Peptide-Based Nanoparticles for αvβ3 Integrin-Targeted DNA Delivery to Cancer and Uterine Leiomyoma Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Design of Carriers
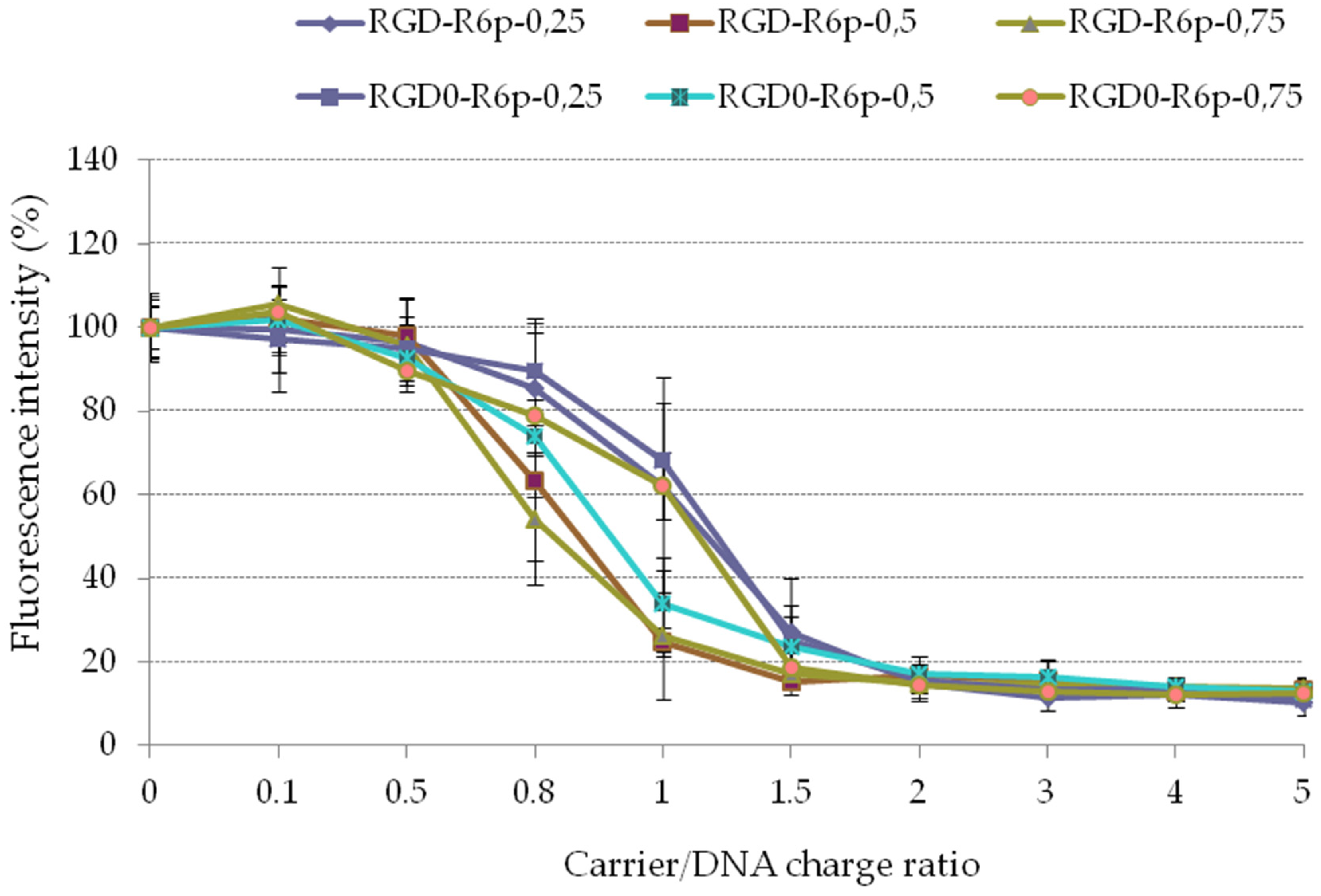
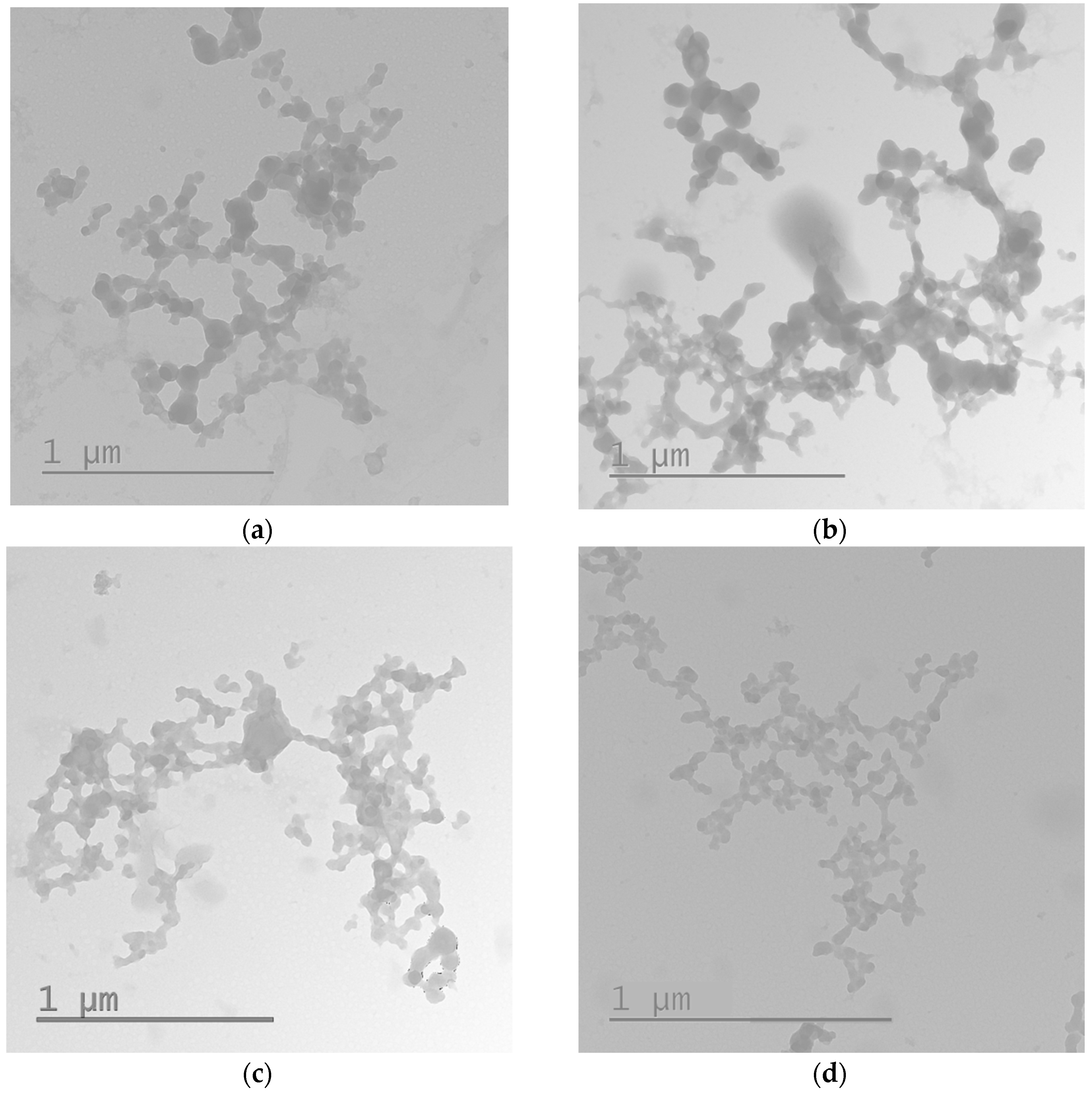
2.2. Physicochemical Characterization of the Peptide/DNA Complexes
2.3. Cytotoxicity of Peptide/DNA Polyplexes
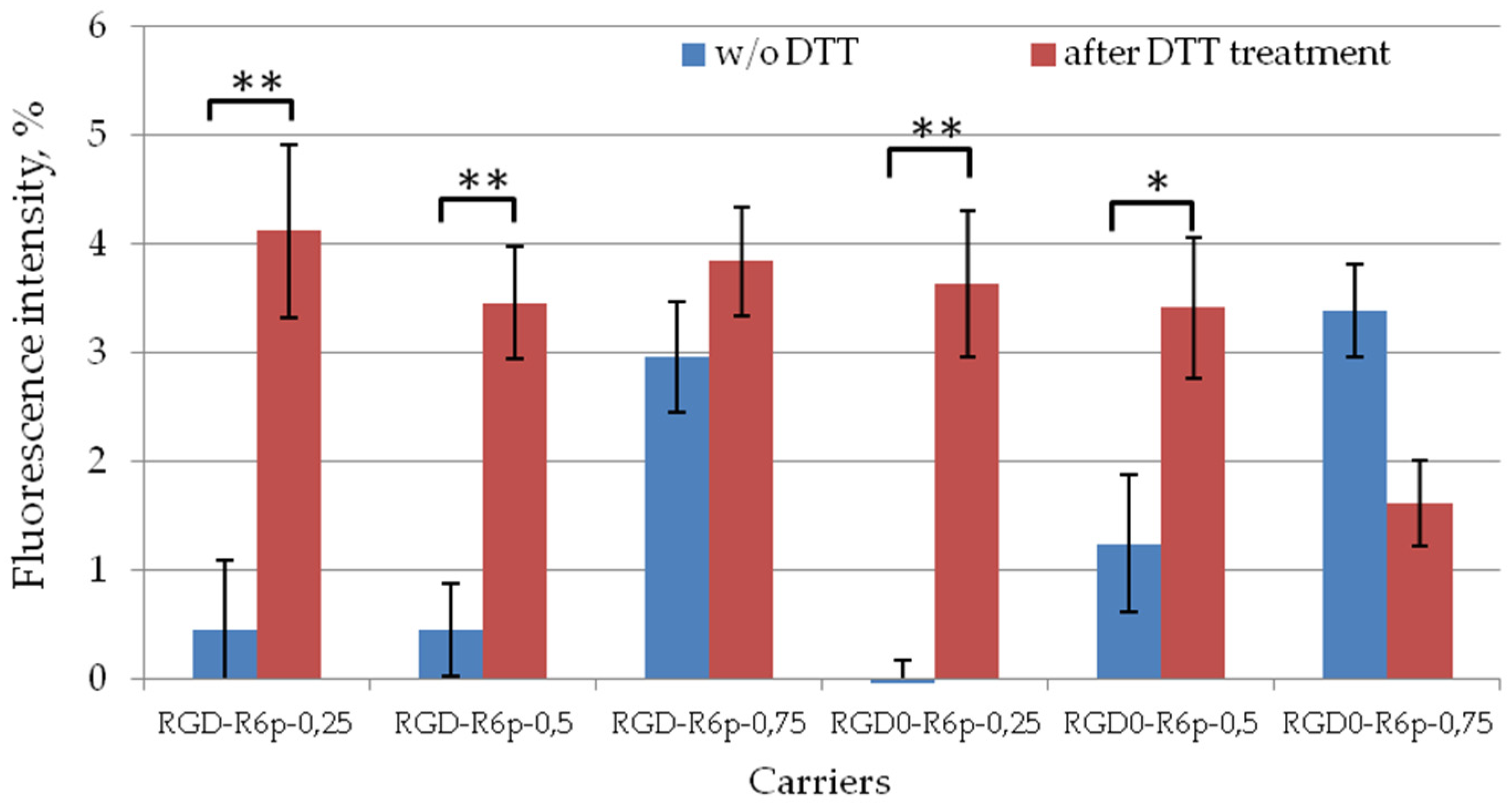
2.4. Cellular Uptake of Peptide/DNA Polyplexes
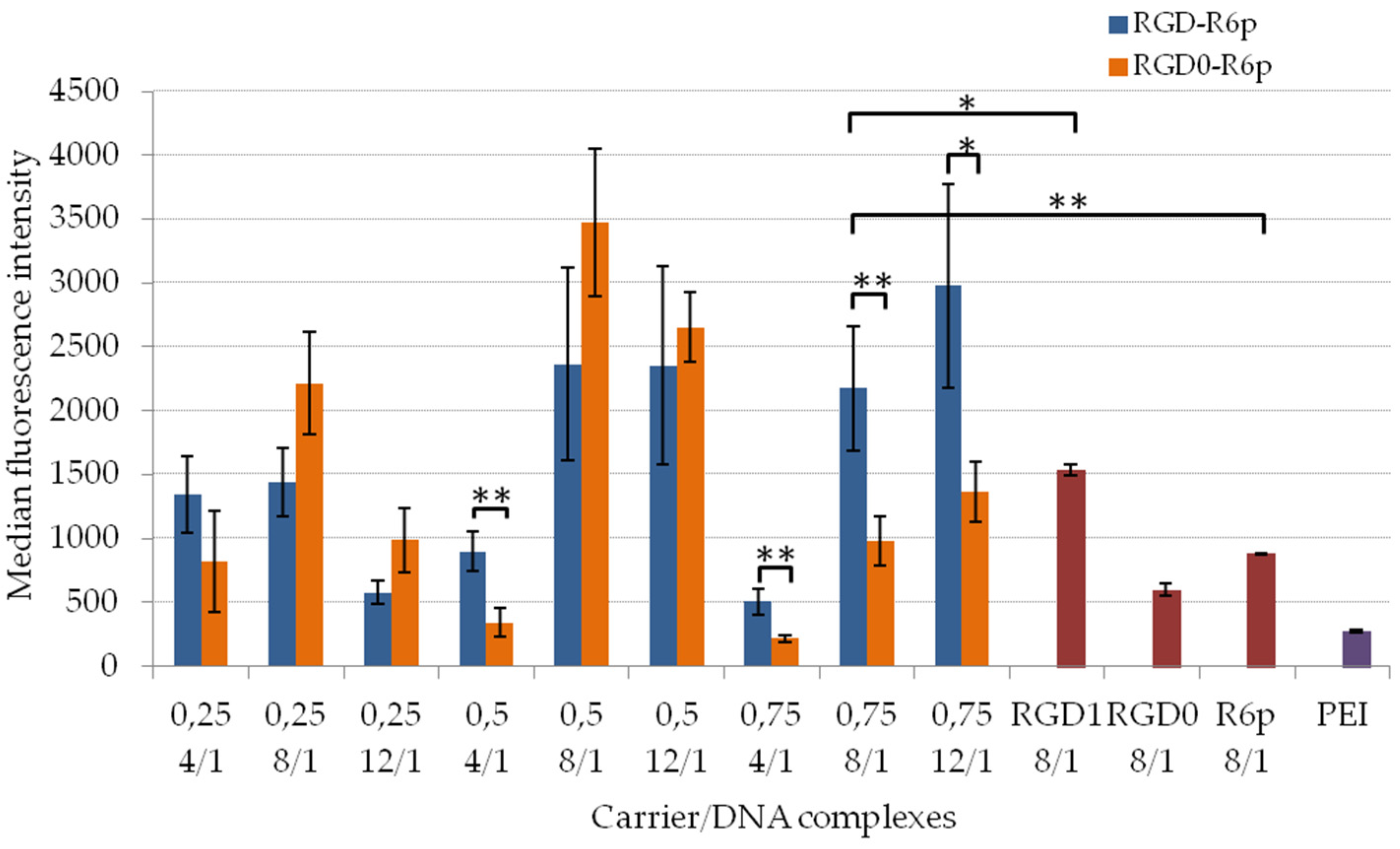
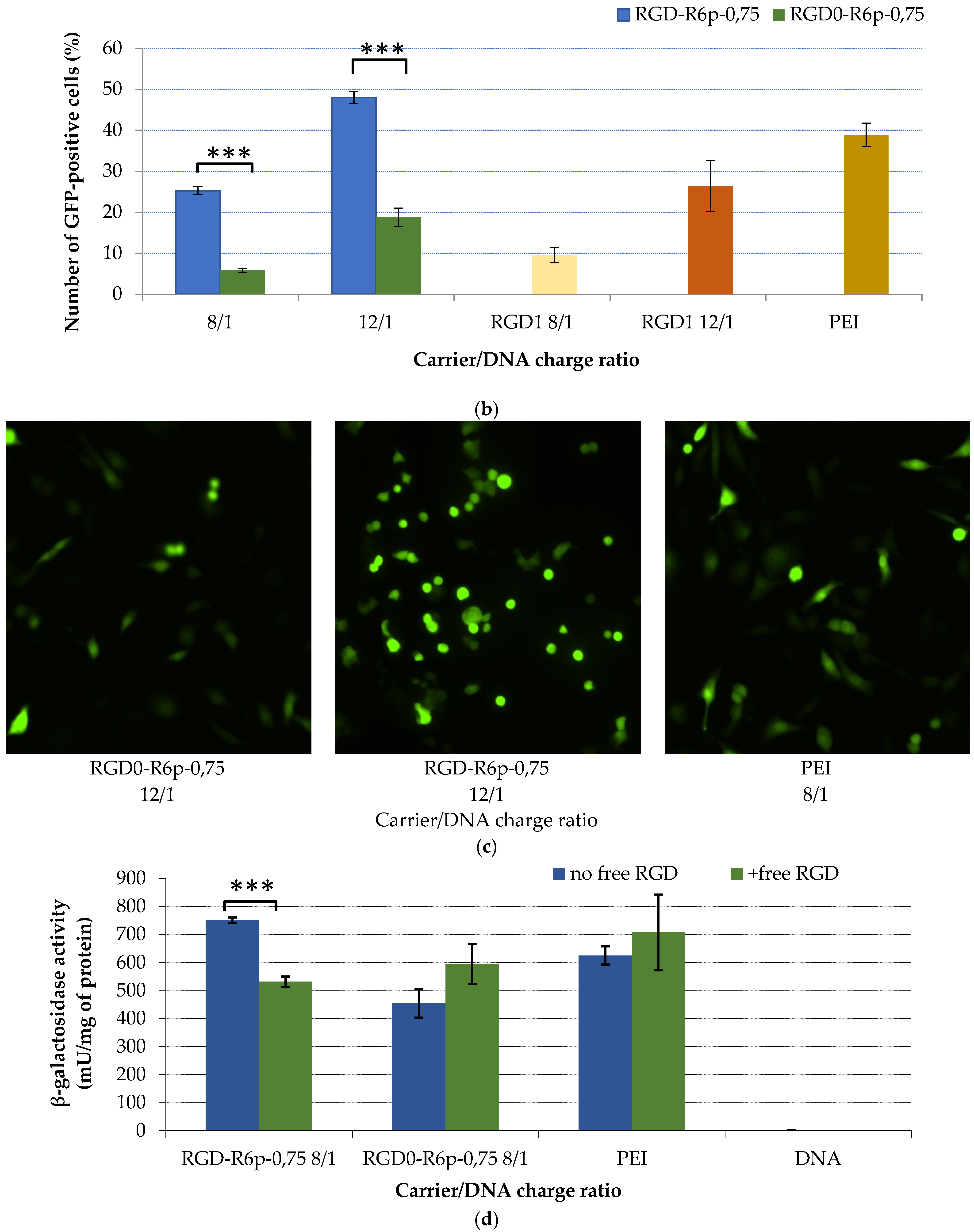
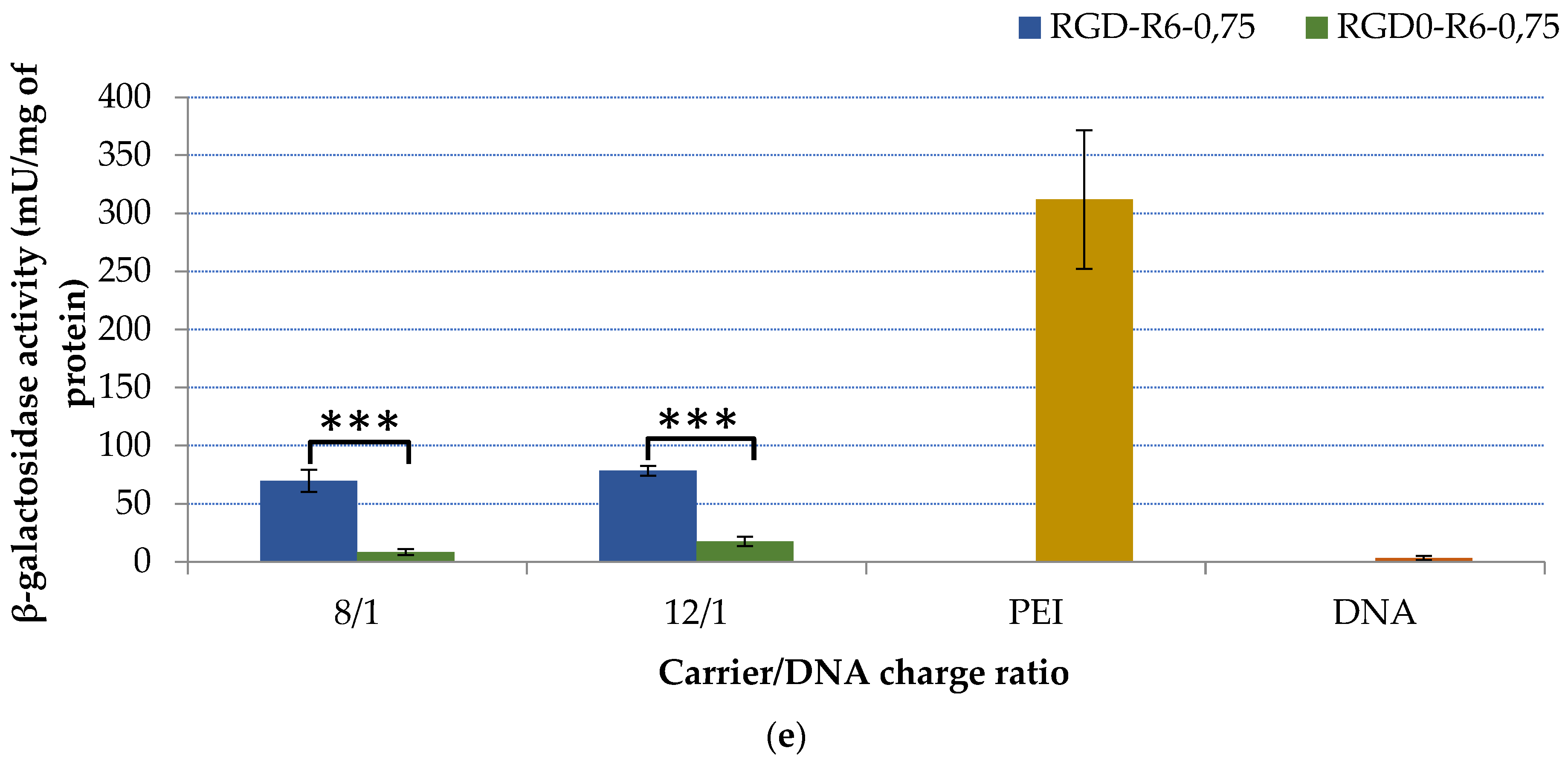
2.5. Transfection Studies
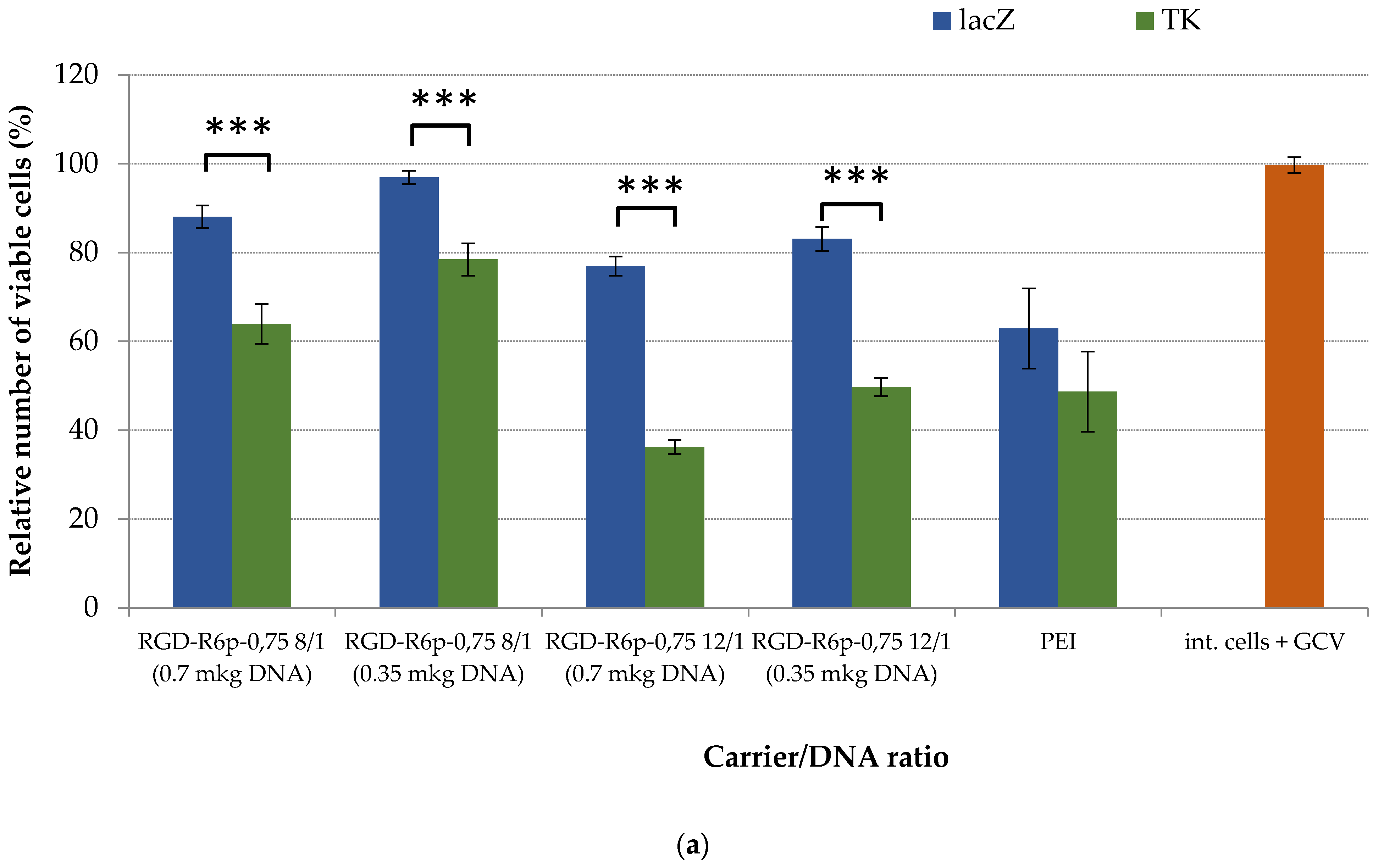
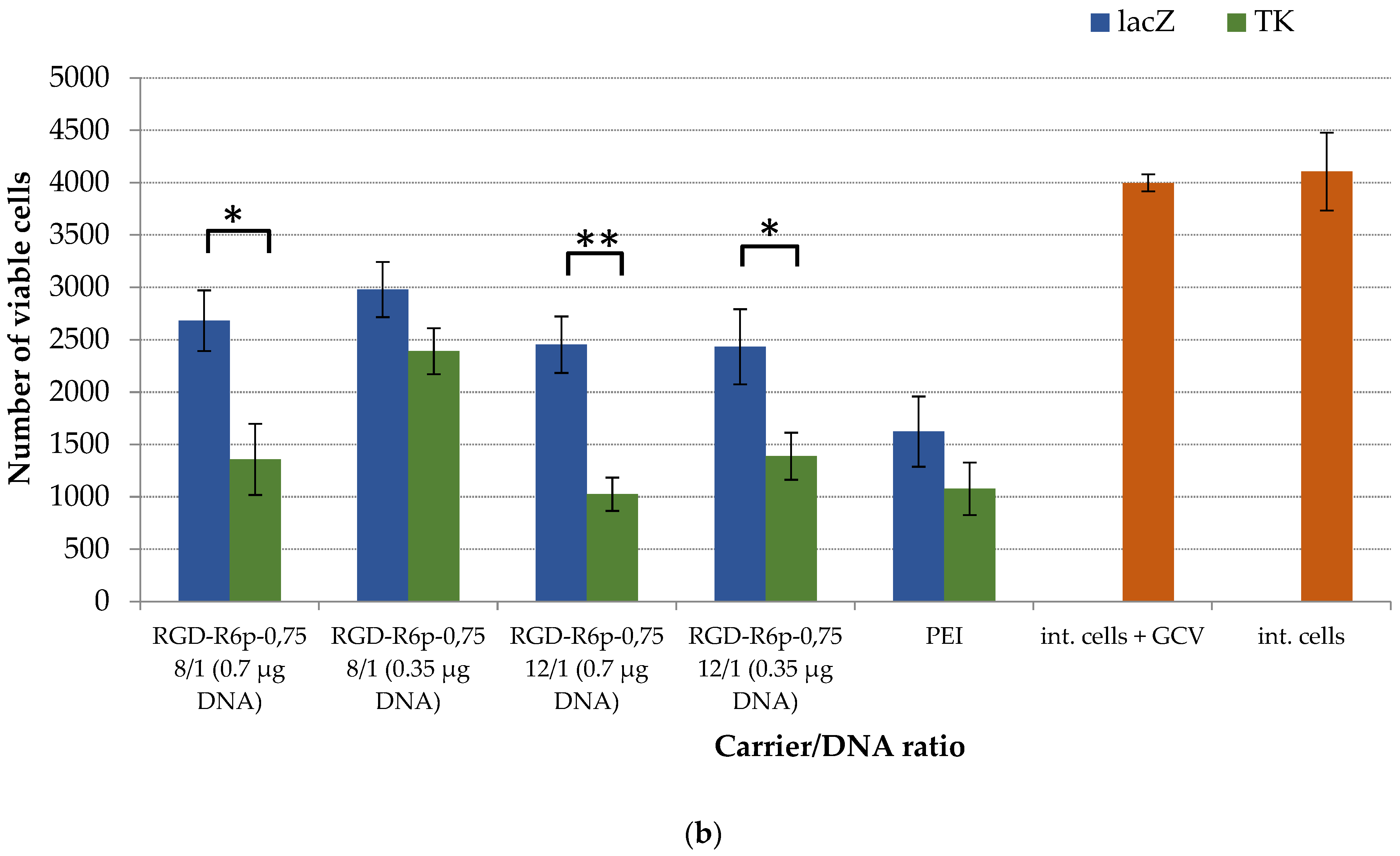
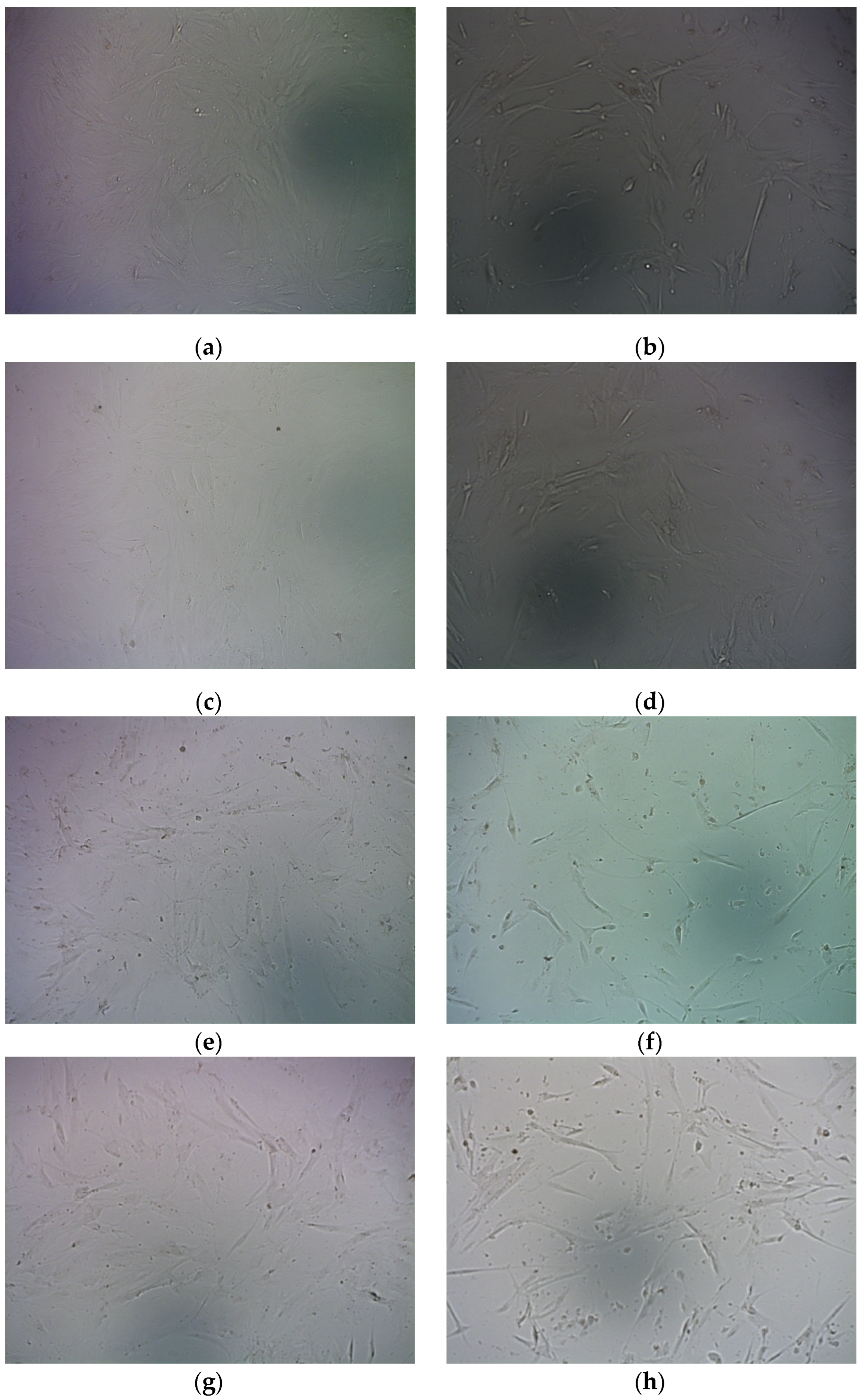
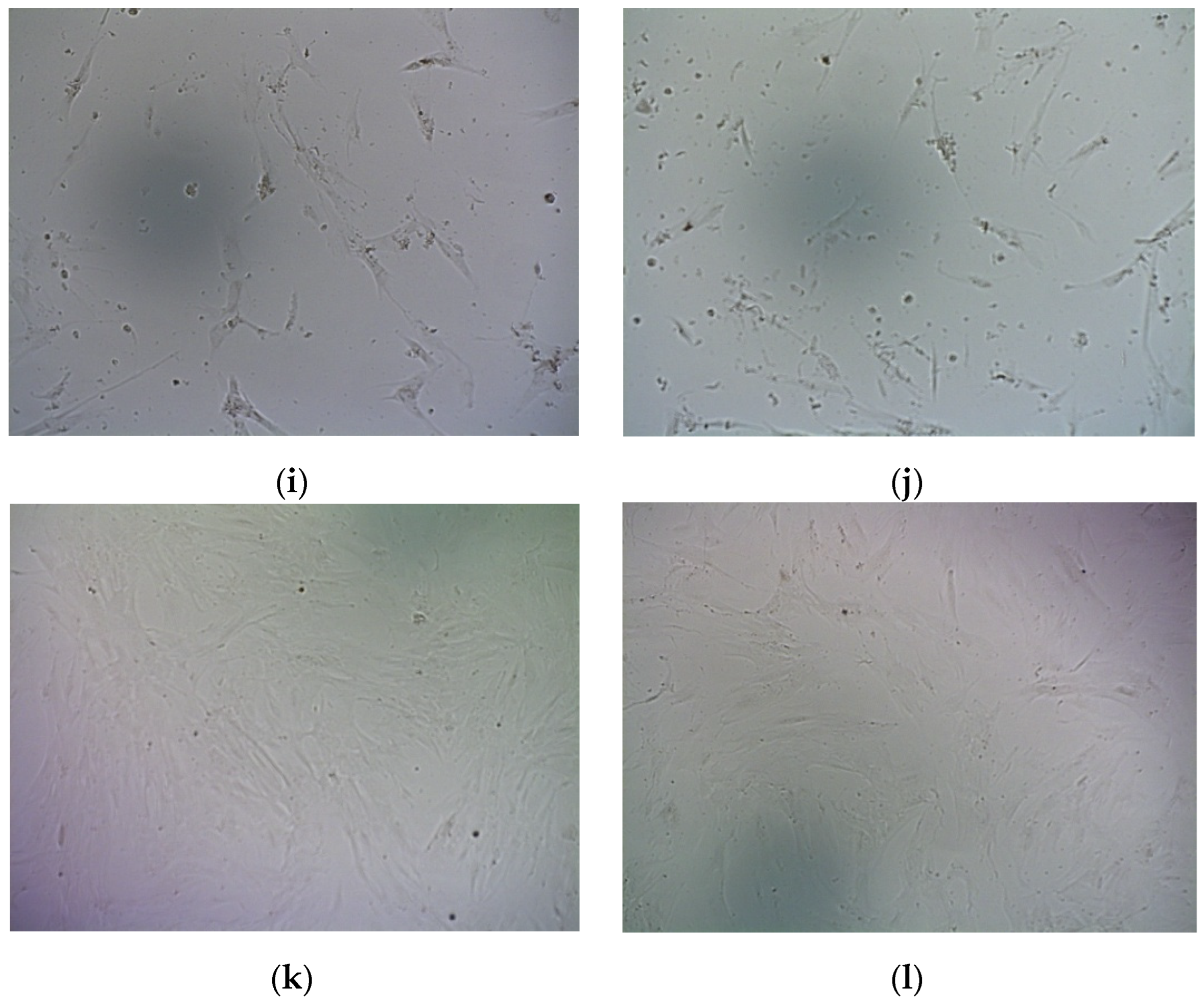
2.6. Suicide Effect of RGD-R6p-0,75/pPTK1 Polyplexes with GSV Treatment in Uterine Leiomyoma Cells
3. Materials and Methods
3.1. Cell Lines
3.2. Peptides Synthesis and Characterization
3.3. Reporter Plasmids
3.4. Nucleopeptide Complexes Preparation and Their Physico-Chemical Characterization
3.5. Transmission Electronic Microscopy
3.6. Cellular Uptake of Peptide/DNA Complexes
3.7. Gene Transfer
3.8. Cytotoxicity Assay
3.9. Suicide Gene Therapy
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ibraheem, D.; Elaissari, A.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Morgan, R.A. Gene Therapy for Infectious Diseases. Clin. Microbiol. Rev. 1998, 11, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Dishart, K.L.; Work, L.M.; Denby, L.; Baker, A.H. Gene therapy for cardiovascular disease. J. Biomed. Biotechnol. 2003, 2003, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Abreu, N.J.; Waldrop, M.A. Overview of gene therapy in spinal muscular atrophy and Duchenne muscular dystrophy. Pediatr. Pulmonol. 2021, 56, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Maule, G.; Arosio, D.; Cereseto, A. Gene Therapy for Cystic Fibrosis: Progress and Challenges of Genome Editing. Int. J. Mol. Sci. 2020, 21, 3903. [Google Scholar] [CrossRef]
