High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent
Abstract
1. Introduction
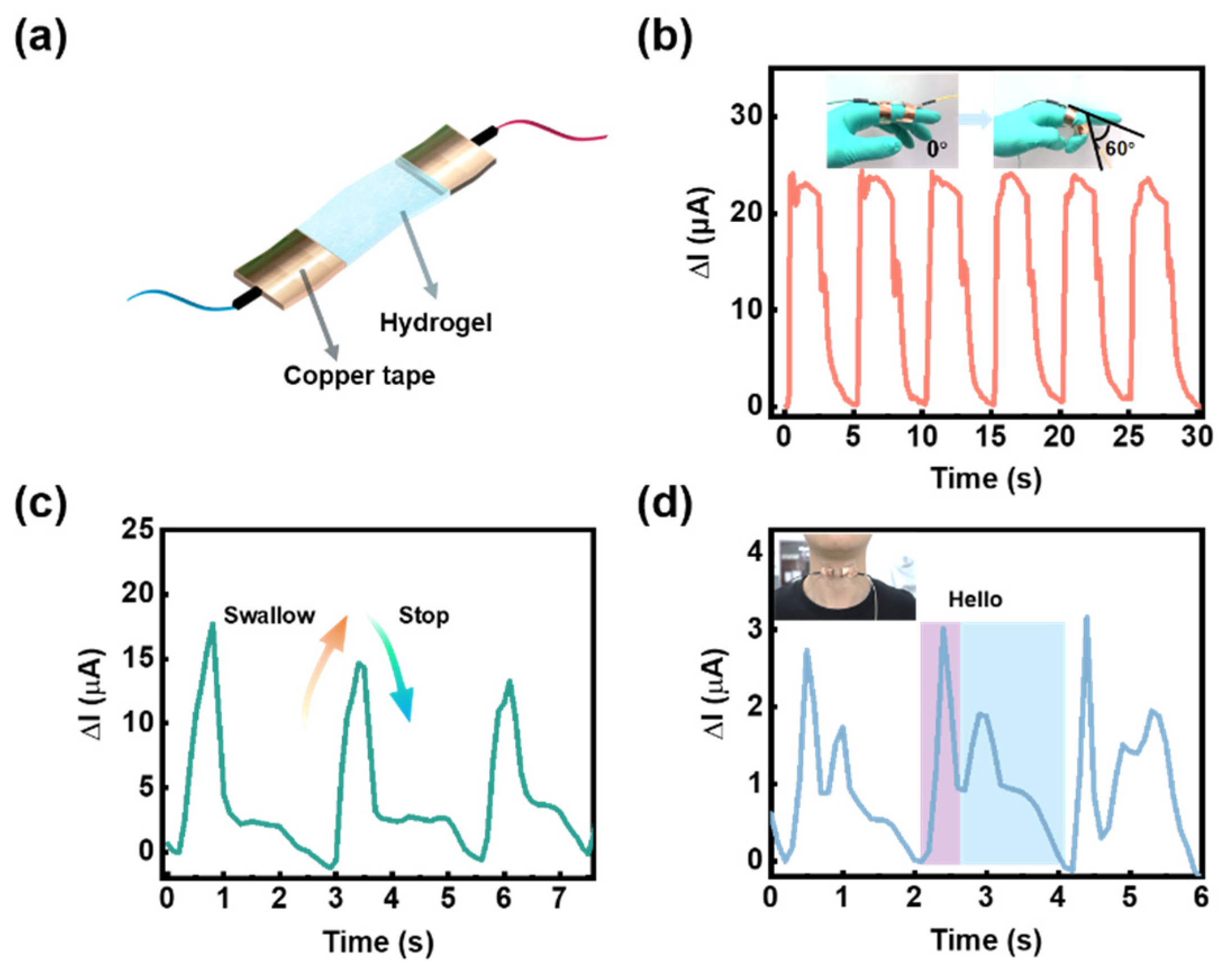
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Preparation of Hydrogels
3.2.1. Preparation of DES (ChCl/AA)
3.2.2. Preparation of PVA-DES Hydrogels
3.3. Construction of Strain Sensor and Its Integration as a Wearable Sensor
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Qiu, Z.; Wan, Y.; Zhou, W.; Yang, J.; Yang, J.; Huang, J.; Zhang, J.; Liu, Q.; Huang, S.; Bai, N.; et al. Ionic skin with biomimetic dielectric layer templated from calathea zebrine leaf. Adv. Funct. Mater. 2018, 28, 1802343. [Google Scholar] [CrossRef]
- Yue, O.; Wang, X.; Liu, X.; Hou, M.; Zheng, M.; Wang, Y.; Cui, B. Spider-web and ant-tentacle doubly bio-inspired multifunctional self-poweredelectronic skin with hierarchical nanostructure. Adv. Sci. 2021, 8, 2004377. [Google Scholar] [CrossRef]
- Qin, Z.; Chen, X.; Lv, Y.; Zhao, B.; Fang, X.; Pan, K. Wearable and high-performance piezoresistive sensor based on nanofiber/sodium alginate synergistically enhanced MXene composite aerogel. Chem. Eng. J. 2023, 451, 138586. [Google Scholar] [CrossRef]
- Zhai, Y.; Yu, Y.; Zhou, K.; Yun, Z.; Huang, W.; Liu, H.; Xia, Q.; Dai, K.; Zheng, G.; Liu, C.; et al. Flexible and wearable carbon black/thermoplastic polyurethane foam with a pinnate-veined aligned porous structure for multifunctional piezoresistive sensors. Chem. Eng. J. 2020, 382, 122985. [Google Scholar] [CrossRef]
- Wu, H.; Qi, H.; Wang, X.; Qiu, Y.; Shi, K.; Zhang, H.; Zhang, Z.; Zhang, W.; Tian, Y. Stretchable, sensitive, flexible strain sensor incorporated with patterned liquid metal on hydrogel for human motion monitoring and human–machine interaction. J. Mater. Chem. C 2022, 10, 8206–8217. [Google Scholar] [CrossRef]
- Wu, M.; Pan, M.; Qiao, C.; Ma, Y.; Yan, B.; Yang, W.; Peng, Q.; Han, L.; Zeng, H. Ultra stretchable, tough, elastic and transparent hydrogel skins integrated with intelligent sensing functions enabled by machine learning algorithms. Chem. Eng. J. 2022, 450, 138212. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, Q.; Mugo, S.M.; An, L.; Zhang, Q.; Lu, Y. Self-healing andshape-editable wearable supercapacitors based on highly stretchable hydrogel electrolytes. Adv. Sci. 2022, 9, 2201039. [Google Scholar] [CrossRef]
