Construction of Microporous Zincophilic Interface for Stable Zn Anode
Abstract
:1. Introduction
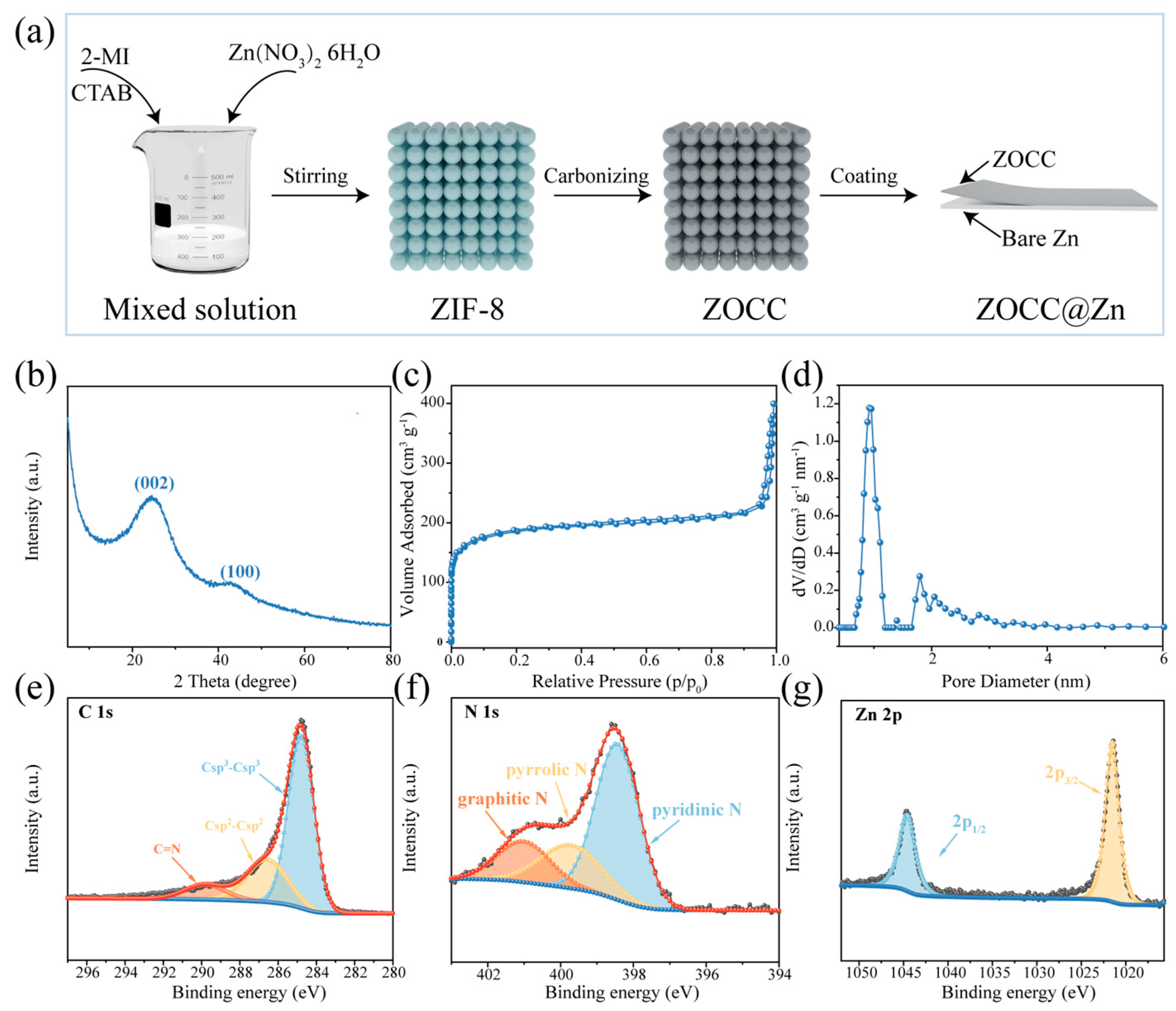
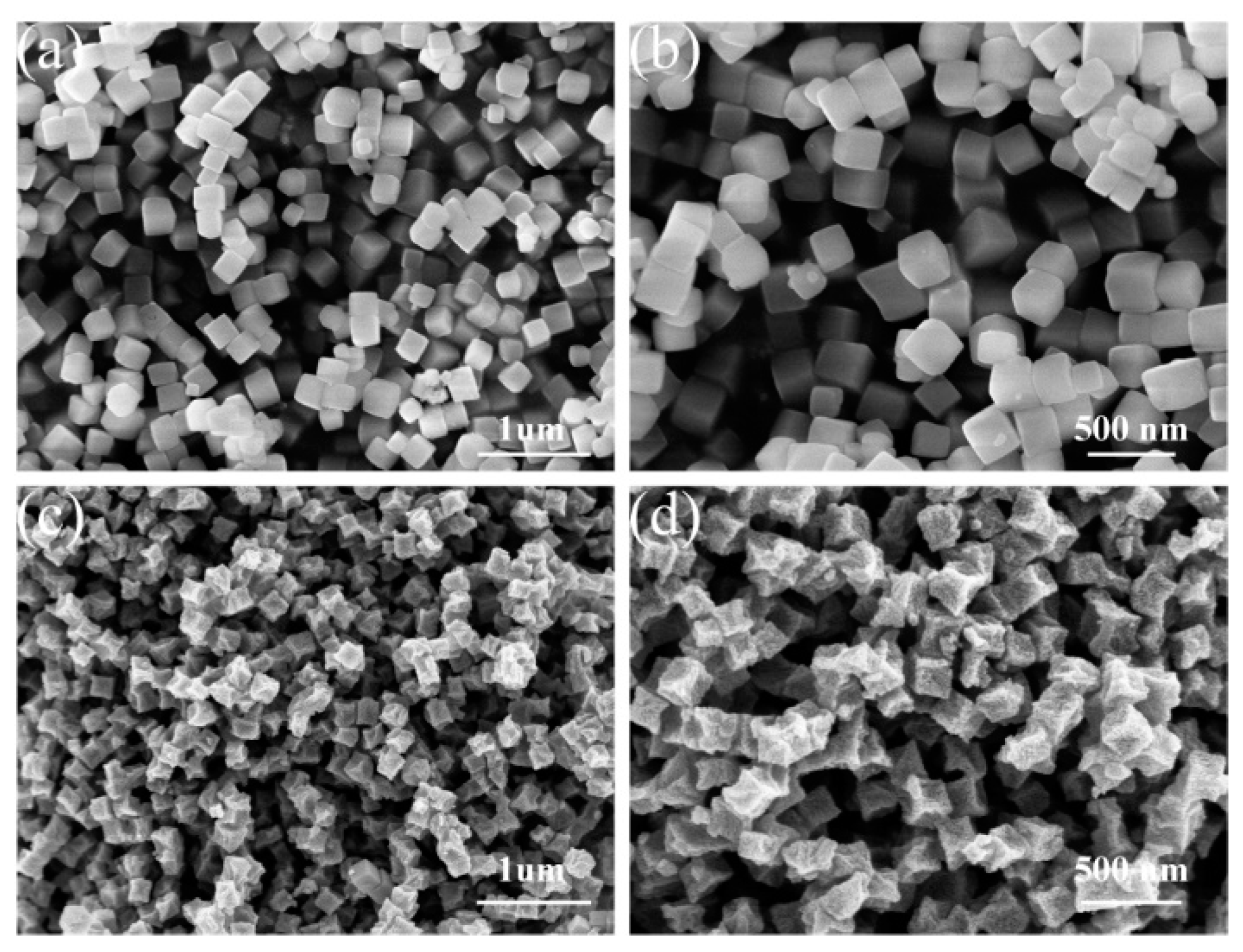
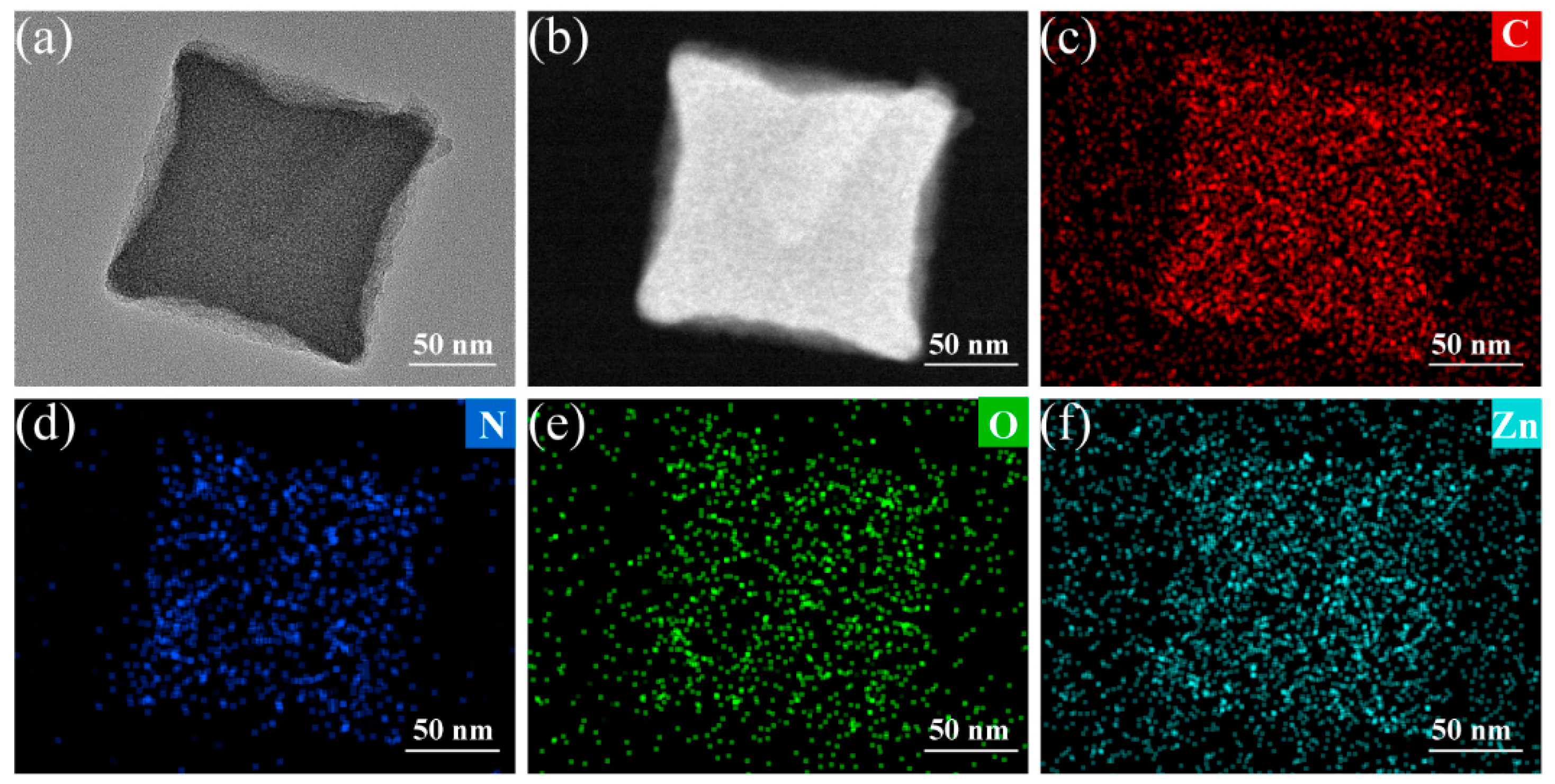
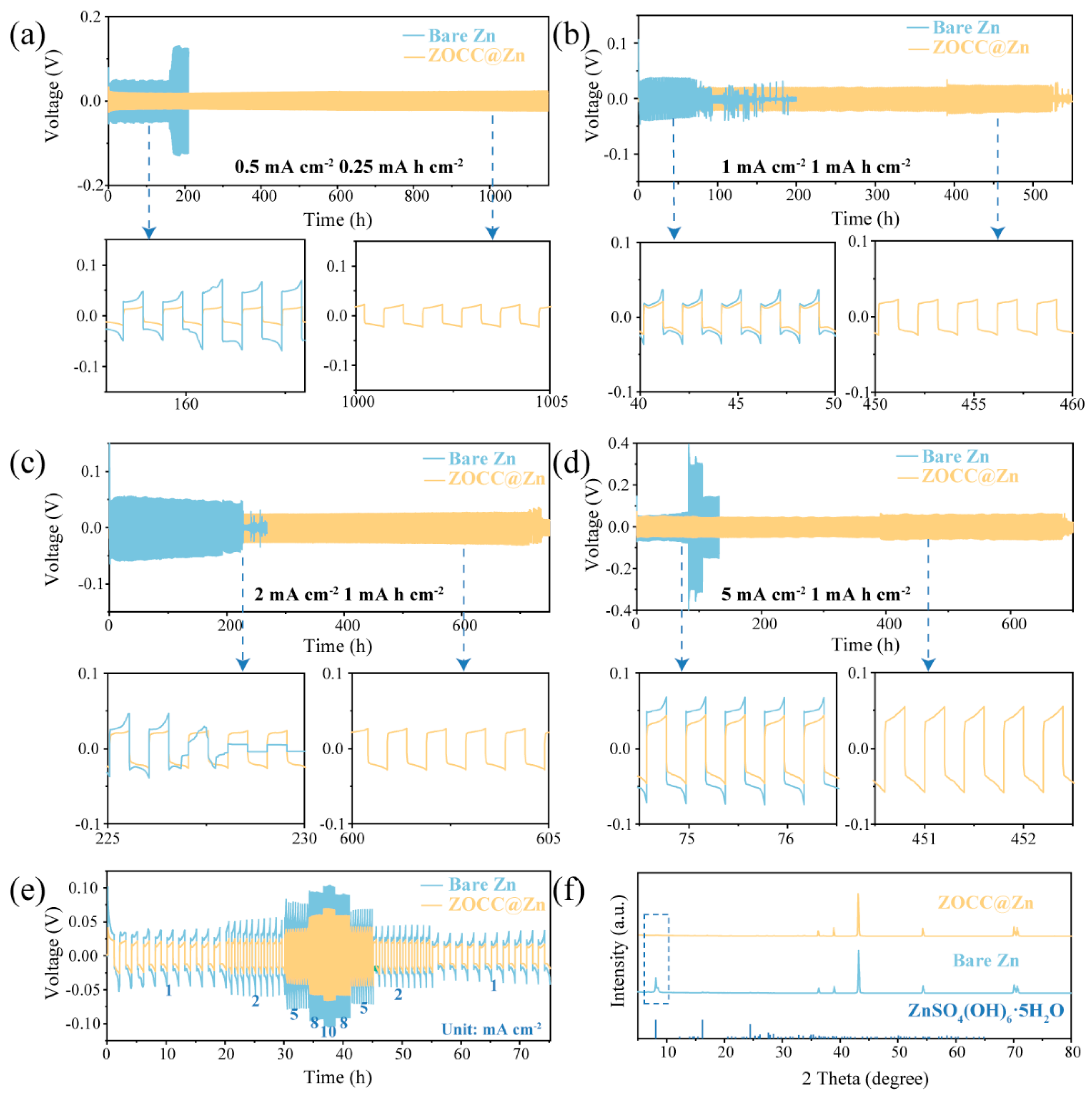
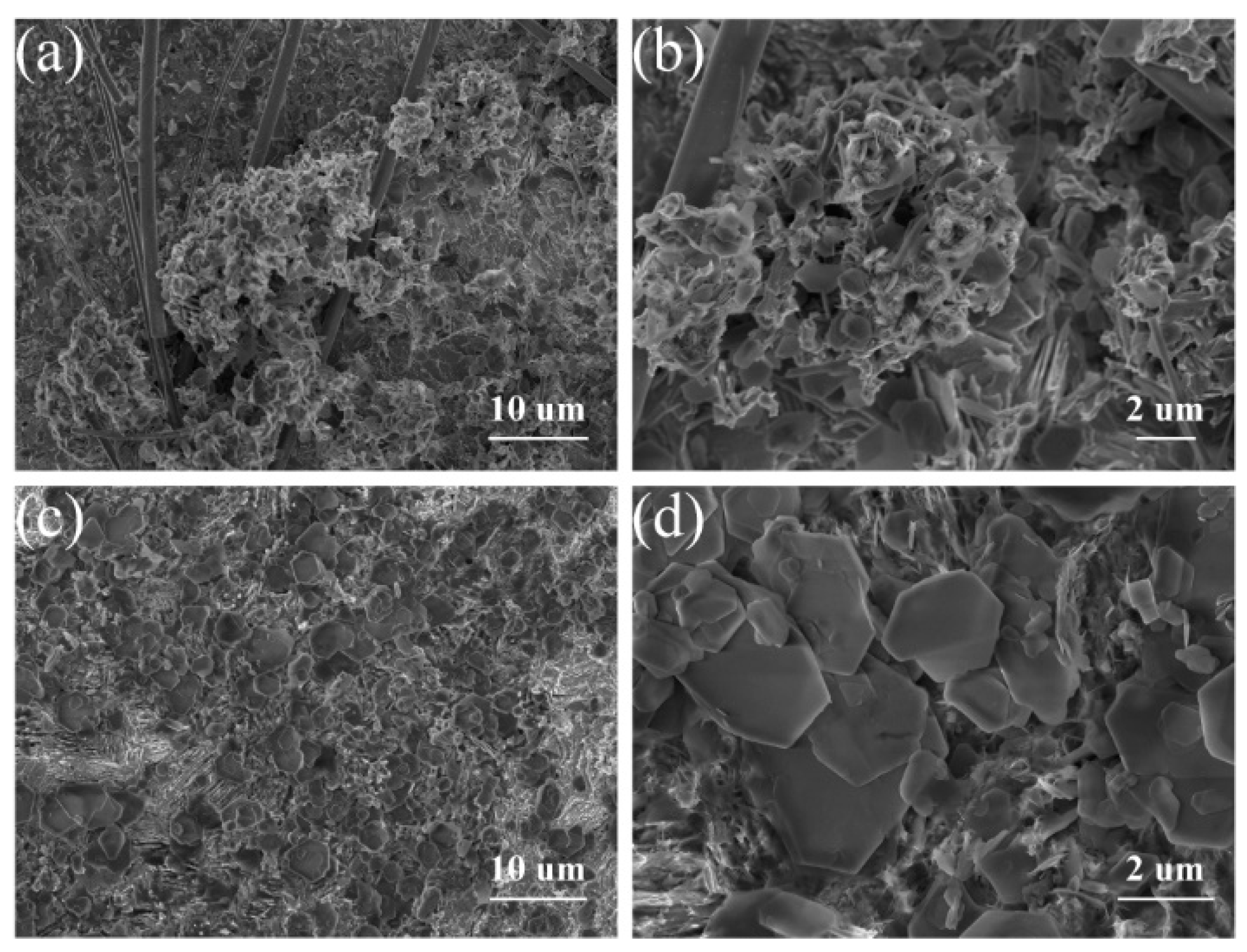
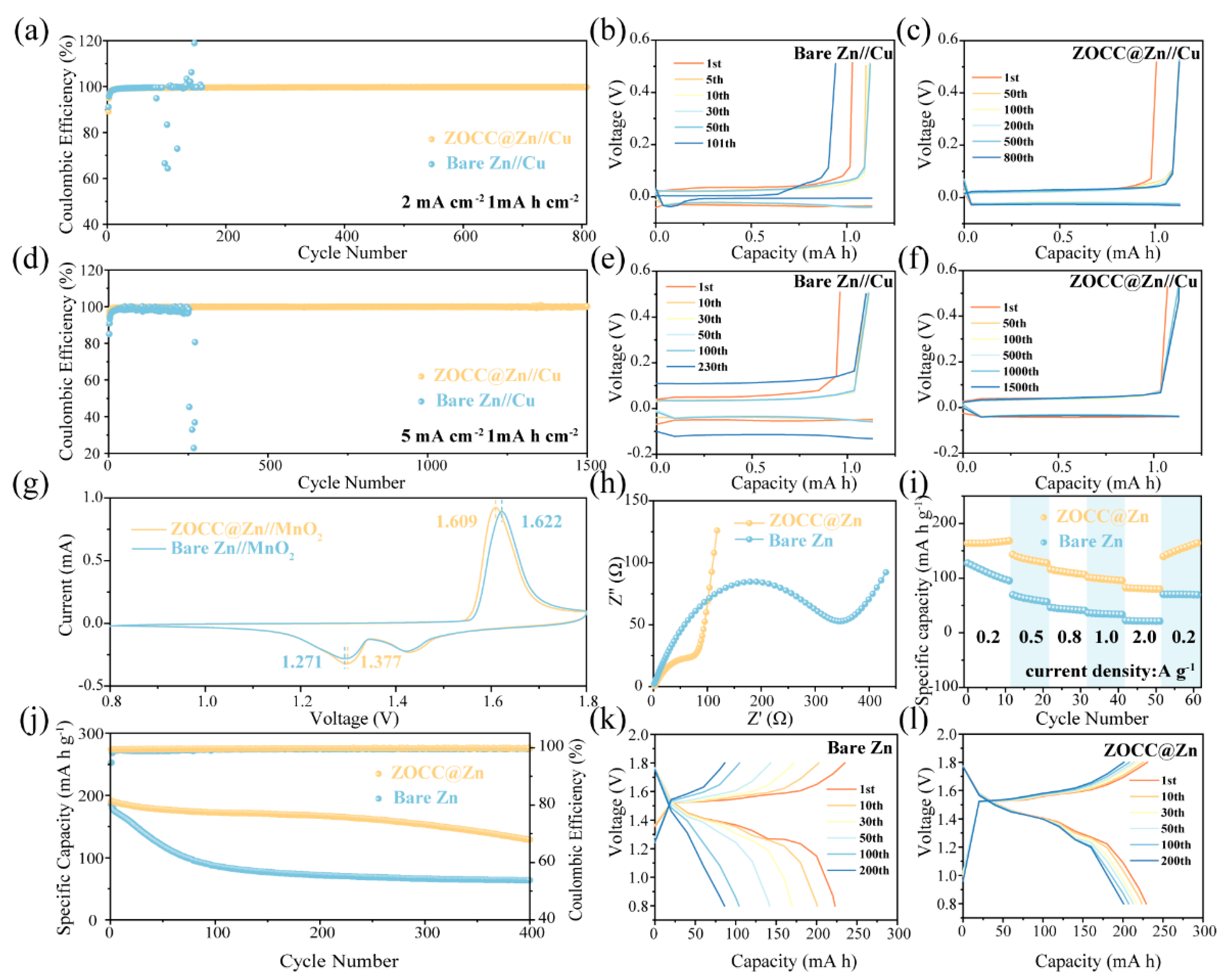
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of ZIF-8 Precursor and ZOCC
3.3. Preparation of ZOCC@Zn
3.4. Preparation of MnO2 Electrode
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Choi, N.S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Sun, S. Advanced phosphorus-based materials for lithium/sodium-ion batteries: Recent developments and future perspectives. Adv. Energy Mater. 2018, 8, 1703058. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cheng, Z.; Zhang, C. Improved Strategies for Separators in Zinc-Ion Batteries. ChemSusChem 2023, 16, e202202330. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Open challenges and good experimental practices in the research field of aqueous Zn-ion batteries. Nat. Commun. 2022, 13, 687. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Tang, Y.; Ji, X.; Wang, H. Interfacial design of dendrite-free zinc anodes for aqueous zinc-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 13180–13191. