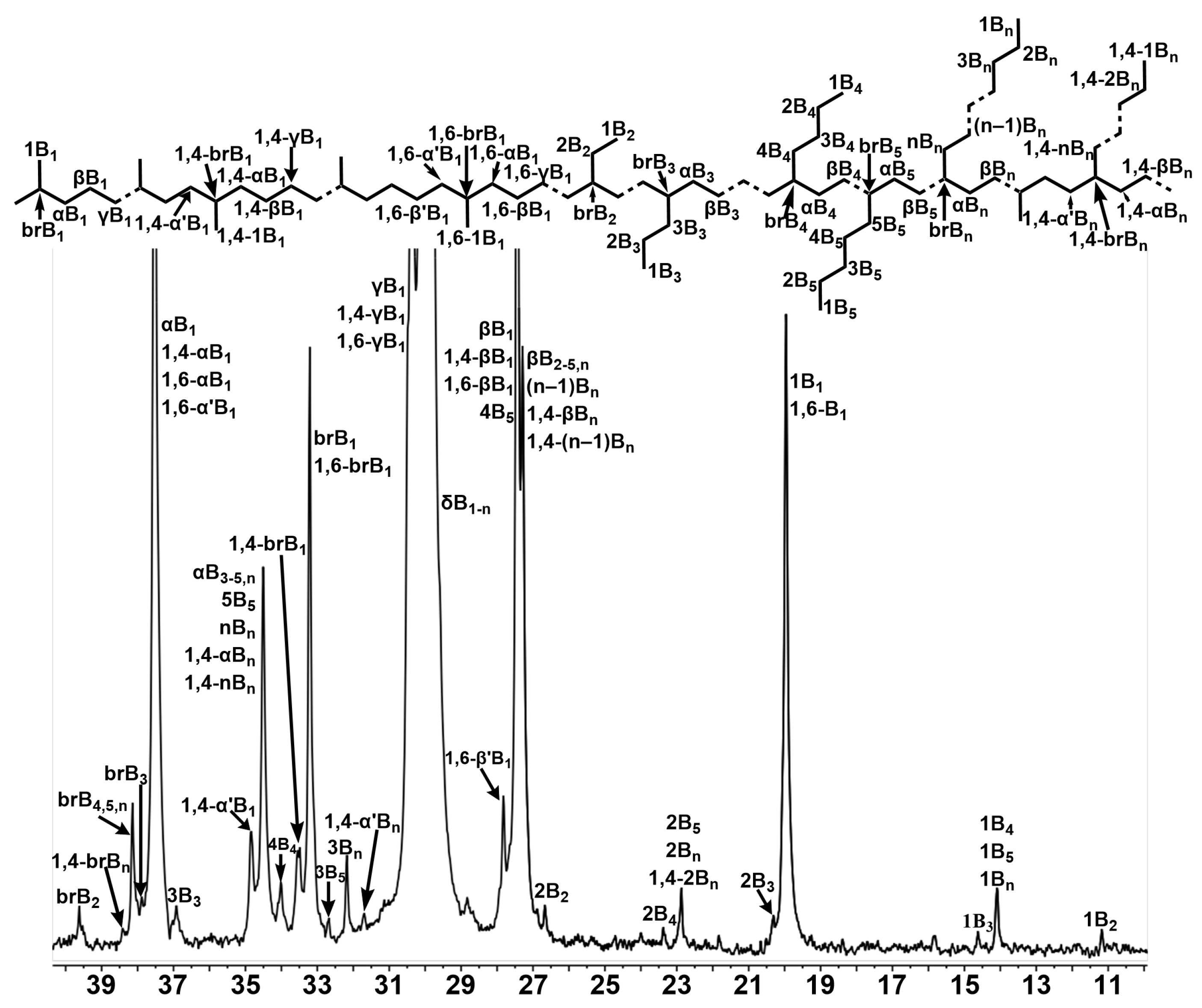
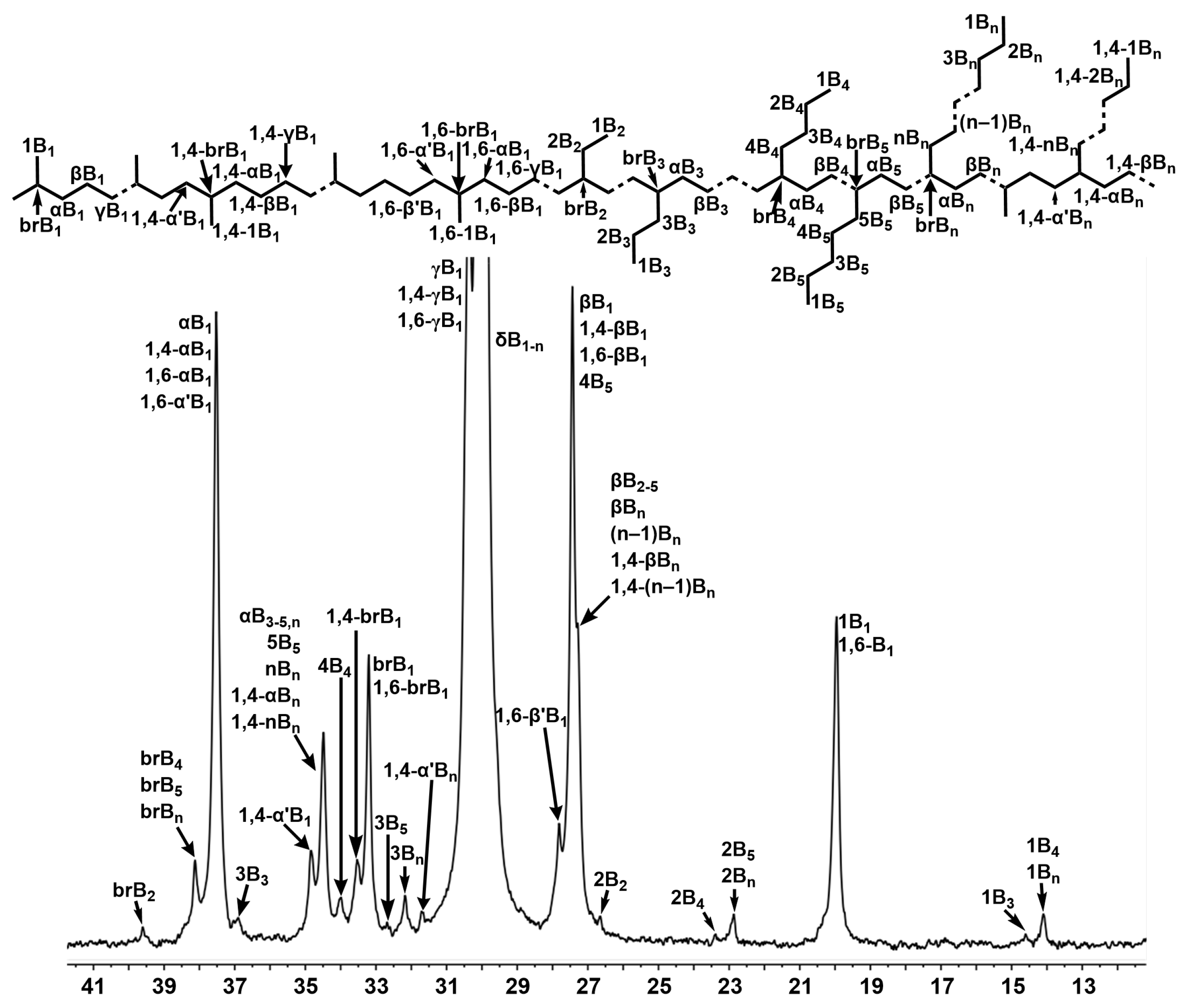
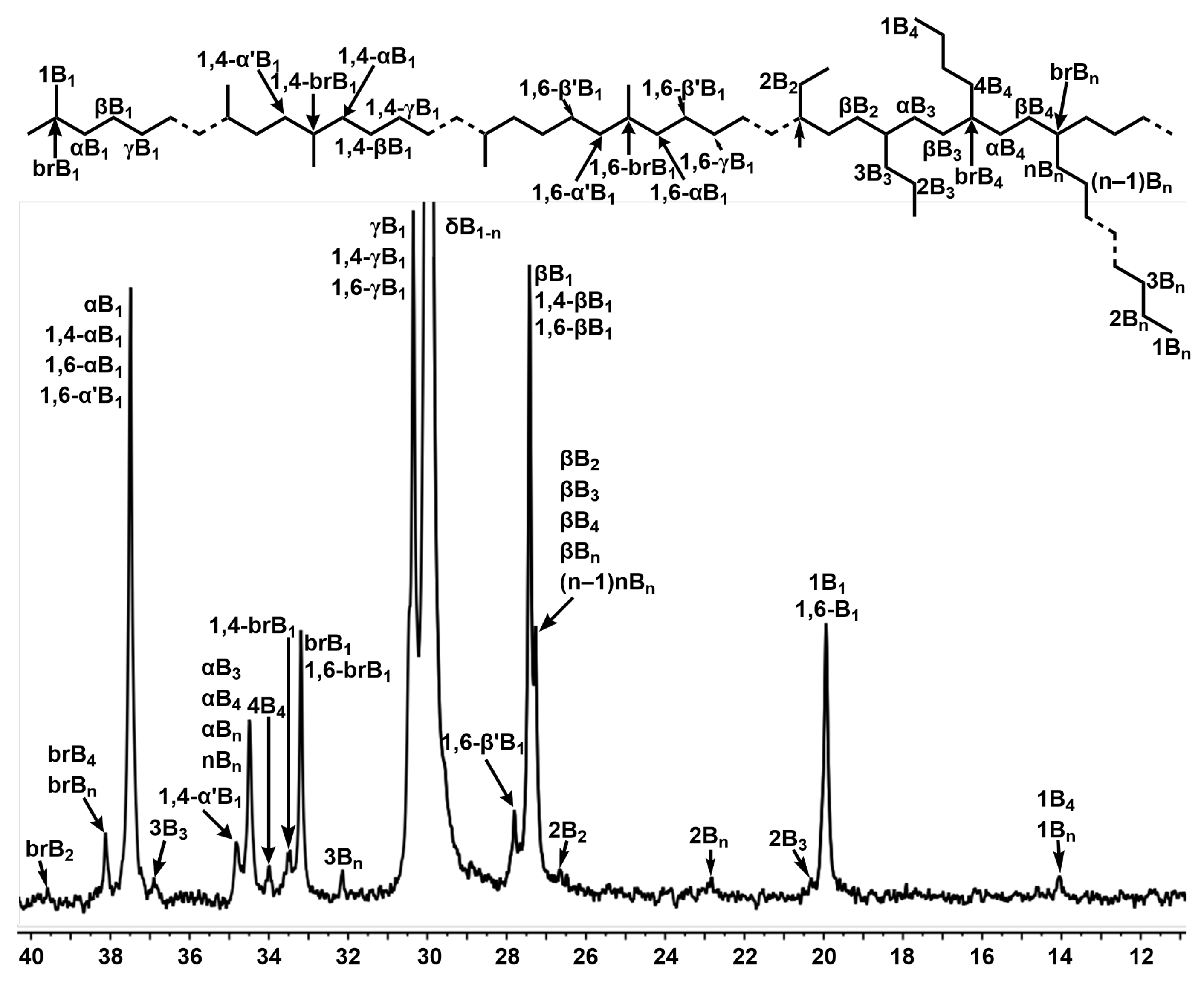

| Ni2 | Ni4 | |
|---|---|---|
| Bond lengths (Å) | ||
| Ni1-N1 | 2.0221(11) | 2.014(5) |
| Ni1-N2 | 2.0081(10) | 2.004(5) |
| Ni1-Br1 | 2.3331(3) | 2.3304(13) |
| Ni1-Br2 | 2.3418(3) | 2.3361(13) |
| C3-N2 | 1.2840(17) | 1.285(8) |
| C2-N1 | 1.2856(17) | 1.280(8) |
| Bond angles (°) | ||
| N1-Ni1-N2 | 81.36(4) | 80.6(2) |
| N1-Ni1-Br1 | 113.49(3) | 100.68(15) |
| N1-Ni1-Br2 | 109.52(3) | 121.18(15) |
| N2-Ni1-Br1 | 112.24(3) | 121.51(15) |
| N2-Ni1-Br2 | 108.12(3) | 99.74(15) |
| Br1-Ni1-Br2 | 123.970(11) | 125.57(5) |
| Run | Activator | Al:Ni | T (°C) | PE (g) | Activity b | Mwc | Mw/Mnc | Tm (°C) d |
|---|---|---|---|---|---|---|---|---|
| 1 | MAO | 2000 | 30 | 3.57 | 7.14 | 10.1 | 2.5 | 92.96 |
| 2 | MMAO | 2000 | 30 | 3.37 | 6.74 | 9.18 | 2.9 | 89.75 |
| 3 | Et2AlCl | 500 | 30 | 5.03 | 10.1 | 9.98 | 2.5 | 75.28 |
| 4 | EtAlCl2 | 500 | 30 | 5.15 | 10.3 | 8.22 | 2.9 | 80.59 |
| 5 | EASC | 500 | 30 | 0.95 | 1.90 | 7.61 | 2.9 | 79.59 |
| Run | Precat. | Al:Ni | T (°C) | t (min) | PE (g) | Activity b | Mwc | Mw/Mnc | Tm (°C) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni2 | 500 | 20 | 30 | 3.87 | 7.74 | 12.5 | 2.9 | 104.57 |