- Wang, D.; Yang, F.; Cong, L.; Feng, W.; Wang, C.; Chu, F.; Nan, J.; Chen, R. Lignin-containing hydrogel matrices with enhanced adhesion and toughness for all-hydrogel supercapacitors. Chem. Eng. J. 2022, 450, 138025. [Google Scholar] [CrossRef]
- Lee, J.; Tan, M.W.M.; Parida, K.; Thangavel, G.; Park, S.A.; Park, T.; Lee, P.S. Water-processable, stretchable, self-healable, thermally stable, and transparent ionic conductors for actuators and sensors. Adv. Mater. 2020, 32, 1906679. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Wang, S.; Wang, L.; Wu, M. Neuron-inspired multifunctional conductive hydrogels for flexible wearable sensors. J. Mater. Chem. C 2022, 10, 4327–4335. [Google Scholar] [CrossRef]
- Ge, G.; Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Muscle-inspired self-healing hydrogels for strain and temperature sensor. ACS Nano 2020, 14, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Honda, W.; Harada, S.; Arie, T.; Akita, S.; Takei, K. Wearable, human-interactive, health-monitoring, wireless devices fabricated by macroscale printing techniques. Adv. Funct. Mater. 2014, 24, 3299–3304. [Google Scholar] [CrossRef]
- Wang, R.; Chi, W.; Wan, F.; Wei, J.; Ping, H.; Zou, Z.; Xie, J.; Wang, W.; Fu, Z. Nanocage ferritin reinforced polyacrylamide hydrogel for wearable flexible strain sensors. ACS Appl. Mater. Interfaces 2022, 14, 21278–21286. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zhong, Q.; Hu, Q.; Wu, N.; Li, W.; Wang, B.; Hu, B.; Zhou, J. Stretchable self-powered fiber-based strain sensor. Adv. Funct. Mater. 2015, 25, 1798–1803. [Google Scholar] [CrossRef]
- Hines, L.; Petersen, K.H.; Lum, G.Z.; Sitti, M. Soft actuators for small-scale robotics. Adv. Mater. 2017, 29, 1603483. [Google Scholar] [CrossRef]
