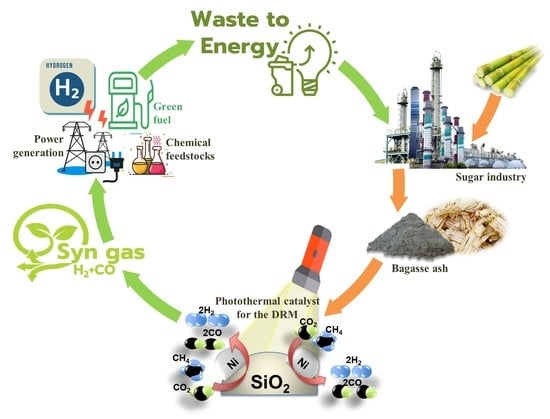
Development of Photothermal Catalyst from Biomass Ash (Bagasse) for Hydrogen Production via Dry Reforming of Methane (DRM): An Experimental Study
Abstract
1. Introduction
2. Results and Discussion
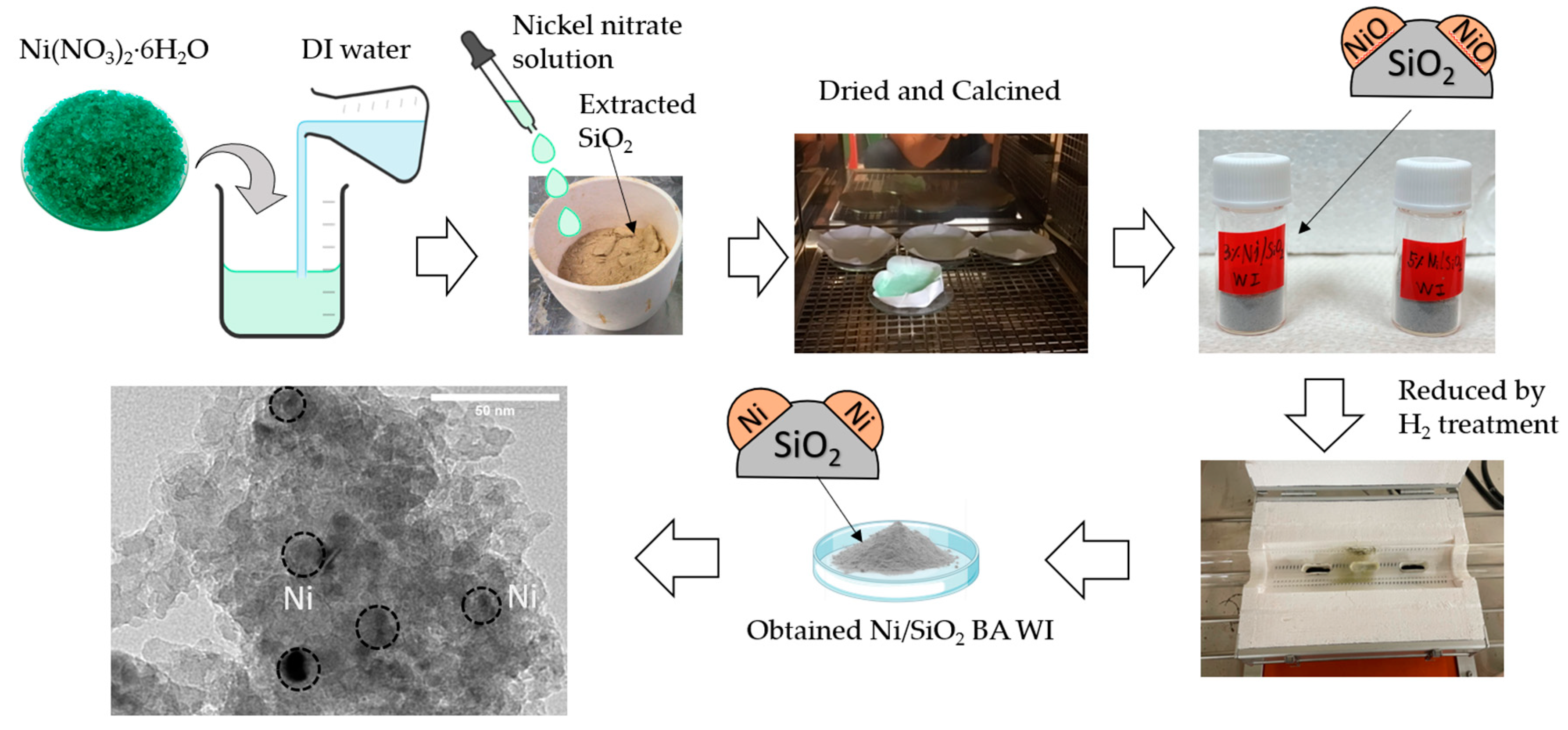
2.1. Fabrication of the Catalyst Support Preparation and Synthesis Catalyst
2.2. Characterization
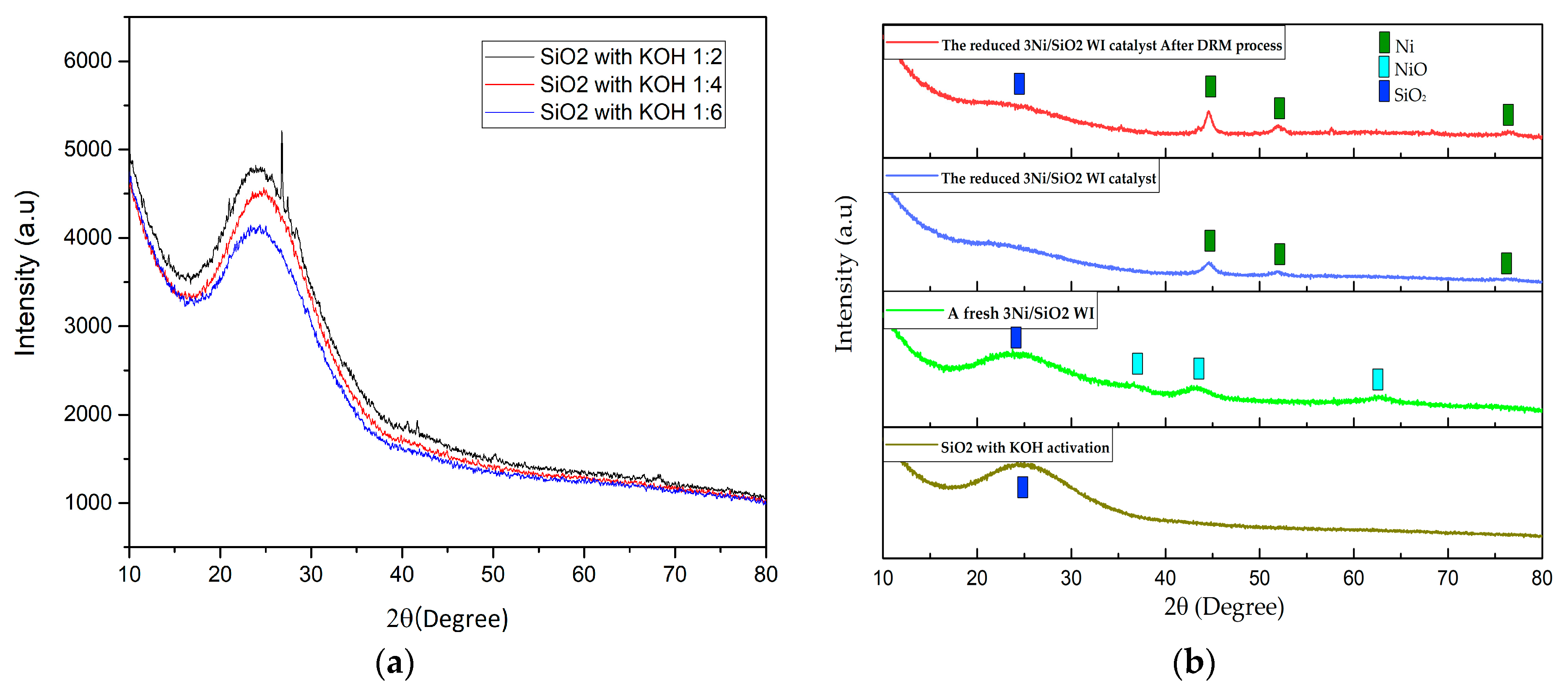
