Functionalized and Nonfunctionalized Nanosystems for Mitochondrial Drug Delivery with Metallic Nanoparticles
Abstract
1. Introduction
2. Mitochondrial Dysfunction and Diseases
3. Therapeutic Approaches
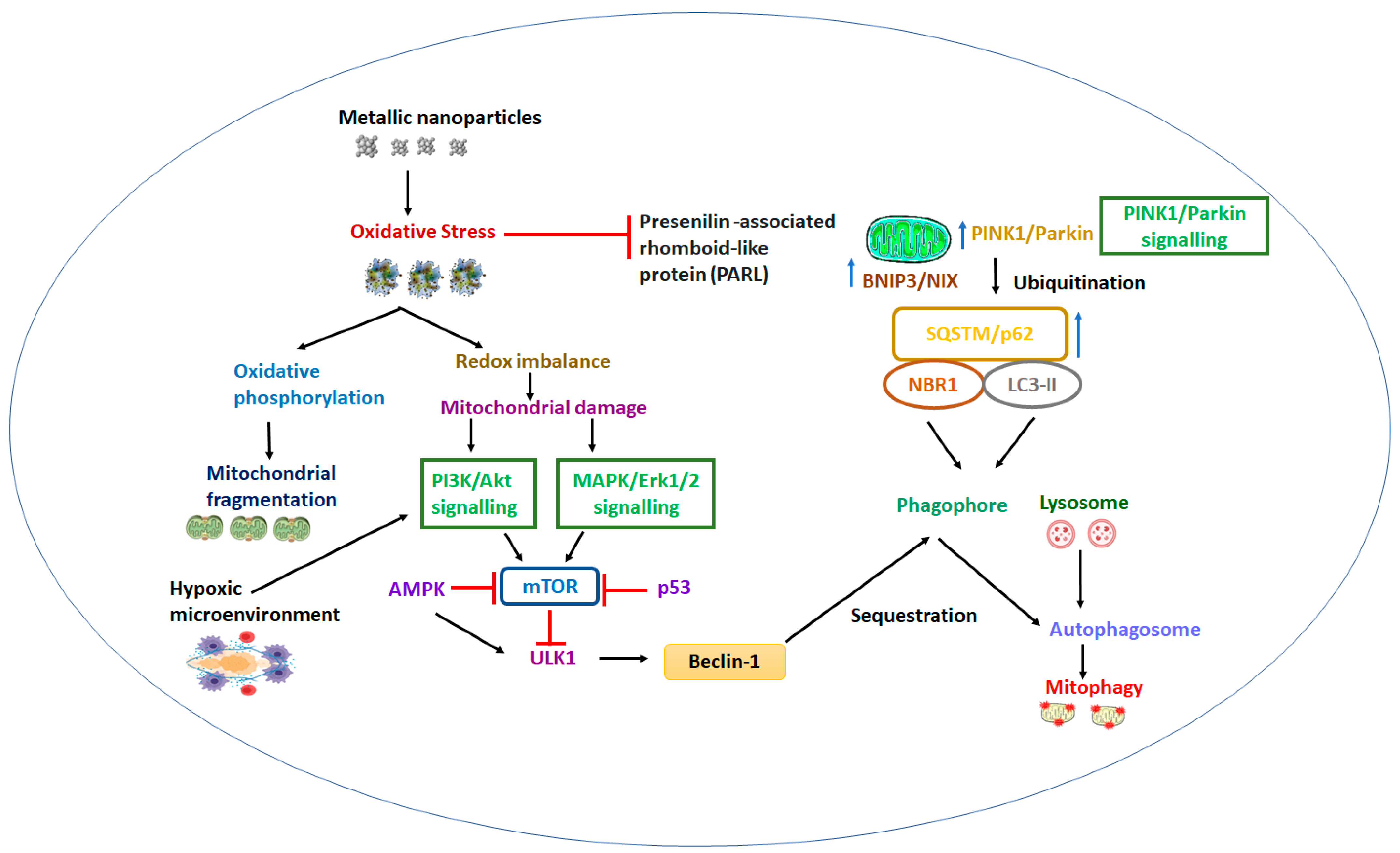
4. Biological Barrier and Toxicity
5. Nanoengineered Mitochondria Targeted Approaches
5.1. Gold Nanoparticles (AuNPs)
5.2. Iron Oxide Nanoparticles
5.3. Silver Nanoparticles
5.4. Titanium Dioxide (TiO2) Nanoparticles
5.5. Zinc Oxide (ZnO) Nanoparticles
5.6. Selenium Nanoparticles
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, P.; Chan, D.C. Mitochondrial dynamics and inheritance during cell division, development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 634–646. [Google Scholar] [CrossRef]
- Kühlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef]
- Calvo, S.E.; Mootha, V.K. The mitochondrial proteome and human disease. Annu. Rev. Genom. Hum. Genet. 2010, 11, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age-related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef]
- Calabrese, V.; Lodi, R.; Tonon, C. D’Agata. V.; Sapienza, M.; Scapagnini, G.; Mangiameli, A.; Pennisi, G.; Stella, A.M.; Butterfield, D.A. Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich’s ataxia. J. Neurol. Sci. 2005, 233, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Hellebrekers, D.M.; Wolfe, R.; Hendrickx, A.T.; de Coo, I.F.; de Die, C.E.; Geraedts, J.P.; Chinnery, P.F.; Smeets, H.J. PGD and heteroplasmic mitochondrial DNA point mutations: A systematic review estimating the chance of healthy offspring. Hum. Reprod. Update 2012, 18, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Bisaccia, G.; Ricci, F.; Gallina, S.; Di Baldassarre, A.; Ghinassi, B. Mitochondrial Dysfunction and Heart Disease: Critical Appraisal of an Overlooked Association. Int. J. Mol. Sci. 2021, 22, 614. [Google Scholar] [CrossRef]
- Sergi, D.; Naumovski, N.; Heilbronn, L.K.; Abeywardena, M.; O’Callaghan, N.; Lionetti, L.; Luscombe-Marsh, N. Mitochondrial (Dys)function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Takemura, K.; Nishi, H.; Inagi, R. Mitochondrial Dysfunction in Kidney Disease and Uremic Sarcopenia. Front. Physiol. 2020, 11, 565023. [Google Scholar] [CrossRef]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, Y. Mitochondria as a target in cancer treatment. MedComm 2020, 1, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Kamatani, N.; Kushiyama, A.; Toyo-Oka, L.; Toyo-Oka, T. Treatment of two mitochondrial disease patients with a combination of febuxostat and inosine that enhances cellular ATP. J. Hum. Genet. 2019, 64, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Viscomi, C.; Bottani, E.; Civiletto, G.; Cerutti, R.; Moggio, M.; Fagiolari, G.; Schon, E.A.; Lamperti, C.; Zeviani, M. In vivo correction of COX deficiency by activation of the AMPK/PGC-1α axis. Cell. Metab. 2011, 14, 80–90. [Google Scholar] [CrossRef]
- Golubitzky, A.; Dan, P.; Weissman, S.; Link, G.; Wikstrom, J.D.; Saada, A. Screening for active small molecules in mitochondrial complex I deficient patient’s fibroblasts, reveals AICAR as the most beneficial compound. PLoS ONE 2011, 6, e26883. [Google Scholar] [CrossRef]
- Airhart, S.E.; Shireman, L.M.; Risler, L.J.; Anderson, G.D.; Nagana Gowda, G.A.; Raftery, D.; Tian, R.; Shen, D.D.; O’Brien, K.D. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE 2017, 12, e0186459. [Google Scholar] [CrossRef]
- Sudo, A.; Sano, H.; Kawamura, N. Determination of the critical time point for efficacy of L-arginine infusion therapy in a case of MELAS with frequent stroke-like episodes. No Hattatsu 2014, 46, 39–43. [Google Scholar]
- Lorenz, C.; Lesimple, P.; Bukowiecki, R.; Zink, A.; Inak, G.; Mlody, B.; Singh, M.; Semtner, M.; Mah, N.; Auré, K.; et al. Human iPSC-Derived Neural Progenitors Are an Effective Drug Discovery Model for Neurological mtDNA Disorders. Cell Stem Cell 2017, 20, 659–674.e9. [Google Scholar] [CrossRef]
- Bartsakoulia, M.; Mϋller, J.S.; Gomez-Duran, A.; Yu-Wai-Man, P.; Boczonadi, V.; Horvath, R. Cysteine Supplementation May be Beneficial in a Subgroup of Mitochondrial Translation Deficiencies. J. Neuromuscul. Dis. 2016, 3, 363–379. [Google Scholar] [CrossRef]
- Guha, S.; Konkwo, C.; Lavorato, M.; Mathew, N.D.; Peng, M.; Ostrovsky, J.; Kwon, Y.J.; Polyak, E.; Lightfoot, R.; Seiler, C.; et al. Pre-clinical evaluation of cysteamine bitartrate as a therapeutic agent for mitochondrial respiratory chain disease. Hum. Mol. Genet. 2019, 28, 1837–1852. [Google Scholar] [CrossRef]
- Blankenberg, F.G.; Kinsman, S.L.; Cohen, B.H.; Goris, M.L.; Spicer, K.M.; Perlman, S.L.; Krane, E.J.; Kheifets, V.; Thoolen, M.; Miller, G.; et al. Brain uptake of Tc99m-HMPAO correlates with clinical response to the novel redox modulating agent EPI-743 in patients with mitochondrial disease. Mol. Genet. Metab. 2012, 107, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Kouga, T.; Takagi, M.; Miyauchi, A.; Shimbo, H.; Iai, M.; Yamashita, S.; Murayama, K.; Klein, M.B.; Miller, G.; Goto, T.; et al. Japanese Leigh syndrome case treated with EPI-743. Brain Dev. 2018, 40, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, A.; Travaglione, S.; Maroccia, Z.; Guidotti, M.; Pierri, C.L.; Primiano, G.; Servidei, S.; Loizzo, S.; Fiorentini, C. The Bacterial Protein CNF1 as a Potential Therapeutic Strategy against Mitochondrial Diseases: A Pilot Study. Int. J. Mol. Sci. 2018, 19, 1825. [Google Scholar] [CrossRef]