- Nojoomi, A.; Arslan, H.; Lee, K.; Yum, K. Bioinspired 3D structures with programmable morphologies and motions. Nat. Commun. 2018, 9, 3705. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Ma, Z.; Bai, Y.; Wang, J.; Zeng, P.; Gong, P.; Shi, F.; Ji, Z.; Qiao, Y.; et al. Highly sensitive strain sensor based on a stretchable and conductive poly(vinyl alcohol)/phytic acid/NH2-POSS hydrogel with a 3D microporous structure. ACS Appl. Mater. Interfaces 2020, 12, 26496–26508. [Google Scholar] [CrossRef]
- Roh, E.; Hwang, B.U.; Kim, D.; Kim, B.Y.; Lee, N.E. Stretchable, transparent, ultrasensitive, and patchable strain sensor for human–machine interfaces comprising a nanohybrid of carbon nanotubes and conductive elastomers. ACS Nano 2015, 9, 6252–6261. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Moon, H.C. Ionoskins: Nonvolatile, highly transparent, ultrastretchable ionic sensory platforms for wearable electronics. Adv. Funct. Mater. 2020, 30, 1907290. [Google Scholar] [CrossRef]
- Wei, H.; Kong, D.; Li, T.; Xue, Q.; Wang, S.; Cui, D.; Huang, Y.; Wang, L.; Hu, S.; Wan, T.; et al. Solution-processable conductive composite hydrogels with multiple synergetic networks toward wearable pressure/strain sensors. ACS Sens. 2021, 6, 2938–2951. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, M.; Moo, O.S.; Choi, S.M.; Zo, S.; Rao, K.M.; Han, S.S. Tissue-adhesive, stretchable, and self-healable hydrogels based on carboxymethyl cellulose-dopamine/PEDOT:PSS via mussel-inspired chemistry for bioelectronic applications. Chem. Eng. J. 2021, 426, 130847. [Google Scholar] [CrossRef]
- Wang, P.; Li, G.; Liu, J.; Hou, Z.; Meng, C.; Guo, S.; Liu, C.; Fan, S. Tailorable capacitive tactile sensor based on stretchable and dissolvable porous silver nanowire/polyvinyl alcohol nanocomposite hydrogel for wearable human motion detection. Adv. Mater. Interfaces 2021, 8, 2100998. [Google Scholar] [CrossRef]
- Fan, L.; Hu, L.; Xie, J.; He, Z.; Zheng, Y.; Wei, D.; Yao, D.; Su, F. Biosafe, self-adhesive, recyclable, tough, and conductive hydrogels for multifunctional sensors. Biomater. Sci. 2021, 9, 5884–5896. [Google Scholar] [CrossRef]
