Are (Co)Polymers of 1,1,3,3,3-Pentafluoropropene Possible?
Abstract
1. Introduction
2. Results and Discussion
2.1. Radical Copolymerization of PFP
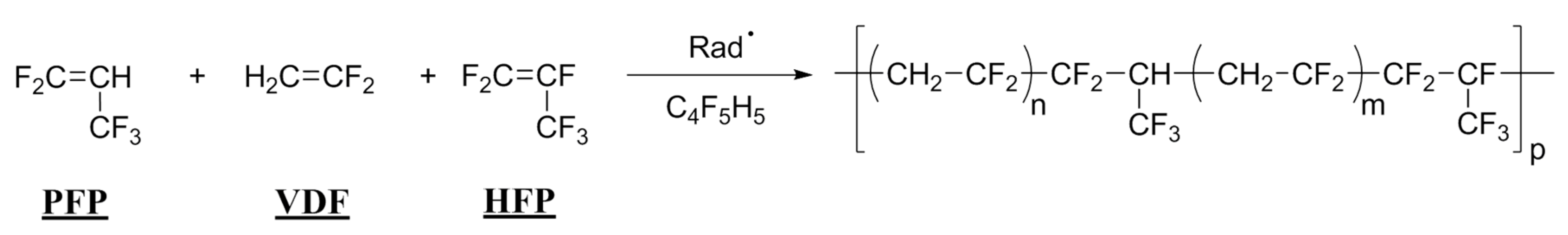
2.1.1. Radical Copolymerization of PFP with VDF
2.1.2. Radical Copolymerization of PFP with MAF-TBE
2.1.3. Radical Copolymerization of PFP with HFP
2.1.4. Radical Copolymerization of PFP with PMVE
2.1.5. Radical Copolymerization of PFP and TFP
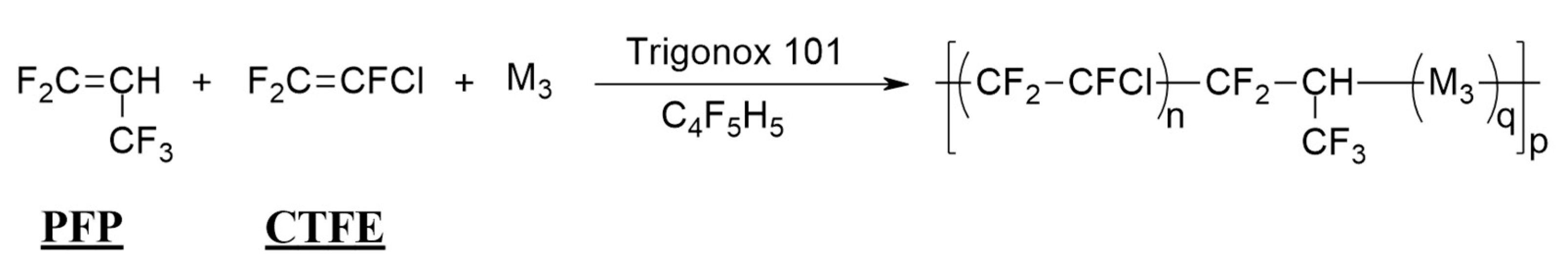
2.1.6. Radical Copolymerization of PFP with CTFE
2.1.7. Radical Copolymerization of VDF with MAF-TBE
2.1.8. Radical Copolymerization of VDF with HFP
2.2. Radical Terpolymerization of PFP with VDF and Fluorinated M3 Monomer
2.2.1. Radical Terpolymerization of PFP with VDF and MAF-TBE
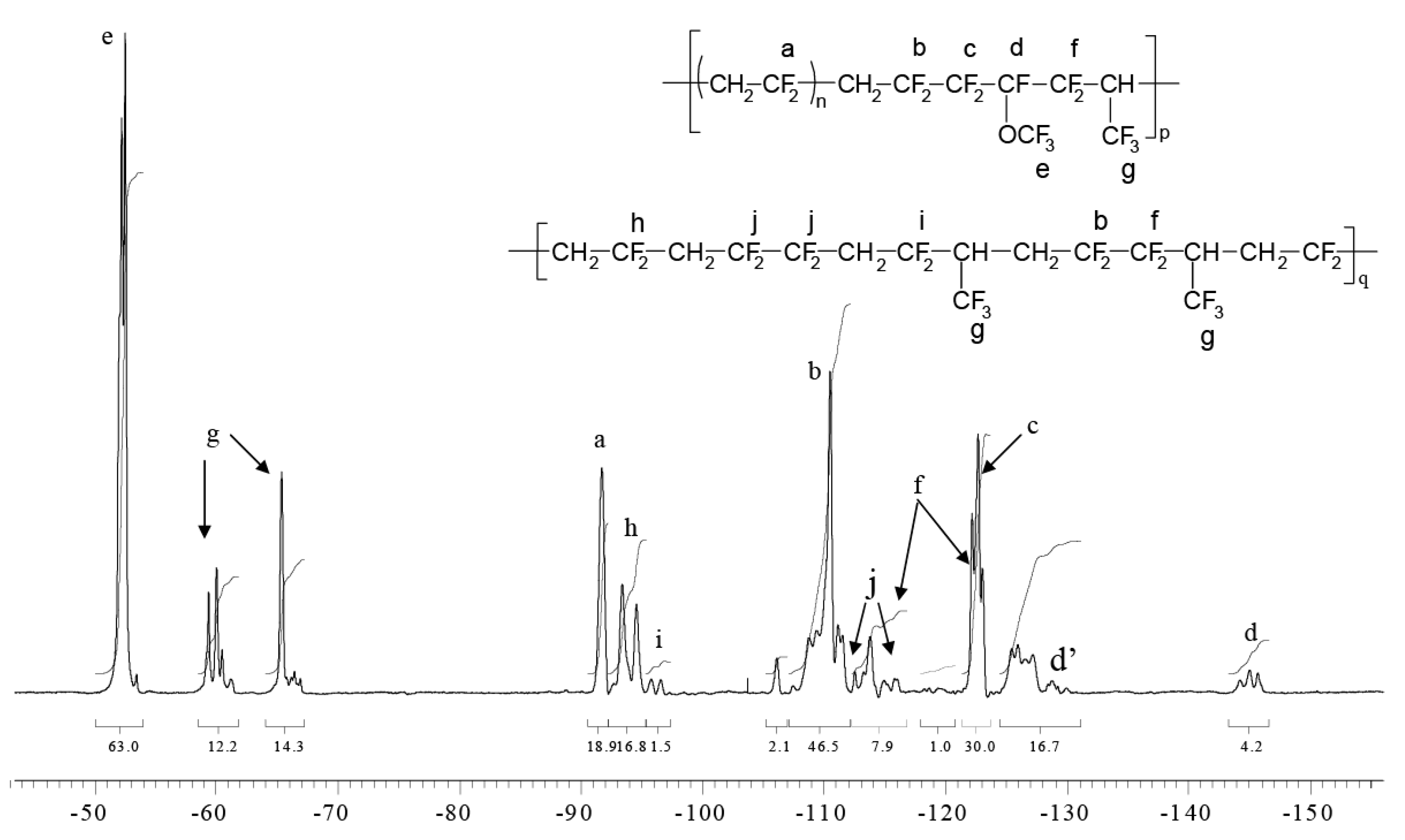
2.2.2. Radical Terpolymerization of PFP with VDF and HFP
2.2.3. Radical Terpolymerization of PFP with VDF and PMVE
2.2.4. Radical Terpolymerization of PFP with VDF and TFP
2.2.5. Radical Terpolymerization of PFP with CTFE and M3 Monomer
2.2.6. Radical Terpolymerization of PFP with CTFE and m-TMI
2.2.7. Radical Terpolymerization of PFP with CTFE and VDF
2.2.8. Radical Terpolymerization of PFP with CTFE and VCA
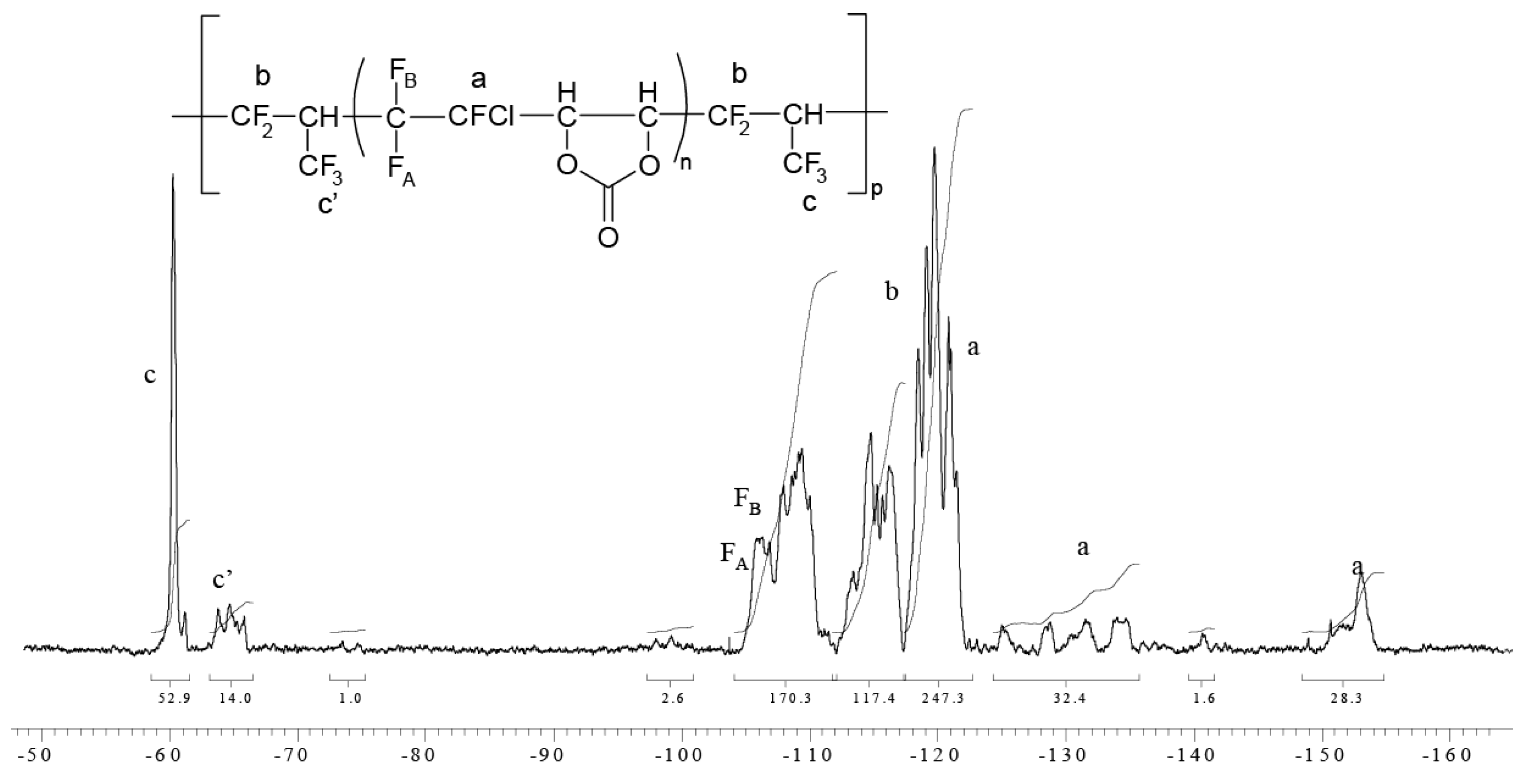
2.2.9. Radical Terpolymerization of PFP with CTFE and EVE
2.3. Thermal Properties of Co- and Terpolymers
3. Experimental Section
Materials
4. Characterization
4.1. NMR
4.2. SEC
4.3. FTIR
4.4. TGA
4.5. DSC
5. Synthesis
5.1. Autoclave
5.2. Radical Copolymerization of PFP
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ameduri, B.; Boutevin, B. Well-Architectured Fluoropolymers: Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Haszeldine, R.N. Reactions of fluorocarbon radicals. V. Alternative syntheses for (trifluoroethyl)acetylene (3,3,3-trifluoropropyne) and the influence of polyfluoro groups on the adjacent hydrogen and halogen atoms. J. Chem. Soc. Abstr. 1951, 2495–2504. [Google Scholar] [CrossRef]
- Maxson, M.T.; Norris, A.W.; Owen, M.J. Fluorosilicones. In Modern Fluoropolymers; Scheirs, J., Ed.; Wiley: New York, NY, USA, 1997; Chapter 20; pp. 359–372. [Google Scholar]
- Boschet, F.; Kostov, G.; Raynova, H.; Ameduri, B. Telomers of 1,1,3,3,3-pentafluoropropylene. Eur. Polym. J. 2015, 73, 487–499. [Google Scholar] [CrossRef]
- Rexford, D.R. Copolymer Elastomer of Vinylidenefluoride and Hexafluoropropene. US3051677A, 28 August 1962. [Google Scholar]
- Dixon, S.; Rexford, D.R.; Rugg, J.S. Vinylidene Fluoride—Hexafluoropropylene Copolymer. Indus. Eng. Chem. 1957, 49, 1687–1690. [Google Scholar] [CrossRef]