- Johnson, S.C.; Yanos, M.E.; Kayser, E.B.; Quintana, A.; Sangesland, M.; Castanza, A.; Uhde, L.; Hui, J.; Wall, V.Z.; Gagnidze, A.; et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 2013, 342, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.; Soikkeli, M.; Portero-Otín, M.; Wilson, A.; Kemppainen, E.; McIlroy, G.; Ellilä, S.; Kemppainen, K.K.; Tuomela, T.; Lakanmaa, M.; et al. Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proc. Natl. Acad. Sci. USA 2010, 107, 9105–9110. [Google Scholar] [CrossRef]
- Burkhardt, C.; Kelly, J.P.; Lim, Y.H.; Filley, C.M.; Parker, W.D., Jr. Neuroleptic medications inhibit complex I of the electron transport chain. Ann. Neurol. 1993, 33, 512–517. [Google Scholar] [CrossRef]
- Pathak, R.K.; Kolishetti, N.; Dhar, S. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 315–329. [Google Scholar] [CrossRef]
- Yu, H.; Koilkonda, R.D.; Chou, T.-H.; Porciatti, V.; Ozdemir, S.S.; Chiodo, V.; Boye, S.L.; Boye, S.E.; Hauswirth, W.W.; Lewin, A.S. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber’s hereditary optic neuropathy in a mouse model. Proc. Natl. Acad. Sci. USA 2012, 109, E1238–E1247. [Google Scholar] [CrossRef]
- Kagan, V.E.; Borisenko, G.G.; Tyurina, Y.Y.; Tyurin, V.A.; Jiang, J.; Potapovich, A.I.; Kini, V.; Amoscato, A.A.; Fujii, Y. Oxidative lipidomics of apoptosis: Redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free. Radic. Biol. Med. 2004, 37, 1963–1985. [Google Scholar] [CrossRef]
- Wallace, D.C.; Fan, W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion 2010, 10, 12–31. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K. How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp. Gerontol. 2010, 45, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Will, Y.; Shields, J.E.; Wallace, K.B. Drug-Induced Mitochondrial Toxicity in the Geriatric Population: Challenges and Future Directions. Biology 2019, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Nadanaciva, S.; Dykens, J.A.; Bernal, A.; Capaldi, R.A.; Will, Y. Mitochondrial impairment by PPAR agonists and statins identified via immunocaptured OXPHOS complex activities and respiration. Toxicol. Appl. Pharmacol. 2007, 223, 277–287. [Google Scholar] [CrossRef]
- Dykens, J.A.; Jamieson, J.D.; Marroquin, L.D.; Nadanaciva, S.; Xu, J.J.; Dunn, M.C.; Smith, A.R.; Will, Y. In vitro assessment of mitochondrial dysfunction and cytotoxicity of nefazodone, trazodone, and buspirone. Toxicol. Sci. 2008, 103, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Dykens, J.A.; Jamieson, J.; Marroquin, L.; Nadanaciva, S.; Billis, P.A.; Will, Y. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically poised HepG2 cells and human hepatocytes in vitro. Toxicol. Appl. Pharmacol. 2008, 233, 203–210. [Google Scholar] [CrossRef]
- Raza, H.; John, A. Implications of altered glutathione metabolism in aspirin-induced oxidative stress and mitochondrial dysfunction in HepG2 cells. PLoS ONE 2012, 7, e36325. [Google Scholar] [CrossRef]
- Bonifacio, A.; Mullen, P.J.; Mityko, I.S.; Navegantes, L.C.; Bouitbir, J.; Krähenbühl, S. Simvastatin induces mitochondrial dysfunction and increased atrogin-1 expression in H9c2 cardiomyocytes and mice in vivo. Arch. Toxicol. 2016, 90, 203–215. [Google Scholar] [CrossRef]
- Marrache, S.; Pathak, R.K.; Darley, K.L.; Choi, J.H.; Zaver, D.; Kolishetti, N.; Dhar, S. Nanocarriers for tracking and treating diseases. Curr. Med. Chem. 2013, 20, 3500–3514. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Marrache, S.; Dhar, S. Engineering of blended nanoparticle platform for delivery of mitochondriaacting therapeutics. Proc. Natl. Acad. Sci. USA 2012, 109, 16288–16293. [Google Scholar] [CrossRef]
- Bae, Y.; Jung, M.K.; Lee, S.; Song, S.J.; Mun, J.Y.; Green, E.S.; Han, J.; Ko, K.S.; Choi, J.S. Dequalinium-based functional nanosomes show increased mitochondria targeting and anticancer effect. Eur. J. Pharm. Biopharm. 2018, 124, 104–115. [Google Scholar] [CrossRef]
- Vaidya, B.; Paliwal, R.; Rai, S.; Khatri, K.; Goyal, A.K.; Mishra, N.; Vyas, S.P. Cell-selective mitochondrial targeting: A new approach for cancer therapy. Cancer Ther. 2009, 7, 141–148. [Google Scholar]
- Choi, Y.S.; Cho, T.S.; Kim, J.M.; Han, S.W.; Kim, S.K. Amine terminated G-6 PAMAM dendrimer and its interaction with DNA probed by Hoechst 33258. Biophys. Chem. 2006, 121, 142–149. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Z.H.; Zhang, L.; Yao, H.J.; Zhang, Y.; Li, R.J.; Ju, R.J.; Wang, X.X.; Zhou, J.; Li, N.; et al. Mitochondrial targeting topotecan-loaded liposomes for treating drug-resistant breast cancer and inhibiting invasive metastases of melanoma. Biomaterials 2012, 33, 1808–1820. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, F.; Wen, H.; Shi, W.; Huang, Q.; Huang, Y.; Xie, J.; Li, P.; Chen, J.; Qin, L.; et al. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J. Nanobiotechnol. 2020, 18, 8. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Pellitteri, R.; Ruozi, B.; Puglia, C.; Santonocito, D.; Pignatello, R.; Musumeci, T. Curcumin Loaded Polymeric vs. Lipid Nanoparticles: Antioxidant Effect on Normal and Hypoxic Olfactory Ensheathing Cells. Nanomaterials 2021, 11, 159. [Google Scholar] [CrossRef]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting Mitochondrial Dysfunction and Oxidative Stress in Activated Microglia using Dendrimer-Based Therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Wang, X.; Wang, J.; Hao, Q.; Hao, J.; Hou, X. Osthole-Loaded Nanoemulsion Enhances Brain Target in the Treatment of Alzheimer’s Disease via Intranasal Administration. Oxidative Med. Cell. Longev. 2021, 2021, 8844455. [Google Scholar] [CrossRef] [PubMed]
