Metabolic Fingerprinting of Muscat of Alexandria Grape Musts during Industrial Alcoholic Fermentation Using HS-SPME and Liquid Injection with TMS Derivatization GC-MS Methods
Abstract
1. Introduction
2. Results and Discussion
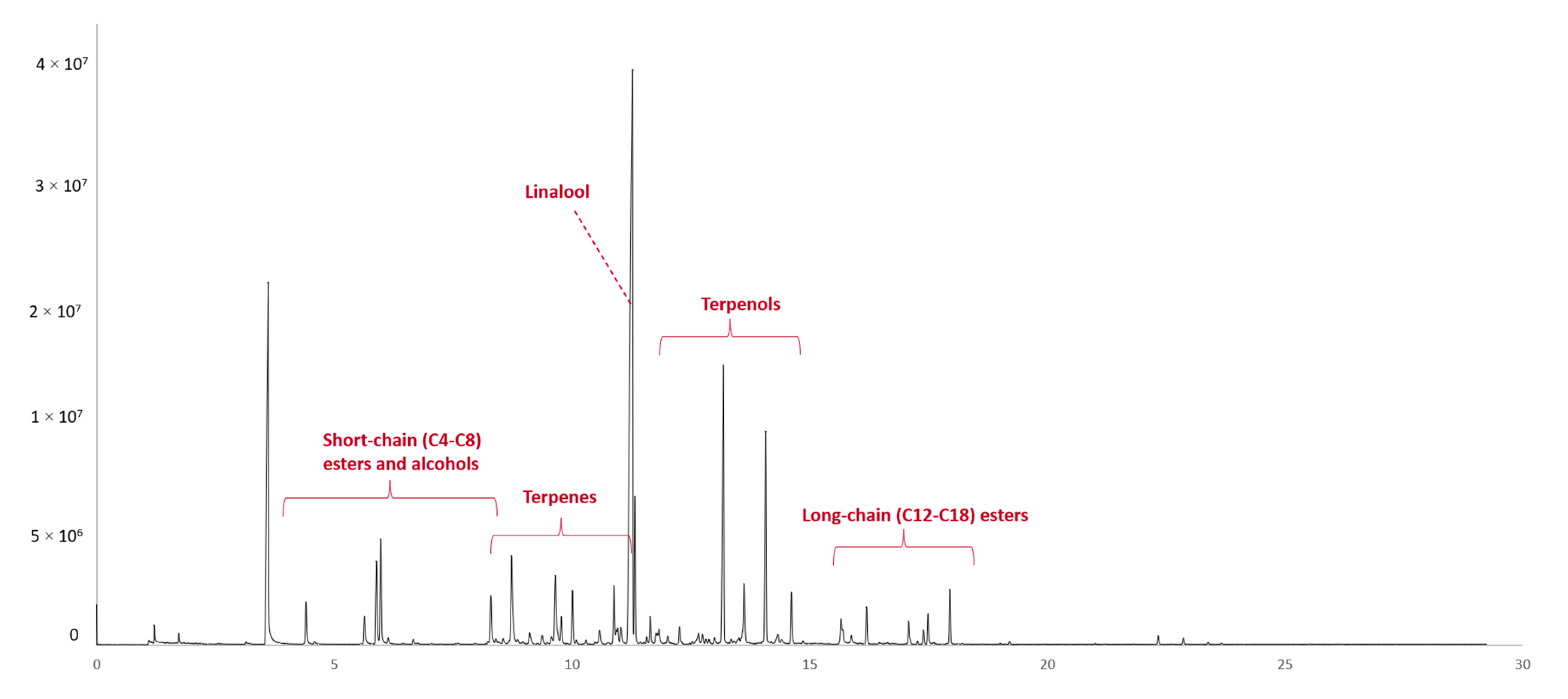
2.1. Volatile Composition of Grape Must—Changes during Fermentation
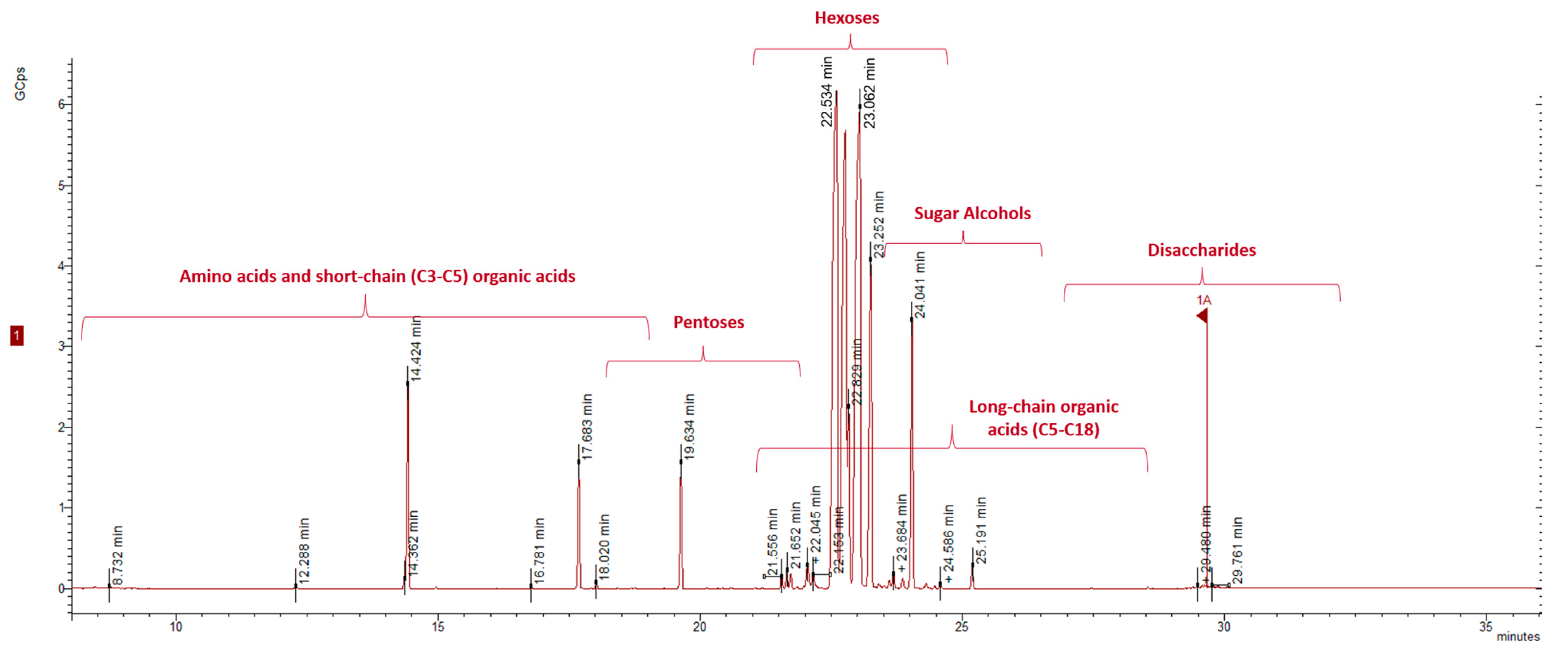
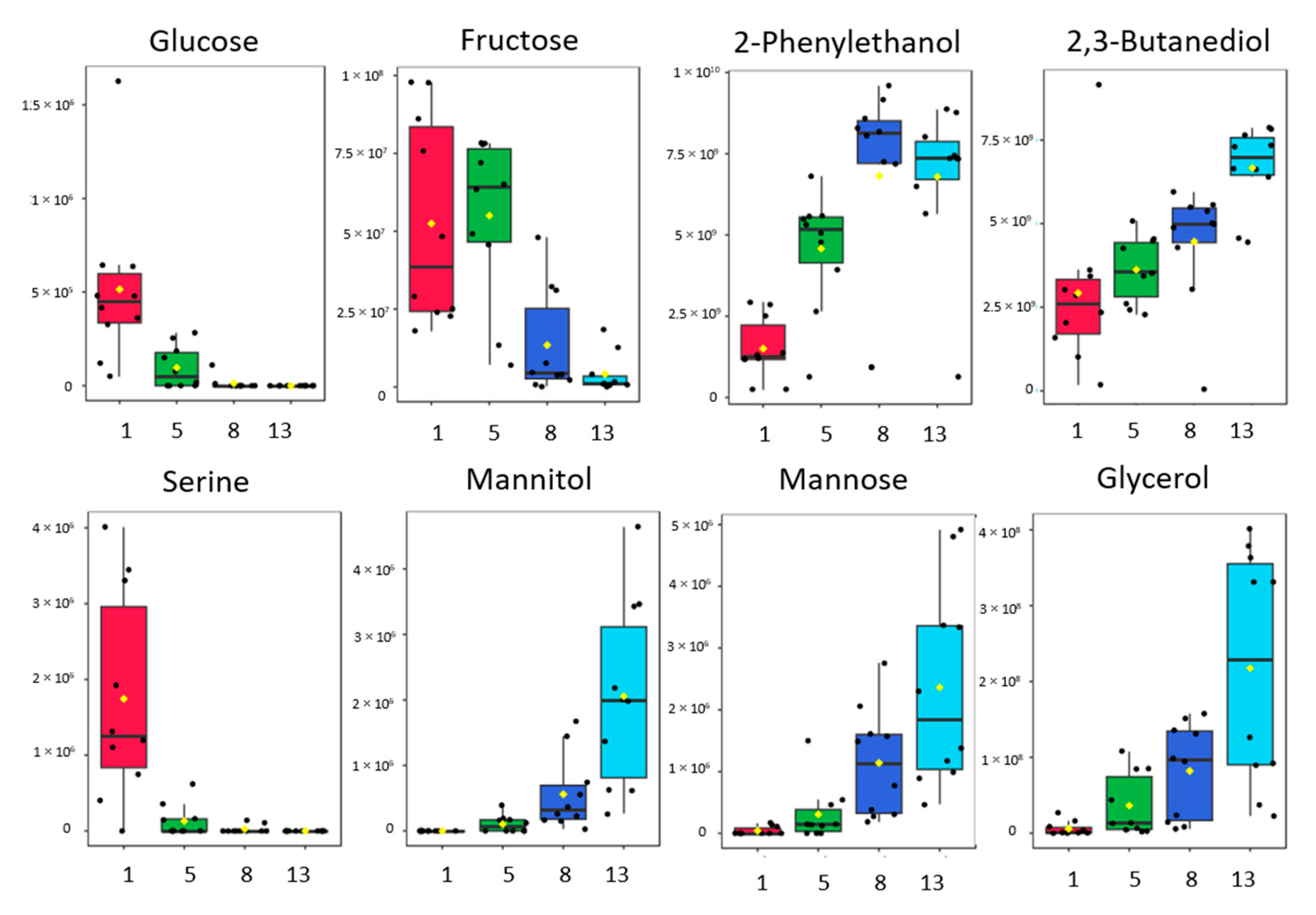
2.2. Metabolic Profiling of Sugars and Organic Acids during Fermentation
2.3. Multivariate Statistical Analysis during Alcoholic Fermentation for the Proposed Methods
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. HS-SPME-GC-MS Method
3.3.1. Sample Preparation and Extraction
3.3.2. GC-MS Conditions
3.4. GC-MS Method using Liquid Injection after Derivatization
3.4.1. Sample Extraction and Derivatization
3.4.2. GC-MS Conditions
3.5. Data Processing and Chemometrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Buesa, I.; Intrigliolo, D.S.; Castel, J.R.; Vilanova, M. Influence of water regime on grape aromatic composition of Muscat of Alexandria in a semiarid climate. Sci. Hortic. 2021, 290, 110525. [Google Scholar] [CrossRef]
- Lanaridis, P.; Salaha, M.-J.; Tzourou, I.; Tsoutsouras, E.; Karagiannis, S. Volatile compounds in grapes and wines from two Muscat varieties cultivated in Greek islands. OENO One 2002, 36, 39–47. [Google Scholar] [CrossRef]
- Muscat of Alexandria, Wines Greece. (n.d.). Available online: https://winesofgreece.org/varieties/muscat-of-alexandria/ (accessed on 22 March 2023).
- Muscat of Lemnos, Wines Greece. (n.d.). Available online: https://winesofgreece.org/articles/muscat-of-lemnos/ (accessed on 22 March 2023).
- Bordiga, M.; Rinaldi, M.; Locatelli, M.; Piana, G.; Travaglia, F.; Coïsson, J.D.; Arlorio, M. Characterization of Muscat wines aroma evolution using comprehensive gas chromatography followed by a post-analytic approach to 2D contour plots comparison. Food Chem. 2013, 140, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Licen, S.; Muzic, E.; Briguglio, S.; Tolloi, A.; Barbieri, P.; Giungato, P. Derivatized volatile organic compound characterization of Friulano wine from Collio (Italy–Slovenia) by HS-SPME-GC-MS and discrimination from other varieties by chemometrics. Br. Food J. 2021, 123, 2844–2855. [Google Scholar] [CrossRef]
- Setkova, L.; Risticevic, S.; Pawliszyn, J. Rapid headspace solid-phase microextraction-gas chromatographic–time-of-flight mass spectrometric method for qualitative profiling of ice wine volatile fraction: II: Classification of Canadian and Czech ice wines using statistical evaluation of the data. J. Chromatogr. A 2007, 1147, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa-López, M.D.C.; Aragón-García, F.; Ruíz-Rodríguez, A.; Piñeiro, Z.; Durán-Guerrero, E.; Palma, M. Effects from the Freezing of Either Whole or Crushed Grapes on the Volatile Compounds Contents in Muscat Wines. Foods 2022, 11, 1782. [Google Scholar] [CrossRef]