- Yu, M.; Poeschla, E.; Wong-Staal, F. Progress towards gene therapy for HIV-infection. Gene Ther. 1994, 1, 13–26. [Google Scholar]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef]
- Moolten, F.L.; Wells, J.M. Curability of Tumors Bearing Herpes Thymidine Kinase Genes Transfered by Retroviral Vectors. JNCI J. Natl. Cancer Inst. 1990, 82, 297–300. [Google Scholar] [CrossRef]
- Abdelaziz, M.; Sherif, L.; ElKhiary, M.; Nair, S.; Shalaby, S.; Mohamed, S.; Eziba, N.; El-Lakany, M.; Curiel, D.; Ismail, N.; et al. Targeted Adenoviral Vector Demonstrates Enhanced Efficacy for In Vivo Gene Therapy of Uterine Leiomyoma. Reprod. Sci. 2016, 23, 464–474. [Google Scholar] [CrossRef]
- Shubina, A.; Egorova, A.; Baranov, V.; Kiselev, A. Recent Advances in Gene Therapy of Endometriosis. Recent Pat. DNA Gene Seq. 2014, 7, 169–178. [Google Scholar] [CrossRef]
- Montini, E.; Cesana, D.; Schmidt, M.; Sanvito, F.; Ponzoni, M.; Bartholomae, C.; Sergi, L.S.; Benedicenti, F.; Ambrosi, A.; Di Serio, C.; et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006, 24, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Lukashev, A.N.; Zamyatnin, A.A. Viral vectors for gene therapy: Current state and clinical perspectives. Biochem. Biokhimiia 2016, 81, 700–708. [Google Scholar] [CrossRef]
- Athanasopoulos, T.; Munye, M.M.; Yáñez-Muñoz, R.J. Nonintegrating Gene Therapy Vectors. Hematol. Oncol. Clin. North Am. 2017, 31, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Boulaiz, H.; Marchal, J.A.; Prados, J.; Melguizo, C.; Aránega, A. Non-viral and viral vectors for gene therapy. Cell. Mol. Biol. 2005, 51, 3–22. [Google Scholar] [PubMed]