- Dittman, A.; Passino, H.J.; Wrightson, J.M. Polymerization of Fluorine-Containing Monomers in Aqueous Redox System. US-1954/2,689,241, 14 September 1954. [Google Scholar]
- Sianesi, D.; Bernardi, C.; Regio, A. Copolymers of Vinylidene Fluoride with 1, 2, 3, 3, 3-pentafluoropropylene. US-1967/3,331,823, 18 July 1967. [Google Scholar]
- Sianesi, D.; Bernardi, C.; Diotallevi, G. Copolymers from 1, 2, 3, 3, 3-pentafluoropropylene and Tetrafluoroethylene and Process for Preparing Same. US-1967/3350373A, 31 October 1967. [Google Scholar]
- Sianesi, D.; Caporiccio, G. Process for the Polymerization of Fluorinated Olefins and Polymeric Products Obtained therefrom. US-1962/3,287,339, 22 November 1966. [Google Scholar]
- Bolstad, A. Polymerization Process for Unsaturated Fluoroolefins. US-1964/3,163,628, 29 December 1964. [Google Scholar]
- Duvalsaint, F.; Moore, A.L. Process for Producing Fluorelastomers. US-2001/6,277,937, 21 August 2001. [Google Scholar]
- Schmiegel, W.W.; Tang, P.L. Curable Base-Resistant Fluoroelastomers. WO-2002/092687A1, 9 March 2002. [Google Scholar]
- Sato, H.; Sawada, Y.; Kasai, S. Modified Polytetrafluoroethylene Particles, Methods for Producing the Same and Modified Polytetrafluoroethylene Molded Product. US-2015/8,969,432B2, 3 March 2015. [Google Scholar]
- Usmanov, K.U.; Yul’Chibaev, A.A.; Mukhamadaliev, N.; Sarros, T.K. Radiation copolymerization of 2-hydropentafluoropropylene with vinylidene fluoride. Izv. Vys. Zhim. Khim. Tekhnol. 1975, 18, 464–472. [Google Scholar]
- Otazaghine, B.; Sauguet, L.; Ameduri, B. Synthesis and copolymerisation of fluorinated monomers bearing a reactive lateral group: Part 21. Radical copolymerisation of vinylidene fluoride with 2-hydroperfluorooct-1-ene. J. Fluor. Chem. 2005, 126, 1009–1016. [Google Scholar] [CrossRef]
- Asandei, A.D.; Adebolu, O.I.; Simpson, C.P. Mild-Temperature Mn2(CO)10-Photomediated Controlled Radical Polymerization of Vinylidene Fluoride and Synthesis of Well-Defined Poly(vinylidene fluoride) Block Copolymers. J. Am. Chem. Soc. 2012, 134, 6080–6083. [Google Scholar] [CrossRef]
- Asandei, A.D.; Adebolu, O.I.; Simpson, C.P.; Kim, J.-S. Visible-Light Hypervalent Iodide Carboxylate Photo(trifluoro)methylations and Controlled Radical Polymerization of Fluorinated Alkenes. Angew. Chem. Int. Ed. 2013, 52, 10027–10030. [Google Scholar] [CrossRef]
- Twum, E.B.; Gao, C.; Li, X.; McCord, E.F.; Fox, P.A.; Lyons, D.F.; Rinaldi, P.L. Characterization of the Chain-Ends and Branching Structures in Polyvinylidene Fluoride with Multidimensional NMR. Macromolecules 2012, 45, 5501–5512. [Google Scholar] [CrossRef]