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent advances in aqueous zinc-ion batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Gao, T.; Fan, X.; Sun, W.; Han, F.; Faraone, A.; Dura, J.A.; Xu, K.; Wang, C. Highly reversible zinc metal anode for aqueous batteries. Nat. Mater. 2018, 17, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Yi, J.; Liu, X.; Wu, K.; Wang, Z.; Cui, J.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Highly reversible Zn anode enabled by controllable formation of nucleation sites for Zn-based batteries. Adv. Funct. Mater. 2020, 30, 1908528. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Gou, Q.; Luo, H.; Zheng, Y.; Zhang, Q.; Li, C.; Wang, J.; Odunmbaku, O.; Zheng, J.; Xue, J.; Sun, K. Construction of Bio-inspired Film with Engineered Hydrophobicity to Boost Interfacial Reaction Kinetics of Aqueous Zinc–Ion Batteries. Small 2022, 18, 2201732. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, Y.; Wei, W.; Ji, X.; Chen, L. A Functional Organic Zinc-Chelate Formation with Nanoscaled Granular Structure Enabling Long-Term and Dendrite-Free Zn Anodes. ACS Nano 2022, 16, 9736–9747. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Yu, H.; Liu, W.; Kuang, G.; Mei, L.; Wu, Z.; Wei, W.; Ji, X.; Qu, B. A Multifunctional Artificial Interphase with Fluorine-Doped Amorphous Carbon layer for Ultra-Stable Zn Anode. Adv. Funct. Mater. 2022, 32, 2205600. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, X.; Luo, M.; Cao, K.; Lu, Y.; Xu, B.B.; Pan, H.; Tao, K.; Jiang, Y. Amino acid-induced interface charge engineering enables highly reversible Zn anode. Adv. Funct. Mater. 2021, 31, 2103514. [Google Scholar] [CrossRef]
- Mitha, A.; Yazdi, A.Z.; Ahmed, M.; Chen, P. Surface adsorption of polyethylene glycol to suppress dendrite formation on zinc anodes in rechargeable aqueous batteries. ChemElectroChem 2018, 5, 2409–2418. [Google Scholar] [CrossRef]
- Gou, Q.; Luo, H.; Zhang, Q.; Deng, J.; Zhao, R.; Odunmbaku, O.; Wang, L.; Li, L.; Zheng, Y.; Li, J. Electrolyte Regulation of Bio-Inspired Zincophilic Additive toward High-Performance Dendrite-Free Aqueous Zinc-Ion Batteries. Small 2023, 19, 2207502. [Google Scholar] [CrossRef]
- Du, W.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Li, C.C. Challenges in the material and structural design of zinc anode towards high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2020, 13, 3330–3360. [Google Scholar] [CrossRef]