- Porter, T.J.; Divol, B.; Setati, M.E. Investigating the biochemical and fermentation attributes of Lachancea species and strains: Deciphering the potential contribution to wine chemical composition. Int. J. Food Microbiol. 2018, 290, 273–287. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Lavilla, M.; Amárita, F. Wine aroma profile modification by Oenococcus oeni strains from Rioja Alavesa region: Selection of potential malolactic starters. Int. J. Food Microbiol. 2021, 356, 109324. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Soares, R.D.; Welke, J.E.; Nicolli, K.P.; Zanus, M.; Caramão, E.B.; Manfroi, V.; Zini, C.A. Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine. Food Chem. 2015, 183, 291–304. [Google Scholar] [CrossRef]
- Lukić, I.; Lotti, C.; Vrhovsek, U. Evolution of free and bound volatile aroma compounds and phenols during fermentation of Muscat blanc grape juice with and without skins. Food Chem. 2017, 232, 25–35. [Google Scholar] [CrossRef]
- Schievano, E.; D’Ambrosio, M.; Mazzaretto, I.; Ferrarini, R.; Magno, F.; Mammi, S.; Favaro, G. Identification of wine aroma precursors in Moscato Giallo grape juice: A nuclear magnetic resonance and liquid chromatography–mass spectrometry tandem study. Talanta 2013, 116, 841–851. [Google Scholar] [CrossRef]
- Gunata, Y.; Bayonove, C.; Baumes, R.; Cordonnier, R. The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J. Chromatogr. A 1985, 331, 83–89. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. The aroma of grapes. Localisation and evolution of free and bound fractions of some grape aroma components c.v. Muscat during first development and maturation. J. Sci. Food Agric. 1985, 36, 857–862. [Google Scholar] [CrossRef]
- Zemni, H.; Souid, I.; Fathalli, N.; Ben Salem, A.; Hammami, M.; Ghorbel, A.; Hellali, R. Aromatic Composition of Two Muscat Grape Cultivars Cultivated in Two Different Regions of Tunisia. Int. J. Fruit Sci. 2007, 7, 97–112. [Google Scholar] [CrossRef]
- Louw, C.; La Grange, D.; Pretorius, I.; van Rensburg, P. The effect of polysaccharide-degrading wine yeast transformants on the efficiency of wine processing and wine flavour. J. Biotechnol. 2006, 125, 447–461. [Google Scholar] [CrossRef]
- Bakker, J.; Clarke, R.J. Wine: Flavour Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Oliveira, A.S.; Furtado, I.; Bastos, M.D.L.; de Pinho, P.G.; Pinto, J. The influence of different closures on volatile composition of a white wine. Food Packag. Shelf Life 2020, 23, 100465. [Google Scholar] [CrossRef]