2.2.1. XRD Analysis
2.2.2. BET Surface Analysis
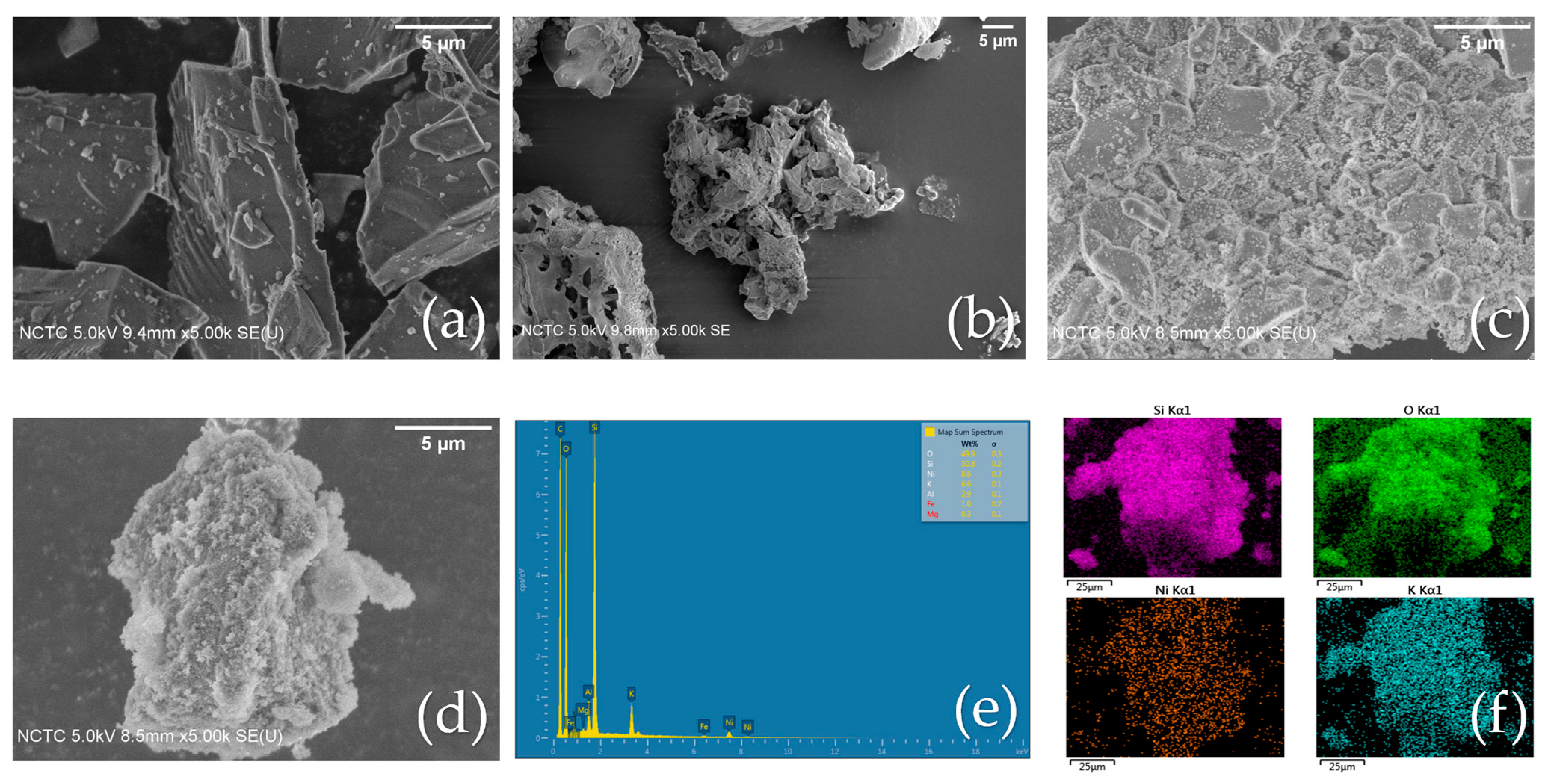
2.2.3. SEM Analysis
2.2.4. XRF Analysis
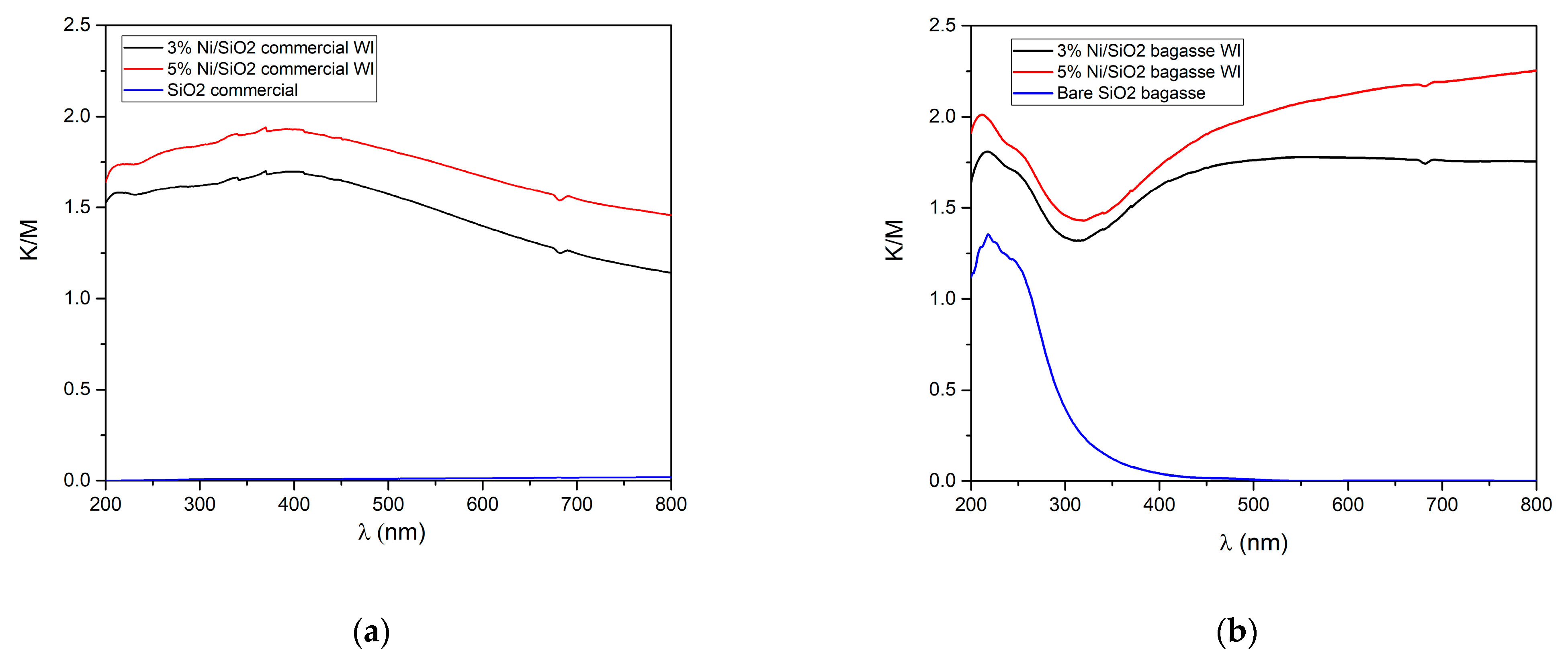
2.2.5. Optical Properties