- Elekofehinti, O.O.; Ayodele, O.C.; Iwaloye, O. Momordica charantia nanoparticles promote mitochondria biogenesis in the pancreas of diabetic-induced rats: Gene expression study. Egypt. J. Med. Hum. Genet. 2021, 22, 80. [Google Scholar] [CrossRef]
- Weissig, V.; Lasch, J.; Erdos, G.; Meyer, H.W.; Rowe, T.C.; Hughes, J. DQAsomes: A novel potential drug and gene delivery system made from Dequalinium. Pharm. Res. 1998, 15, 334–337. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Li, W.; Jiang, X.; Ji, Y.; Wu, X.; Xu, L.; Qiu, Y.; Zhao, K.; Wei, T.; et al. Selective targeting of gold nanorods at the mitochondria of cancer cells: Implications for cancer therapy. Nano Lett. 2011, 11, 772–780. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Semmler-Behnke, M.; Seitz, J.; Scymczak, W.; Wenk, A.; Mayer, P.; Takenaka, S.; Oberdörster, G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 2009, 21, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part. Fibre Toxicol. 2016, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Zoroddu, M.A.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef]
- Paek, H.J.; Lee, Y.J.; Chung, H.E.; Yoo, N.H.; Lee, J.A.; Kim, M.K.; Lee, J.K.; Jeong, J.; Choi, S.J. Modulation of the pharmacokinetics of zinc oxide nanoparticles and their fates in vivo. Nanoscale 2013, 5, 11416–11427. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomedicine 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Salnikov, V.; Lukyánenko, Y.O.; Frederick, C.A.; Lederer, W.J.; Lukyánenko, V. Probing the outer mitochondrial membrane in cardiac mitochondria with nanoparticles. Biophys. J. 2007, 92, 1058–1071. [Google Scholar] [CrossRef]
- Gallud, A.; Klöditz, K.; Ytterberg, J.; Östberg, N.; Katayama, S.; Skoog, T.; Gogvadze, V.; Chen, Y.Z.; Xue, D.; Moya, S.; et al. Cationic gold nanoparticles elicit mitochondrial dysfunction: A multi-omics study. Sci. Rep. 2019, 9, 4366. [Google Scholar] [CrossRef]
- Chen, S.; Lei, Q.; Qiu, W.X.; Liu, L.H.; Zheng, D.W.; Fan, J.X.; Rong, L.; Sun, Y.X.; Zhang, X.Z. Mitochondria-targeting “Nanoheater” for enhanced photothermal/chemotherapy. Biomaterials 2017, 117, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Hamblin, M.R. Mitochondria-targeted nanoparticles (mitoNANO): An emerging therapeutic shortcut for cancer. Biomater. Biosyst. 2021, 3, 100023. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, I.; Chakrabarti, M.; Mukherjee, A. Genotoxicity and biocompatibility of superparamagnetic iron oxide nanoparticles: Influence of surface modification on biodistribution, retention, DNA damage and oxidative stress. Food Chem. Toxicol. 2020, 136, 110989. [Google Scholar] [CrossRef]
- Khan, M.I.; Mohammad, A.; Patil, G.; Naqvi, S.A.; Chauhan, L.K.; Ahmad, I. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 2012, 33, 1477–1488. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Liang, X.; Zhang, J.; Tao, W.; Zhu, X.; Chang, D.; Zeng, X.; Liu, G.; Mei, L. Iron Oxide Nanoparticles Induce Autophagosome Accumulation through Multiple Mechanisms: Lysosome Impairment, Mitochondrial Damage, and ER Stress. Mol. Pharm. 2016, 13, 2578–2587. [Google Scholar] [CrossRef]
- Rivas-García, L.; Quiles, J.L.; Varela-López, A.; Giampieri, F.; Battino, M.; Bettmer, J.; Montes-Bayón, M.; Llopis, J.; Sánchez-González, C. Ultra-Small Iron Nanoparticles Target Mitochondria Inducing Autophagy, Acting on Mitochondrial DNA and Reducing Respiration. Pharmaceutics 2021, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Luo, R.; Bai, Y.; Dou, J.; Zhang, Z.; Liu, F.; Feng, F.; Xu, J.; Liu, W. Mitochondrial membrane anchored photosensitive nano-device for lipid hydroperoxides burst and inducing ferroptosis to surmount therapy-resistant cancer. Theranostics 2019, 9, 6209–6223. [Google Scholar] [CrossRef]
- AshaRani, P.; Hande, M.P.; Valiyaveettil, S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar] [CrossRef]
- Gopisetty, M.K.; Kovács, D.; Igaz, N.; Rónavári, A.; Bélteky, P.; Rázga, Z.; Venglovecz, V.; Csoboz, B.; Boros, I.M.; Kónya, Z.; et al. Endoplasmic reticulum stress: Major player in size-dependent inhibition of P-glycoprotein by silver nanoparticles in multidrug-resistant breast cancer cells. J. Nanobiotechnol. 2019, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, L.; Tang, H.; He, X.; Ye, G.; Shi, F.; Kang, M.; Chen, H.; Li, Y. Silver Nanoparticles Induced Oxidative Stress and Mitochondrial Injuries Mediated Autophagy in HC11 Cells Through Akt/AMPK/mTOR Pathway. Biol. Trace Element Res. 2021, 199, 1062–1073. [Google Scholar] [CrossRef]
