Improving the Cellulose Enzymatic Digestibility of Sugarcane Bagasse by Atmospheric Acetic Acid Pretreatment and Peracetic Acid Post-Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of AA Pretreatment on Chemical Compositions
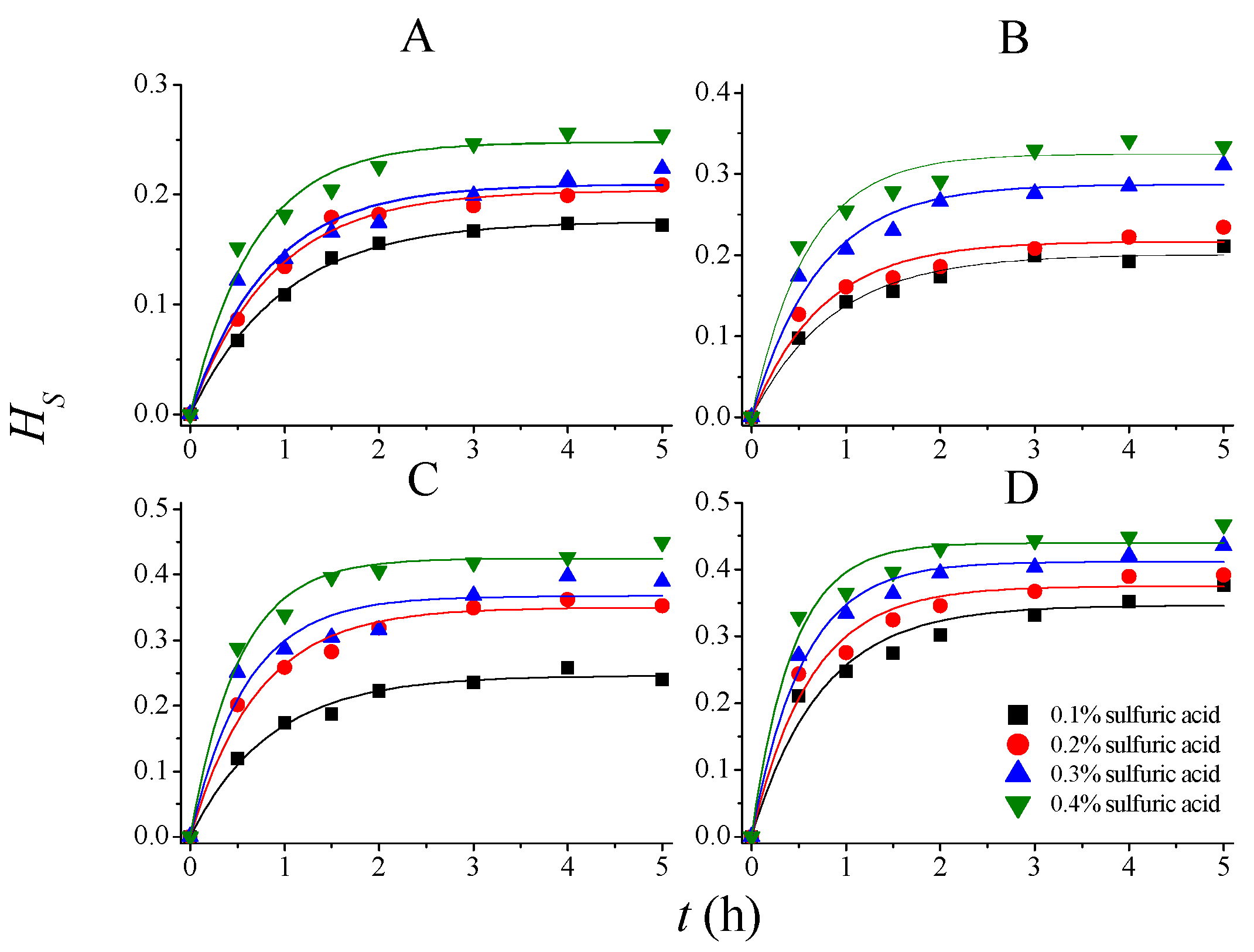
2.2. Kinetics of Delignification and Solubilization of Holocellulose
2.2.1. Saeman’s Model
2.2.2. The “Potential Degree of Reaction (PDR)” Model
2.3. Effects of AA Pretreatment on the Enzymatic Hydrolysis of Pretreated Solid
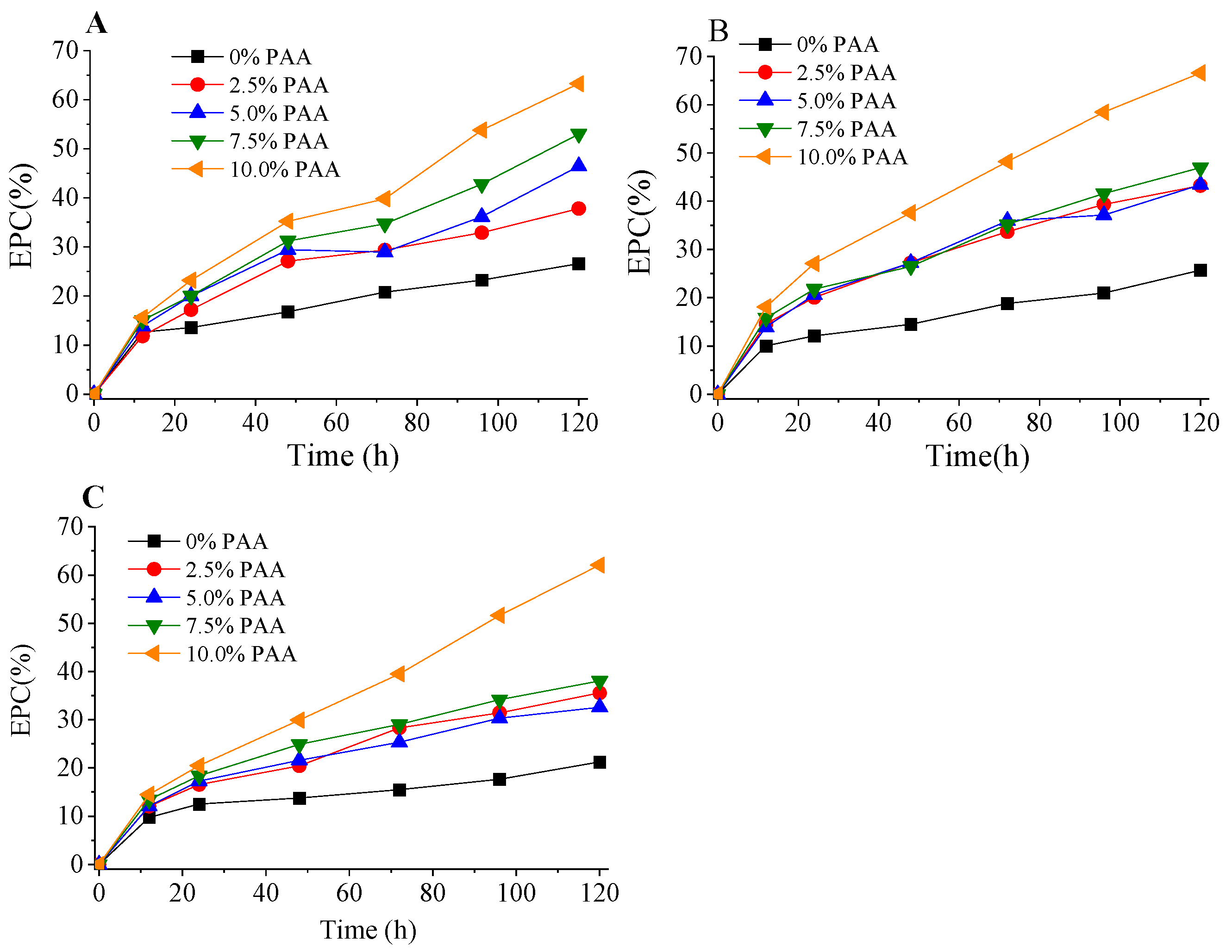
2.4. Effect of PAA Post-Treatment on Cellulose Hydrolysis
3. Materials and Methods
3.1. Materials
3.2. AA Pretreatment and PAA Post-Treatment
3.3. Enzymatic Hydrolysis of Pretrereated and Post-Treated Substrates
3.4. Experimental Design
3.5. Analytic Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Reena, R.; Alphy, M.P.; Reshmy, R.; Thomas, D.; Madhavan, A.; Chaturvedi, P.; Pugazhendhi, A.; Awasthi, M.K.; Ruiz, H.; Kumar, V.; et al. Sustainable Valorization of Sugarcane Residues: Efficient Deconstruction Strategies for Fuels and Chemicals Production. Bioresour. Technol. 2022, 361, 127759. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Yusaf, T.; Hamza, N.H.; Wandel, A.P.; Fattah, I.M.R.; Laimon, M.; Rahman, S.M.A. Sugarcane Biomass as a Source of Biofuel for Internal Combustion Engines (Ethanol and Acetone-Butanol-Ethanol): A Review of Economic Challenges. Energies 2022, 15, 8644. [Google Scholar] [CrossRef]
- Shabbirahmed, A.M.; Haldar, D.; Dey, P.; Patel, A.K.; Singhania, R.R.; Dong, C.-D.; Purkait, M.K. Sugarcane Bagasse into Value-Added Products: A Review. Environ. Sci. Pollut. Res. 2022, 29, 62785–62806. [Google Scholar] [CrossRef]
- Alokika; Anu; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and Hemicellulosic Fractions of Sugarcane Bagasse: Potential, Challenges and Future Perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different Pretreatment Technologies of Lignocellulosic Biomass for Bioethanol Production: An Overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Marques, P.F.; Soares, A.K.L.; Lomonaco, D.; Alexandre e Silva, L.M.; Santaella, S.T.; de Freitas Rosa, M.; Carrhá Leitão, R. Steam Explosion Pretreatment Improves Acetic Acid Organosolv Delignification of Oil Palm Mesocarp Fibers and Sugarcane Bagasse. Int. J. Biol. Macromol. 