- Park, J.-E.; Kang, H.S.; Baek, J.; Park, T.H.; Oh, S.; Lee, H.; Koo, M.; Park, C. Rewritable, printable conducting liquid metal hydrogel. ACS Nano 2019, 13, 9122–9130. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, J.; Zhan, W.; Yuan, H.; Wu, L.; Sui, G.; Zhang, H. A multifunctional hydrogel fabricated via ultra-fast polymerization by graphene oxide-adsorbed liquid metal nanodroplets. Chem. Eng. J. 2022, 435, 135018. [Google Scholar] [CrossRef]
- Deng, Y.; Shang, T.; Wu, Z.; Tao, Y.; Luo, C.; Liang, J.; Han, D.; Lyu, R.; Qi, C.; Lv, W.; et al. Fast gelation of Ti3C2Tx MXene initiated by metal ions. Adv. Mater. 2019, 31, 1902432. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; El-Demellawi, J.K.; Jiang, Q.; Ge, G.; Liang, H.; Lee, K.; Dong, X.; Alshareef, H.N. MXene hydrogels: Fundamentals and applications. Chem. Soc. Rev. 2020, 49, 7229–7251. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, Y.; Ding, Q.; Mei, C.; Xu, X.; Wu, Q.; Xiao, H.; Han, J. Self-recovery, fatigue-resistant, and multifunctional sensor assembled by a nanocellulose/carbon nanotube nanocomplex-mediated hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 50281–50297. [Google Scholar] [CrossRef]
- Crowhurst, L.; Lancaster, N.L.; Arlandis, J.M.P.; Welton, T. Manipulating solute nucleophilicity with room temperature ionic liquids. J. Am. Chem. Soc. 2004, 126, 11549–11555. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zakeeruddin, S.M.; Exnar, I.; Grätzel, M. High efficiency dye-sensitized nanocrystalline solar cells based on ionic liquid polymer gel electrolyte. Chem. Commun. 2002, 24, 2972–2973. [Google Scholar] [CrossRef]
- Kamio, E.; Minakata, M.; Iida, Y.; Yasui, T.; Matsuoka, A.; Matsuyama, H. Inorganic/organic double-network ion gel membrane with a high ionic liquid content for CO2 separation. Polym. J. 2021, 53, 137–147. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, J.; Chang, L.; Zhang, X.; Liu, H.; Jiang, L. Preparation of high-performance ionogels with excellent transparency, good mechanical strength, and high conductivity. Adv. Mater. 2017, 29, 1704253. [Google Scholar] [CrossRef] [PubMed]