- Holmila, R.J.; Vance, S.A.; King, S.B.; Tsang, A.W.; Singh, R.; Furdui, C.M. Silver Nanoparticles Induce Mitochondrial Protein Oxidation in Lung Cells Impacting Cell Cycle and Proliferation. Antioxidants 2019, 8, 552. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Chang, X.; Gan, J.; Li, W.; Niu, S.; Kong, L.; Wu, T.; Zhang, T.; Tang, M.; et al. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ. Pollut. 2020, 256, 113430. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-G.; Han, Y.-H.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Subcellular performance of nanoparticles in cancer therapy. Int. J. Nanomed. 2020, 15, 675. [Google Scholar] [CrossRef] [PubMed]
- Buchke, S.; Sharma, M.; Bora, A.; Relekar, M.; Bhanu, P.; Kumar, J. Mitochondria-Targeted, Nanoparticle-Based Drug-Delivery Systems: Therapeutics for Mitochondrial Disorders. Life 2022, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Skalska, J.; Dąbrowska-Bouta, B.; Frontczak-Baniewicz, M.; Sulkowski, G.; Strużyńska, L. A Low Dose of Nanoparticulate Silver Induces Mitochondrial Dysfunction and Autophagy in Adult Rat Brain. Neurotox. Res. 2020, 38, 650–664. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Behera, B.P.; Mishra, S.R.; Bhutia, S.K. The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin. Cancer Biol. 2020, 66, 45–58. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef]
- Dey, S.; Fageria, L.; Sharma, A.; Mukherjee, S.; Pande, S.; Chowdhury, R.; Chowdhury, S. Silver nanoparticle-induced alteration of mitochondrial and ER homeostasis affects human breast cancer cell fate. Toxicol. Rep. 2022, 9, 1977–1984. [Google Scholar] [CrossRef]
- Qiu, K.; Du, Y.; Liu, J.; Guan, J.L.; Chao, H.; Diao, J. Super-resolution observation of lysosomal dynamics with fluorescent gold nanoparticles. Theranostics 2020, 10, 6072–6081. [Google Scholar] [CrossRef]
- Ke, S.; Zhou, T.; Yang, P.; Wang, Y.; Zhang, P.; Chen, K.; Ren, L.; Ye, S. Gold nanoparticles enhance TRAIL sensitivity through Drp1-mediated apoptotic and autophagic mitochondrial fission in NSCLC cells. Int. J. Nanomed. 2017, 12, 2531–2551. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, O.; Akinyelu, J.; Singh, M. Co-Polymer Functionalised Gold Nanoparticles Show Efficient Mitochondrial Targeted Drug Delivery in Cervical Carcinoma Cells. J. Biomed. Nanotechnol. 2020, 16, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, O.; Akinyelu, J.; Daniels, A.; Singh, M. Modified Gold Nanoparticles for Efficient Delivery of Betulinic Acid to Cancer Cell Mitochondria. Int. J. Mol. Sci. 2021, 22, 5072. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, J.; Lee, J.-H.; Lee, J.H.; Choi, J.M.; Kweon, H.S.; Han, J.H.; Kim, J.H.; Byun, K.M.; Jung, J.H.; et al. Enhanced NIR radiation-triggered hyperthermia by mitochondrial targeting. J. Am. Chem. Soc. 2015, 137, 3017–3023. [Google Scholar] [CrossRef]
- Hauser, A.K.; Mitov, M.I.; Daley, E.F.; McGarry, R.C.; Anderson, K.W.; Hilt, J.Z. Targeted iron oxide nanoparticles for the enhancement of radiation therapy. Biomaterials 2016, 105, 127–135. [Google Scholar] [CrossRef]
- Wang, X.S.; Zeng, J.Y.; Li, M.; Li, Q.R.; Gao, F.; Zhang, X.Z. Highly Stable Iron Carbonyl Complex Delivery Nanosystem for Improving Cancer Therapy. ACS Nano 2020, 14, 9848–9860. [Google Scholar] [CrossRef]
- Morais, M.; Machado, V.; Dias, F.; Figueiredo, P.; Palmeira, C.; Martins, G.; Fernandes, R.; Malheiro, A.R.; Mikkonen, K.S.; Teixeira, A.L.; et al. Glucose-Functionalized Silver Nanoparticles as a Potential New Therapy Agent Targeting Hormone-Resistant Prostate Cancer cells. Int. J. Nanomed. 2022, 17, 4321–4337. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Zhao, H.; Song, W.; Gu, M.; Li, Q.; Liu, Y.; Li, C.; Wang, J.; Zhan, H. Silver nanoparticles functionalized Paclitaxel nanocrystals enhance overall anti-cancer effect on human cancer cells. Nanotechnology 2021, 32, 085105. [Google Scholar] [CrossRef]
- Varadharajaperumal, P.; Muthuswamy, S.; Thiruvengadam, S.; Muthuswamy, S.; Mahalingam, S. Biosynthesised Drug-Loaded Silver Nanoparticles: A Vivid Agent for Drug Delivery On Human Breast Carcinoma. Biosci. Biotechnol. Res. Commun. 2021, 14, 1839–1846. [Google Scholar] [CrossRef]
- Zuo, S.; Zhang, Y.; Wang, Z.; Wang, J. Mitochondria-Targeted Mesoporous Titanium Dioxide Nanoplatform for Synergistic Nitric Oxide Gas-Sonodynamic Therapy of Breast Cancer. Int. J. Nanomed. 2022, 17, 989–1002. [Google Scholar] [CrossRef]
- Abdel-Ghany, S.; Raslan, S.; Tombuloglu, H.; Shamseddin, A.; Cevik, E.; Said, O.A.; Madyan, E.F.; Senel, M.; Bozkurt, A.; Rehman, S.; et al. Vorinostat-loaded titanium oxide nanoparticles (anatase) induce G2/M cell cycle arrest in breast cancer cells via PALB2 upregulation. 3 Biotech 2020, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Waseem, M.; Kaushik, P.; Dutta, S.; Chakraborty, R.; Hassan, M.I.; Parvez, S. Modulatory Role of Quercetin in Mitochondrial Dysfunction in Titanium Dioxide Nanoparticle-Induced Hepatotoxicity. ACS Omega 2022, 7, 3192–3202. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, Y.; Zhang, J.; Zhang, W.; Foda, M.F.; Dai, X.; Han, H. Ultrasmall Peptide-Coated Platinum Nanoparticles for Precise NIR-II Photothermal Therapy by Mitochondrial Targeting. ACS Appl. Mater. Interfaces 2020, 12, 39434–39443. [Google Scholar] [CrossRef]