| 2 | Ni2 | 500 | 30 | 30 | 5.15 | 10.3 | 8.22 | 2.9 | 80.59 |
| 3 | Ni2 | 500 | 40 | 30 | 4.84 | 9.68 | 7.51 | 2.8 | 70.10 |
| 4 | Ni2 | 500 | 50 | 30 | 4.62 | 9.24 | 5.66 | 2.7 | 55.44 |
| 5 | Ni2 | 500 | 60 | 30 | 3.30 | 6.60 | 4.64 | 2.7 | 46.27 |
| 6 | Ni2 | 500 | 70 | 30 | 1.13 | 2.26 | 3.89 | 2.6 | 44.54 |
| 7 | Ni2 | 300 | 30 | 30 | 1.95 | 3.90 | 5.71 | 2.4 | 76.78 |
| 8 | Ni2 | 400 | 30 | 30 | 3.28 | 6.56 | 5.74 | 3.1 | 68.45 |
| 9 | Ni2 | 600 | 30 | 30 | 4.86 | 9.72 | 7.08 | 2.9 | 90.43 |
| 10 | Ni2 | 700 | 30 | 30 | 4.56 | 9.12 | 6.68 | 2.9 | 73.68 |
| 11 | Ni2 | 500 | 30 | 5 | 0.91 | 10.9 | 3.10 | 2.2 | 77.26 |
| 12 | Ni2 | 500 | 30 | 15 | 2.61 | 10.4 | 6.48 | 2.1 | 82.04 |
| 13 | Ni2 | 500 | 30 | 45 | 7.11 | 9.48 | 10.1 | 2.8 | 82.24 |
| 14 | Ni2 | 500 | 30 | 60 | 9.24 | 9.24 | 12.1 | 2.2 | 72.26 |
| 15 e | Ni2 | 500 | 30 | 30 | 1.21 | 2.42 | 6.10 | 1.9 | 65.07 |
| 16 f | Ni2 | 500 | 30 | 30 | 0.21 | 0.42 | 3.52 | 1.9 | 33.75 |
| 17 | Ni1 | 500 | 30 | 30 | 4.30 | 8.60 | 5.35 | 2.6 | 103.41 |
| 18 | Ni3 | 500 | 30 | 30 | 0.13 | 0.26 | 4.37 | 2.2 | 85.62 |
| 19 | Ni4 | 500 | 30 | 30 | 1.62 | 3.24 | 5.90 | 2.1 | 62.82 |