- Robles, A.D.; Fabjanowicz, M.; Płotka-Wasylka, J.; Konieczka, P. Organic Acids and Polyphenols Determination in Polish Wines by Ultrasound-Assisted Solvent Extraction of Porous Membrane-Packed Liquid Samples. Molecules 2019, 24, 4376. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Zubiaguirre, L.; Olivares, M.; Castro, K.; Carrero, J.A.; García-Benito, C.; García-Serrano, J.; Pérez-Pérez, J.; Pérez-Arantegui, J. Wine markers in archeological potteries: Detection by GC-MS at ultratrace levels. Anal. Bioanal. Chem. 2019, 411, 6711–6722. [Google Scholar] [CrossRef] [PubMed]
- Wirth, J.; Guo, W.; Baumes, R.; Günata, Z. Volatile Compounds Released by Enzymatic Hydrolysis of Glycoconjugates of Leaves and Grape Berries from Vitis vinifera Muscat of Alexandria and Shiraz Cultivars. J. Agric. Food Chem. 2001, 49, 2917–2923. [Google Scholar] [CrossRef]
- Hardy, P. Changes in volatiles of muscat grapes during ripening. Phytochemistry 1970, 9, 709–715. [Google Scholar] [CrossRef]
- Van Rensburg, P.; Strauss, M.; Lambrechts, M.; Otero, R.C.; Pretorius, I. The heterologous expression of polysaccharidase-encoding genes with oenological relevance in Saccharomyces cerevisiae. J. Appl. Microbiol. 2007, 103, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, M.A.; Loira, I.; Guamis, B.; Escott, C.; Del Fresno, J.M.; Codina-Torrella, I.; Quevedo, J.M.; Gervilla, R.; Chavarría, J.M.R.; de Lamo, S.; et al. White wine processing by UHPH without SO2. Elimination of microbial populations and effect in oxidative enzymes, colloidal stability and sensory quality. Food Chem. 2020, 332, 127417. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, A.; Durán-Guerrero, E.; Natera, R.; Palma, M.; Barroso, C.G. Influence of Two Different Cryoextraction Procedures on the Quality of Wine Produced from Muscat Grapes. Foods 2020, 9, 1529. [Google Scholar] [CrossRef]
- Smit, S.J.; Vivier, M.A.; Young, P.R. Linking Terpene Synthases to Sesquiterpene Metabolism in Grapevine Flowers. Front. Plant Sci. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Sollazzo, M.; Baccelloni, S.; D’Onofrio, C.; Bellincontro, A. Combining color chart, colorimetric measurement and chemical compounds for postharvest quality of white wine grapes. J. Sci. Food Agric. 2018, 98, 3532–3541. [Google Scholar] [CrossRef]
- Wu, B.H.; Yang, C.X.; Liang, Z.C.; Liu, W.; Wang, Y.J.; Liu, C.Y.; Li, S.H. Inheritance of berry volatile compounds in two half-sib grape (Vitis vinifera) populations. Euphytica 2012, 189, 351–364. [Google Scholar] [CrossRef]
- Lioupi, A.; Marinaki, M.; Virgiliou, C.; Gika, H.; Wilson, I.; Theodoridis, G. State-of-the-art in LC–MS Approaches for Probing the Polar Metabolome. In Advanced Mass Spectrometry-based Analytical Separation Techniques for Probing the Polar Metabolome; Royal Society of Chemistry: London, UK, 2021; Volume 10, pp. 1–26. [Google Scholar] [CrossRef]
