Synergistic Influence of Yeast Extract and Calcium Oxide Nanoparticles on the Synthesis of Bioactive Antioxidants and Metabolites in Swertia chirata In Vitro Callus Cultures
Abstract
1. Introduction
2. Results
2.1. Impact on Callus Nature Response and Color
2.2. Impact of Elicitation on Callus Growth and Biomass
2.3. Efficacy of Elicitors in Improving Callus Moisture Contents, DPPH Scavenging Percentage, Tocopherols and Anthocyanin Values
2.4. Effect of Callus Elicitation on Production of Amarogentin, Mangiferin, Total Flavonoids and Total Phenolics
2.5. Elicitation Impact on Bioactive Antioxidants
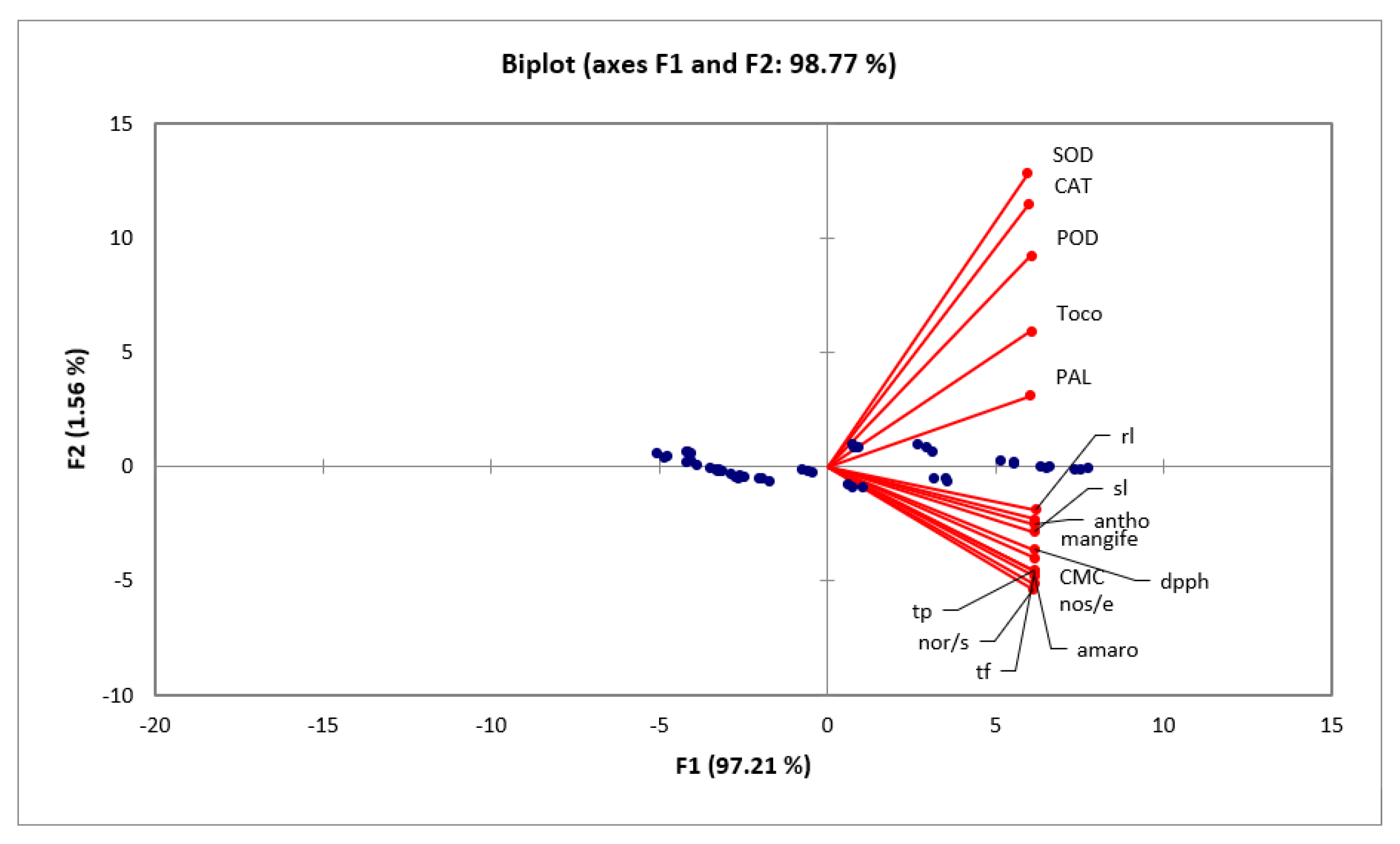
2.6. Statistical Assessment on the Studied Variables
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Material
4.2. Elicitation Experiments
4.3. Extract Preparation
4.4. Quantification of Amarogentin and Mangiferin
4.5. Determination of Total Phenolic Content
4.6. Determination of Total Flavonoid Content
4.7. Determination of DPPH Scavenging Percentage
4.8. Determination of Activities of Bioactive Antioxidants
4.9. Determination of Anthocyanin and Tocopherol Contents
4.10. Statistical Analysis and Experimental Layout
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, K.; Dhande, S.; Kadam, V. Therapeutic Swertia chirata-an overview. Res. J. Pharmacogn. Phytochem. 2013, 5, 199–207. [Google Scholar]
- Parihar, S. Swertia chirata—A Wonderful Herb. Indian J. Pharm. Biol. Res. 2021, 9, 10–15. [Google Scholar]
- Tabassum, S.; Mahmood, S.; Hanif, J.; Hina, M.; Uzair, B. An overview of medicinal importance of Swertia chirata. Int. J. Appl. 2012, 2, 1. [Google Scholar]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Hakkim, F.L.; Shankar, C.G.; Girija, S. Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J. Agric. Food Chem. 2007, 55, 9109–9117. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Rab, A.; Ahmad, N. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J. Photochem. Photobiol. B Biol. 2016, 154, 51–56. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Ahmed, O.K.; El-Desouky, W. Effect of low doses γ-irradiation on oxidative stress and secondary metabolites production of rosemary (Rosmarinus officinalis L.) callus culture. Radiat. Phys. Chem. 2011, 80, 968–976. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M. Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl. Biochem. Biotechnol. 2016, 180, 1076–1092. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Atiq Hussain, S.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed Nano-Priming with Calcium Oxide Maintains the Redox State by Boosting the Antioxidant Defense System in Water-Stressed Carom (Trachyspermum ammi L.) Plants to Confer Drought Tolerance. Nanomaterials 2023, 13, 1453. [Google Scholar] [CrossRef]