2.3. Photothermal Catalytic Activity
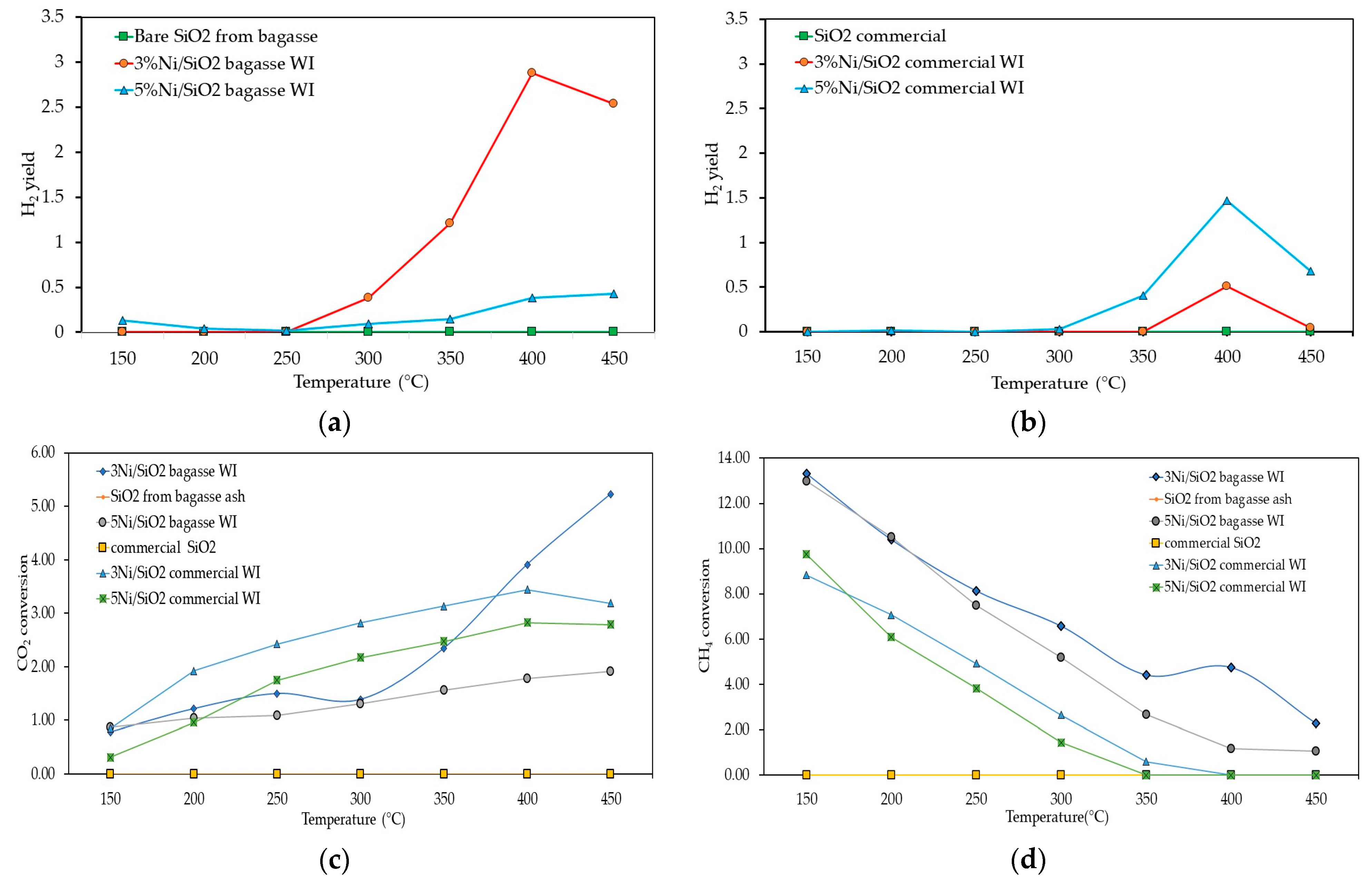
2.3.1. Photothermal Catalytic Hydrogen Generation Results and Analysis
2.3.2. Band Alignment and Proposed Photothermal Catalytic Mechanism