- Somia, N.; Verma, I.M. Gene therapy: Trials and tribulations. Nat. Rev. Genet. 2000, 1, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Stribley, J.M.; Rehman, K.S.; Niu, H.; Christman, G.M. Gene therapy and reproductive medicine. Fertil. Steril. 2002, 77, 645–657. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Dowaidar, M.; Hällbrink, M.; Langel, Ü. Gene delivery using cell penetrating peptides-zeolitic imidazolate frameworks. Microporous Mesoporous Mater. 2020, 300, 110173. [Google Scholar] [CrossRef]
- Egorova, A.; Kiselev, A. Peptide modules for overcoming barriers of nucleic acids transport to cells. Curr. Top. Med. Chem. 2016, 16, 330–342. [Google Scholar] [CrossRef]
- Rothbard, J.B.; Jessop, T.C.; Lewis, R.S.; Murray, B.A.; Wender, P.A. Role of Membrane Potential and Hydrogen Bonding in the Mechanism of Translocation of Guanidinium-Rich Peptides into Cells. J. Am. Chem. Soc. 2004, 126, 9506–9507. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef]
- Sakai, N.; Takeuchi, T.; Futaki, S.; Matile, S. Direct Observation of Anion-Mediated Translocation of Fluorescent Oligoarginine Carriers into and across Bulk Liquid and Anionic Bilayer Membranes. ChemBioChem 2005, 6, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Calnan, B.J.; Biancalana, S.; Hudson, D.; Frankel, A.D. Analysis of arginine-rich peptides from the HIV Tat protein reveals unusual features of RNA-protein recognition. Genes Dev. 1991, 5, 201–210. [Google Scholar] [CrossRef]
- Mason, A.J.; Leborgne, C.; Moulay, G.; Martinez, A.; Danos, O.; Bechinger, B.; Kichler, A. Optimising histidine rich peptides for efficient DNA delivery in the presence of serum. J. Control. Release 2007, 118, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Read, M.L.; Singh, S.; Ahmed, Z.; Stevenson, M.; Briggs, S.S.; Oupicky, D.; Barrett, L.B.; Spice, R.; Kendall, M.; Berry, M.; et al. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005, 33, e86. [Google Scholar] [CrossRef] [PubMed]