- Buzzeo, M.C.; Evans, R.G.; Compton, R.G. Non-haloaluminate room-temperature ionic liquids in electrochemistry—A review. ChemPhysChem 2004, 5, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Owyeung, R.E.; Sonkusale, S.R.; Panzer, M.J. Highly stretchable and nonvolatile gelatin-supported deep eutectic solvent gel electrolyte-based ionic skins for strain and pressure sensing. J. Mater. Chem. C 2019, 7, 601–608. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- del Monte, F.; Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L. Deep eutectic solvents in polymerizations: A greener alternative to conventional syntheses. ChemSusChem 2014, 7, 999–1009. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Weaver, K.D.; Kim, H.J.; Sun, J.; MacFarlane, D.R.; Elliott, G.D. Cyto-toxicity and biocompatibility of a family of choline phosphate ionic liquids designed for pharmaceutical applications. Green Chem. 2010, 12, 507–513. [Google Scholar] [CrossRef]
- Ilgen, F.; Ott, D.; Kralisch, D.; Reil, C.; Palmberger, A.; König, B. Conversion of carbohydrates into 5-hydroxymethylfurfural in highly concentrated low melting mixtures. Green Chem. 2009, 11, 1948–1954. [Google Scholar] [CrossRef]
- Wang, M.; Li, R.; Chen, G.; Zhou, S.; Feng, X.; Chen, Y.; He, M.; Liu, D.; Song, T.; Qi, H. Highly stretchable, transparent, and conductive wood fabricated by in situ photopolymerization with polymerizable deep eutectic solvents. ACS Appl. Mater. Interfaces 2019, 11, 14313–14321. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, X.; Xu, M.; Zhao, L.; Huang, D.; Wei, X.; Chen, W. Carbon nanotube reinforced polyvinyl alcohol/biphasic calcium phosphate scaffold for bone tissue engineering. RSC Advances 2019, 9, 38998–39010. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.H.A.; Saad, A.P.M.; Harun, M.N.; Syahrom, A.; Ramlee, M.H.; Sulong, M.A.; Kadir, M.R.A. Developing functionally graded PVA hydrogel using simple freeze-thaw method for artificial glenoid labrum. J. Mech. Behav. Biomed. Mater. 2019, 91, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Morton, L.M.; Phillips, T.J. Dressings for chronic wounds. Dermatol. Ther. 2013, 26, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Miano, F.; D’Antona, P.; Paradossi, G. Study of gelling behavior of poly(vinyl alcohol)-methacrylate for potential utilizations in tissue replacement and drug delivery. Biomacromolecules 2004, 5, 2439–2446. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. X-ray diffraction analysis of poly(vinyl alcohol) hydrogels, obtained by freezing and thawing techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Pathak, S.K.; Cui, J.; Jeon, I. Hydrogels with superior mechanical properties from the synergistic effect in hydrophobic–hydrophilic copolymers. Chem. Eng. J. 2019, 362, 325–338. [Google Scholar] [CrossRef]
- Tran, V.T.; Mredha, M.T.I.; Pathak, S.K.; Yoon, H.; Cui, J.; Jeon, I. Conductive Tough Hydrogels with a Staggered Ion-Coordinating Structure for High Self-Recovery Rate. ACS Appl. Mater. Interfaces 2019, 11, 24598–24608. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Nonoyama, T.; Wada, S.; Kiyama, R.; Kitamura, N.; Mredha, M.T.I.; Zhang, X.; Kurokawa, T.; Nakajima, T.; Takagi, Y.; Yasuda, K.; et al. Double-Network Hydrogels Strongly Bondable to Bones by Spontaneous Osteogenesis Penetration. Adv. Mater. 2016, 28, 6740–6745. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; Palmese, G.R. The role of crystallization and phase separation in the formation of physically cross-linked PVA hydrogels. Soft Matter 2013, 9, 826–833. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Gutiérrez, M.C.; Sanchez, I.C.; Luna-Bárcenas, G.; del Monte, F. Frontal polymerizations carried out in deep-eutectic mixtures providing both the monomers and the polymerization medium. Chem. Commun. 2011, 47, 5328–5330. [Google Scholar] [CrossRef]
- Li, R.; Chen, G.; He, M.; Tian, J.; Su, B. Patternable transparent and conductive elastomers towards flexible tactile/strain sensors. J. Mater. Chem. C 2017, 5, 8475–8481. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.; Cai, L.; Li, R.A.; He, M. Wearable transparent conductive fibers with harsh environment tolerance. ACS Appl. Mater. Interfaces 2021, 13, 8952–8959. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Li, X.; Liu, W.; Yu, X.; Yan, F.; Sun, J. Dynamic hydrophobic domains enable the fabrication of mechanically robust and highly elastic poly(vinyl alcohol)-based hydrogels with excellent self-healing ability. ACS Mater. Lett. 2020, 2, 764–770. [Google Scholar] [CrossRef]