- Guo, W.; Cong, Z.; Guo, Z.; Chang, C.; Liang, X.; Liu, Y.; Hu, W.; Pu, X. Dendrite-free Zn anode with dual channel 3D porous frameworks for rechargeable Zn batteries. Energy Storage Mater. 2020, 30, 104–112. [Google Scholar] [CrossRef]
- Wei, Y.; Tang, B.; Liang, X.; Zhang, F.; Tang, Y. Ultrahigh Mass-Loading Integrated Free-Standing Functional All-Carbon Positive Electrode Prepared Using Architecture Tailoring Strategy for High-Energy-Density Dual Ion Battery. Adv. Mater. 2023, 2302086. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, J.; Han, X.; Wang, Y.; Ni, Q.; Hu, Z.; Wu, C.; Bai, Y. Stabilizing Zn Metal Anodes via Cation/Anion Regulation toward High Energy Density Zn-Ion Batteries. Adv. Energy Mater. 2023, 13, 2203542. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Xu, L.; Yang, C.; Hong, M.; Cui, M.; Clifford, B.C.; He, S.; Jing, S.; Yao, Y. A sustainable chitosan-zinc electrolyte for high-rate zinc-metal batteries. Matter 2022, 5, 3402–3416. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, F.; Zhao, H.; Du, M.; Li, H.; Jiang, H.; Li, W.; Sang, Y.; Liu, H.; Wang, S. Tailoring Local Electrolyte Solvation Structure via a Mesoporous Molecular Sieve for Dendrite-Free Zinc Batteries. Adv. Funct. Mater. 2022, 32, 2111635. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.; Huang, R.; Wei, G.; Liu, A.; Li, L.; Chen, R. Ultrathin surface coating of nitrogen-doped graphene enables stable zinc anodes for aqueous zinc-Ion batteries. Adv. Mater. 2021, 33, 2101649. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yang, K.; Zhao, S.; Xie, Y.; Liu, C.; Chen, N.; Wang, C.; Wang, D.; Zhang, D.; Shen, Z.X. Recyclable and Ultrafast Fabrication of Zinc Oxide Interface Layer Enabling Highly Reversible Dendrite-Free Zn Anode. ACS Energy Lett. 2023, 8, 1201–1208. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.-J.; Shao-Horn, Y.; Dincă, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shen, M.; Zhou, H.; Meng, C.; Yuan, A. MOF-Derived CuS@Cu-BTC Composites as High-Performance Anodes for Lithium-Ion Batteries. Small 2019, 15, 1903522. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent progress in MOF-derived, heteroatom-doped porous carbons as highly efficient electrocatalysts for oxygen reduction reaction in fuel cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Wang, X.; Zhang, J.; Hu, Y.; Wang, D.; Zuin, L.; Zhou, T.; Wu, Y. High-Performance Reversible Aqueous Zn-Ion Battery Based on Porous MnOx Nanorods Coated by MOF-Derived N-Doped Carbon. Adv. Energy Mater. 2018, 8, 1801445. [Google Scholar] [CrossRef]