- Guiot, J.; Ameduri, B.; Boutevin, B. Radical Homopolymerization of Vinylidene Fluoride Initiated by tert-Butyl Peroxypivalate. Investigation of the Microstructure by 19F and 1H NMR Spectroscopies and Mechanisms. Macromolecules 2002, 35, 8694–8707. [Google Scholar]
- Ameduri, B.; Bauduin, G.; Boutevin, B.; Kostov, G.K.; Petrova, P. Synthesis and Polymerization of Fluorinated Monomers Bearing a Reactive Lateral Group. 9. Bulk copolymerization of Vinylidene Fluoride with 4,5,5-Trifluoro-4-ene Pentyl Acetate. Macromolecules 1999, 32, 4544–4550. [Google Scholar] [CrossRef]
- Ameduri, B. Copolymers of vinylidene fluoride with functional comonomers and applications therefrom: Recent developments, challenges and future trends. Prog. Polym. Sci. 2022, 133, 101591. [Google Scholar] [CrossRef]
- Aglietto, M.; Passaglia, E.; di Mirabello, L.M.; Botteghi, C.; Paganelli, S.; Matteoli, U.; Menchi, G. Synthesis of new polymers containing α-(trifluoromethyl)-acrylate units. Macromol. Chem. Phys. 1995, 196, 2843–2853. [Google Scholar] [CrossRef]
- Balagué, J.; Améduri, B.; Boutevin, B.; Caporiccio, G. Synthèse de télomères fluorés. Partie III. Télomérisation de l’hexafluoropropène avec des iodures de perfluoroalkyle. J. Fluor. Chem. 1995, 74, 49–58. [Google Scholar] [CrossRef]
- Souzy, R.; Ameduri, B.; Boutevin, B. Radical copolymerization of a-trifluoromethylacrylic acid with vinylidene fluoride and vinylidene fluoride/hexafluoropropene. Macromol. Chem. Phys. 2004, 205, 476–485. [Google Scholar] [CrossRef]
- Tournut, C. New Copolymers of vinylidene fluoride. Macromol. Symp. 1994, 82, 99–110. [Google Scholar] [CrossRef]
- Schmiegel, W.W. Crosslinking of Elastomeric Vinylidene Fluoride Copolymers with Nucleophiles. Angew. Makromol. Chem. 1979, 76, 39–65. [Google Scholar] [CrossRef]
- Bargain, F.; Soulestin, T.; Domingues Dos Santos, F.; Ladmiral, V.; Améduri, B.; Tencé-Girault, S. Semicrystalline Organization of VDF- and TrFE-Based Electroactive Terpolymers: Impact of the trans-1,3,3,3-Tetrafluoropropene Termonomer. Macromolecules 2017, 50, 3313–3322. [Google Scholar] [CrossRef]
- Otazaghine, B.; Sauguet, L.; Boucher, M.; Ameduri, B. Radical copolymerization of vinylidene fluoride with perfluoroalkylvinyl ethers. Eur. Polym. J. 2005, 41, 1747–1756. [Google Scholar] [CrossRef]
- Boyer, C.; Améduri, B.; Hung, M.H. Telechelic Diiodopoly(VDF-co-PMVE) Copolymers by Iodine Transfer Copolymerization of Vinylidene Fluoride (VDF) with Perfluoromethyl Vinyl Ether (PMVE). Macromolecules 2010, 43, 3652–3663. [Google Scholar] [CrossRef]