- Torrano, A.A.; Herrmann, R.; Strobel, C.; Rennhak, M.; Engelke, H.; Reller, A.; Hilger, I.; Wixforth, A.; Bräuchle, C. Cell membrane penetration and mitochondrial targeting by platinum-decorated ceria nanoparticles. Nanoscale 2016, 8, 13352–13367. [Google Scholar] [CrossRef]
- Su, Y.; Tu, Y.; Lin, H.; Wang, M.M.; Zhang, G.D.; Yang, J.; Liu, H.K.; Su, Z. Mitochondria-targeted Pt (IV) prodrugs conjugated with an aggregation-induced emission luminogen against breast cancer cells by dual modulation of apoptosis and autophagy inhibition. J. Inorg. Biochem. 2022, 226, 111653. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, P.; Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Targeted delivery of quercetin via pH-responsive zinc oxide nanoparticles for breast cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 129–140. [Google Scholar] [CrossRef]
- Hariharan, R.; Senthilkumar, S.; Suganthi, A.; Rajarajan, M. Synthesis and characterization of doxorubicin modified ZnO/PEG nanomaterials and its photodynamic action. J. Photochem. Photobiol. B 2012, 116, 56–65. [Google Scholar] [CrossRef]
- Ren, Z.; Han, X.; Wang, L.; Wang, Y. Hyaluronic acid functionalized ZnO nanoparticles co-deliver AS and GOD for synergistic cancer starvation and oxidative damage. Sci. Rep. 2022, 12, 4574. [Google Scholar] [CrossRef]
- Patil, N.; Gade, W.N.; Deobagkar, D.D. Epigenetic modulation upon exposure of lung fibroblasts to TiO2 and ZnO nanoparticles: Alterations in DNA methylation. Int. J. Nanomed. 2016, 11, 4509–4519. [Google Scholar]
- Grohm, J.; Plesnila, N.; Culmsee, C. Bid mediates fission, membrane permeabilization and peri-nuclear accumulation of mitochondria as a prerequisite for oxidative neuronal cell death. Brain Behav. Immun. 2010, 24, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-N.; Chang, S.-H.; Park, S.J.; Lim, J.; Lee, J.; Yoon, T.-J.; Kim, J.-S.; Cho, M.-H. Titanium Dioxide Nanoparticles Induce Endoplasmic Reticulum Stress-Mediated Autophagic Cell Death via Mitochondria-Associated Endoplasmic Reticulum Membrane Disruption in Normal Lung Cells. PLoS ONE 2015, 10, e0131208. [Google Scholar] [CrossRef]
- Brassolatti, P.; de Almeida Rodolpho, J.M.; Franco de Godoy, K.; de Castro, C.A.; Flores Luna, G.L.; Dias de Lima Fragelli, B.; Pedrino, M.; Assis, M.; Nani Leite, M.; Cancino-Bernardi, J.; et al. Functionalized Titanium Nanoparticles Induce Oxidative Stress and Cell Death in Human Skin Cells. Int. J. Nanomed. 2022, 17, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Huerta-García, E.; Pérez-Arizti, J.A.; Márquez-Ramírez, S.G.; Delgado-Buenrostro, N.L.; Chirino, Y.I.; Iglesias, G.G.; López-Marure, R. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free. Radic. Biol. Med. 2014, 73, 84–94. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, L.; Zhong, G.; Huang, Y.; Zhang, X.; Chu, C.; Chen, L.; Wang, M. Titanium Dioxide Nanoparticles Induce Mitochondrial Dynamic Imbalance and Damage in HT22 Cells. J. Nanomater. 2019, 2019, 4607531. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, N.; Zhu, M.; Lu, J.; Zhong, H.; Xue, X.; Guo, S.; Li, M.; Wei, X.; Tao, Y.; et al. TiO2 nanoparticles cause mitochondrial dysfunction, activate inflammatory responses, and attenuate phagocytosis in macrophages: A proteomic and metabolomic insight. Redox Biol. 2018, 15, 266–276. [Google Scholar] [CrossRef]
- Raja, G.; Cao, S.; Kim, D.H.; Kim, T.J. Mechanoregulation of titanium dioxide nanoparticles in cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110303. [Google Scholar] [CrossRef]
- Danielsen, P.H.; Cao, Y.; Roursgaard, M.; Moller, P.; Loft, S. Endothelial cell activation, oxidative stress and inflammation induced by a panel of metal-based nanomaterials. Nanotoxicology 2015, 9, 813–824. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef] [PubMed]
- Baky, N.A.; Faddah, L.M.; Al-Rasheed, N.M.; Al-Rasheed, N.M.; Fatani, A.J. Induction of inflammation, DNA damage and apoptosis in rat heart after oral exposure to zinc oxide nanoparticles and the cardioprotective role of α-lipoic acid and vitamin E. Drug Res. 2013, 63, 228–236. [Google Scholar] [CrossRef]
- Chuang, H.C.; Juan, H.T.; Chang, C.N.; Yan, Y.H.; Yuan, T.H.; Wang, J.S.; Chen, H.C.; Hwang, Y.H.; Lee, C.H.; Cheng, T.J. Cardiopulmonary toxicity of pulmonary exposure to occupationally relevant zinc oxide nanoparticles. Nanotoxicology 2014, 8, 593–604. [Google Scholar] [CrossRef]
- Liang, S.; Sun, K.; Wang, Y.; Dong, S.; Wang, C.; Liu, L.; Wu, Y. Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS death receptor pathway in apoptosis induced by zinc oxide nanoparticles in human aortic endothelial cells and the protective effect by alpha-lipoic acid. Chem. Biol. Interact. 2016, 258, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Dwivedi, S.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Shin, H.S. ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf. B 2014, 117, 267–276. [Google Scholar] [CrossRef]
- Bai, D.P.; Zhang, X.F.; Zhang, G.L.; Huang, Y.F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521–6535. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Yang, Y.W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 28370555. [Google Scholar] [CrossRef]
- Zhang, H. Delivery of Encapsulated Mitochondria as Novel Pharmaceutical Solution. Available online: https://sparkfinland.fi/projects/ (accessed on 3 May 2023).