| Run | Precat. | Al:Ni | T (°C) | t (min) | PE (g) | Activity b | Mwc | Mw/Mnc | Tm (°C) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni2 | 500 | 30 | 30 | 5.03 | 10.1 | 9.98 | 2.5 | 75.28 |
| 2 | Ni2 | 500 | 40 | 30 | 5.33 | 10.7 | 8.85 | 2.5 | 63.72 |
| 3 | Ni2 | 500 | 50 | 30 | 3.58 | 7.16 | 7.97 | 2.4 | 55.77 |
| 4 | Ni2 | 500 | 60 | 30 | 3.33 | 6.66 | 5.50 | 2.2 | 43.99 |
| 5 | Ni2 | 500 | 70 | 30 | 3.20 | 6.40 | 5.09 | 2.2 | 38.77 |
| 6 | Ni2 | 300 | 40 | 30 | 1.62 | 3.24 | 6.30 | 2.4 | 67.54 |
| 7 | Ni2 | 400 | 40 | 30 | 4.61 | 9.22 | 7.00 | 3.3 | 63.67 |
| 8 | Ni2 | 600 | 40 | 30 | 4.87 | 9.74 | 5.56 | 2.6 | 62.21 |
| 9 | Ni2 | 700 | 40 | 30 | 2.77 | 5.54 | 5.43 | 2.6 | 65.86 |
| 10 | Ni2 | 500 | 40 | 5 | 0.64 | 7.68 | 3.06 | 2.2 | 73.52 |
| 11 | Ni2 | 500 | 40 | 15 | 3.41 | 13.6 | 4.80 | 2.2 | 57.56 |
| 12 | Ni2 | 500 | 40 | 45 | 5.94 | 7.92 | 10.1 | 2.3 | 66.89 |
| 13 | Ni2 | 500 | 40 | 60 | 6.43 | 6.43 | 10.9 | 2.5 | 66.44 |
| 14 e | Ni2 | 500 | 40 | 30 | 0.40 | 0.80 | 3.08 | 1.8 | 55.00 |
| 15 f | Ni2 | 500 | 40 | 30 | 0.18 | 0.36 | 2.79 | 1.5 | 24.27 |
| 16 | Ni1 | 500 | 40 | 30 | 2.06 | 4.12 | 3.69 | 2.1 | 87.80 |
| 17 | Ni3 | 500 | 40 | 30 | 0.02 | 0.04 | 6.26 | 2.4 | 72.35 |