- Noreen, Z.; Ashraf, M. Changes in antioxidant enzymes and some key metabolites in some genetically diverse cultivars of radish (Raphanus sativus L.). Environ. Exp. Bot. 2009, 67, 395–402. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Pandey, D.K.; Gupta, R.C.; Dey, A. Simultaneous microwave-assisted extraction and HPTLC quantification of mangiferin, amarogentin, and swertiamarin in Swertia species from Western Himalayas. Ind. Crops Prod. 2019, 132, 449–459. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. Quantitative determination of swertiamarin, mangiferin, and amarogentin in callus culture of Swertia chirata by HPLC analysis. J. Pharmacol. Res. 2015, 4, 1270–1279. [Google Scholar]
- Patel, K.; Patel, D.K. Secoiridoid Amarogentin from ‘Gentianaceae’ with their Health Promotion, Disease Prevention and Modern Analytical Aspects. Curr. Bioact. Compd. 2020, 16, 191–200. [Google Scholar] [CrossRef]
- Rivero-Montejo, S.D.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as novel elicitors to improve bioactive compounds in plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R. Seed priming with Calcium oxide nanoparticles improves germination, biomass, antioxidant defense, and yield traits of canola plants under drought stress. S. Afr. J. Bot. 2022, 151, 889–899. [Google Scholar] [CrossRef]
- Lala, S. Nanoparticles as elicitors and harvesters of economically important secondary metabolites in higher plants: A review. IET Nanobiotechnol. 2021, 15, 28–57. [Google Scholar] [CrossRef]
- Sørensen, J.L.; Sondergaard, T.E. The effects of different yeast extracts on secondary metabolite production in Fusarium. Int. J. Food Microbiol. 2014, 170, 55–60. [Google Scholar] [CrossRef]
- Pathak, A.R.; Patel, S.R.; Joshi, A.G.; Shrivastava, N.; Sindhav, G.; Sharma, S.; Ansari, H. Elicitor mediated enhancement of shoot biomass and lupeol production in Hemidesmus indicus (L.) R. Br. ex Schult. and Tylophora indica (Burm. F.) Merrill using yeast extract and salicylic acid. Nat. Prod. Res. 2022, 37, 1767–1773. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.; Kuenz, A.; Prüße, U. Nutritional requirements and the impact of yeast extract on the d-lactic acid production by Sporolactobacillus inulinus. Green Chem. 2017, 19, 4633–4641. [Google Scholar] [CrossRef]