- Soundara Manickam, D.; Bisht, H.S.; Wan, L.; Mao, G.; Oupicky, D. Influence of TAT-peptide polymerization on properties and transfection activity of TAT/DNA polyplexes. J. Control. Release 2005, 102, 293–306. [Google Scholar] [CrossRef]
- Kiselev, A.; Egorova, A.; Laukkanen, A.; Baranov, V.; Urtti, A. Characterization of reducible peptide oligomers as carriers for gene delivery. Int. J. Pharm. 2013, 441, 736–747. [Google Scholar] [CrossRef]
- Egorova, A.; Selutin, A.; Maretina, M.; Selkov, S.; Baranov, V.; Kiselev, A. Characterization of iRGD-Ligand Modified Arginine-Histidine-Rich Peptides for Nucleic Acid Therapeutics Delivery to αvβ3 Integrin-Expressing Cancer Cells. Pharmaceuticals 2020, 13, 300. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Prakash Karmali, P.; Ramana Kotamraju, V.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef]
- Ruoslahti, E. Drug targeting to specific vascular sites. Drug Discov. Today 2002, 7, 1138–1143. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef]
- Zuo, H. iRGD: A Promising Peptide for Cancer Imaging and a Potential Therapeutic Agent for Various Cancers. J. Oncol. 2019, 2019, 9367845. [Google Scholar] [CrossRef] [PubMed]
- Erel-Akbaba, G.; Carvalho, L.A.; Tian, T.; Zinter, M.; Akbaba, H.; Obeid, P.J.; Chiocca, E.A.; Weissleder, R.; Kantarci, A.G.; Tannous, B.A. Radiation-Induced Targeted Nanoparticle-Based Gene Delivery for Brain Tumor Therapy. ACS Nano 2019, 13, 4028–4040. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Shtykalova, S.; Selutin, A.; Shved, N.; Maretina, M.; Selkov, S.; Baranov, V.; Kiselev, A. Development of iRGD-Modified Peptide Carriers for Suicide Gene Therapy of Uterine Leiomyoma. Pharmaceutics 2021, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Naeye, B.; Høgset, A.; Demeester, J.; De Smedt, S.C.; Xu, L.; Anchordoquy, T.; Ah, H.; Nam, K.; Wan, S.; et al. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 281–288. [Google Scholar] [CrossRef]
- Okuda, T.; Niidome, T.; Aoyagi, H. Cytosolic soluble proteins induce DNA release from DNA—Gene carrier complexes. J. Control. Release 2004, 98, 325–332. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, H.H.; Yang, J.M.; Shin, S. An insight into the gene delivery mechanism of the arginine peptide system: Role of the peptide/DNA complex size. Biochim. Biophys. Acta-Gen. Subj. 2006, 1760, 1604–1612. [Google Scholar] [CrossRef]
- Niidome, T.; Ohmori, N.; Ichinose, A.; Wada, A.; Mihara, H.; Hirayama, T.; Aoyagi, H. Binding of Cationic α-Helical Peptides to Plasmid DNA and Their Gene Transfer Abilities into Cells. J. Biol. Chem. 1997, 272, 15307–15312. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Bosman, F.T.; Stamenkovic, I. Functional structure and composition of the extracellular matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef]
- Mislick, K.A.; Baldeschwieler, J.D. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc. Natl. Acad. Sci. USA 1996, 93, 12349–12354. [Google Scholar] [CrossRef]
- Ruponen, M.; Ylä-Herttuala, S.; Urtti, A. Interactions of polymeric and liposomal gene delivery systems with extracellular glycosaminoglycans: Physicochemical and transfection studies. Biochim. Biophys. Acta-Biomembr. 1999, 1415, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials 2001, 22, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Oba, M.; Fukushima, S.; Kanayama, N.; Aoyagi, K.; Nishiyama, N.; Koyama, H.; Kataoka, K. Cyclic RGD Peptide-Conjugated Polyplex Micelles as a Targetable Gene Delivery System Directed to Cells Possessing α v β 3 and α v β 5 Integrins. Bioconjugate Chem. 2007, 18, 1415–1423. [Google Scholar] [CrossRef]