- Lioupi, A.; Marinaki, M.; Virgiliou, C.; Begou, O.; Gika, H.; Wilson, I.; Theodoridis, G. Probing the polar metabolome by UHPLC-MS. TrAC Trends Anal. Chem. 2023, 161, 117014. [Google Scholar] [CrossRef]
- Maicas, S. Advances in Wine Fermentation. Fermentation 2021, 7, 187. [Google Scholar] [CrossRef]
- Zarate, E.; Boyle, V.; Rupprecht, U.; Green, S.; Villas-Boas, S.G.; Baker, P.; Pinu, F.R. Fully Automated Trimethylsilyl (TMS) Derivatisation Protocol for Metabolite Profiling by GC-MS. Metabolites 2016, 7, 1. [Google Scholar] [CrossRef]
- Gracia-Moreno, E.; Lopez, R.; Ferreira, V. Quantitative determination of five hydroxy acids, precursors of relevant wine aroma compounds in wine and other alcoholic beverages. Anal. Bioanal. Chem. 2015, 407, 7925–7934. [Google Scholar] [CrossRef]
- Fabjanowicz, M.; Różańska, A.; Kalinowska, K.; Płotka-Wasylka, J. Miniaturized, green salting-out liquid–liquid microextraction coupled with GC–MS used to evaluate biogenic amines in wine samples. Microchem. J. 2022, 180, 107616. [Google Scholar] [CrossRef]
- Soleas, G.J.; Diamandis, E.P.; Karumanchiri, A.; Goldberg, D.M. A Multiresidue Derivatization Gas Chromatographic Assay for Fifteen Phenolic Constituents with Mass Selective Detection. Anal. Chem. 1997, 69, 4405–4409. [Google Scholar] [CrossRef]
- Tashakkorï, P.; Tağaç, A.A.; Merdïvan, M. Graphene Oxide-Ionic Liquid Used as Solid-Phase Microextraction Coating for Polyphenolic Compounds’ Extraction and Determination with GC-MS after on-Fiber Derivatization in Wine. J. Turk. Chem. Soc. Sect. A Chem. 2020, 7, 411–426. [Google Scholar] [CrossRef]
- Villas-Bôas, S.G.; Noel, S.; Lane, G.A.; Attwood, G.; Cookson, A. Extracellular metabolomics: A metabolic footprinting approach to assess fiber degradation in complex media. Anal. Biochem. 2006, 349, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Edwards, P.J.B.; Jouanneau, S.; Kilmartin, P.A.; Gardner, R.C.; Villas-Boas, S.G. Sauvignon blanc metabolomics: Grape juice metabolites affecting the development of varietal thiols and other aroma compounds in wines. Metabolomics 2013, 10, 556–573. [Google Scholar] [CrossRef]
- Prusova, B.; Humaj, J.; Kulhankova, M.; Kumsta, M.; Sochor, J.; Baron, M. Capture of Fermentation Gas from Fermentation of Grape Must. Foods 2023, 12, 574. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, Q.; Yan, G.; Duan, C. Using headspace solid phase micro-extraction for analysis of aromatic compounds during alcoholic fermentation of red wine. Food Chem. 2011, 125, 743–749. [Google Scholar] [CrossRef]
- Stevens, K.L.; Bomben, J.; Lee, A.; McFadden, W.H. Volatiles from Grapes. Muscat of Alexandria. J. Agric. Food Chem. 1966, 14, 249–252. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Moio, L. Influence of Yeast Strain on Odor-Active Compounds in Fiano Wine. Appl. Sci. 2021, 11, 7767. [Google Scholar] [CrossRef]