- Atawia, A.A.R.; El-Desouqi, S.A. Trials for improving fruit set, yield and fruit quality of Washington navel orange by application of some growth regulators and yeast extract as a natural source of phytohormones. Ann. Agric. Sci. Moshtohor 1997, 34, 1613–1632. [Google Scholar]
- Alsohaimi, I.H.; Nassar, A.M.; Elnasr, T.A.S.; amar Cheba, B. A novel composite silver nanoparticles loaded calcium oxide stemming from egg shell recycling: A potent photocatalytic and antibacterial activities. J. Clean. Prod. 2020, 248, 119274. [Google Scholar] [CrossRef]
- Gandhi, N.; Shruthi, Y.; Sirisha, G.; Anusha, C.R. Facile and eco-friendly method for synthesis of calcium oxide (CaO) nanoparticles and its potential application in agriculture. Haya Saudi J. Life Sci. 2021, 6, 89–103. [Google Scholar]
- Khalaf, B.M.; Mohsen, A.A.; El-Kemary, M.; Dowidar, S.M.; Abo-Hamad, S.A.; Elhaak, M.A.; Elsherif, D.E. Influence of Calcium Nanoparticles on Antioxidant Enzymes, Total Carbohydrates Content and Isozymes of In Vitro Cultured Lupinus Termis under Cadmium Stress. Available online: https://www.researchsquare.com/article/rs-2167818/v1 (accessed on 5 May 2023).
- Yazicilar, B.; Bezirganoglu, İ.; Chang, Y.L.; Nadar, M. CaO and Graphene Oxide Enhances Drought Stress from Callus Tissues of Medicago Sativa L. Cultivars. J. Inst. Sci. Technol. 2022, 12, 2450–2458. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Baldoni, G. Factors influencing anthocyanin content in red cabbage (Brassica oleracea var capitata L f rubra (L) Thell). J. Sci. Food Agric. 2002, 82, 1504–1509. [Google Scholar] [CrossRef]
- Syu, Y.Y.; Hung, J.H.; Chen, J.C.; Chuang, H.W. Impacts of size and shape of silver nanoparticles on Ara-bidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Veerashree, V.; Anuradha, C.M.; Kumar, V. Elicitor-enhanced production of gymnemic acid in cell suspension cultures of Gymnema sylvestre R. Br. Plant Cell Tissue Organ Cult. 2012, 108, 27–35. [Google Scholar] [CrossRef]
- Ge, X.; Wu, J. Tanshinone production and isoprenoid pathways in Salvia miltiorrhiza hairy roots induced by Ag+ and yeast elicitor. Plant Sci. 2005, 168, 487–491. [Google Scholar] [CrossRef]
- Pirian, K.; Piri, K. Influence of yeast extract as a biotic elicitor on noradrenaline production in hairy root culture of Portulaca oleracea L. Int. J. Agron. Plant Prod. 2013, 4, 2960–2964. [Google Scholar]