2021, 175, 304–312. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Tang, B.; Zeng, M.; Liang, Z.; Jiang, C.; Lin, J.; Xiao, W.; Liu, Z. Enhanced Saccharification of Sugarcane Bagasse by the Optimization of Low Concentration of Naoh and Ammonia Pretreatment. Ind. Crops Prod. 2021, 172, 114016. [Google Scholar] [CrossRef]
- Hashmi, M.; Sun, Q.; Tao, J.; Wells, T.; Shah, A.A.; Labbé, N.; Ragauskas, A.J. Comparison of Autohydrolysis and Ionic Liquid 1-Butyl-3methylimidazolium Acetate Pretreatment to Enhance Enzymatic Hydrolysis of Sugarcane Bagasse. Bioresour. Technol. 2017, 224, 714–720. [Google Scholar] [CrossRef]
- Pin, T.C.; Nakasu, P.Y.S.; Mattedi, S.; Rabelo, S.C.; Costa, A.C. Screening of Protic Ionic Liquids for Sugarcane Bagasse Pretreatment. Fuel 2019, 235, 1506–1514. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Xie, J.; Qin, Y. Effects of Naoh-Catalyzed Organosolv Pretreatment and Surfactant on the Sugar Production from Sugarcane Bagasse. Bioresour. Technol. 2020, 312, 123601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fan, M.; Li, X.; Zhang, A.; Xie, J. Enhancing Enzymatic Hydrolysis of Sugarcane Bagasse by Ferric Chloride Catalyzed Organosolv Pretreatment and Tween 80. Bioresour. Technol. 2018, 258, 295–301. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv Pretreatment of Lignocellulosic Biomass for Enzymatic Hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Savou, V.; Kumagai, S.; Saito, Y.; Kameda, T.; Yoshioka, T. Effects of Acetic Acid Pretreatment and Pyrolysis Temperatures on Product Recovery from Fijian Sugarcane Bagasse. Waste Biomass Valorization 2019, 11, 6347–6357. [Google Scholar] [CrossRef]