- Kostov, G.K.; Ameduri, B.; Brandstadter, S.M. Telomerization of 3,3,3-trifluoroprop-1-ene and functionaolization of its telomers. Coll. Czech. Chem. Commun. 2008, 73, 1747–1763. [Google Scholar] [CrossRef]
- Kostov, G.; Ameduri, B. Telomerisation Reaction of 3,3,3-Trifluoropropene. In Modern Synthesis Processes and Reactivity of Fluorinated Compounds; Groult, H., Leroux, F., Tressaud, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Chapter 18; pp. 533–559. [Google Scholar]
- Boschet, F.; Kostov, G.; Ameduri, B.; Jackson, A.; Boutevin, B. Synthesis of 3,3,3-trifluoropropene telomers and their modification into fluorosurfactants. Polym. Chem. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Soulestin, T.; Ladmiral, V.; Lannuzel, T.; Dos Santos, F.D.; Ameduri, B. Importance of Microstructure Control for Designing New Electroactive Terpolymers Based on Vinylidene Fluoride and Trifluoroethylene. Macromolecules 2015, 48, 7861–7871. [Google Scholar] [CrossRef]
- Boschet, F.; Ameduri, B. (Co)polymers of Chlorotrifluoroethylene: Synthesis, Properties, and Applications. Chem. Rev. 2014, 114, 927–980. [Google Scholar] [CrossRef] [PubMed]
- Takakura, T. CTFE/vinyl ether copolymers. In Modern Fluoropolymers; Scheirs, J., Ed.; Wiley Interscience: New York, NY, USA, 1997; Chapter 29; pp. 557–564. [Google Scholar]
- Kyulavska, M.; Kostov, G.; Ameduri, B.; Mateva, R. Unexpected alternating radical copolymerization of chlorotrifluoroethylene with 3-isopropenyl-α,α’-dimethylbenzyl isocyanate. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2681–2697. [Google Scholar] [CrossRef]
- Weise, J.K. Termonomer induced copolymerization of methyl methacrylate and tetrafluoroethylene. Polym. Prepr. (ACS Div. Polym. Chem.) 1971, 12, 512–520. [Google Scholar]
- Tiers, G.V.D.; Bovey, B.A. Polymer NMR spectroscopy: VII. The stereochemical configuration of poly(trifluorochloroethylene). J. Polym. Sci. Part A Gen. Pap. 1963, 1, 833–841. [Google Scholar] [CrossRef]
- Kumar, K.S.S.; Ameduri, B. Synthesis and characterization of epoxy functionalized cooligomers based on chlorotrifluoroethylene and allyl glycidyl ether. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3587–3595. [Google Scholar] [CrossRef]
- Falireas, P.G.; Wehbi, M.; Alaaeddine, A.; Améduri, B. Kinetics of radical copolymerization of vinylidene fluoride with tert-butyl 2-trifluoromethyl acrylate: A suitable pair for the synthesis of alternating fluorinated copolymers. Polym. Chem. 2018, 9, 3754–3761. [Google Scholar] [CrossRef]
- Patil, Y.; Ameduri, B. Advances in the (co)polymerization of alkyl 2-trifluoromethacrylates and 2-(trifluoromethyl)acrylic acid. Prog. Polym. Sci. 2013, 38, 703–739. [Google Scholar] [CrossRef]