| 18 | Ni4 | 500 | 40 | 30 | 0.15 | 0.30 | 5.29 | 2.4 | 51.86 |
| Run | Precat. | Al:Ni | T (°C) | t (min) | PE (g) | Activity b | Mwc | Mw/Mnc | Tm (°C) d |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ni2 | 2000 | 30 | 30 | 3.57 | 7.14 | 10.1 | 2.5 | 92.99 |
| 2 | Ni2 | 2000 | 40 | 30 | 6.60 | 13.2 | 9.68 | 2.0 | 66.47 |
| 3 | Ni2 | 2000 | 50 | 30 | 5.78 | 11.6 | 7.95 | 2.1 | 54.58 |
| 4 | Ni2 | 2000 | 60 | 30 | 3.91 | 7.82 | 7.24 | 2.1 | 43.40 |
| 5 | Ni2 | 2000 | 70 | 30 | 3.27 | 6.54 | 5.75 | 1.9 | 36.67 |
| 6 | Ni2 | 1500 | 40 | 30 | 4.75 | 9.50 | 9.33 | 2.0 | 70.24 |
| 7 | Ni2 | 1750 | 40 | 30 | 5.85 | 11.7 | 10.4 | 2.2 | 69.31 |
| 8 | Ni2 | 2250 | 40 | 30 | 6.21 | 12.4 | 10.9 | 2.4 | 67.72 |
| 9 | Ni2 | 2500 | 40 | 30 | 6.00 | 12.0 | 9.58 | 2.1 | 64.40 |
| 10 | Ni2 | 2000 | 40 | 5 | 1.15 | 13.8 | 7.24 | 1.9 | 66.03 |
| 11 | Ni2 | 2000 | 40 | 15 | 3.35 | 13.4 | 7.28 | 1.9 | 64.97 |
| 12 | Ni2 | 2000 | 40 | 45 | 8.57 | 11.4 | 8.69 | 2.0 | 63.71 |
| 13 | Ni2 | 2000 | 40 | 60 | 10.7 | 10.7 | 7.78 | 2.0 | 62.05 |
| 14 e | Ni2 | 2000 | 40 | 30 | 1.56 | 3.12 | 7.87 | 1.9 | 54.73 |
| 15 f | Ni2 | 2000 | 40 | 30 | 0.31 | 0.62 | 3.53 | 1.5 | 17.35 |