- Pan, X.-J.; Sano, Y. Atmospheric Acetic Acid Pulping of Rice Straw Iv: Physico-Chemical Characterization of Acetic Acid Lignins from Rice Straw and Woods. Part 1. Physical Characteristics. Holzforschung 1999, 53, 511–518. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D. Kinetic Modeling and Mechanisms of Acid-Catalyzed Delignification of Sugarcane Bagasse by Aqueous Acetic Acid. Bioenergy Res. 2013, 6, 436–447. [Google Scholar] [CrossRef]
- Muurinen, E. Organosolv Pulping: A Review and Distillation Study Related to Peroxyacid Pulping. Ph.D. Thesis, University of Oulu, Oulu, Finland, 2000. [Google Scholar]
- Zhang, K.; Pei, Z.; Wang, D. Organic Solvent Pretreatment of Lignocellulosic Biomass for Biofuels and Biochemicals: A Review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Gilkes, N.; Saddler, J.N. Effect of Acetyl Groups on Enzymatic Hydrolysis of Cellulosic Substrates. Holzforschung 2006, 60, 398–401. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D. Fractionating Pretreatment of Sugarcane Bagasse for Increasing the Enzymatic Digestibility of Cellulose. Chin. J. Biotechnol. 2011, 27, 384–392. [Google Scholar]
- Zhou, Z.; Ouyang, D.; Liu, D.; Zhao, X. Oxidative Pretreatment of Lignocellulosic Biomass for Enzymatic Hydrolysis: Progress and Challenges. Bioresour. Technol. 2023, 367, 128208. [Google Scholar] [CrossRef] [PubMed]
- Saeman, J.F. Kinetics of Wood Saccharification—Hydrolysis of Cellulose and Decomposition of Sugars in Dilute Acid at High Temperature. Ind. Eng. Chem. 1945, 37, 43–52. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Liu, D. Kinetic Model for Glycan Hydrolysis and Formation of Monosaccharides During Dilute Acid Hydrolysis of Sugarcane Bagasse. Bioresour. Technol. 2012, 105, 160–168. [Google Scholar] [CrossRef]
- Zhao, X.; Morikawa, Y.; Qi, F.; Zeng, J.; Liu, D. A Novel Kinetic Model for Polysaccharide Dissolution During Atmospheric Acetic Acid Pretreatment of Sugarcane Bagasse. Bioresour. Technol. 2014, 151, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhao, X.B.; Liu, D. Kinetic Modeling of Atmospheric Formic Acid Pretreatment of Wheat Straw with “Potential Degree of Reaction” Models. Rsc. Adv. 2015, 5, 20992–21000. [Google Scholar] [CrossRef]