- Banerjee, S.; Wehbi, M.; Manseri, A.; Mehdi, A.; Alaaeddine, A.; Hachem, A.; Ameduri, B. Poly(vinylidene fluoride) Containing Phosphonic Acid as Anticorrosion Coating for Steel. ACS Appl. Mater. Interfaces 2017, 9, 6433–6443. [Google Scholar] [CrossRef]
- Wehbi, M.; Banerjee, S.; Mehdi, A.; Alaaeddine, A.; Hachem, A.; Ameduri, B. Vinylidene Fluoride-Based Polymer Network via Cross-Linking of Pendant Triethoxysilane Functionality for Potential Applications in Coatings. Macromolecules 2017, 50, 9329–9339. [Google Scholar] [CrossRef]
- Wehbi, M.; Dolphijn, G.; Brassinne, J.; Gohy, J.-F.; Ameduri, B. Synthesis of Vinylidene Fluoride-Based Copolymers Bearing Perfluorinated Ether Pendant Groups and Their Application in Gel Polymer Electrolytes. Macromolecules 2019, 52, 3056–3065. [Google Scholar] [CrossRef]
- Boyer, C.; Ameduri, B. Iodine transfer copolymerization of vinylidene fluoride and a-trifluoromethacrylic acid in emulsion process without any surfactants. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 4710–4722. [Google Scholar] [CrossRef]
- Tournut, C. Thermoplastic Copolymers of Vinylidene Fluoride. In Modern Fluoropolymers; Scheirs, J., Ed.; Wiley Interscience: New York, NY, USA, 1997; Chapter 31; pp. 577–596. [Google Scholar]
- Ameduri, B.; Boutevin, B.; Kostov, G.K. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Moore, A.L. Fluoroelastomers Handbook; the Definitive User’s Guide and Data Book; Plastic Design Library, William Andrew Publishing: Norwich, NY, USA, 2006. [Google Scholar]
- Ameduri, B.; Sawada, H. Fluorinated Polymers: Volume 1: Synthesis, Properties, Processing and Simulation; RSC: Oxford, UK, 2017; Volume 1. [Google Scholar]
- Ameduri, B.; Sawada, H. Fluorinated Polymers: Volume 2: Applications; RSC: Oxford, UK, 2017. [Google Scholar]
- Ameduri, B.; Fomin, S. Fascinating Fluoropolymers and Their Applications; Progress in Fluorine Science Vol. 2; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Apostolo, M.; Arcella, V.; Storti, G.; Morbidelli, M. Kinetics of the Emulsion Polymerization of Vinylidene Fluoride and Hexafluoropropylene. Macromolecules 1999, 32, 989–1003. [Google Scholar] [CrossRef]
- Moggi, G.; Bonardelli, P.; Bart, J.C.J. Synthesis and properties of some hexafluoropropene-1, 1-difluoroethene copolymers. Polym. Bull. 1982, 7, 115. [Google Scholar] [CrossRef]
- Patil, Y.; Ameduri, B. First RAFT/MADIX radical copolymerization of tert-butyl 2-trifluoromethacrylate with vinylidene fluoride controlled by xanthat. Polym. Chem. 2013, 4, 2783–2799. [Google Scholar] [CrossRef]