- Morris, W.; Stevens, C.J.; Taylor, R.; Dybowski, C.; Yaghi, O.M.; Garcia-Garibay, M.A. NMR and X-ray study revealing the rigidity of zeolitic imidazolate frameworks. J. Phys. Chem. C 2012, 116, 13307–13312. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, X.; Mijowska, E. Insight into the Effect of ZIF-8 Particle Size on the Performance in Nanocarbon-Based Supercapacitors. Chem. Eur. J. 2020, 26, 16328–16337. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Teng, Y.; Cai, K.; Chen, H. Honeycomb ZnO/N/C obtained from cornsilk and ZIF-8 dual induced method for long-life aqueous zinc-ion batteries. J. Alloys Compd. 2021, 855, 157398. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, X.; Ai, F.; Yang, X.; Zhu, G.; Wang, F. Graphitic carbon nanocubes derived from ZIF-8 for photothermal therapy. Inorg. Chem. 2016, 55, 5750–5752. [Google Scholar] [CrossRef]
- Shuai, C.; Yuan, X.; Shuai, Y.; Qian, G.; Yao, J.; Xu, W.; Peng, S.; Yang, W. Nitrogen-doped carbon-ZnO heterojunction derived from ZIF-8: A photocatalytic antibacterial strategy for scaffold. Mater. Today Nano 2022, 18, 100210. [Google Scholar] [CrossRef]
- Wang, B.; Ji, L.; Yu, Y.; Wang, N.; Wang, J.; Zhao, J. A simple and universal method for preparing N, S co-doped biomass derived carbon with superior performance in supercapacitors. Electrochim. Acta 2019, 309, 34–43. [Google Scholar] [CrossRef]