| 16 | Ni1 | 2000 | 40 | 30 | 2.89 | 5.78 | 8.22 | 2.0 | 91.10 |
| 17 | Ni3 | 2000 | 40 | 30 | 0.26 | 0.52 | 15.0 | 2.0 | 78.19 |
| 18 | Ni4 | 2000 | 40 | 30 | 2.09 | 4.18 | 12.2 | 2.1 | 49.43 |
| Sample | Branches/1000 Cs | Me/% | Et/% | Pr/% | Bu/% | Am/% | Longer Branches/% | Methyl (1,4-Paired) | Methyl (1,6-Paired) |
|---|---|---|---|---|---|---|---|---|---|
| PE30E|Ni2 | 73 | 81.8 | 2.13 | 4.79 | 4.03 | 2.11 | 5.17 | 7.55 | 6.69 |
| PE40D|Ni2 | 104 | 81.1 | 1.17 | 3.93 | 3.54 | 2.97 | 7.23 | 9.74 | 9.55 |
| PE40M|Ni2 | 99 | 82.9 | 2.53 | 6.01 | 3.31 | 0 | 5.29 | 8.92 | 12.9 |
| Sample | Tm (°C) a | Mwb | Xc (%) c | Stress (MPa) d | Strain (%) d |
|---|---|---|---|---|---|
| PE30E|Ni2 | 80.59 | 8.22 | 16.2 | 6.98 | 353 |
| PE30D|Ni2 | 75.28 | 9.98 | 15.6 | 10.72 | 612 |
| PE30M|Ni2 | 92.99 | 10.1 | 22.3 | 15.91 | 713 |
| PE60E|Ni2 | 46.27 | 4.64 | 6.3 | 4.29 | 462 |
| PE60D|Ni2 | 43.99 | 5.50 | 5.5 | 8.59 | 861 |
| PE60M|Ni2 | 43.40 | 7.24 | 5.1 | 9.02 | 764 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Zheng, Y.; Oleynik, I.V.; Yu, Z.; Solan, G.A.; Oleynik, I.I.; Liu, M.; Ma, Y.; Liang, T.; Sun, W.-H. N,N-Bis(2,4-Dibenzhydryl-6-cycloalkylphenyl)butane-2,3-diimine–Nickel Complexes as Tunable and Effective Catalysts for High-Molecular-Weight PE Elastomers. Molecules 2023, 28, 4852. https://doi.org/10.3390/molecules28124852