- Banerjee, S.; Soulestin, T.; Patil, Y.; Ladmiral, V.; Ameduri, B. Towards new strategies for the synthesis of functional vinylidene fluoride-based copolymers with tunable wettability. Polym. Chem. 2016, 7, 4004–4015. [Google Scholar] [CrossRef]
- Walkowiak-Kulikowska, J.; Boschet, F.; Kostov, G.; Gouverneur, V.; Ameduri, B. On the reactivity of α-trifluoromethylstyrene in radical copolymerizations with various fluoroalkenes. Eur. Polym. J. 2016, 84, 612–621. [Google Scholar] [CrossRef]
- Sawada, H.; Tashima, T.; Nishiyama, Y.; Kikuchi, M.; Goto, Y.; Kostov, G.; Ameduri, B. Iodine Transfer Terpolymerization of Vinylidene Fluoride, α-Trifluoromethacrylic Acid and Hexafluoropropylene for Exceptional Thermostable Fluoropolymers/Silica Nanocomposites. Macromolecules 2011, 44, 1114–1124. [Google Scholar] [CrossRef]
- Nakamura, T.; Busfield, W.K.; Jenkins, I.D.; Rizzardo, E.; Thang, S.H.; Suyama, S. Initiation Mechanisms for Radical Polymerization of Methyl Methacrylate with tert-Butyl Peroxypivalate. J. Am. Chem. Soc. 1996, 118, 10824–10828. [Google Scholar] [CrossRef]
- Loginova, N.N.; Podlesskaya, N.K.; Berezina, G.G. USSR Plast. Massy 1990, 19, 102888. [Google Scholar]
- Wadekar, M.N.; Patil, Y.R.; Ameduri, B. Superior Thermostability and Hydrophobicity of Poly(vinylidene fluoride-co-fluoroalkyl 2-trifluoromethacrylate). Macromolecules 2014, 47, 13–25. [Google Scholar] [CrossRef]
- Carnevale, D.; Wormald, P.; Ameduri, B.; Tayouo, R.; Ashbrook, S.E. Multinuclear Magnetic Resonance and DFT Studies of the Poly(chlorotrifluoroethylene-alt-ethyl vinyl ether) Copolymers. Macromolecules 2009, 42, 5652–5659. [Google Scholar] [CrossRef]
- Tayouo, R.; David, G.; Ameduri, B.; Roualdès, S.; Rozière, J. New fluorinated polymers bearing pendant phosphonic acid groups. Proton conductivity for fuel cell membranes: Part 2. Synthesis and Characterizations of the fluorinated polymeric backbone. Macromolecules 2010, 43, 5269–5276. [Google Scholar] [CrossRef]
- Boutevin, B.; Cersosimo, F.; Youssef, B. Studies of the alternating copolymerization of vinyl ethers with chlorotrifluoroethylene. Macromolecules 1992, 25, 2842–2846. [Google Scholar] [CrossRef]
- Kostov, G.; Rousseau, A.; Boutevin, B.; Pascal, T. Novel fluoroacrylated copolymers: Synthesis, characterization and properties. J. Fluor. Chem. 2005, 126, 231–240. [Google Scholar] [CrossRef]
- Moggi, G.; Bonardelli, P.; Bart, J.C. Copolymers of 1,1-difluoroethene with tetrafluoroethene, chlorotrifluoroethene, and bromotrifluoroethene. J. Polym. Sci. Part B Polym. Phys. 1984, 22, 357–365. [Google Scholar] [CrossRef]