- Read, M.L.; Bremner, K.H.; Oupický, D.; Green, N.K.; Searle, P.F.; Seymour, L.W. Vectors based on reducible polycations facilitate intracellular release of nucleic acids. J. Gene Med. 2003, 5, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Haberland, A.; Cartier, R.; Heuer, D.; Zaitsev, S.; Paulke, B.-R.; Schäfer-Korting, M.; Böttger, M. Structural aspects of histone H1–DNA complexes and their relation to transfection efficiency. Biotechnol. Appl. Biochem. 2005, 42, 107. [Google Scholar] [CrossRef] [PubMed]
- Vachutinsky, Y.; Oba, M.; Miyata, K.; Hiki, S.; Kano, M.R.; Nishiyama, N.; Koyama, H.; Miyazono, K.; Kataoka, K. Antiangiogenic gene therapy of experimental pancreatic tumor by sFlt-1 plasmid DNA carried by RGD-modified crosslinked polyplex micelles. J. Control. Release 2011, 149, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A. Uterine fibroids. Lancet 2001, 357, 293–298. [Google Scholar] [CrossRef]
- Walker, C.L. Uterine Fibroids: The Elephant in the Room. Science 2005, 308, 1589–1592. [Google Scholar] [CrossRef]
- Gingold, J.A.; Gueye, N.-A.; Falcone, T. Minimally Invasive Approaches to Myoma Management. J. Minim. Invasive Gynecol. 2018, 25, 237–250. [Google Scholar] [CrossRef]
- Dubuisson, J. The current place of mini-invasive surgery in uterine leiomyoma management. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 77–81. [Google Scholar] [CrossRef]