- Corona, O.; Planeta, D.; Bambina, P.; Giacosa, S.; Paissoni, M.A.; Squadrito, M.; Torchio, F.; Segade, S.R.; Cinquanta, L.; Gerbi, V.; et al. Influence of Different Dehydration Levels on Volatile Profiles, Phenolic Contents and Skin Hardness of Alkaline Pre-Treated Grapes c.v. Muscat of Alexandria (Vitis vinifera L.). Foods 2020, 9, 666. [Google Scholar] [CrossRef]
- Pascual, G.A.; Serra, I.; Calderón-Orellana, A.; Laurie, V.F.; Lopéz, M.D. Changes in concentration of volatile compounds in response to defoliation of Muscat of Alexandria grapevines grown under a traditional farming system. Chil. J. Agric. Res. 2017, 77, 373–381. [Google Scholar] [CrossRef]
- Williams, P.J.; Strauss, C.R.; Wilson, B. Hydroxylated linalool derivatives as precursors of volatile monoterpenes of muscat grapes. J. Agric. Food Chem. 1980, 28, 766–771. [Google Scholar] [CrossRef]
- De-La-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Valentin, D.; Ferreira, V. Fourteen ethyl esters of wine can be replaced by simpler ester vectors without compromising quality but at the expense of increasing aroma concentration. Food Chem. 2019, 307, 125553. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, M.; Viana, R.; Granja-Soares, J.; Martins, M.; Ribeiro, H.; Malfeito-Ferreira, M. Adaptation of Ancient Techniques to Recreate ‘Wines’ and ‘Beverages’ Using Withered Grapes of Muscat of Alexandria. Fermentation 2022, 8, 85. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Strauss, C.R.; Wilson, B.; Williams, P.J. The actinidols: Nor-isoprenoid compounds in grapes, wines and spirits. Phytochemistry 1985, 24, 767–770. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Rioseras, A.T.; Gomez, D.G.; Ebert, B.E.; Blank, L.M.; Ibáñez, A.J.; Sinues, P.M.-L. Comprehensive Real-Time Analysis of the Yeast Volatilome. Sci. Rep. 2017, 7, 14236. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Araujo, L.D.; Tian, B. Varietal Aromas of Sauvignon Blanc: Impact of Oxidation and Antioxidants Used in Winemaking. Fermentation 2022, 8, 686. [Google Scholar] [CrossRef]
- Smid, E.; Kleerebezem, M. Production of Aroma Compounds in Lactic Fermentations. Annu. Rev. Food Sci. Technol. 2014, 5, 313–326. [Google Scholar] [CrossRef]
- Del Barrio-Galán, R.; del Valle-Herrero, H.; Bueno-Herrera, M.; López-De-La-Cuesta, P.; Pérez-Magariño, S. Volatile and Non-Volatile Characterization of White and Rosé Wines from Different Spanish Protected Designations of Origin. Beverages 2021, 7, 49. [Google Scholar] [CrossRef]
- Ikeda, R.M.; Webb, A.D.; Kepner, R.E. Wine Constituents, Comparative Analysis of Fusel Oils from Thompson Seedless, Emperor, and Muscat of Alexandria Wines. J. Agric. Food Chem. 1956, 4, 355–363. [Google Scholar] [CrossRef]