Jiang S, Zheng Y, Oleynik IV, Yu Z, Solan GA, Oleynik II, Liu M, Ma Y, Liang T, Sun W-H. N,N-Bis(2,4-Dibenzhydryl-6-cycloalkylphenyl)butane-2,3-diimine–Nickel Complexes as Tunable and Effective Catalysts for High-Molecular-Weight PE Elastomers. Molecules. 2023; 28(12):4852. https://doi.org/10.3390/molecules28124852
Chicago/Turabian StyleJiang, Shu, Yuting Zheng, Irina V. Oleynik, Zhixin Yu, Gregory A. Solan, Ivan I. Oleynik, Ming Liu, Yanping Ma, Tongling Liang, and Wen-Hua Sun. 2023. "N,N-Bis(2,4-Dibenzhydryl-6-cycloalkylphenyl)butane-2,3-diimine–Nickel Complexes as Tunable and Effective Catalysts for High-Molecular-Weight PE Elastomers" Molecules 28, no. 12: 4852. https://doi.org/10.3390/molecules28124852
APA StyleJiang, S., Zheng, Y., Oleynik, I. V., Yu, Z., Solan, G. A., Oleynik, I. I., Liu, M., Ma, Y., Liang, T., & Sun, W.-H. (2023). N,N-Bis(2,4-Dibenzhydryl-6-cycloalkylphenyl)butane-2,3-diimine–Nickel Complexes as Tunable and Effective Catalysts for High-Molecular-Weight PE Elastomers. Molecules, 28(12), 4852. https://doi.org/10.3390/molecules28124852