- Lu, L.; Sun, H.; Peng, F.; Jiang, Z. Novel graphite-filled PVA/CS hybrid membrane for pervaporation of benzene/cyclohexane mixtures. J. Membr. Sci. 2006, 281, 245–252. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martínez, G.; Gómez, M.A. Synthesis of poly(vinyl alcohol)/reduced graphite oxide nanocomposites with improved thermal and electrical properties. J. Mater. Chem. 2009, 19, 5027–5032. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Li, B.; Zhang, P.; Kan, L.; Wang, G.; Wei, H.; Zhang, X.; Ma, N. One-step preparation of a highly stretchable, conductive, and transparent poly(vinyl alcohol)–phytic acid hydrogel for casual writing circuits. ACS Appl. Mater. Interfaces 2019, 11, 32441–32448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-N.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly(vinyl alcohol)–Tannic Acid Hydrogels with Excellent Mechanical Properties and Shape Memory Behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, X.; Chen, Y.-N.; Bai, Q.-W.; Shang, C.; Zhang, L.; Wang, H. Hydrogen-Bonded Polymer-Small Molecule Complexes with Tunable Mechanical Properties. Macromol. Rapid Commun. 2018, 39, 1800050. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Wan, P.; Wen, J.; Gong, M.; Wu, X.; Wang, Y.; Shi, R.; Zhang, L. Wearable, healable, and adhesive epidermal sensors assembled from mussel-inspired conductive hybrid hydrogel framework. Adv. Funct. Mater. 2017, 27, 1703852. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Bioinspired double network hydrogels: From covalent double network hydrogels via hybrid double network hydrogels to physical double network hydrogels. Mater. Horiz. 2021, 8, 1173–1188. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, B.; Mao, Z.; Sui, X.; Feng, X. Nonvolatile, stretchable and adhesive ionogel fiber sensor designed for extreme environments. Chem. Eng. J. 2022, 433, 133500. [Google Scholar] [CrossRef]