- de Lorenzis, G.; Squadrito, M.; Rossoni, M.; di Lorenzo, G.S.; Brancadoro, L.; Scienza, A. Study of intra-varietal diversity in biotypes of Aglianico and Muscat of Alexandria (Vitis vinifera L.) cultivars. Aust. J. Grape Wine Res. 2016, 23, 132–142. [Google Scholar] [CrossRef]
- Strauss, C.R.; Gooley, P.R.; Wilson, B.; Williams, P.J. Application of droplet countercurrent chromatography to the analysis of conjugated forms of terpenoids, phenols, and other constituents of grape juice. J. Agric. Food Chem. 1987, 35, 519–524. [Google Scholar] [CrossRef]
- Pinto, J.; Oliveira, A.S.; Azevedo, J.; Freitas, V.; Lopes, P.; Roseira, I.; Cabral, M.; de Pinho, P.G. Assessment of oxidation compounds in oaked Chardonnay wines: A GC–MS and 1 H NMR metabolomics approach. Food Chem. 2018, 257, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.D.; Kepner, R.E. Some Volatile Aroma Constituents of Vitis vinifera var. Muscat of Alexandria. J. Food Sci. 1957, 22, 384–394. [Google Scholar] [CrossRef]
- Mouskeftara, T.; Virgiliou, C.; Theodoridis, G.; Gika, H. Analysis of urinary organic acids by gas chromatography tandem mass spectrometry method for metabolic profiling applications. J. Chromatogr. A 2021, 1658, 462590. [Google Scholar] [CrossRef]
- Abbiss, H.; Rawlinson, C.; Maker, G.L.; Trengove, R. Assessment of automated trimethylsilyl derivatization protocols for GC–MS-based untargeted metabolomic analysis of urine. Metabolomics 2015, 11, 1908–1921. [Google Scholar] [CrossRef]
- Skogerson, K.; Runnebaum, R.; Wohlgemuth, G.; de Ropp, J.; Heymann, H.; Fiehn, O. Comparison of Gas Chromatography-Coupled Time-of-Flight Mass Spectrometry and 1H Nuclear Magnetic Resonance Spectroscopy Metabolite Identification in White Wines from a Sensory Study Investigating Wine Body. J. Agric. Food Chem. 2009, 57, 6899–6907. [Google Scholar] [CrossRef]
- Howell, K.S.; Cozzolino, D.; Bartowsky, E.J.; Fleet, G.H.; Henschke, P.A. Metabolic profiling as a tool for revealing Saccharomyces interactions during wine fermentation. FEMS Yeast Res. 2006, 6, 91–101. [Google Scholar] [CrossRef]
- Miranda, A.; Pereira, V.; Jardim, H.; Malfeito-Ferreira, M.; Marques, J.C. Impact of Non-Saccharomyces Yeast Fermentation in Madeira Wine Chemical Composition. Processes 2023, 11, 482. [Google Scholar] [CrossRef]
- International Code of Oenlogical Practices.pdf, (n.d.). Available online: https://www.oiv.int/sites/default/files/publication/2022-10/International%20Code%20of%20oenlogical%20practices.pdf (accessed on 30 March 2023).
- International-Oenological-Codex.pdf, (n.d.). Available online: https://www.oiv.int/sites/default/files/publication/2022-10/international-oenological-codex.pdf (accessed on 30 March 2023).
- Technical_Review_Issue_223_Wilkes.pdf, (n.d.). Available online: https://www.awri.com.au/wp-content/uploads/2011/07/Technical_Review_Issue_223_Wilkes.pdf (accessed on 30 March 2023).