- Zhuang, Y.; Li, L.; Feng, L.; Wang, S.; Su, H.; Liu, H.; Wu, Y. Mitochondrion-targeted selenium nanoparticles enhance reactive oxygen species-mediated cell death. Nanoscale 2020, 12, 1389–1396. [Google Scholar] [CrossRef]
- Yue, D.; Zeng, C.; Okyere, S.K.; Chen, Z.; Hu, Y. Glycine nano-selenium prevents brain oxidative stress and neurobehavioral abnormalities caused by MPTP in rats. J. Trace Elem. Med. Biol. 2021, 64, 126680. [Google Scholar] [CrossRef] [PubMed]
- Mal’tseva, V.N.; Gudkov, S.V.; Turovsky, E.A. Modulation of the Functional State of Mouse Neutrophils by Selenium Nanoparticles In Vivo. Int. J. Mol. Sci. 2022, 23, 13651. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Khabatova, V.V.; Gudkov, S.V.; Plotnikov, E.Y.; Turovsky, E.A. Cytoprotective Properties of a New Nanocomplex of Selenium with Taxifolin in the Cells of the Cerebral Cortex Exposed to Ischemia/Reoxygenation. Pharmaceutics 2022, 14, 2477. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J. The NHS crisis has been years in the making. BMJ 2023, 380, 96. [Google Scholar] [CrossRef]
- Liu, H.J.; Qin, Y.; Zhao, Z.H.; Zhang, Y.; Yang, J.H.; Zhai, D.H.; Cui, F.; Luo, C.; Lu, M.X.; Liu, P.P.; et al. Lentinan-functionalized Selenium Nanoparticles target Tumor Cell Mitochondria via TLR4/TRAF3/MFN1 pathway. Theranostics 2020, 10, 9083–9099. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Chen, Q.; You, Y.; Li, X.; Chen, T. Functionalized Selenium Nanoparticles Synergizes with Metformin to Treat Breast Cancer Cells Through Regulation of Selenoproteins. Front. Bioeng. Biotechnol. 2021, 9, 758482. [Google Scholar] [CrossRef]



| Disease | Mitochondrial Abnormalities | Management | Ref. |
|---|---|---|---|
| Cardiovascular diseases | Impaired mitochondrial electron transport chain due to elevated LDL, greater ROS production disturbed cardiac tension, altered cytosolic Ca2+ flux, ischemia-reperfusion injury, and other diseases (such as diabetes mellitus) | Control fatty acids and cholesterol level. Regulate enzymes i.e., mitochondrial creatin phosphate, creatin kinase, and ATP synthase. Modulate Ca2+ concentration in myocardium. | [8] |
| Diabetes | MtDNA mutation Delayed Electron transport chain Increased beta oxidation and lipid accumulation Higher ROS overproduction Disturbed insulin signal pathway Increased intracellular glucose content, and constrained metabolism of glucose | Prevention of ROS and lipid peroxidation. Reverse MtDNA change. Enhance glucose metabolism by inhibiting acetyl-CoA in mitochondria. | [9] |
| Kidney | Mitochondrial DNA mutation (complex I–V) Hypomagnesemia Hypokalemia Hypoparathyroidasim Uremic toxins Kidney diseases | Manage Erythropoetin signaling for normal mitochondrial biogenesis and metabolism. Hypoxia-inducible factor prolyl hydroxylase (HIF-PH) inhibitors, Nrf2-activating triterpenoid, sodium–glucose transporter 2 (SGLT2) inhibitors, and control of carnitine level | [10] |
| Alzheimer’s disease | Aggregated Aβ peptide bond with a component that controls mitochondrial permeability ‘cyclophilin D’ Resultant reduction in membrane potential due to opening of pores. Free energy and ROS generated by beta amyloid peptide. Leads to MtDNA mutation and neuronal toxicity. | Inhibition ofAβ peptide clusters binding with cyclophilin D. Restoration of MtDNA and enzyme replacement Inhibit cytochrome c and caspase activity Decrease mitochondrial fission | [11] |
| Cancer | Enhanced mitochondrial complex I activity. Mutations in oncogenes Expression of oncoproteins (IDH 1 and 2, SDH and FH) Apoptosis induced factors released. Defective oxidative phosphorylation Hypoxic milieu proliferate condition | Isocitrate dehydrogenase (IDH) 1 and 2 inhibitors Oxidative phosphorylation suppression Antioxidants Mitochondrial complex I and V inhibitors Lactate dehydrogenase (LDH) inhibitors | [12] |
| Therapeutics | Approach | Model | Outcome | Ref. |
|---|---|---|---|---|
| Upsurging ATP levels | ||||
| Inosine and Febuxostat | To increase ATP level and hypoxanthine in peripheral blood | Patients with homoplasmic and heteroplasmic mutations | After oral administration, brain natriuretic peptide (specific marker for heart failure) was reduced up to 31%. Moreover, 3.1-fold insulinogenic index was improved, suggesting a promising action of the given treatment. | [13] |
| Stimulating mitochondrial biogenesis | ||||
| Bezafibrate and AMPK agonist 5-aminoimidazole-4-carboxamide ribonucleotide | To initiate biogenesis and activate AMP protein kinase/PGC-1α-dependent pathway | Double recombinant mice overexpressing PGC-1α in skeletal muscles | Stimulation of PPAR/AMPK/PGC-1 alpha increased mitochondrial biogenesis, which monitors the homeostatic pathway and motor improvement in Sco2(KO/KI) animal model | [14] |
| 5-Aminoimidazole-4-carboxamide ribotide (AICAR) | ATP content and mitochondrial growth were aimed without disturbing membrane potential | CI deficient fibroblasts, such as NDUFS2 and C20ORF7 | Fluorescence microscopy detailed the activation of AMP protein kinase with AICAR | [15] |
| Nicotinamide riboside (NAD+ precursor) | Effect of nicotinamide in pharmacokinetic parameters and NAD+ level in blood for the treatment of genetic or acquired mitochondrial abnormalities | Impaired mitochondrial murine model | Orally administered nicotinamide riboside was well tolerated, and an increased mean steady state concentration (Css, p = 0.03) two times greater than the baseline NAD+ concentration in blood was observed, whereas average circulating level of NAD+ at day 1 was 27 ± 6 µM. | [16] |
| Modulation of the Nitric acid or cGMP/PKG pathway | ||||
| L-arginine (Nitric acid precursor) | To reduce capacity for nitric acid-based vasodilation | MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) patients | Obtained data suggested that prepared L-Arg infusion was most effective when administered within 4 hrs of the onset of brain disorder symptoms in the acute phase of MELAS sufferers | [17] |
| Neural progenitor cells (NPC) | To preserve parental mtDNA and show metabolic shift toward oxidative phosphorylation | Homoplasmic mutation in the mitochondrial gene (MT-ATP6) | Human induced pluripotent stem cells originated NPC, providing a potential tool for mtDNA targeted drug screening to avoid nervous system disorder. | [18] |
| Antioxidant therapy | ||||
| N-acetyl cysteine | Supplementation with N-acetyl cysteine to modify the mitochondrial respiratory chain function | MELAS patients containing common mutation, i.e., m.3243A>G, m.8344A>G | Supplementation with N-acetyl cysteine improved 2-thiomodificationof tRNA, thus regulating protein synthesis. | [19] |
| Cysteamine bitartrate | Enhancement of glutathione biosynthesis for the reduction of oxidative stress associated with mitochondrial diseases | Zebrafish model and Caenorhabditis elegans model carrying Complex I defect | Cysteamine bitartrate improved mitochondrial membrane potential in nephropathic cystinosis and cured multiple RC complex diseases in FBXL4 human at 10 to 100 μm concentrations. | [20] |