- Hasegawa, H.; Shimada, M.; Yonemitsu, Y.; Utsunomiya, T.; Gion, T.; Kaneda, Y.; Sugimachi, K. Preclinical and therapeutic utility of HVJ liposomes as a gene transfer vector for hepatocellular carcinoma using herpes simplex virus thymidine kinase. Cancer Gene Ther. 2001, 8, 252–258. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shved, N.; Egorova, A.; Osinovskaya, N.; Kiselev, A. Development of primary monolayer cell model and organotypic model of uterine leiomyoma. Methods Protoc. 2022, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Shtykalova, S.; Maretina, M.; Selutin, A.; Shved, N.; Deviatkin, D.; Selkov, S.; Baranov, V.; Kiselev, A. Polycondensed Peptide Carriers Modified with Cyclic RGD Ligand for Targeted Suicide Gene Delivery to Uterine Fibroid Cells. Int. J. Mol. Sci. 2022, 23, 1164. [Google Scholar] [CrossRef]
- Shtykalova, S.; Egorova, A.; Maretina, M.; Baranov, V.; Kiselev, A. Magnetic Nanoparticles as a Component of Peptide-Based DNA Delivery System for Suicide Gene Therapy of Uterine Leiomyoma. Bioengineering 2022, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Gustafsson, K.; Fabre, J.W. Tissue-binding properties of a synthetic peptide DNA vector targeted to cell membrane integrins: A possible universal nonviral vector for organ and tissue transplantation. Transplantation 2000, 69, 1041–1050. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar] [CrossRef]
- Kiselev, A.V.; Il’ina, P.L.; Egorova, A.A.; Baranov, A.N.; Guryanov, I.A.; Bayanova, N.V.; Tarasenko, I.I.; Lesina, E.A.; Vlasov, G.P.; Baranov, V.S. Lysine dendrimers as vectors for delivery of genetic constructs to eukaryotic cells. Russ. J. Genet. 2007, 43, 593–600. [Google Scholar] [CrossRef]
- Egorova, A.; Bogacheva, M.; Shubina, A.; Baranov, V.; Kiselev, A. Development of a receptor-targeted gene delivery system using CXCR4 ligand-conjugated cross-linking peptides. J. Gene Med. 2014, 16, 336–351. [Google Scholar] [CrossRef]




| Name | Composition (mol%) |
|---|---|
| RGD0-R6p-0,25 | RRRRRRRRRHHHH (25 mol%) + (-CHRRRRRRHC-)n (75 mol%) |