- Xue, P.; Guo, C.; Li, L.; Li, H.; Luo, D.; Tan, L.; Chen, Z. A MOF-Derivative Decorated Hierarchical Porous Host Enabling Ultrahigh Rates and Superior Long-Term Cycling of Dendrite-Free Zn Metal Anodes. Adv. Mater. 2022, 34, 2110047. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Q.; He, J.; Wu, C.; Xie, K.; Liu, Y.; Zhang, W.; Cheng, H.; Hu, H.; Wang, C. Rational design of unique ZnO/ZnS@ NC heterostructures for high-performance lithium-ion batteries. J. Phys. Chem. Lett. 2020, 11, 905–912. [Google Scholar] [CrossRef]
- Jian, Q.; Wan, Y.; Sun, J.; Wu, M.; Zhao, T. A dendrite-free zinc anode for rechargeable aqueous batteries. J. Mater. Chem. A 2020, 8, 20175–20184. [Google Scholar] [CrossRef]
- Jiao, S.; Fu, J.; Wu, M.; Hua, T.; Hu, H. Ion sieve: Tailoring Zn2+ desolvation kinetics and flux toward dendrite-free metallic zinc anodes. ACS Nano 2021, 16, 1013–1024. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Wei, F.; Sui, Y.; Qi, J. Controllable synthesis of ZIF-derived nano-hexahedron porous carbon for supercapacitor electrodes. Mater. Lett. 2020, 258, 126761. [Google Scholar] [CrossRef]