| Inclusion of Redox-Active Molecules | ||||
| Tc99m-HMPAO | To explore Tc99m-HMPAO for determination of glutathione/protein thiol levels in cerebral blood flow | Pediatric mitochondrial diseased patients | The patients showed improvement in Newcastle score (n = 5, p = 0.028), confirming the potential of Tc99m-HMPAO as a bioimaging marker for the oxidative state of brain. | [21] |
| EPI-743 | Management of cellular oxidative stress in mitochondrial respiratory chain disease | 5-month-old girl suffering from Leigh syndrome | EPI-743 (a potent stress protectant) exhibited improvement in mitochondrial associated issues, i.e., improvement in eye, motor, and bowel movements | [22] |
| Monitoring of mitochondrial dynamics | ||||
| Cytotoxic Necrotizing Factor-1 | To control mitochondrial impairment and cell damage | Patient sufferer from m.8344A>G gene | CNF-1 triggered energetic content of mitochondria via activation of actin cycloskeleton in Myoclinic epilepsy with ragged red fibers (MERRF) and increased mitochondrial marker tomo20 | [23] |
| Modulating mitochondrial autophagy | ||||
| Rapamycin | Inhibition of mTOR in mitochondrial defect in Leigh syndrome | Ndufs4 wild type mouse | Rapamycin slowed down neurological symptoms and minimized neuroinflammation in brain lesions. It also slowed the formation of glycolytic intermediates. | [24] |
| NADH dehydrogenase Ndi1 | To replace mitochondrial complex 1 for reoxidation of NADH intramitochondrially. | Transgenic strains of Drosophila | Overexpression of NDI1 relieves aging and manages production of ROS. Deposition of these oxidative damaged markers is declined in aged flies. | [25] |
| Nanodrug Delivery System | Purpose | Model | Outcomes | Relevance | Ref. |
|---|---|---|---|---|---|
| Topotecan loaded liposome | Mitochondrial targeted system to overcome resistant related metastases | Multi drug Resistant MCF-7/ADR cell xenografts | Mitochondrial targeted liposomes were 64.84 nm with −0.52 ± 0.08 mV membrane potential. Encapsulation efficiency was ≥95%. They were stable in physiological blood system and exhibited minimal leakage. The system led to release cytochrome C, stimulated caspase 9 and 3, and exhibited superior inhibitory action on the resistant B16 melanoma metastatic mice. | Topotecan localized in mitochondria that exhibited potent inhibitory action on the on the resistant B16 metastatic melanoma. | [44] |
| Paclitaxel loaded triphenylphosphine nanomicelles | Inhibition of antiapoptotic Bcl-2 | Drug-resistant breast cancer-bearing mouse model with lung metastasis (A549/ADRcells) | The nanomicelles were small (142 ± 8.35 nm) with PDI 0.235 and negative zeta potential (−24.65 ). This system significantly hampered A549/ADR cells and deposited over mitochondria surface. Inhibition of Bcl-2 led to release cytochrome C and triggered caspase 3 and 9, mediating mitochondrial outer membrane permeabilization. | Positively charged nanomicelles adhered inside mitochondria and exhibited apparent multidrug resistant tumor targeting efficacy | [45] |
| Curcumin loaded polymeric and lipid nanosuspensions | To neutralize generation of reactive oxygen species due to disturbance of signal proteins in mitochondria. | Olfactory ensheathing cells | Uniform and spherical polymeric and lipid nanosuspensions were of mean sizes 338 nm and 127 nm, respectively, with negatively charged zeta potential. The nanosuspensions were quite stable for more than 135 days. Improved cell viability suggested potential incorporation of curcumin in nanosuspension against hypoxic cells of Olfactory ensheathing cells at the concentration of 5 µM. | Potential intranasal polymeric and lipid nanosuspention was advised for neuroprotective action due to antioxidant property of curcumin. | [46] |
| Hydroxyl-terminated polyamidoamine (PAMAM)-N-acetyl cysteine dendrimers | Mitochondrial targeted delivery in oxidative stress-induced glial cell | Rabbit traumatic brain injury (TBI) model | Significantly high localization of drug in mitochondria than nonmodified dendrimer due to potential for attenuation of oxidative stress. Systemic administration in TBI model of rabbit exhibited capability to penetrate BBB and target glial cells due to localization in the white matter of the injured hemisphere. | Colocalization of dendrimer in mitochondria of glial cells in traumatic brain injury | [47] |
| Osthole nanoemulsion (OST-NE) | Regulation of apoptosis pathway and mitochondrial oxidative stress. | Alzheimer’s disease model mice | Intranasal delivery of osthole nanoemulsion (mean particle size 2.33 nm) enhanced bioavailability, regulated cholinergic system, maintained mitochondrial potential, and inhibited apoptosis and oxidative stress. | OST-NE lessened upstream modulator Bax, that depolarized mitochondrial membrane potentail and exhibited antiapoptotic effect or neuroprotective action in Alzheimer disease | [48] |
| Momordica charantia silver nanoparticles | Uphold mitochondria biogenesis and enhanced the expression of PPARϒ, an energy metabolism coordinator | Pancrease of diabetic rats | Silver nanoparticles possessed irregular/uneven surface and diameter. Contained Momordica charantia enhanced glucose sensitivity in the dibetic rat model at a lower dose of 50 mg/kg by slowing down JAK/STAT and AKT/PI3K pathways in mitochondria. | Developed nanoparticles promoted glucose uptake and insulin secretion via improving mitochondrial biogenesis in pancreas of diabetic rats. | [49] |
| Dequalinium embedded DQAsomes | Dicationic amphiphilic was investigated for affinity towards binding with mitochondria DNA. | Plasmid DNA firefly luciferase | Developed DQAsomes created a liposome-like aggregate system in aqueous media that were able to bind with DNA and had efficiency to transfect cells compared to Lipofectin™ reagent. | DQAsomes selectively accumulated in mitochondria of cancerous cells, and suggeted potential use as a nonviral transfection vector in gene delivery system. | [50] |
| Metallic Nanoparticles | Conjugated with | Mechanism and Model | Outcomes | Ref. |
|---|---|---|---|---|
| Functionalized gold nanoparticles | ||||
| Fluorescent gold nanoprobe | Fluorophore cyanine 5 | Mitophagy, breast cancer cell line (MDA-MB-231) | Oxidation and polarization in the mitochondrial membrane, used for tracking lysosomes to image mitophagy | [78] |
| Gold nanoparticles | Tumor necrosis factor-related apoptosis-inducing ligand | Autophagy, Excessive Drp1-dependent mitochondrial fragmentation, and dysfunction in tumor cells | Promoted apoptosis in nonsmall cell lung cancer (NSCLC) cells | [79] |
| Epigallocatechin gallate capped gold nanoparticles | Poly-D-lysine-PEG functionalized with mitochondria targeting cation (triphenylphosphonium) | Mitochondrial Caspasedependent apoptosis in HeLa cell line. The laminin receptor dependent uptake of nanoparticles | Preferentially localized in mitochondrial and enhanced delivery of paclitaxel to the Cervical carcinoma cells | [80] |