| RGD-R6p-0,25 | 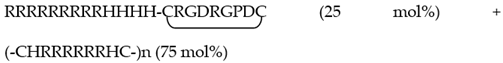 |
| RGD0-R6p-0,5 | RRRRRRRRRHHHH (50 mol%) + (-CHRRRRRRHC-)n (50 mol%) |
| RGD-R6p-0,5 |  |
| RGD0-R6p-0,75 | RRRRRRRRRHHHH (75 mol%) + (-CHRRRRRRHC-)n (25 mol%) |
| RGD-R6p-0,75 |  |
| Carrier | Size ± SD | Z-Potential ± SD |
|---|---|---|
| RGD-R6p-0,25 | 102.7 ± 2.81 | 14.9 ± 2.57 |
| RGD-R6p-0,5 | 165.6 ± 0.51 | 13.4 ± 0.61 |
| RGD-R6p-0,75 | 627.2 ± 18.36 | −0.1 ± 0.26 |
| RGD0-R6p-0,25 | 94.9 ± 2.50 | 13.9 ± 1.04 |
| RGD0-R6p-0,5 | 104.0 ± 4.61 | 13.6 ± 2.50 |
| RGD0-R6p-0,75 | 968.7 ± 45.64 | 5.4 ± 0.25 |
| Carrier | Carrier/DNA Charge Ratio |
|---|---|
| RGD-R6p-0,25 | 2/1 |
| RGD-R6p-0,5 | 2/1 |
| RGD-R6p-0,75 | 2/1 |
| RGD0-R6p-0,25 | 2/1 |
| RGD0-R6p-0,5 | 1.5/1 |
| RGD0-R6p-0,75 | 2/1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorova, A.; Selutin, A.; Maretina, M.; Selkov, S.; Kiselev, A. Peptide-Based Nanoparticles for αvβ3 Integrin-Targeted DNA Delivery to Cancer and Uterine Leiomyoma Cells. Molecules 2022, 27, 8363. https://doi.org/10.3390/molecules27238363
Egorova A, Selutin A, Maretina M, Selkov S, Kiselev A. Peptide-Based Nanoparticles for αvβ3 Integrin-Targeted DNA Delivery to Cancer and Uterine Leiomyoma Cells. Molecules. 2022; 27(23):8363. https://doi.org/10.3390/molecules27238363
Chicago/Turabian StyleEgorova, Anna, Alexander Selutin, Marianna Maretina, Sergei Selkov, and Anton Kiselev. 2022. "Peptide-Based Nanoparticles for αvβ3 Integrin-Targeted DNA Delivery to Cancer and Uterine Leiomyoma Cells" Molecules 27, no. 23: 8363. https://doi.org/10.3390/molecules27238363
APA StyleEgorova, A., Selutin, A., Maretina, M., Selkov, S., & Kiselev, A. (2022). Peptide-Based Nanoparticles for αvβ3 Integrin-Targeted DNA Delivery to Cancer and Uterine Leiomyoma Cells. Molecules, 27(23), 8363. https://doi.org/10.3390/molecules27238363