- Mcdonough, T.J. The Chemistry of Organosolv Delignification. Tappi J. 1994, 76, 186–193. [Google Scholar]
- Vazquez, G.; Antorrena, G.; Gonzalez, J. Kinetics of Acid-Catalyzed Delignification of Eucalyptus-Globulus Wood by Acetic-Acid. Wood Sci. Technol. 1995, 29, 267–275. [Google Scholar] [CrossRef]
- Vázquez, G.; Antorrena, G.; González, J.; Freire, S.; López, S. Acetosolv Pulping of Pine Wood. Kinetic Modelling of Lignin Solubilization and Condensation. Bioresour. Technol. 1997, 59, 121–127. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D. Fractionating Pretreatment of Sugarcane Bagasse by Aqueous Formic Acid with Direct Recycle of Spent Liquor to Increase Cellulose Digestibility-the Formiline Process. Bioresour. Technol. 2012, 117, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Van der Heide, E.; Zhang, T.; Liu, D. Single-Stage Pulping of Sugarcane Bagasse with Peracetic Acid. J. Wood Chem. Technol. 2011, 31, 1–25. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, H.; Liu, N.; Zhang, J.; Zhao, X. Insight into the Negative Effects of Lignin on Enzymatic Hydrolysis of Cellulose for Biofuel Production Via Selective Oxidative Delignification and Inhibitive Actions of Phenolic Model Compounds. Renew. Energy 2022, 185, 196–207. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, T.; Zhou, Y.; Liu, D. Preparation of peracetic acid from hydrogen peroxide: Part I: Kinetics for peracetic acid synthesis and hydrolysis. J. Mol. Catal. A Chem. 2007, 271, 246–252. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Hao, J.; Liu, D. Preparation of Peracetic Acid from Hydrogen Peroxide, Part II: Kinetics for Spontaneous Decomposition of Peracetic Acid in the Liquid Phase. J. Mol. Catal. A Chem. 2008, 284, 58–68. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, T.; Zhou, Y.; Liu, D. Preparation of Peracetic Acid from Acetic Acid and Hydrogen Peroxide: Experimentation and Modeling. Chin. J. Process Eng. 2008, 1, 35–41. [Google Scholar]
- Shi, S.; He, F. Analysis and Detection in Pulping and Papermaking; China Light Industry Press: Beijing, China, 2003. [Google Scholar]