- Grant, C.L.; Pramer, D. Minor element composition of yeast extract. J. Bacteriol. 1962, 84, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cai, X.; Yu, A.; Xi, Y.; Zhai, G. Redox-sensitive self-assembled nanoparticles based on al-pha-tocopherol succinate-modified heparin for intracellular delivery of paclitaxel. J. Colloid Interface Sci. 2017, 496, 311–326. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Semchuk, N.M. Tocopherol biosynthesis: Chemistry, regulation and effects of environmental factors. Acta Physiol. Plant. 2012, 34, 1607–1628. [Google Scholar] [CrossRef]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, M.; Mazhar, M.W.; Maqbool, M.; Hussain, T.; Hussain, S.A.; Casini, R.; Abd-ElGawad, A.M.; Elansary, H.O. Seed Priming with the Selenium Nanoparticles Maintains the Redox Status in the Water Stressed Tomato Plants by Modulating the Antioxidant Defense Enzymes. Plants 2023, 12, 1556. [Google Scholar] [CrossRef]
- Zaman, G.; Farooq, U.; Bajwa, M.N.; Jan, H.; Shah, M.; Ahmad, R.; Andleeb, A.; Drouet, S.; Hano, C.; Abbasi, B.H. Effects of yeast extract on the production of phenylpropanoid metabolites in callus culture of purple basil (Ocimum Basilicum L. var purpurascens) and their in-vitro evaluation for antioxidant potential. Plant Cell Tissue Organ Cult. 2022, 150, 543–553. [Google Scholar] [CrossRef]
- Sarkate, A.; Banerjee, S.; Mir, J.I.; Roy, P.; Sircar, D. Antioxidant and cytotoxic activity of bioactive phenolic metabolites isolated from the yeast-extract treated cell culture of apple. Plant Cell Tissue Organ Cult. 2017, 130, 641–649. [Google Scholar] [CrossRef]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.S.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Veeramani, C.; El Newehy, A.S.; Aloud, A.A.; Alsaif, M.A.; Al-Numair, K.S. Effect of calcium oxide nanopar-ticles produced from Lavetira critica leaf extract on the freshness of fresh-sliced fruits. J. Food Process. Preserv. 2022, 46, e17234. [Google Scholar] [CrossRef]
- Akçay, F.A.; Avcı, A. Effects of process conditions and yeast extract on the synthesis of selenium nanoparticles by a novel indigenous isolate Bacillus sp. EKT1 and characterization of nanoparticles. Arch. Microbiol. 2020, 202, 2233–2243. [Google Scholar] [CrossRef]
- Ames, J.M.; Leod, G.M. Volatile components of a yeast extract composition. J. Food Sci. 1985, 50, 125–131. [Google Scholar] [CrossRef]
- Phoboo, S.; Pinto, M.D.S.; Barbosa, A.C.L.; Sarkar, D.; Bhowmik, P.C.; Jha, P.K.; Shetty, K. Phenolic-Linked Biochemical Rationale for the Anti-Diabetic Properties of Swertia chirata (Roxb. ex Flem.). Karst. Phytother. Res. 2013, 27, 227–235. [Google Scholar] [CrossRef]