3. Materials and Methods
3.1. Preparation of Bagasse Fly Ash
3.2. Preparation of Silicon Dioxide Extraction
3.3. Preparation of KOH Activation for Silicon Dioxide
3.4. Preparation of Catalysts
Wet Impregnation Method
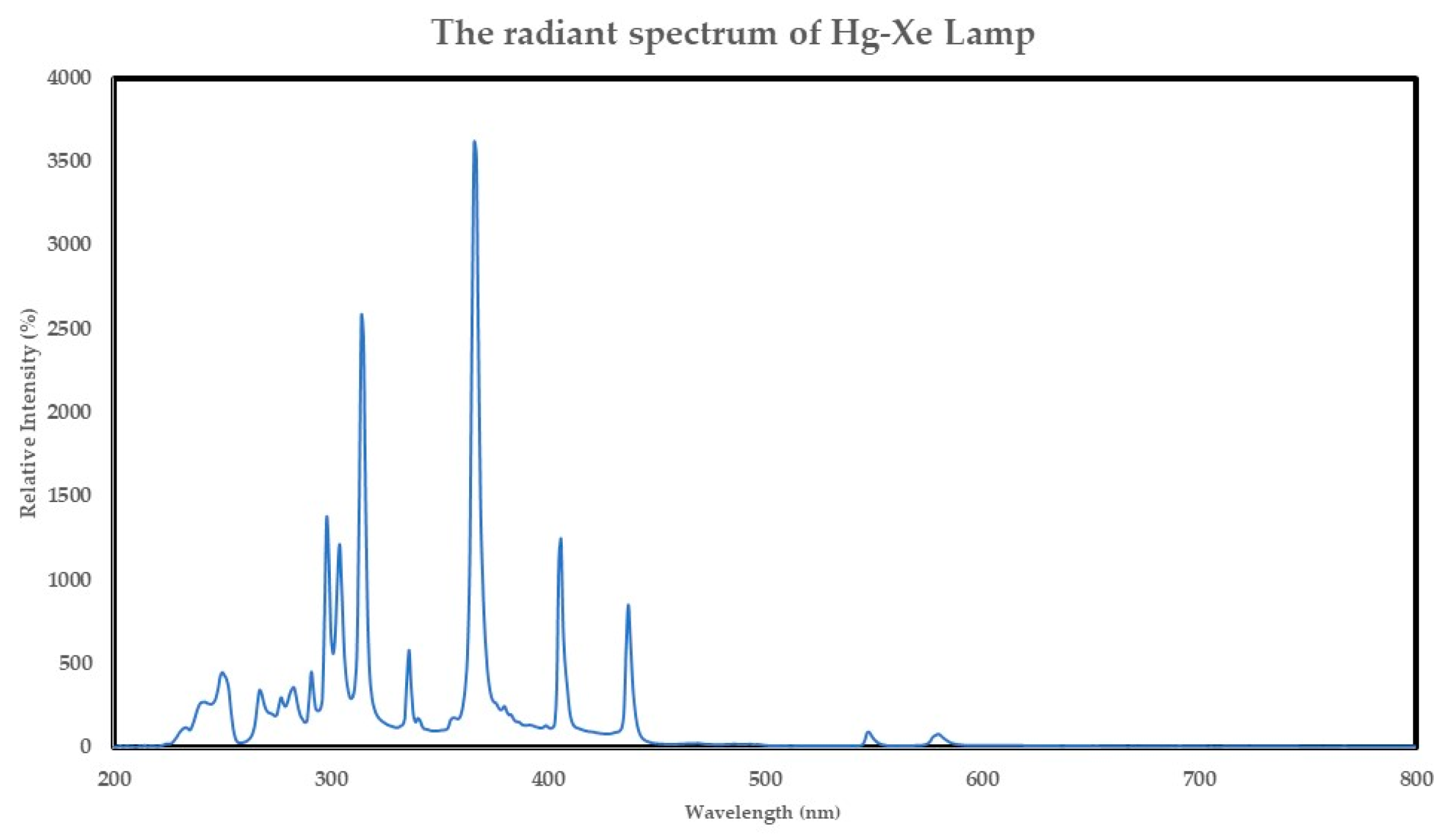
3.5. Photothermal DRM Activity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bennett, S.; Remme, U. The Future of Hydrogen; Technology Report; IEA: Paris, France, 2019.