- Kang, L.; Cui, M.; Jiang, F.; Gao, Y.; Luo, H.; Liu, J.; Liang, W.; Zhi, C. Nanoporous CaCO3 coatings enabled uniform Zn stripping/plating for long-life zinc rechargeable aqueous batteries. Adv. Energy Mater. 2018, 8, 1801090. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Shu, T.; Huang, H.; Yi, H.; Zhang, Y.; Xiao, W.; Li, L.; Zhang, Y.; Ma, M.; Liu, X.; et al. Construction of Microporous Zincophilic Interface for Stable Zn Anode. Molecules 2023, 28, 4789. https://doi.org/10.3390/molecules28124789
Yang X, Shu T, Huang H, Yi H, Zhang Y, Xiao W, Li L, Zhang Y, Ma M, Liu X, et al. Construction of Microporous Zincophilic Interface for Stable Zn Anode. Molecules. 2023; 28(12):4789. https://doi.org/10.3390/molecules28124789
Chicago/Turabian StyleYang, Xin, Tie Shu, Haoyu Huang, Hongquan Yi, Yanchi Zhang, Wei Xiao, Liang Li, Yuxin Zhang, Minghao Ma, Xingyuan Liu, and et al. 2023. "Construction of Microporous Zincophilic Interface for Stable Zn Anode" Molecules 28, no. 12: 4789. https://doi.org/10.3390/molecules28124789
APA StyleYang, X., Shu, T., Huang, H., Yi, H., Zhang, Y., Xiao, W., Li, L., Zhang, Y., Ma, M., Liu, X., & Yao, K. (2023). Construction of Microporous Zincophilic Interface for Stable Zn Anode. Molecules, 28(12), 4789. https://doi.org/10.3390/molecules28124789