- Huang, H.; Han, L.; Li, J.; Fu, X.; Wang, Y.; Yang, Z.; Xu, X.; Pan, L.; Xu, M. Super-stretchable, elastic and recoverable ionic conductive hydrogel for wireless wearable, stretchable sensor. J. Mater. Chem. A 2020, 8, 10291–10300. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, S.; Qian, L.; Wei, N.; Nica, V.; Coseri, S.; Han, F. Super stretchable, self-healing, adhesive ionic conductive hydrogels based on tailor-made ionic liquid for high-performance strain sensors. Adv. Funct. Mater. 2022, 32, 2204565. [Google Scholar] [CrossRef]
- Xu, J.; Wang, G.; Wu, Y.; Ren, X.; Gao, G. Ultrastretchable wearable strain and pressure sensors based on adhesive, tough, and self-healing hydrogels for human motion monitoring. ACS Appl. Mater. Interfaces 2019, 11, 25613–25623. [Google Scholar] [CrossRef]
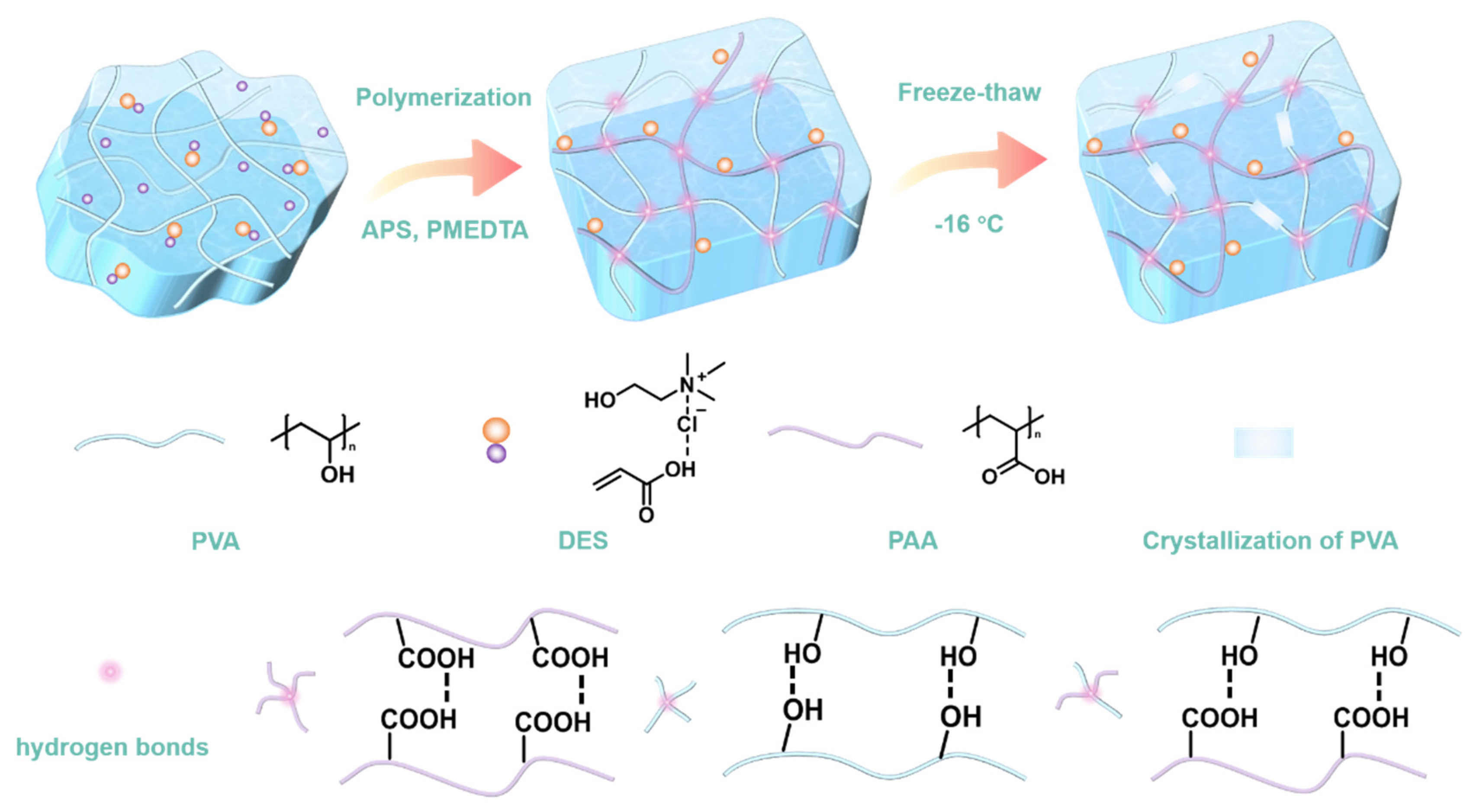
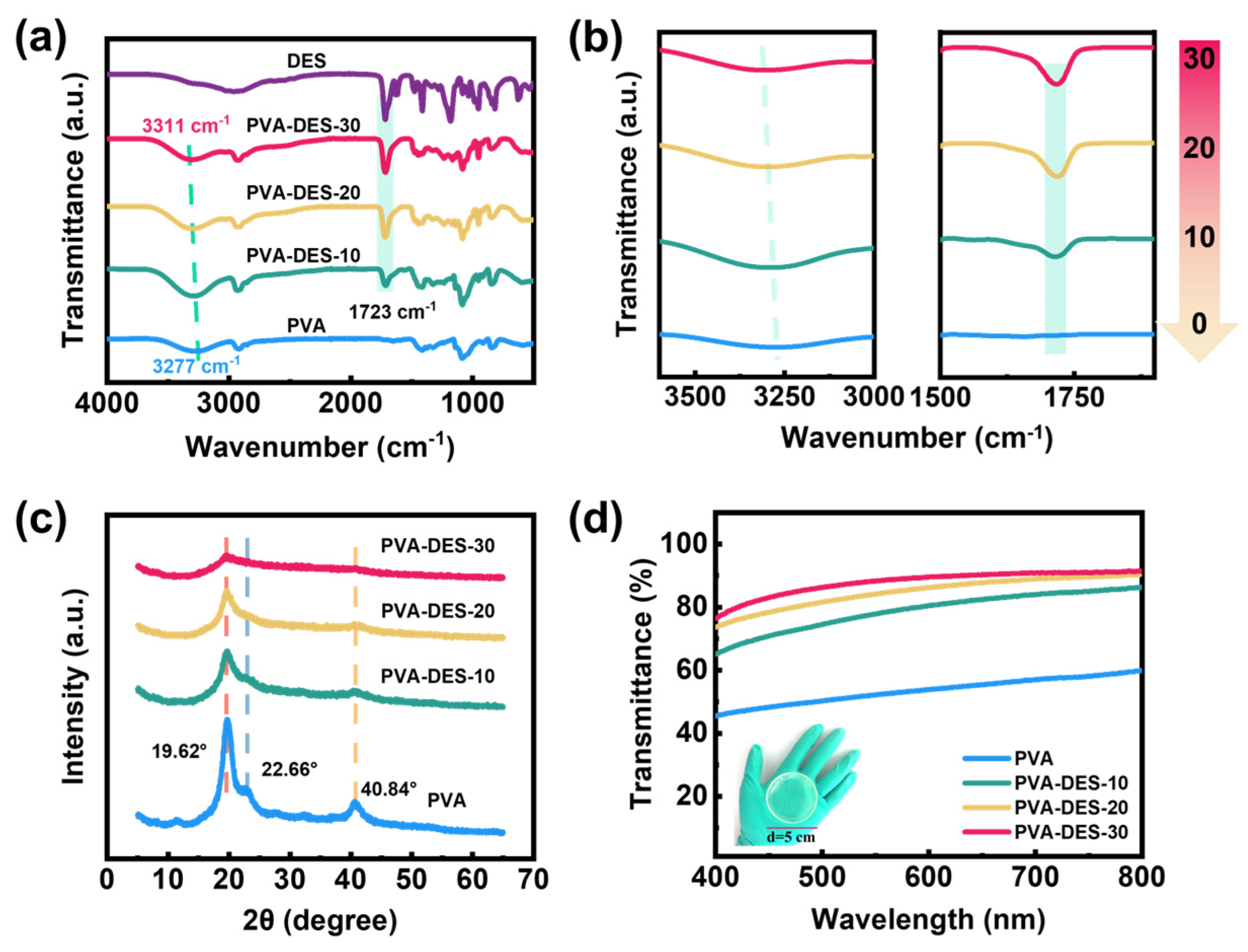
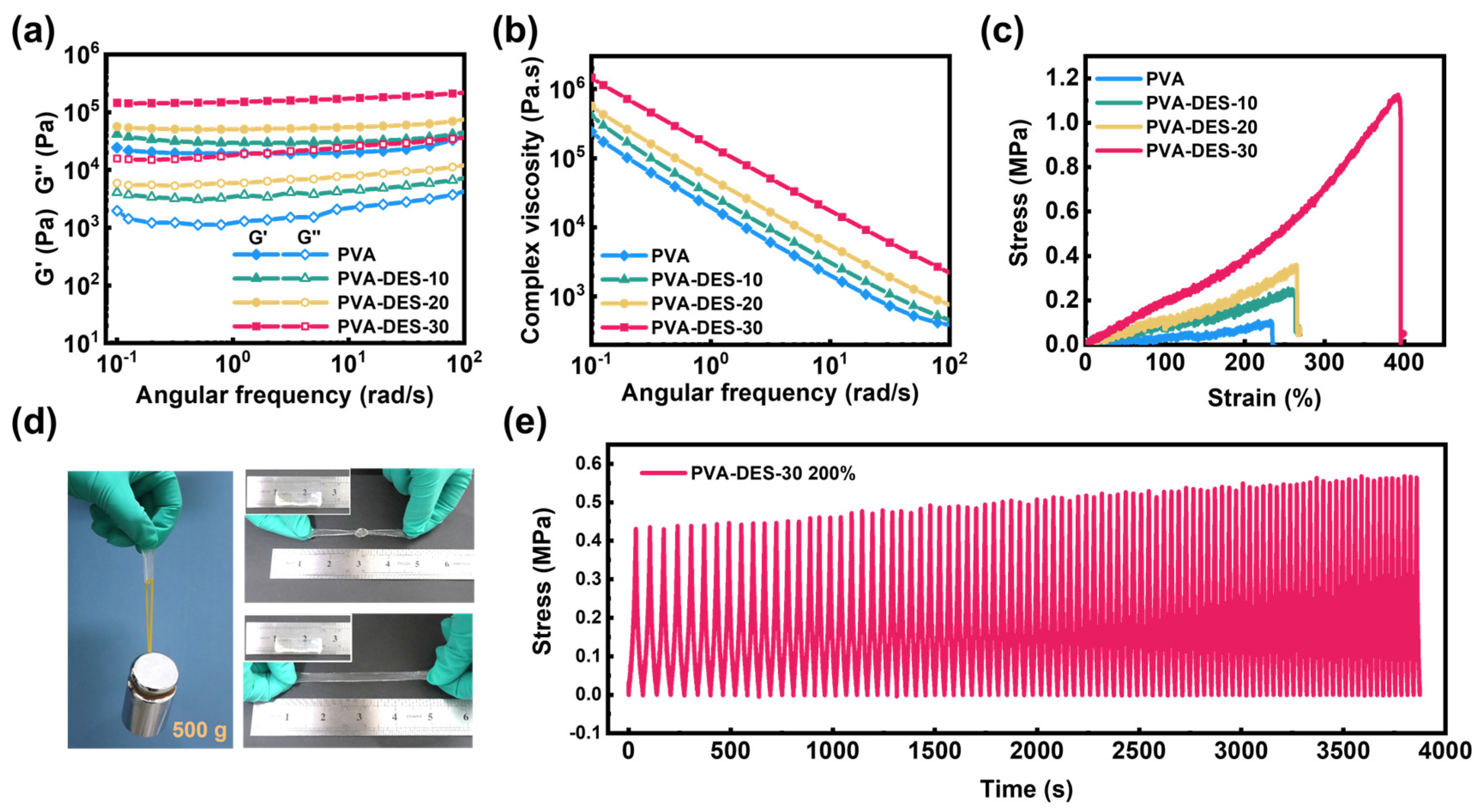
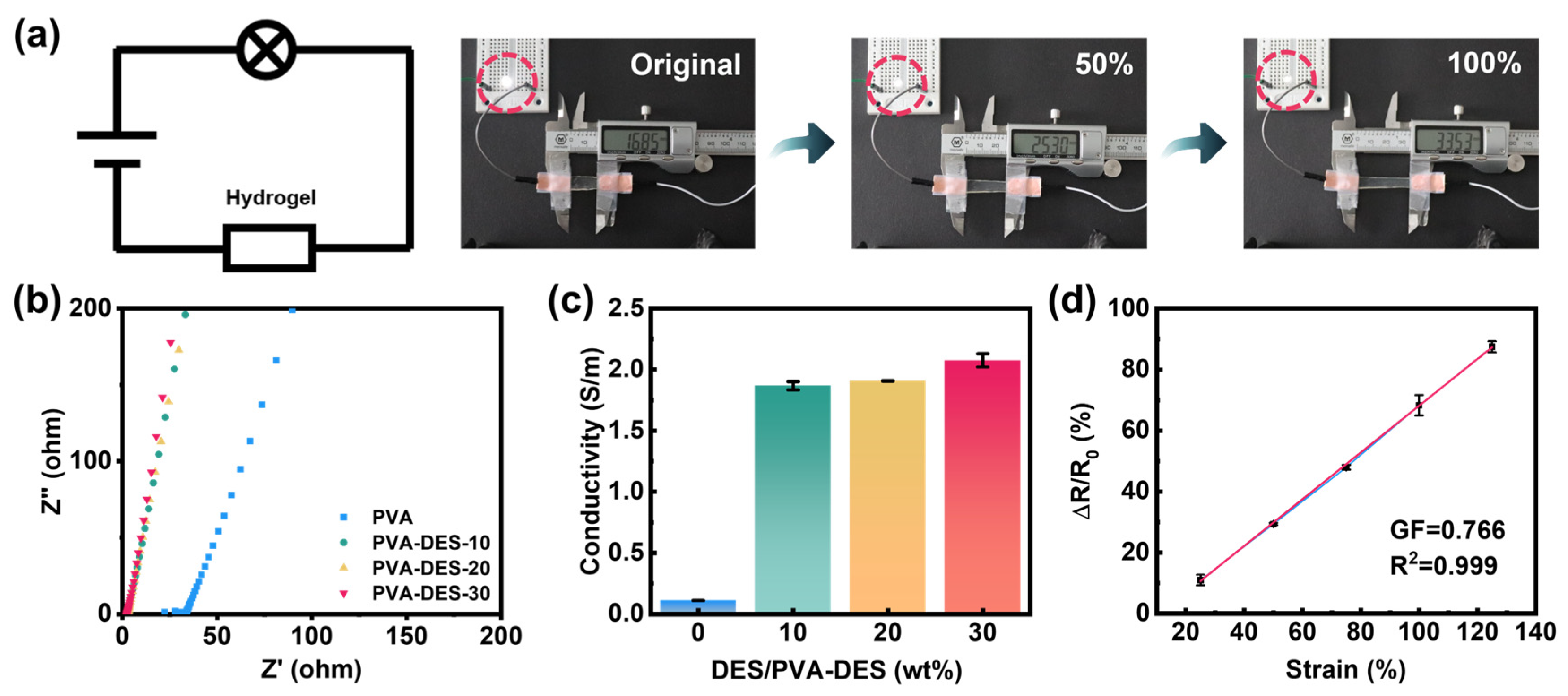
| Hydrogels | PVA (g) | DI (g) | DES (g) | APS (g) | PMDETA (µL) | Content of DI (wt%) |
|---|---|---|---|---|---|---|
| PVA | 1.5 | 10.5 | 0 | 0 | 0 | 87.5 |
| PVA-DES-10 | 1.5 | 10.5 | 1.33 | 0.014 | 5.6 | 78.7 |
| PVA-DES-20 | 1.5 | 10.5 | 3.00 | 0.031 | 12.4 | 69.8 |
| PVA-DES-30 | 1.5 | 10.5 | 5.14 | 0.052 | 21.2 | 61.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jiang, L.; Zhang, H.; Li, Q.; Ma, N.; Zhang, X.; Ma, L. High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent. Molecules 2023, 28, 4690. https://doi.org/10.3390/molecules28124690
Zhang Y, Jiang L, Zhang H, Li Q, Ma N, Zhang X, Ma L. High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent. Molecules. 2023; 28(12):4690. https://doi.org/10.3390/molecules28124690
Chicago/Turabian StyleZhang, Yihan, Lei Jiang, Haibing Zhang, Qingyin Li, Ning Ma, Xinyue Zhang, and Li Ma. 2023. "High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent" Molecules 28, no. 12: 4690. https://doi.org/10.3390/molecules28124690
APA StyleZhang, Y., Jiang, L., Zhang, H., Li, Q., Ma, N., Zhang, X., & Ma, L. (2023). High-Strength Double-Network Conductive Hydrogels Based on Polyvinyl Alcohol and Polymerizable Deep Eutectic Solvent. Molecules, 28(12), 4690. https://doi.org/10.3390/molecules28124690