- Dohany, J.E.; Humphrey, J.S. Vinylidene Fluoride Polymers. In Encyclopedia in Polymer Sciences and Engineering; Mark, H.F., Bikales, N.M., Overberger, C.G., Menges, G., Eds.; Wiley: New York, NY, USA, 1989; Chapter 17; pp. 532–548. [Google Scholar]
- Alaaeddine, A.; Boschet, F.; Ameduri, B.; Boutevin, B. New Fluoropolymers Bearing Glycidyl Carbonate Side-groups as Polymer Electrolytes for Lithium Ion Batteries. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3303–3312. [Google Scholar] [CrossRef]
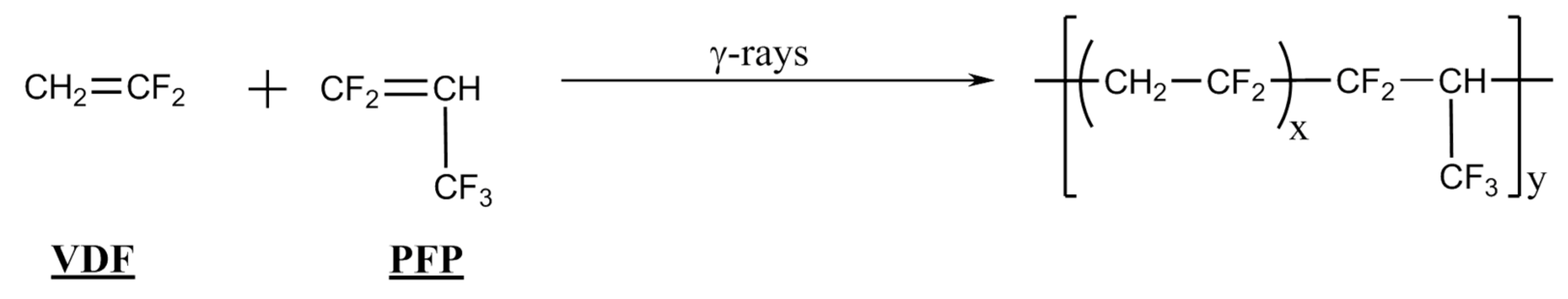

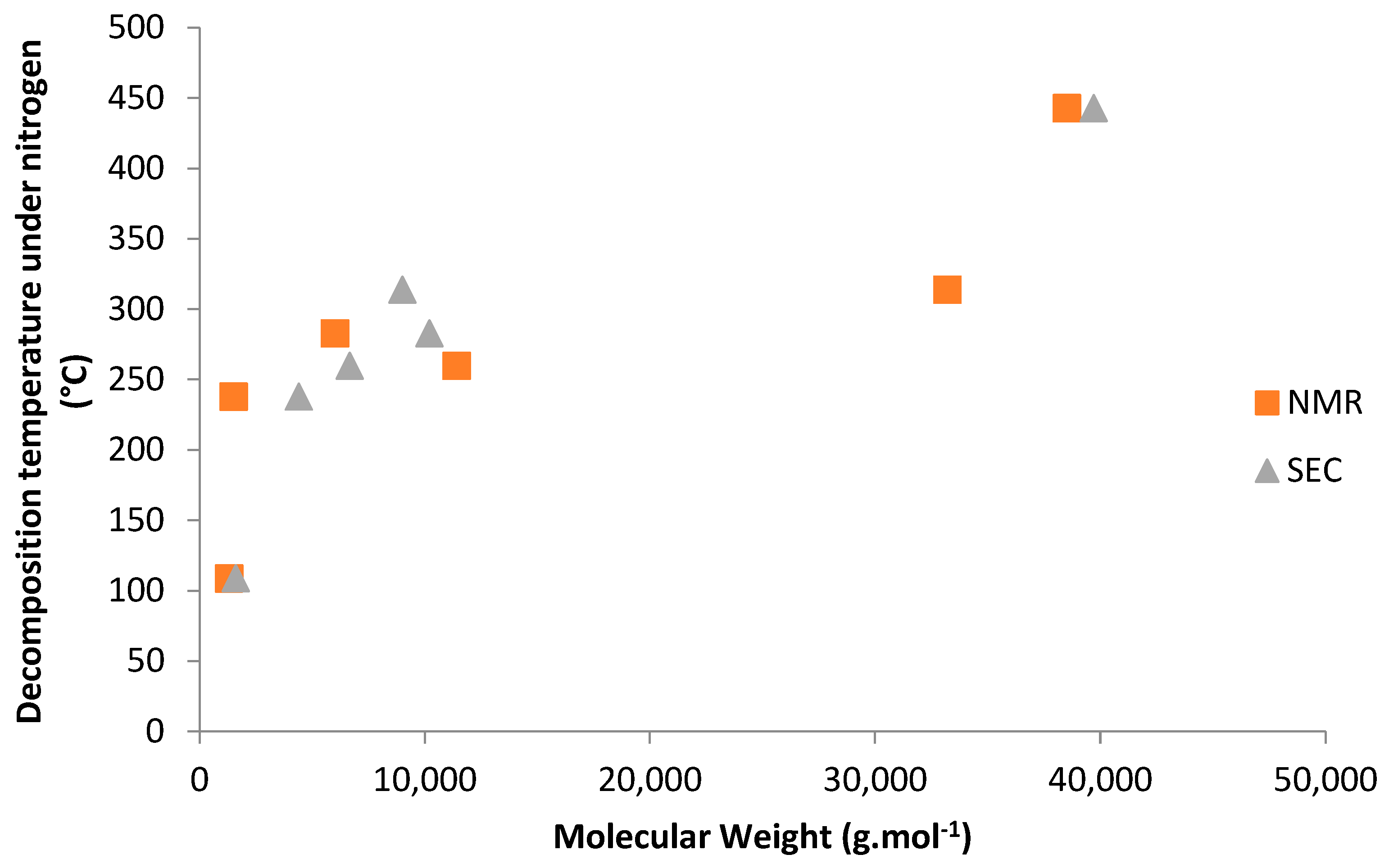
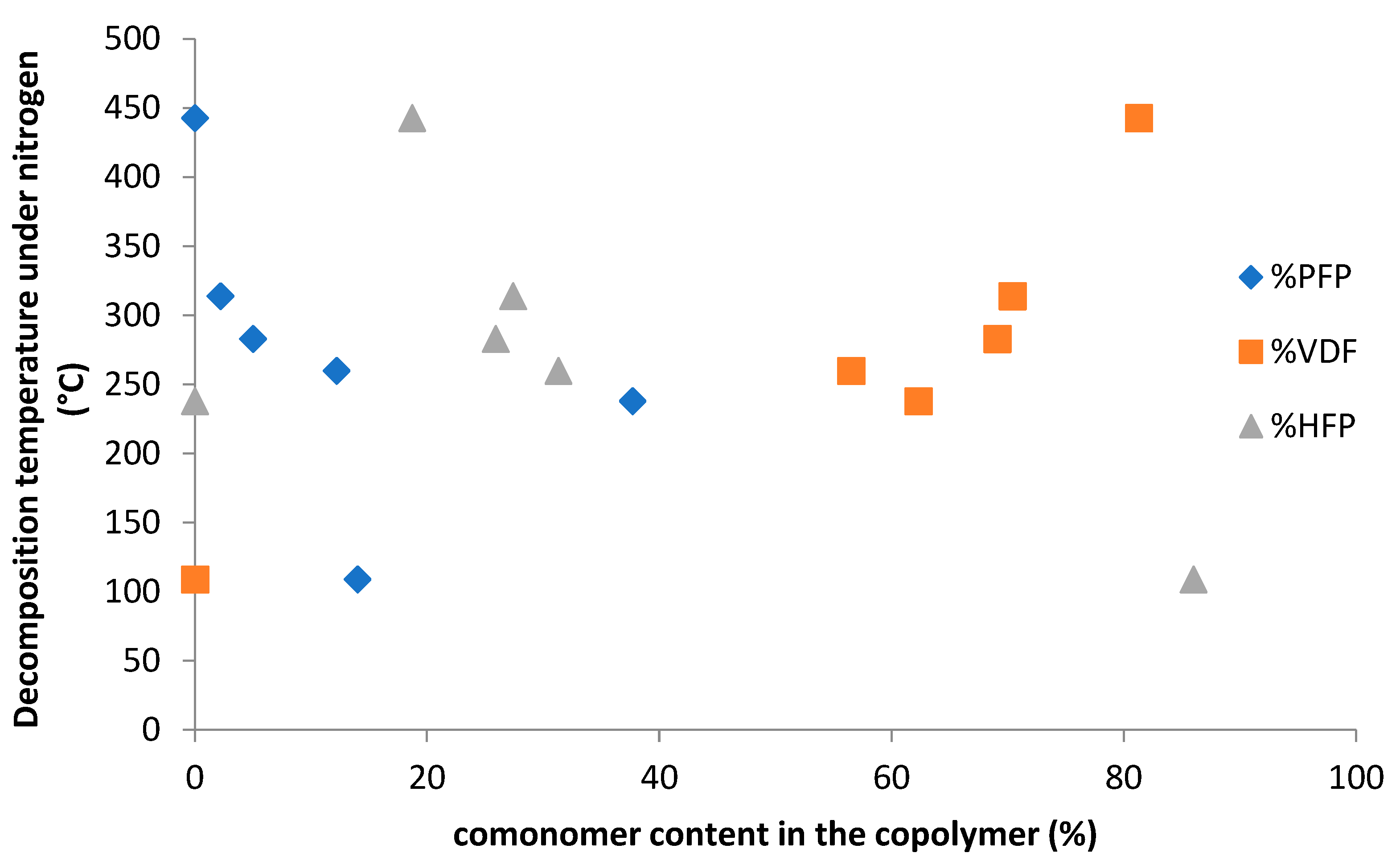


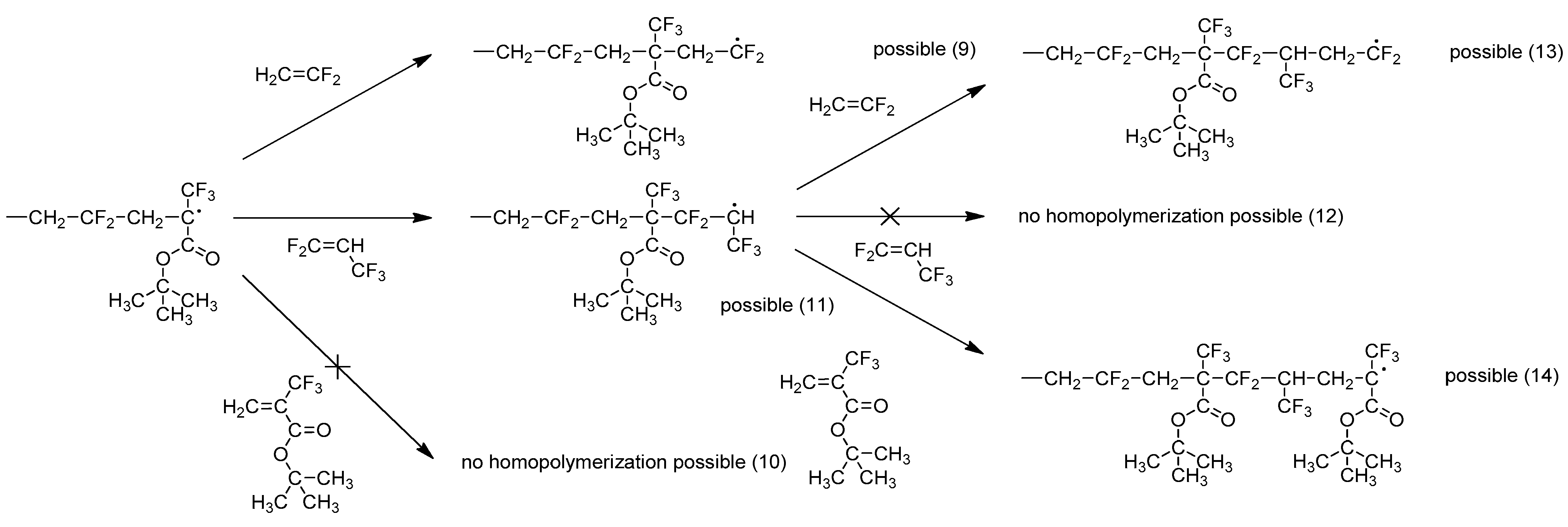



| Entry | Feed Composition (mol%) | Solvent | Initiators a). | C0 | T (°C) | τconv. (wt%) | Yield (wt%) | Copolymer Composition (mol%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFP | VDF | MAF-TBE | PFP | VDF | MAF-TBE | |||||||
| 1 | 80 | 20 | - | C4F5H5 | Trig. 101 | 0.020 | 130 | 32 | 11 | 37.7 | 62.3 | 0 |
| 2 | 50 | - | 50 | CH3CN | Trig. 101 | 0.030 | 130 | 35 | 14 | 1.3 | 0 | 98.7 |
| 3 | - | 74 | 26 | C4F5H5 | Trig. 101 | 0.020 | 130 | 88 | 84 | 0 | 43.0 | 57.0 |
| 4 | 80 | - | 20 | CH3CN | Trig. 101 | 0.030 | 130 | 20 | 10 | 5.4 | 0 | 94.6 |
| 5 | 80 | - | 20 | C4F5H5 | Trig. 101 | 0.020 | 130 | 35 | 26 | 0.9 | 0 | 99.1 |
| 6 | 68.7 | 21.3 | 10.0 | C4F5H5 | Trig.101/DTBP | 0.015 | 130/140 | 30 | 16 | 4.8 | 62.5 | 32.7 |
| 7 | 50 | 35 | 15 | CH3CN | Trig.101/DTBP | 0.015 | 130/140 | 45 | 20 | 8.6 | 72.9 | 8.5 |
| 8 | 50 | 35 | 15 | C4F5H5 | Trig.101/DTBP | 0.015 | 130/140 | 21 | 20 | 5.1 | 41.5 | 53.4 |
| 9 | 25.5 | 67.2 | 7.3 | C4F5H5 | Trig.101/DTBP | 0.015 | 130/140 | 32 | 30 | 4.4 | 65.5 | 30.1 |
| 10 | 25 | 50 | 25 | C4F5H5 | Trig.101/DTBP | 0.015 | 130/140 | 49 | 47 | 8.0 | 42.0 | 50.0 |
| Entry | M3 | Feed Composition mol% | C0 | τconv. wt% | Yield wt% | Copolymer Composition mol% | Mn g·mol−1 | PDI | Tg °C | Td,10% °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFP | VDF | M3 | PFP | VDF | M3 | NMR | SEC | N2 | Air | |||||||
| 1 | HFP | 80 | 20 | 0 | 0.020 | 32 | 11 | 37.7 | 62.3 | 0 | 1500 | 4400 | 1.5 | −29 | 238 | 234 |
| 2 | HFP | 69.4 | 0 | 30.6 | 0.020 | 27 | 3 | 14.0 | 0 | 86.0 | 1300 | 1600 | 1.3 | −46 | 109 | 95 |
| 3 | HFP | 0 | 70.0 | 30.0 | 0.020 | 90 | 87 | 0 | 81.3 | 18.7 | 38,500 | 39,700 | 1.4 | −28 | 443 | 378 |
| 4 | HFP | 46.4 | 31.9 | 21.7 | 0.020 | 32 | 27 | 12.2 | 56.5 | 31.3 | 11,400 | 6660 | 1.5 | −34 | 260 | 257 |
| 5 | HFP | 21.2 | 52.6 | 26.2 | 0.017 | 81 | 62 | 5.0 | 69.1 | 25.9 | 6000 | 10,200 | 1.6 | −36 | 283 | 300 |
| 6 | HFP | 10.1 | 54.3 | 35.6 | 0.020 | 87 | 65 | 2.2 | 70.4 | 27.4 | 33,200 | 9000 | 1.8 | −33 | 314 | 317 |