- Balmaseda, A.; Aniballi, L.; Rozès, N.; Bordons, A.; Reguant, C. Use of Yeast Mannoproteins by Oenococcusoeni during Malolactic Fermentation under Different Oenological Conditions. Foods 2021, 10, 1540. [Google Scholar] [CrossRef]
- Karpe, A.V.; Beale, D.; Morrison, P.D.; Harding, I.H.; Palombo, E.A. Untargeted metabolic profiling of Vitis vinifera during fungal degradation. FEMS Microbiol. Lett. 2015, 362, fnv060. [Google Scholar] [CrossRef]
- Karpe, A.V.; Beale, D.J.; Godhani, N.B.; Morrison, P.D.; Harding, I.H.; Palombo, E.A. Untargeted metabolic profiling of winery-derived biomass waste degradation by Aspergillus niger. J. Chem. Technol. Biotechnol. 2015, 91, 1505–1516. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. Mixed alcoholic fermentation of Schizosaccharomyces pombe and Lachanceathermotolerans and its influence on mannose-containing polysaccharides wine Composition. AMB Express 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Lapuente, L.M.; Guadalupe, Z.; Ayestarán, B.; Pérez-Porras, P.; Bautista-Ortín, A.B.; Gómez-Plaza, E. Ultrasound treatment of crushed grapes: Effect on the must and red wine polysaccharide composition. Food Chem. 2021, 356, 129669. [Google Scholar] [CrossRef] [PubMed]
- Dupin, I.V.S.; McKinnon, B.M.; Ryan, C.; Boulay, M.; Markides, A.J.; Jones, G.P.; Williams, P.J.; Waters, E.J. Saccharomyces cerevisiae Mannoproteins that Protect Wine from Protein Haze: Their Release during Fermentation and Lees Contact and a Proposal for Their Mechanism of Action. J. Agric. Food Chem. 2000, 48, 3098–3105. [Google Scholar] [CrossRef]
- Marangon, M.; Vegro, M.; Vincenzi, S.; Lomolino, G.; De Iseppi, A.; Curioni, A. A Novel Method for the Quantification of White Wine Mannoproteins by a Competitive Indirect Enzyme-Linked Lectin Sorbent Assay (CI-ELLSA). Molecules 2018, 23, 3070. [Google Scholar] [CrossRef]
- Gonçalves, F.; Heyraud, A.; de Pinho, M.N.; Rinaudo, M. Characterization of White Wine Mannoproteins. J. Agric. Food Chem. 2002, 50, 6097–6101. [Google Scholar] [CrossRef]
- Cuadros-Inostroza, A.; Ruíz-Lara, S.; González, E.; Eckardt, A.; Willmitzer, L.; Peña-Cortés, H. GC–MS metabolic profiling of Cabernet Sauvignon and Merlot cultivars during grapevine berry development and network analysis reveals a stage- and cultivar-dependent connectivity of primary metabolites. Metabolomics 2016, 12, 39. [Google Scholar] [CrossRef]