| Epigallocatechin gallate-capped gold nanoparticles | Cationic triphenylphosphonium functionalized | Predominant mitochondrial depolarization, Activation of caspases 3 and 7, and G0/G1 phase arrest of the cell cycle. Cellular uptake and mitochondrial localization in human Caco-2, MCF-7, and HeLa cancers cell lines | Mitochondrial targeted delivery botulinic acid gold nanoparticles with lesser IC50 values (3.12–13.2 µM) compared to the bare one (9.74–36.31 µM). Mitochondrial apoptosis in cancer cells. | [81] |
| Functionalized iron oxide nanoparticles | ||||
| Iron oxide nanoparticle (fluorescent) | Coumarin | In HeLa cells, intracellular temperature was increased by 2.1 °C in five minutes on laser irradiation of 740 nm that targeted mitochondrial cells. | The system particularly released coumarin-derived iron oxide NPs (fluorescent) to the mitochondria of HeLa cells. Further, elevated temperature and more cytotoxicity mediated photothermal therapy and lead to better targeting in cancerous cells. | [82] |
| Iron oxide nanoparticles | TAT, a cell penetrating peptide | Encouraged leakage of electrons from the mitochondrial electron transport chain that led to amplified production of reactive hydroxyl radicals and permeabilized lysosomal membrane of cancerous A549 cells. | TAT modified iron oxide nanoparticles along with radiation therapy synergized effects of chemotherapy | [83] |
| Iron oxide | Carbonyl monoxide complex | Generation of carbon monoxide from complex initiated mitophagy/autophagy | Developed iron pentacarbonyl system specifically triggered under near-infrared irradiation in the tumor environment. Carbon monoxide and iron oxide damaged mitochondria and synergized effect of cancer cells. | [84] |
| Functionalized silver nanoparticles | ||||
| Silver nanoparticles | Glucose | Castrate-resistant prostate cancers cell line was selected, due to its capacity for high glucose consumption. | Developed system was stable, spherical, and had average size 61 nm. It produced ROS, depolarized mitochondrial membrane potential, and led to cancerous cell apoptosis. | [85] |
| Silver nanoparticles | Polydopamine-coated paclitaxel for targeting tumor peptide NR1 | Augmented Bax-to-Bcl-2 ratio and the stimulation of proapoptotic P53 and caspase 3 pathways resulted in intense ROS release and a break down of double-stranded DNA. | NR1 decorated paclitaxel-silver nanoparticles displayed pH responsive drug release and superior apoptosis via mitochondrial membrane lysis and nucleus damage. | [86] |
| Silver nanoparticles | Tamoxifen | The conjugate stimulated cell apoptosis via mediating Bax/Bcl2 and caspase–cascade signal pathways in mitochondria. | Tamoxifen conjugated silver nanoparticles (440 nm) were amorphous, disrupted the mitochondrial membrane, and induced apoptosis signal in human breast cancer cells. | [87] |
| Titanium dioxide nanoparticles | ||||
| Mesoporous titanium dioxide | Nitric acid decorated L-arginine and mitochondrial targeting ligand (triphenyl phosphonium) | Conjugated nitric oxide inhibited cell respiration and released reactive nitric species targeted mitochondrial sites of cancerous cells (MCF-7). | Developed system efficiently accumulated inside the mitochondria and generated Nitric oxide gas and ROS. A synergized effect of sonodynamic therapy on the management of breast cancer achieved. | [88] |
| Titanium dioxide | Erlotinib and Vorinostat | Different cancerous cells, including MDA-MB-231, MCF-7 and cancerous amniotic cells. Depicted G2/M phase cell arrest. | Designed nanoconjugate exhibited induction of apoptosis due to deposition of p53. Increased ROS and disrupted mitochondrial DNA were exhibited in treated lymphocytes. | [89] |
| Titanium dioxide | Quercetin | The level of mitochondrial complex I–V dropped with ATP depletion in rodent model. in rodents. Enhanced levels of protein carbonyl and lipid peroxidation at 10 and 50 μg/mL concentration. | The conjugate acted as potential antioxidant and caused mitochondrial dysfunction and oxidative stress in liver mitochondria. | [90] |
| Functionalized platinum nanoparticles | ||||
| Platinum nanoparticles | Peptide coated TPP-Pt | Deeper tissue penetration with lesser photon scattering at 1.0 W cm−2 permissible exposure. | Developed conjugate was monodispersed, stable, and exhibited precise NIR II (1000–1350 nm) phototherapy in a low amount to a thermally susceptible mitochondrion. Photothermal therapy for the management of cancer | [91] |
| Pt-Ceria-8-atto nanoparticles (8 nm) | Conjugated with triphenyl phosphonium and Atto 647 N fluorescent dye | HMEC1 cell line. Conjugated lipophilic cationic molecule (triphenyl phosphonium) and dye (Atto 647 N) both attracted and accumulated on the mitochondrial negative membrane potential surface. | Selectively accumulated in the mitochondria. Nanoparticles larger than 150 nm (PDI < 0.1) were internalized via conventional process of endocytosis. | [92] |
| Aggregation-induced emission-based platinum IV | Triphenyl phosphonium | Augmented reactive oxygen species and lessened mitochondrial membrane potential. Cell cycle arrest in the S-phase and DNA disruption. | Potentially active for the management of solid tumor spheroids. | [93] |
| Functionalized zinc oxide nanoparticles | ||||
| Quercetin-decorated zinc oxide nanoparticles | Phenylboronic acid | pH responsive release pattern was observed using 40 nm sized nanoparticles (−10.2 mV zeta potential) in MCF-7 cells | The nanoconjugate induced enhanced oxidative stress, damaged mitochondria, and induced apoptosis in breast cancer cells. | [94] |
| PEG coated zinc oxide | Doxorubicin | Nanocomposite induced cell injury due to generation of ROS under UV irradiation in HeLa cell lines. | Photocleavage of DNA and photodynamic therapy in cancer management. | [95] |
| Zinc oxide nanoparticles with artesunate and glucose oxidase | Hyaluronic acid | Generated hydrogen peroxide caused oxidative damage and accelerated glucose oxidase in cancerous cells | Nanocomposite (163 nm) exhibited intrinsic affinity with CD44 receptors and produced cancer starvation | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misra, S.K.; Rosenholm, J.M.; Pathak, K. Functionalized and Nonfunctionalized Nanosystems for Mitochondrial Drug Delivery with Metallic Nanoparticles. Molecules 2023, 28, 4701. https://doi.org/10.3390/molecules28124701
Misra SK, Rosenholm JM, Pathak K. Functionalized and Nonfunctionalized Nanosystems for Mitochondrial Drug Delivery with Metallic Nanoparticles. Molecules. 2023; 28(12):4701. https://doi.org/10.3390/molecules28124701
Chicago/Turabian StyleMisra, Shashi Kiran, Jessica M. Rosenholm, and Kamla Pathak. 2023. "Functionalized and Nonfunctionalized Nanosystems for Mitochondrial Drug Delivery with Metallic Nanoparticles" Molecules 28, no. 12: 4701. https://doi.org/10.3390/molecules28124701
APA StyleMisra, S. K., Rosenholm, J. M., & Pathak, K. (2023). Functionalized and Nonfunctionalized Nanosystems for Mitochondrial Drug Delivery with Metallic Nanoparticles. Molecules, 28(12), 4701. https://doi.org/10.3390/molecules28124701