| 7 | PMVE | 75.0 | 0 | 25.0 | 0.020 | 22 | 7 | 32.8 | 0 | 67.2 | 2090 | 1.2 | −56 | 118 | 119 | |
| 8 | PMVE | 50.0 | 30.0 | 20.0 | 0.020 | 47 | 35 | 14.7 | 50.2 | 35.1 | 6430 | 1.6 | −42 | 255 | 252 | |
| 9 | TFP | 75.0 | 0 | 25.0 | 0.020 | 43 | 12 | 18.9 | 0 | 81.1 | 1600 | 1.2 | −53 | 121 | 126 | |
| 10 | TFP | 50.0 | 30.0 | 20.0 | 0.020 | 30 | 12 | 10.7 | 40.7 | 48.6 | 4400 | 1.3 | −28 | 280 | 273 | |
| Entry | Copolymer Composition (mol%) | Mn SEC (g mol−1) | PDI | Tg (°C) | Td,10 (°C) | |||
|---|---|---|---|---|---|---|---|---|
| PFP | VDF | MAF-TBE | N2 | Air | ||||
| 1 | 37.7 | 62.3 | 0 | 4400 | 1.5 | −29 | 238 | 234 |
| 2 | 1.3 | 0 | 98.7 | 2000 | 1.1 | +4 | 150 | 156 |
| 3 | 0 | 43.0 | 57.0 | 1900 | 1.4 | +59 | 270 | 272 |
| 4 | 5.4 | 0 | 94.6 | - | - | - | - | - |
| 5 | 0.9 | 0 | 99.1 | 5300 | 2.1 | +53 | 198 | 195 |
| 6 | 4.5 | 65.5 | 30.0 | 1500 | 1.8 | - | 208 | 202 |
| 7 | 8.6 | 72.9 | 18.5 | 5900 | 2.5 | - | 236 | 239 |
| 8 | 5.1 | 41.5 | 53.4 | 3800 | 1.1 | - | 203 | 212 |
| 9 | 4.4 | 65.5 | 30.1 | 6100 | 1.3 | +49 | 262 | 263 |
| 10 | 8.0 | 42.0 | 50.0 | 1700 | 1.3 | +42 | 212 | 218 |
| Entry | M3 | Feed Composition (mol%) | τconv. (wt%) | Yield (wt%) | Copolymer Composition (mol%) | Mn (g mol−1) | PDI | Tg (°C) | Td,10 (°C) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFP | CTFE | M3 | PFP | CTFE | M3 | N2 | Air | |||||||
| 1 | - | 69 | 31 | 0 | 39 | 6 | 1.5 | 98.5 | 0 | 8500 | 1.2 | +8 | 271 | 282 |
| 2 | VDF | 50 | 20 | 30 | 50 | 24 | 0.70 | 46.5 | 52.8 | 990 | 1.8 | - | - | - |
| 3 | m-TMI | 50 | 30 | 20 | 45 | 30 | 34.9 | 60.2 | 4.9 | 2370 | 2.6 | −35 | 193 | 197 |
| 4 | VCA | 50 | 35 | 15 | 40 | 22 | 19.6 | 75.2 | 4.2 | 6840 | 1.9 | +49 | 313 | 312 |
| 5 | EVE | 50 | 35 | 15 | 22 | 14 | 1.6 | 79.3 | 19.1 | 37,120 | 1.6 | - | 323 | 308 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschet, F.; Kostov, G.; Raynova, H.; Améduri, B. Are (Co)Polymers of 1,1,3,3,3-Pentafluoropropene Possible? Molecules 2023, 28, 4618. https://doi.org/10.3390/molecules28124618
Boschet F, Kostov G, Raynova H, Améduri B. Are (Co)Polymers of 1,1,3,3,3-Pentafluoropropene Possible? Molecules. 2023; 28(12):4618. https://doi.org/10.3390/molecules28124618
Chicago/Turabian StyleBoschet, Frédéric, Georgi Kostov, Hristina Raynova, and Bruno Améduri. 2023. "Are (Co)Polymers of 1,1,3,3,3-Pentafluoropropene Possible?" Molecules 28, no. 12: 4618. https://doi.org/10.3390/molecules28124618
APA StyleBoschet, F., Kostov, G., Raynova, H., & Améduri, B. (2023). Are (Co)Polymers of 1,1,3,3,3-Pentafluoropropene Possible? Molecules, 28(12), 4618. https://doi.org/10.3390/molecules28124618