- Degu, A.; Hochberg, U.; Sikron, N.; Venturini, L.; Buson, G.; Ghan, R.; Plaschkes, I.; Batushansky, A.; Chalifa-Caspi, V.; Mattivi, F.; et al. Metabolite and transcript profiling of berry skin during fruit development elucidates differential regulation between Cabernet Sauvignon and Shiraz cultivars at branching points in the polyphenol pathway. BMC Plant Biol. 2014, 14, 188. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Clingeleffer, P.R.; Kerridge, G.; Rühl, E.; Nicholas, P.; Blackmore, D. Effects of the rootstock Ramsey (Vitis champini) on ion and organic acid composition of grapes and wine, and on wine spectral characteristics. Aust. J. Grape Wine Res. 1998, 4, 100–110. [Google Scholar] [CrossRef]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The grape must non-Saccharomyces microbial community: Impact on volatile thiol release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Pinu, F.R.; Edwards, P.J.; Gardner, R.C.; Villas-Boas, S.G. Nitrogen and carbon assimilation by Saccharomyces cerevisiae during Sauvignon blanc juice fermentation. FEMS Yeast Res. 2014, 14, 1206–1222. [Google Scholar] [CrossRef]
- Marinaki, M.; Sampsonidis, I.; Lioupi, A.; Arapitsas, P.; Thomaidis, N.; Zinoviadou, K.; Theodoridis, G. Development of two-level Design of Experiments for the optimization of a HS-SPME-GC-MS method to study Greek monovarietal PDO and PGI wines. Talanta 2023, 253, 123987. [Google Scholar] [CrossRef]
- Deda, O.; Kachrimanidou, M.; Armitage, E.G.; Mouskeftara, T.; Loftus, N.J.; Zervos, I.; Taitzoglou, I.; Gika, H. Metabolic Phenotyping Study of Mouse Brain Following Microbiome Disruption by C. difficile Colonization. Metabolites 2022, 12, 1039. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinaki, M.; Mouskeftara, T.; Arapitsas, P.; Zinoviadou, K.G.; Theodoridis, G. Metabolic Fingerprinting of Muscat of Alexandria Grape Musts during Industrial Alcoholic Fermentation Using HS-SPME and Liquid Injection with TMS Derivatization GC-MS Methods. Molecules 2023, 28, 4653. https://doi.org/10.3390/molecules28124653
Marinaki M, Mouskeftara T, Arapitsas P, Zinoviadou KG, Theodoridis G. Metabolic Fingerprinting of Muscat of Alexandria Grape Musts during Industrial Alcoholic Fermentation Using HS-SPME and Liquid Injection with TMS Derivatization GC-MS Methods. Molecules. 2023; 28(12):4653. https://doi.org/10.3390/molecules28124653
Chicago/Turabian StyleMarinaki, Maria, Thomai Mouskeftara, Panagiotis Arapitsas, Kyriaki G. Zinoviadou, and Georgios Theodoridis. 2023. "Metabolic Fingerprinting of Muscat of Alexandria Grape Musts during Industrial Alcoholic Fermentation Using HS-SPME and Liquid Injection with TMS Derivatization GC-MS Methods" Molecules 28, no. 12: 4653. https://doi.org/10.3390/molecules28124653
APA StyleMarinaki, M., Mouskeftara, T., Arapitsas, P., Zinoviadou, K. G., & Theodoridis, G. (2023). Metabolic Fingerprinting of Muscat of Alexandria Grape Musts during Industrial Alcoholic Fermentation Using HS-SPME and Liquid Injection with TMS Derivatization GC-MS Methods. Molecules, 28(12), 4653. https://doi.org/10.3390/molecules28124653