- Ghose, T.K. Measurement of Cellulase Activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
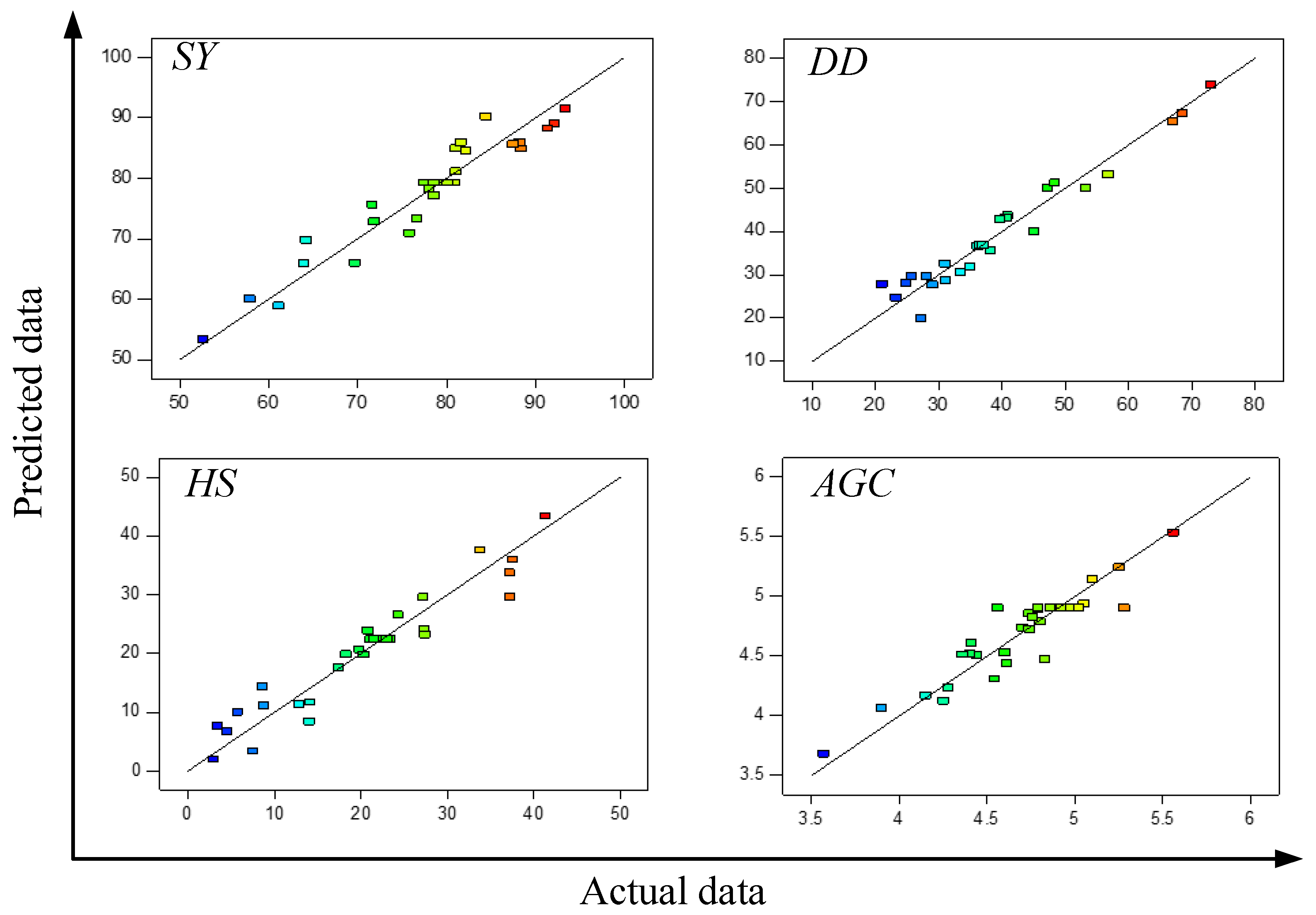
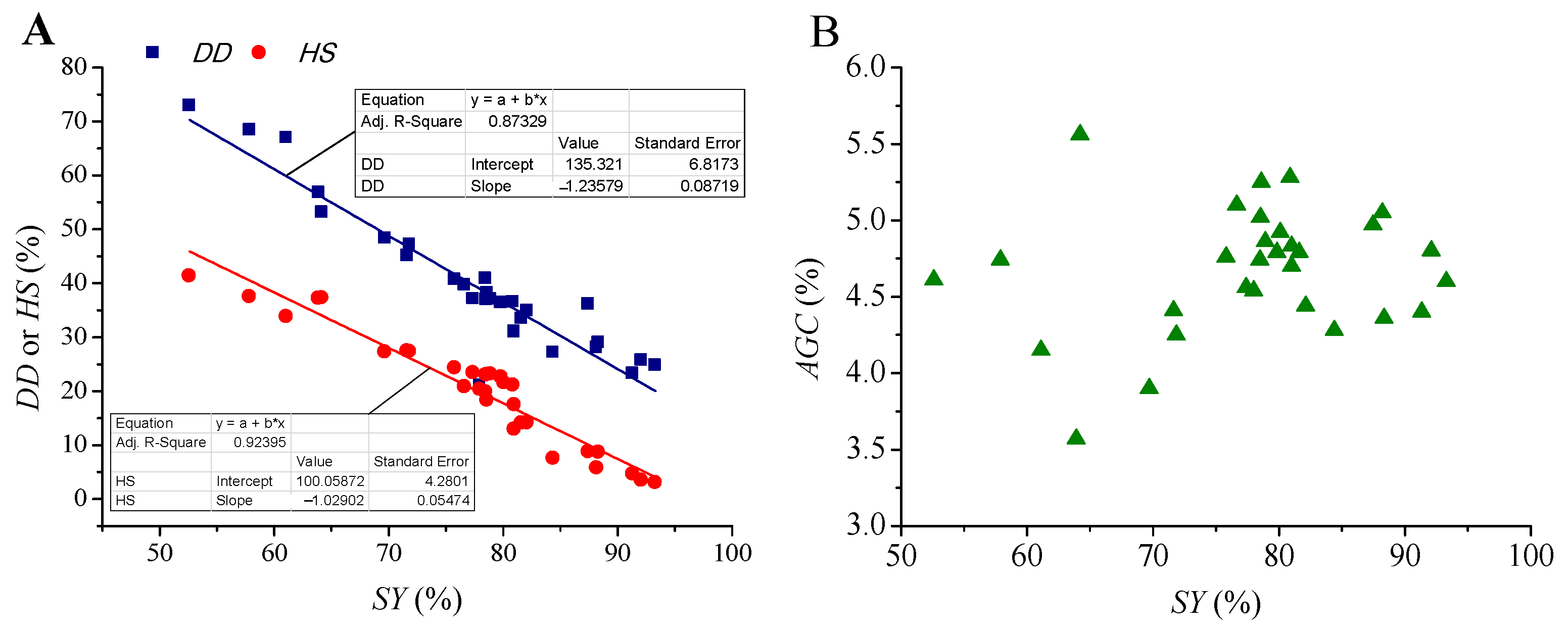

| Run | Variables | SY (%) | DD (%) | HS (%) | AGC (%) | EPC (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | Exp. | |
| 1 | −1 | −1 | −1 | −1 | 93.30 | 91.40 | 24.77 | 28.11 | 3.01 | 2.01 | 4.60 | 4.53 | 7.08 |
| 2 | 1 | −1 | −1 | −1 | 82.13 | 84.46 | 34.88 | 31.81 | 14.10 | 11.71 | 4.44 | 4.52 | 14.43 |
| 3 | −1 | 1 | −1 | −1 | 92.10 | 89.06 | 25.70 | 29.51 | 3.44 | 7.61 | 4.80 | 4.78 | 6.76 |
| 4 | 1 | 1 | −1 | −1 | 78.50 | 77.12 | 40.88 | 43.61 | 19.82 | 20.71 | 4.74 | 4.86 | 18.34 |
| 5 | −1 | −1 | 1 | −1 | 91.35 | 88.28 | 23.23 | 24.73 | 4.57 | 6.75 | 4.40 | 4.52 | 8.95 |
| 6 | 1 | −1 | 1 | −1 | 78.00 | 78.26 | 21.02 | 27.87 | 20.31 | 19.81 | 4.54 | 4.32 | 21.44 |
| 7 | −1 | 1 | 1 | −1 | 88.20 | 85.98 | 28.06 | 29.61 | 5.75 | 10.07 | 5.05 | 4.93 | 9.05 |
| 8 | 1 | 1 | 1 | −1 | 75.80 | 70.96 | 40.65 | 43.15 | 24.29 | 26.53 | 4.76 | 4.83 | 21.16 |
| 9 | −1 | −1 | −1 | 1 | 81.00 | 84.90 | 31.04 | 28.73 | 12.89 | 11.33 | 4.70 | 4.75 | 10.73 |
| 10 | 1 | −1 | −1 | 1 | 71.85 | 72.84 | 47.17 | 49.87 | 27.34 | 24.07 | 4.25 | 4.13 | 23.43 |
| 11 | −1 | 1 | −1 | 1 | 78.60 | 77.12 | 38.17 | 35.61 | 18.28 | 19.85 | 5.25 | 5.24 | 17.07 |
| 12 | 1 | 1 | −1 | 1 | 57.88 | 60.06 | 68.43 | 67.15 | 37.48 | 35.99 | 4.74 | 4.72 | 23.36 |
| 13 | −1 | −1 | 1 | 1 | 81.00 | 81.14 | 30.99 | 32.55 | 17.45 | 17.63 | 4.83 | 4.49 | 14.72 |
| 14 | 1 | −1 | 1 | 1 | 63.90 | 66.00 | 56.75 | 53.13 | 37.18 | 33.73 | 3.57 | 3.69 | 22.47 |
| 15 | −1 | 1 | 1 | 1 | 76.65 | 73.40 | 39.62 | 42.91 | 20.80 | 23.87 | 5.10 | 5.14 | 16.8 |
| 16 | 1 | 1 | 1 | 1 | 52.60 | 53.26 | 72.96 | 73.89 | 41.31 | 43.37 | 4.61 | 4.44 | 21.68 |
| 17 | −2 | 0 | 0 | 0 | 81.60 | 85.98 | 33.45 | 30.60 | 14.03 | 8.41 | 4.79 | 4.90 | 9.95 |
| 18 | 2 | 0 | 0 | 0 | 61.10 | 58.90 | 66.95 | 65.28 | 33.76 | 37.61 | 4.15 | 4.18 | 21.31 |
| 19 | 0 | −2 | 0 | 0 | 88.35 | 84.90 | 28.99 | 27.74 | 8.65 | 14.43 | 4.36 | 4.53 | 10.87 |