- Khanal, S.; Shakya, N.; Thapa, K.; Pant, D.R. Phytochemical investigation of crude methanol extracts of dif-ferent species of Swertia from Nepal. BMC Res. Notes 2015, 8, 821. [Google Scholar] [CrossRef]
- Kumar, V.; Chandra, S. LC-ESI/MS determination of xanthone and secoiridoid glycosides from in vitro re-generated and in vivo Swertia chirata. Physiol. Mol. Biol. Plants 2015, 21, 51–60. [Google Scholar] [CrossRef]
- Gupta, R.; Sood, H. Emerging Technologies for the Production of In Vitro Raised Quality Rich Swertia chirata by Using LED Lights. Sustainability 2023, 15, 1714. [Google Scholar] [CrossRef]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.U.R. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma tuberculata. Artif. Cells Nanomed. Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Ajaib, M.; Hussain, I.; Hussain, T.; Parveen, A.; Thind, S.; Sardar, T.; Akram, R.; et al. Synergistic application of calcium oxide nanoparticles and farmyard manure induces cadmium tolerance in mung bean (Vigna radiata L.) by influencing physiological and biochemical parameters. PLoS ONE 2023, 18, e0282531. [Google Scholar] [CrossRef]
- Rashmi, R.; Trivedi, M.P. Effect of various growth hormone concentration and combination on callus induction, nature of callus and callogenic response of Nerium odorum. Appl. Biochem. Biotechnol. 2014, 172, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Pourmorad, F.; Bekhradnia, A.R. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol. 2008, 7, 18. [Google Scholar]
- Yesmin, M.N.; Uddin, S.N.; Mubassara, S.; Akond, M.A. Antioxidant and antibacterial activities of Calotropis procera Linn. Am.-Eurasian J. Agric. Environ. Sci. 2008, 4, 550–553. [Google Scholar]
- Khan, M.A.; Abbasi, B.H.; Ahmed, N.; Ali, H. Effects of light regimes on in vitro seed germination and silymarin content in Silybum marianum. Ind. Crops Prod. 2013, 46, 105–110. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, O.; De Gara, L.; Tommasi, F.; Liso, R. Changes in the ascorbate system during seed development of Vicia faba L. Plant Physiol. 1992, 99, 235–238. [Google Scholar] [CrossRef]
- Abeles, F.B.; Biles, C.L. Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiol. 1991, 95, 269–273. [Google Scholar] [CrossRef]
- Simoes, C.; Bizarri, C.H.B.; da Silva Cordeiro, L.; de Castro, T.C.; Coutada, L.C.M.; da Silva, A.J.R.; Albarello, N.; Mansur, E. Anthocyanin production in callus cultures of Cleome rosea: Modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiol. Biochem. 2009, 47, 895–903. [Google Scholar] [CrossRef]