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Kee, R.J.; Karakaya, C.; Zhu, H. Process intensification in the catalytic conversion of natural gas to fuels and chemicals. Proc. Combust. Inst. 2017, 36, 51–76. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Tian, D.; Jiang, L.; Li, Z.; Wang, H.; Li, K. Optimization of Ni-Based Catalysts for Dry Reforming of Methane via Alloy Design: A Review. Energy Fuels 2022, 36, 5102–5151. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.-M.; Barkschat, A.; Rodemerck, U. Stable low-temperature dry reforming of methane over mesoporous La2O3-ZrO2 supported Ni catalyst. Appl. Catal. B Environ. 2012, 113–114, 19–30. [Google Scholar] [CrossRef]
- Barama, S.; Dupeyrat-Batiot, C.; Capron, M.; Bordes-Richard, E.; Bakhti-Mohammedi, O. Catalytic properties of Rh, Ni, Pd and Ce supported on Al-pillared montmorillonites in dry reforming of methane. Catal. Today 2009, 141, 385–392. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Comparative study at low and medium reaction temperatures of syngas production by methane reforming with carbon dioxide over silica and alumina supported catalysts. Appl. Catal. A Gen. 1998, 170, 177–187. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, P.; Fang, H.; Zheng, X.; Yashima, T. Production of synthesis gas via methane reforming with CO2 on noble metals and small amount of noble-(Rh-) promoted Ni catalysts. Int. J. Hydrogen Energy 2006, 31, 555–561. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Dry reforming of methane for syngas production: A review and assessment of catalyst development and efficacy. J. Indian Chem. Soc. 2021, 98, 100002. [Google Scholar] [CrossRef]
- Partha, N.; Sivasubramanian, V. Recovery of Chemicals from Pressmud—A Sugar Industry Waste. J. Indian Chem. Eng. 2006, 48, 160–163. [Google Scholar]
- Kanaphan, Y.; Klamchuen, A.; Chaikawang, C.; Harnchana, V.; Srilomsak, S.; Nash, J.; Meethong, N. Interfacially Enhanced Stability and Electrochemical Properties of C/SiOx Nanocomposite Lithium-Ion Battery Anodes. Adv. Mater. Interfaces 2022, 9, 2200303. [Google Scholar] [CrossRef]
- Deraz, N.M. The Comparative Jurisprudence of Catalysts Preparation Methods: I. Precipitation and Impregnation Methods. J. Ind. Environ. Chem. 2018, 2, 19–21. [Google Scholar]
- Ding, F.; Zhang, Y.; Yuan, G.; Wang, K.; Dragutan, I.; Dragutan, V.; Cui, Y.; Wu, J. Synthesis and Catalytic Performance of Ni/SiO2 for Hydrogenation of 2-Methylfuran to 2-Methyltetrahydrofuran. J. Nanomater. 2015, 2015, 791529. [Google Scholar] [CrossRef]
- Güler, E.; Uğur, G.; Uğur, Ş.; Güler, M. A theoretical study for the band gap energies of the most common silica polymorphs. Chin. J. Phys. 2020, 65, 472–480. [Google Scholar] [CrossRef]