| 20 | 0 | 2 | 0 | 0 | 64.20 | 69.82 | 53.14 | 49.90 | 37.23 | 29.67 | 5.56 | 5.53 | 20.67 |
| 21 | 0 | 0 | −2 | 0 | 87.48 | 85.60 | 36.14 | 36.66 | 8.74 | 11.15 | 4.97 | 4.91 | 14.33 |
| 22 | 0 | 0 | 2 | 0 | 71.64 | 75.68 | 45.06 | 40.02 | 27.41 | 23.27 | 4.41 | 4.61 | 22.00 |
| 23 | 0 | 0 | 0 | −2 | 84.40 | 90.22 | 27.14 | 19.78 | 7.51 | 3.41 | 4.28 | 4.24 | 12.10 |
| 24 | 0 | 0 | 0 | 2 | 69.70 | 66.02 | 48.30 | 51.14 | 27.21 | 29.57 | 3.90 | 4.06 | 21.44 |
| 25 | 0 | 0 | 0 | 0 | 77.40 | 79.28 | 37.09 | 36.74 | 23.36 | 22.61 | 4.56 | 4.91 | 17.89 |
| 26 | 0 | 0 | 0 | 0 | 80.88 | 79.28 | 36.55 | 36.74 | 21.09 | 22.61 | 5.28 | 4.91 | 20.43 |
| 27 | 0 | 0 | 0 | 0 | 79.85 | 79.28 | 36.40 | 36.74 | 22.56 | 22.61 | 4.79 | 4.91 | 21.05 |
| 28 | 0 | 0 | 0 | 0 | 78.92 | 79.28 | 37.01 | 36.74 | 23.12 | 22.61 | 4.86 | 4.91 | 18.49 |
| 29 | 0 | 0 | 0 | 0 | 80.10 | 79.28 | 36.42 | 36.74 | 21.55 | 22.61 | 4.92 | 4.91 | 17.01 |
| 30 | 0 | 0 | 0 | 0 | 78.54 | 79.28 | 36.98 | 36.74 | 22.98 | 22.61 | 5.02 | 4.91 | 20.51 |
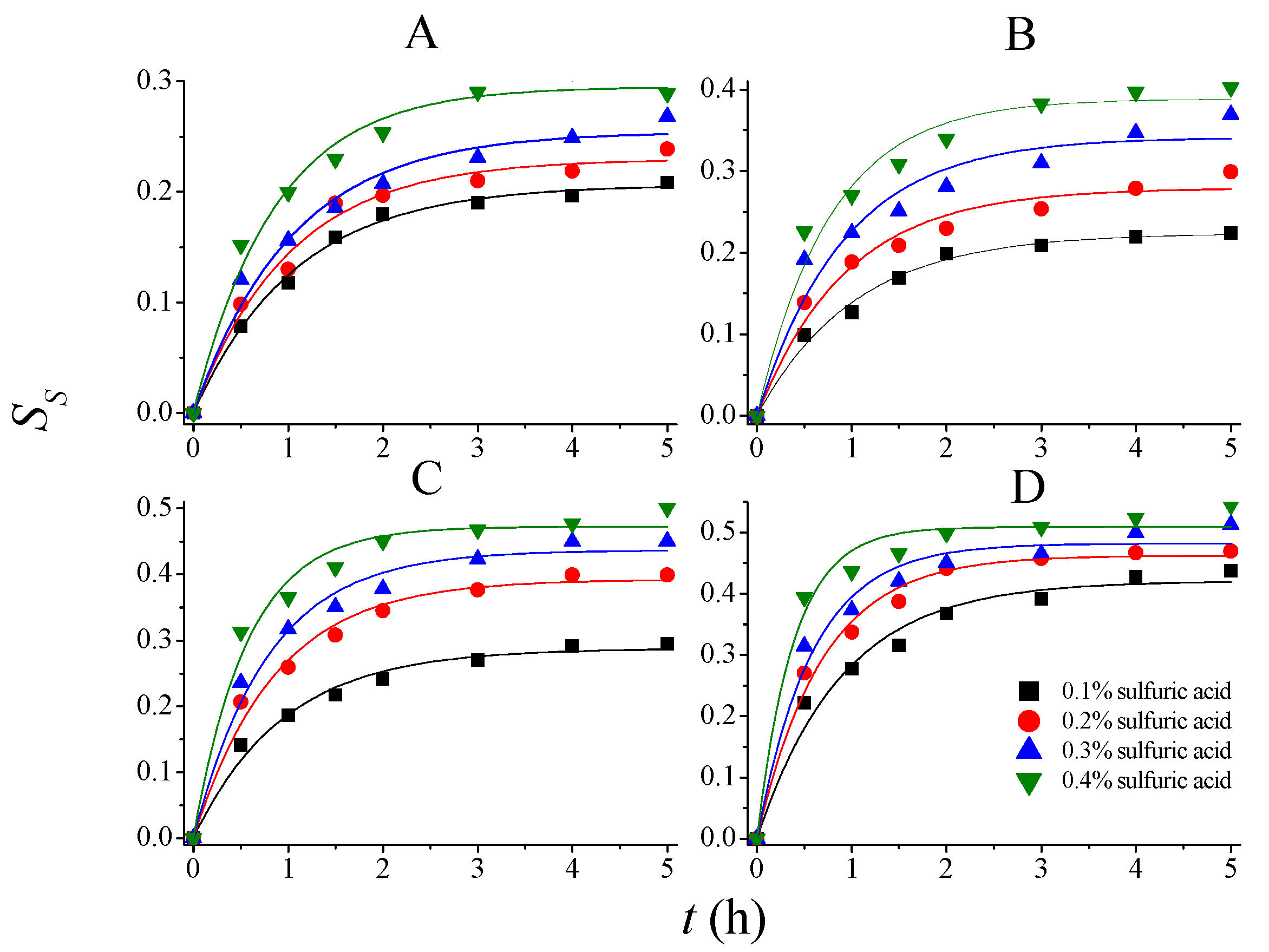
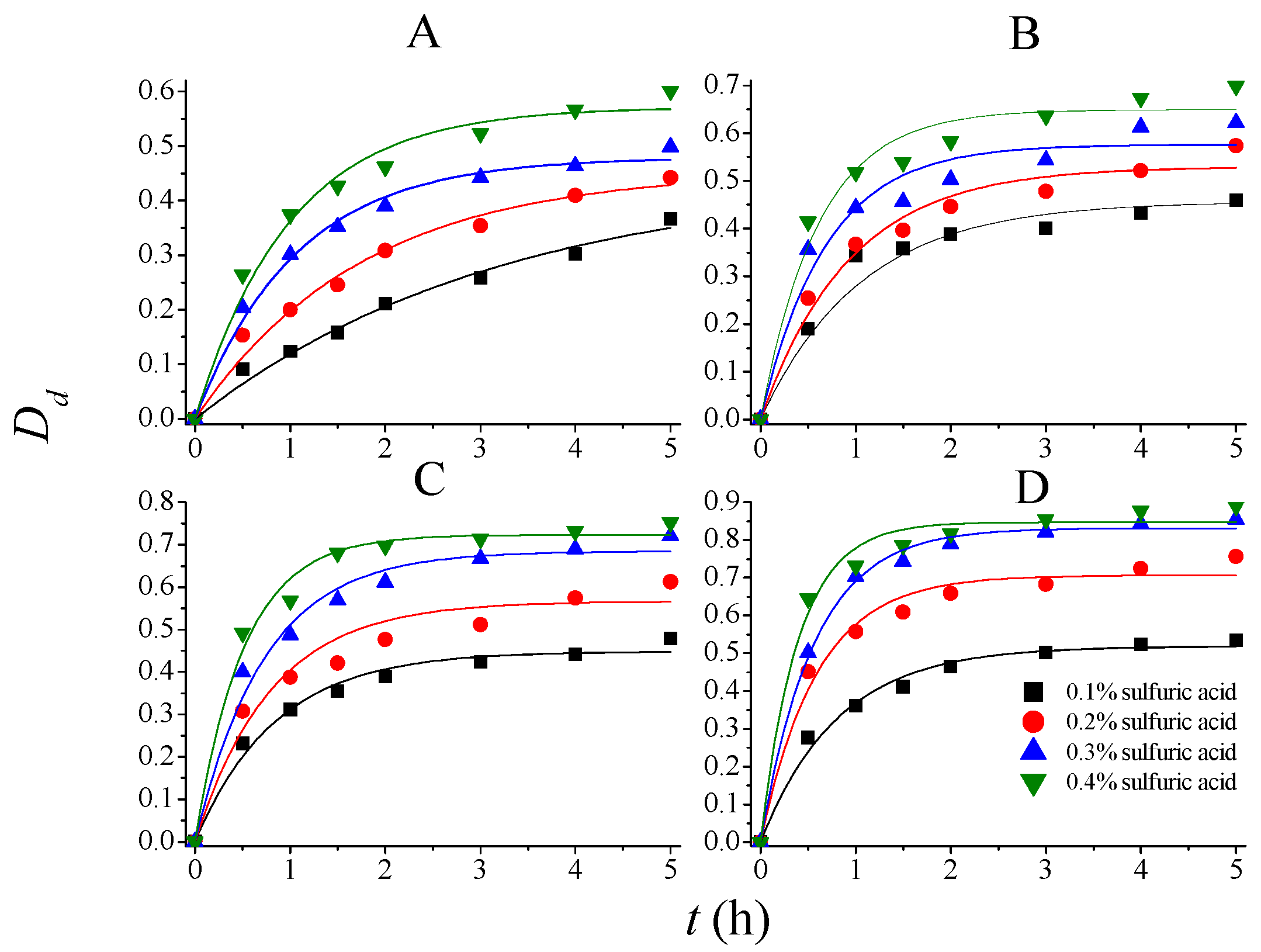
| T (°C) | CSA (mol/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.0102 | 0.0204 | 0.0306 | 0.0408 | |||||
| δ | k (h−1) | δ | k (h−1) | δ | k (h−1) | δ | k (h−1) | |
| For SS | ||||||||
| 80 | 0.2063 | 0.9280 | 0.2297 | 0.9860 | 0.2543 | 0.9598 | 0.2951 | 1.1617 |
| 90 | 0.2239 | 0.9684 | 0.2790 | 1.0650 | 0.3413 | 1.0901 | 0.3884 | 1.2900 |
| 100 | 0.2876 | 1.0570 | 0.3924 | 1.1641 | 0.4367 | 1.2756 | 0.4726 | 1.7496 |
| 110 | 0.4207 | 1.1189 | 0.4625 | 1.4572 | 0.4819 | 1.7093 | 0.5092 | 2.5670 |
| For DD | ||||||||
| 80 | 0.4437 | 0.3116 | 0.4548 | 0.5714 | 0.4794 | 0.9436 | 0.5716 | 1.0047 |
| 90 | 0.4567 | 0.9447 | 0.5297 | 1.0737 | 0.5760 | 1.4615 | 0.6493 | 1.6422 |
| 100 | 0.4485 | 1.1850 | 0.5663 | 1.2515 | 0.6844 | 1.3721 | 0.7232 | 1.9464 |
| 110 | 0.5190 | 1.2391 | 0.7063 | 1.6942 | 0.8315 | 1.7779 | 0.8473 | 2.5142 |
| For HS | ||||||||
| 80 | 0.1759 | 1.0153 | 0.2039 | 1.1438 | 0.2093 | 1.2217 | 0.2477 | 1.4665 |