- Yashin, Y.I.; Yashin, A.Y. Analytical chromatography. Methods, instrumentation and applications. Russ. Chem. Rev. 2006, 75, 329. [Google Scholar] [CrossRef]
- Baker, J.L., Jr. The effectiveness of alpha-tocopherol (vitamin E) in reducing the incidence of spherical contracture around breast implants. Plast. Reconstr. Surg. 1981, 68, 696–698. [Google Scholar] [CrossRef] [PubMed]

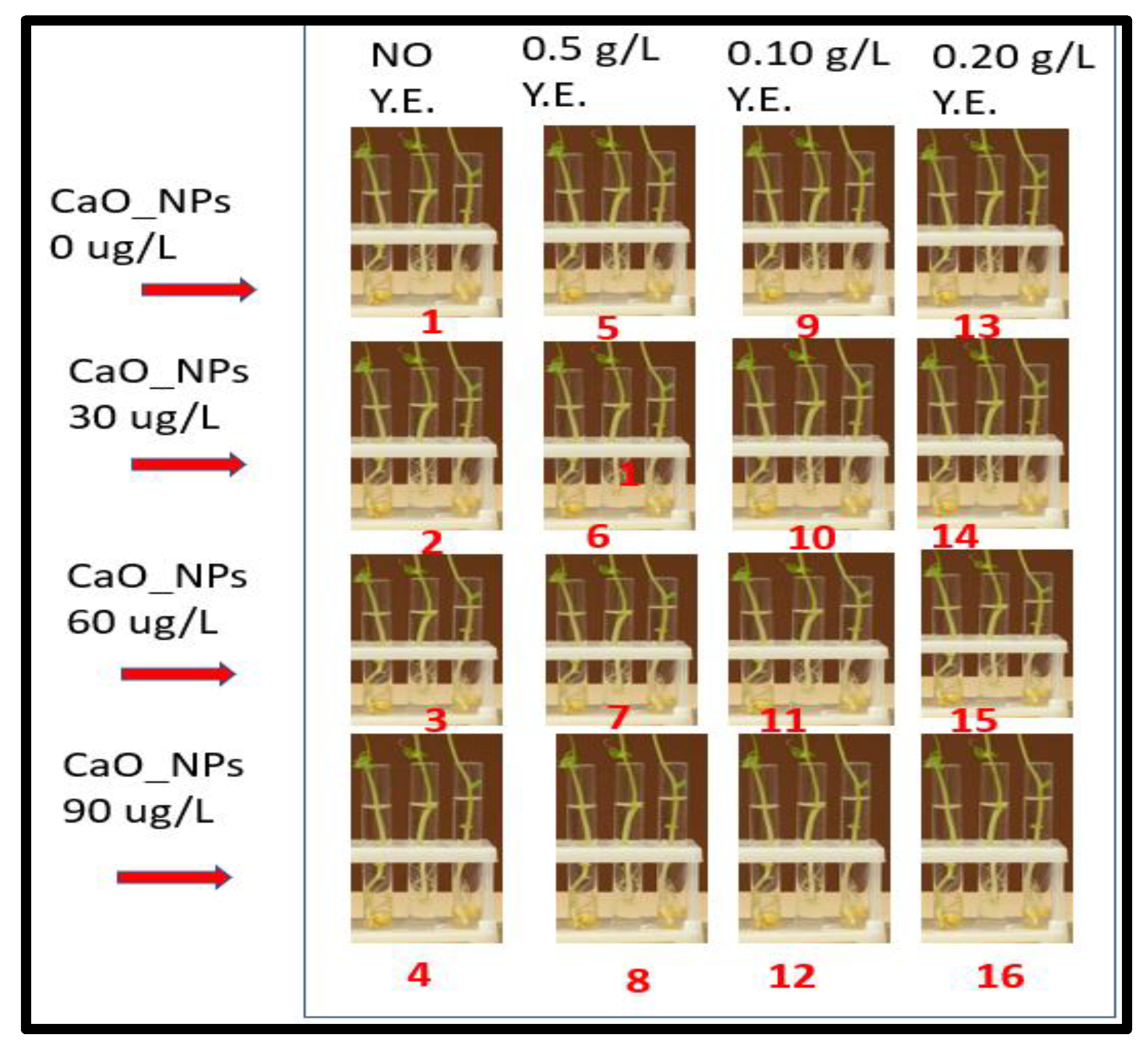
| Treatments | Callus Nature | Callus Response a | Callus Color |
|---|---|---|---|
| T1 | Fragile | ++ | Yellow |
| T2 | Fragile | ++ | Yellow-brown |
| T3 | Fragile | ++ | Yellow-brown |
| T4 | Fragile | ++ | Yellow-brown |
| T5 | Fragile | +++ | Yellow-brown |
| T6 | Semi Compact | ++++ | Yellow greenish |
| T7 | Compact | +++++ | Greenish |
| T8 | Compact | +++++ | Greenish |
| T9 | Fragile | ++ | Yellow-brown |
| T10 | Semi Compact | ++++ | Yellow greenish |
| T11 | Compact | +++++ | Greenish |
| T12 | Compact | +++++ | Greenish |
| T13 | Fragile | ++ | Yellow-brown |
| T14 | Compact | +++++ | Greenish |
| T15 | Compact | +++++ | Greenish |
| T16 | Compact | +++++ | Greenish |
| Treatments | Shoot Length (cm) | Root Length (cm) | No. of Shoots/Explant | No. of Roots per Shoots | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±S.D. | Mean | ±S.D. | Mean | ±S.D. | Mean | ±S.D. | |
| T1 | 2.59 m | 0.020 | 0.99 m | 0.025 | 2.17 l | 0.286 | 1.34 k | 0.040 |
| T2 | 2.66 kl | 0.017 | 1.10 l | 0.015 | 2.60 k | 0.173 | 1.43 j | 0.025 |
| T3 | 2.73 k | 0.021 | 1.24 k | 0.035 | 2.97 j | 0.058 | 1.46 ij | 0.006 |
| T4 | 2.95 j | 0.040 | 1.38 j | 0.036 | 3.23 i | 0.252 | 1.49 lk | 0.012 |
| T5 | 2.64 lm | 0.025 | 1.13 l | 0.015 | 2.30 l | 0.100 | 1.35 k | 0.026 |
| T6 | 3.10 i | 0.032 | 1.51 i | 0.045 | 3.75 g | 0.145 | 1.52 h | 0.020 |
| T7 | 3.24 h | 0.051 | 1.67 h | 0.035 | 4.02 f | 0.076 | 1.58 g | 0.021 |
| T8 | 3.51 f | 0.065 | 1.77 g | 0.026 | 4.23 f | 0.076 | 1.64 f | 0.015 |
| T9 | 2.72 kl | 0.025 | 1.30 k | 0.015 | 2.93 j | 0.058 | 1.47 i | 0.021 |
| T10 | 3.34 g | 0.031 | 1.95 f | 0.045 | 4.48 e | 0.126 | 1.69 e | 0.015 |
| T11 | 3.65 e | 0.095 | 2.12 e | 0.035 | 4.83 d | 0.070 | 1.76 d | 0.015 |
| T12 | 4.06 d | 0.075 | 2.25 d | 0.035 | 5.12 c | 0.104 | 1.82 c | 0.025 |
| T13 | 2.91 i | 0.040 | 1.42 j | 0.035 | 3.47 h | 0.058 | 1.51 h | 0.017 |
| T14 | 4.43 c | 0.072 | 2.42 c | 0.042 | 5.47 b | 0.153 | 1.91 b | 0.021 |