- Jiang, H.; Peng, X.; Yamaguchi, A.; Ueda, S.; Fujita, T.; Abe, H.; Miyauchi, M. Photocatalytic Partial Oxidation of Methane on Palladium-Loaded Strontium Tantalate. Sol. RRL 2019, 3, 1900076. [Google Scholar] [CrossRef]
- Wu, S.; Wu, S.; Sun, Y. Light-Driven Dry Reforming of Methane on Metal Catalysts. Sol. RRL 2021, 5, 2000507. [Google Scholar] [CrossRef]
- Takeda, K.; Yamaguchi, A.; Cho, Y.; Anjaneyulu, O.; Fujita, T.; Abe, H.; Miyauchi, M. Metal Carbide as A Light-Harvesting and Anticoking Catalysis Support for Dry Reforming of Methane. Glob. Chall. 2019, 4, 1900067. [Google Scholar] [CrossRef]
| Samples | BET 1 Surface Area (m2/g) | Average Pore Size (nm) |
|---|---|---|
| Bare commercial SiO2 | 10.7 | 4.47 |
| Bare extracted SiO2 from bagasse ash | 42.3 | 36.0 |
| Extracted SiO2 BA, KOH activation 1:2 | 207 | 13.1 |
| Extracted SiO2 BA, KOH activation 1:4 * | 185 | 20.2 |
| Extracted SiO2 BA, KOH activation 1:6 | 178 | 11.6 |
| 3Ni/SiO2 commercial WI | 11.4 | 4.62 |
| 5Ni/SiO2 commercial WI | 12.6 | 4.85 |
| 3Ni/SiO2 bagasse ash WI | 163 | 20.9 |
| 5Ni/SiO2 bagasse ash WI | 157 | 20.9 |
| Sample | Compounds | |||
|---|---|---|---|---|
| Si | Ni | Al | K | |
| 3Ni/SiO2 BA WI | 71.4% | 11.7% | 5.26% | 11.7% |
| 3Ni/SiO2 commercial WI | 88.0% | 11.7% | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanchanakul, I.; Srinophakun, T.R.; Kuboon, S.; Kaneko, H.; Kraithong, W.; Miyauchi, M.; Yamaguchi, A. Development of Photothermal Catalyst from Biomass Ash (Bagasse) for Hydrogen Production via Dry Reforming of Methane (DRM): An Experimental Study. Molecules 2023, 28, 4578. https://doi.org/10.3390/molecules28124578
Kanchanakul I, Srinophakun TR, Kuboon S, Kaneko H, Kraithong W, Miyauchi M, Yamaguchi A. Development of Photothermal Catalyst from Biomass Ash (Bagasse) for Hydrogen Production via Dry Reforming of Methane (DRM): An Experimental Study. Molecules. 2023; 28(12):4578. https://doi.org/10.3390/molecules28124578
Chicago/Turabian StyleKanchanakul, Ittichai, Thongchai Rohitatisha Srinophakun, Sanchai Kuboon, Hiroaki Kaneko, Wasawat Kraithong, Masahiro Miyauchi, and Akira Yamaguchi. 2023. "Development of Photothermal Catalyst from Biomass Ash (Bagasse) for Hydrogen Production via Dry Reforming of Methane (DRM): An Experimental Study" Molecules 28, no. 12: 4578. https://doi.org/10.3390/molecules28124578
APA StyleKanchanakul, I., Srinophakun, T. R., Kuboon, S., Kaneko, H., Kraithong, W., Miyauchi, M., & Yamaguchi, A. (2023). Development of Photothermal Catalyst from Biomass Ash (Bagasse) for Hydrogen Production via Dry Reforming of Methane (DRM): An Experimental Study. Molecules, 28(12), 4578. https://doi.org/10.3390/molecules28124578