| 90 | 0.2007 | 1.1562 | 0.2170 | 1.3381 | 0.2874 | 1.4000 | 0.3246 | 1.6903 |
| 100 | 0.2460 | 1.1801 | 0.3499 | 1.3930 | 0.3674 | 1.6862 | 0.4250 | 1.9468 |
| 110 | 0.3462 | 1.3510 | 0.3749 | 1.6038 | 0.4118 | 1.8492 | 0.4400 | 2.2970 |
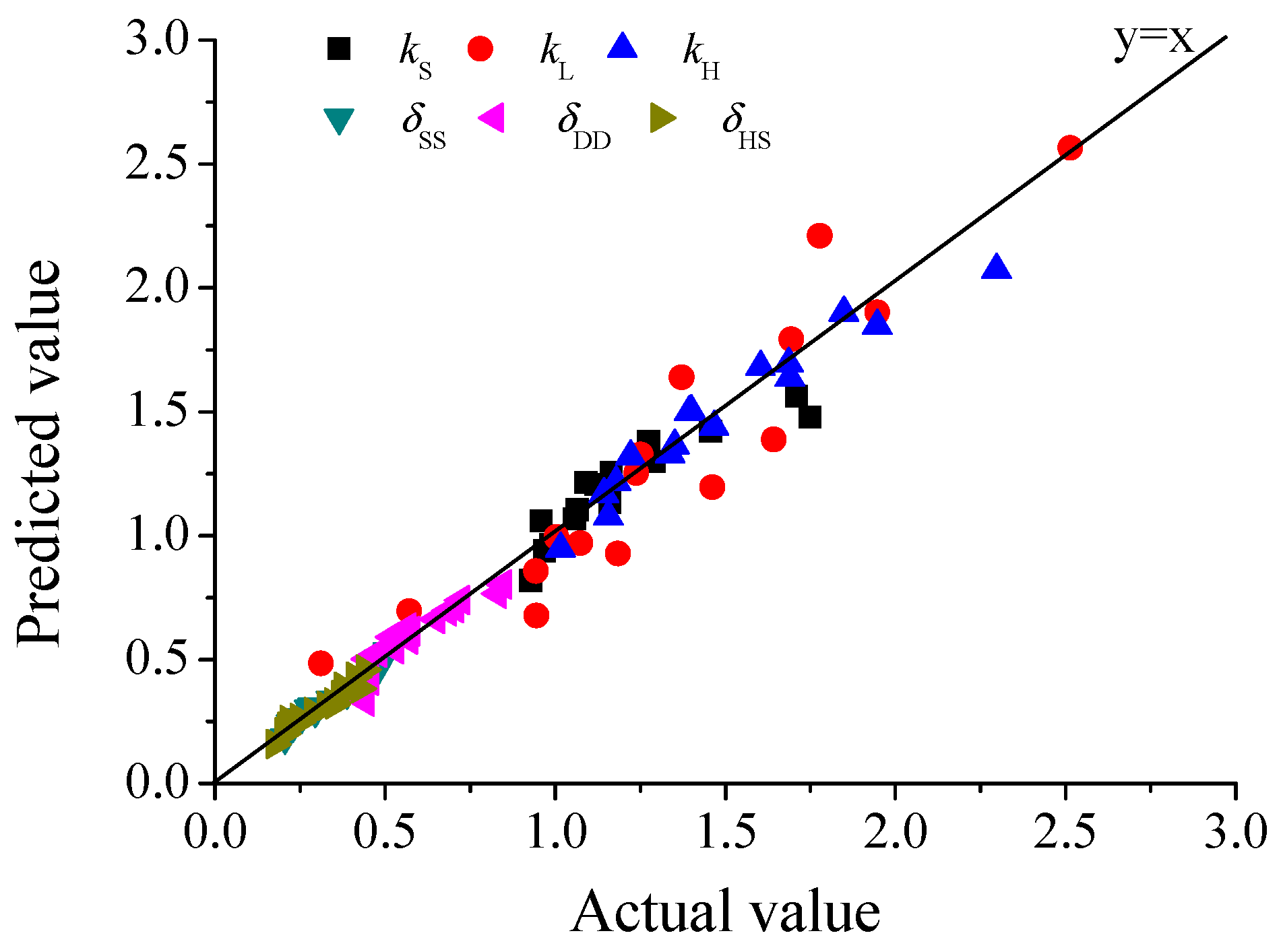
| k | k0 | Ea (kJ/mol) | α | R2 | F | P |
| kS | 343.52 | 14.573 | 0.2348 | 0.8272 | 28.7148 | 0.0000 |
| kL | 9.3887 × 105 | 35.533 | 0.5176 | 0.8448 | 35.3855 | 0.0000 |
| kH | 393.52 | 13.636 | 0.3021 | 0.9386 | 99.3466 | 0.0000 |
| δ | A | m | n | R2 | F | P |
| δSS | 3.2135 | 0.4284 | 0.5011 | 0.9627 | 167.9312 | 0.0000 |
| δDD | 30.3386 | 0.7421 | 0.5281 | 0.8595 | 39.7707 | 0.0000 |
| δHS | 2.2262 | 0.4003 | 0.4619 | 0.9434 | 108.4232 | 0.0000 |
| Source | Sum of Squares | df | Mean Squares | F Value | p-Value p > F |
|---|---|---|---|---|---|
| Model | 713.04 | 14 | 50.93 | 9.57 | <0.0001 |
| X1 | 399.11 | 1 | 399.11 | 74.99 | <0.0001 |
| X2 | 38.94 | 1 | 38.94 | 7.32 | 0.0163 |
| X3 | 38.53 | 1 | 38.53 | 7.24 | 0.0168 |
| X4 | 158.77 | 1 | 158.77 | 29.83 | <0.0001 |
| X1X2 | 1.84 | 1 | 1.84 | 0.35 | 0.5650 |
| X1X3 | 0.030 | 1 | 0.030 | 0.0059 | 0.9414 |
| X1X4 | 8.87 | 1 | 8.87 | 1.67 | 0.2164 |
| X2X3 | 4.79 | 1 | 4.79 | 0.90 | 0.3580 |
| X2X4 | 1.08 | 1 | 1.08 | 0.20 | 0.6593 |
| X3X4 | 10.42 | 1 | 10.42 | 1.96 | 0.1821 |
| X12 | 26.45 | 1 | 26.45 | 4.97 | 0.0415 |
| X22 | 24.60 | 1 | 24.60 | 4.62 | 0.0483 |
| X32 | 3.33 | 1 | 3.33 | 0.62 | 0.4415 |
| X42 | 13.32 | 1 | 13.32 | 2.50 | 0.1344 |
| Residual | 79.83 | 15 | 5.32 | ||
| Lack of Fit | 66.17 | 10 | 6.62 | 2.42 | 0.1705 |
| Pure Error | 13.66 | 5 | 2.73 | ||
| Cor Total | 792.87 | 29 |
| AA Pretreatment | PAA Loading (%) a | SY (%) | Holocellulose (%) | Cellulose (%) | Xylan (%) | Total Lignin (%) | AGC (%) |
|---|---|---|---|---|---|---|---|
| 60% AA, 0.3% SA, 110 °C, 2 h | 0 | 59.8 | 86.0 | 62.6 | 16.1 | 14.7 | 2.46 |
| 2.5 | 57.6 | 88.9 | 67.5 | 15.9 | 12.1 | 1.87 | |
| 5.0 | 55.4 | 90.1 | 68.6 | 16.8 | 10.5 | 1.23 | |
| 7.5 | 53.1 | 90.9 | 70.2 | 17.0 | 8.42 | 0.78 | |
| 10 | 50.1 | 92.3 | 72.5 | 17.4 | 6.23 | 0.58 | |
| 70% AA, 0.3% SA, 110 °C, 2 h | 0 | 54.0 | 88.2 | 65.6 | 15.8 | 13.8 | 3.15 |
| 2.5 | 52.1 | 89.5 | 68.9 | 15.7 | 10.2 | 2.59 | |
| 5.0 | 50.3 | 90.6 | 70.4 | 16.4 | 8.31 | 1.58 | |
| 7.5 | 49.7 | 92.2 | 74.3 | 15.9 | 6.18 | 1.22 | |
| 10 | 48.9 | 93.1 | 75.6 | 16.6 | 5.88 | 0.69 | |
| 80% AA, 0.3% SA, 110 °C, 2 h | 0 | 52.1 | 90.1 | 68.2 | 15.6 | 10.2 | 3.45 |
| 2.5 | 50.5 | 92.4 | 70.6 | 16.6 | 8.27 | 3.00 | |
| 5.0 | 48.7 | 93.2 | 73.4 | 16.0 | 7.01 | 2.45 | |
| 7.5 | 47.2 | 94.0 | 76.5 | 15.4 | 6.23 | 1.66 | |
| 10 | 46.7 | 94.4 | 77.7 | 15.9 | 5.12 | 0.96 |
| Variables, Abbreviation and Units | Code | Levels | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Temperature (T, °C) | X1 | 70 | 80 | 90 | 100 | 110 |
| AA concentration (CAA, wt%) | X2 | 55 | 65 | 75 | 85 | 95 |
| Pretreatment time (t, h) | X3 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| Sulfuric acid concentration (CSA, wt%) | X4 | 0.0 | 0.1 | 0.2 | 0.3 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Tian, M.; Dai, Z.; Zhao, X. Improving the Cellulose Enzymatic Digestibility of Sugarcane Bagasse by Atmospheric Acetic Acid Pretreatment and Peracetic Acid Post-Treatment. Molecules 2023, 28, 4689. https://doi.org/10.3390/molecules28124689
Bai Y, Tian M, Dai Z, Zhao X. Improving the Cellulose Enzymatic Digestibility of Sugarcane Bagasse by Atmospheric Acetic Acid Pretreatment and Peracetic Acid Post-Treatment. Molecules. 2023; 28(12):4689. https://doi.org/10.3390/molecules28124689
Chicago/Turabian StyleBai, Yuchen, Mingke Tian, Zhiwei Dai, and Xuebing Zhao. 2023. "Improving the Cellulose Enzymatic Digestibility of Sugarcane Bagasse by Atmospheric Acetic Acid Pretreatment and Peracetic Acid Post-Treatment" Molecules 28, no. 12: 4689. https://doi.org/10.3390/molecules28124689
APA StyleBai, Y., Tian, M., Dai, Z., & Zhao, X. (2023). Improving the Cellulose Enzymatic Digestibility of Sugarcane Bagasse by Atmospheric Acetic Acid Pretreatment and Peracetic Acid Post-Treatment. Molecules, 28(12), 4689. https://doi.org/10.3390/molecules28124689