| T15 | 4.71 b | 0.045 | 2.61 b | 0.035 | 5.75 a | 0.050 | 1.96 a | 0.015 |
| T16 | 5.07 a | 0.062 | 2.79 a | 0.021 | 5.95 a | 0.050 | 2.00 a | 0.015 |
| LSD = 0.05 | 0.083 | 0.054 | 0.222 | 0.034 | ||||
| Treatments | Total Anthocyanin (mg/100 g FW) | DPPH Scavenging Percentage | Alpha Tocopherol (μg/g FW) | Callus Moisture Content (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±S.D. | Mean | ±S.D. | Mean | ±S.D. | Mean | ±S.D. | |
| T1 | 4.35 m | 0.040 | 28.67 m | 2.082 | 1.67 n | 0.060 | 29.67 n | 1.528 |
| T2 | 4.45 l | 0.025 | 36.33 l | 2.517 | 1.86 m | 0.050 | 35.0 m | 1.732 |
| T3 | 4.66 k | 0.026 | 42.67 k | 1.528 | 1.95 l | 0.026 | 40.33 l | 1.155 |
| T4 | 4.76 j | 0.025 | 46.33 j | 1.528 | 1.98 kl | 0.020 | 43.67 jk | 1.155 |
| T5 | 4.48 l | 0.045 | 34.33 l | 0.577 | 2.07 ij | 0.020 | 33.6 m | 2.082 |
| T6 | 4.93 i | 0.040 | 51.33 i | 1.528 | 2.14 h | 0.035 | 46.33 ij | 1.528 |
| T7 | 5.07 h | 0.049 | 55.67 h | 1.155 | 2.24 g | 0.021 | 51.67 h | 2.082 |
| T8 | 5.24 g | 0.070 | 59.33 g | 1.528 | 2.39 f | 0.045 | 55.00 g | 1.000 |
| T9 | 4.70 jk | 0.067 | 46.67 j | 1.528 | 2.04 jk | 0.045 | 42.3 kl | 1.155 |
| T10 | 5.62 f | 0.070 | 64.33 f | 2.517 | 2.43 f | 0.075 | 59.33 f | 1.528 |
| T11 | 5.88 e | 0.112 | 70.33 e | 2.517 | 2.56 e | 0.031 | 63.33 e | 2.082 |
| T12 | 6.09 d | 0.081 | 76.33 d | 1.528 | 2.68 d | 0.031 | 67.00 d | 1.000 |
| T13 | 4.77 j | 0.032 | 48.33 ij | 1.528 | 2.13 hi | 0.025 | 48.00 i | 1.732 |
| T14 | 6.82 c | 0.035 | 80.33 c | 1.155 | 2.93 c | 0.056 | 74.33 c | 2.887 |
| T15 | 7.05 b | 0.051 | 85.00 b | 2.000 | 3.01 b | 0.026 | 78.33 b | 0.577 |
| T16 | 7.19 a | 0.045 | 88.67 a | 2.517 | 3.19 a | 0.070 | 83.67 a | 3.055 |
| LSD = 0.05 | 0.093 | 3.026 | 0.072 | 2.940 | ||||
| Treatments | Mangiferin | Amarogentin | Total Flavonoids | Total Phenolics | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±S.D. | Mean | ±S.D. | Mean | ±S.D. | Mean | ±S.D. | |
| T1 | 9.12 n | 0.023 | 4.37 m | 0.062 | 12.96 p | 0.059 | 8.20 o | 0.075 |
| T2 | 9.35 m | 0.040 | 4.54 l | 0.025 | 14.15 n | 0.133 | 9.12 m | 0.125 |
| T3 | 9.53 l | 0.021 | 4.74 k | 0.025 | 15.00 m | 0.105 | 9.99 l | 0.100 |
| T4 | 9.67 jk | 0.025 | 4.85 j | 0.030 | 16.43 j | 0.086 | 11.35 j | 0.120 |
| T5 | 9.38 m | 0.025 | 4.44 lm | 0.015 | 13.33 o | 0.111 | 8.69 n | 0.071 |
| T6 | 10.29 i | 0.053 | 5.03 i | 0.057 | 17.32 i | 0.086 | 12.42 i | 0.101 |
| T7 | 10.81 h | 0.055 | 5.24 h | 0.030 | 18.69 h | 0.060 | 13.67 h | 0.105 |
| T8 | 11.30 g | 0.062 | 5.38 g | 0.045 | 19.91 g | 0.062 | 14.20 g | 0.080 |
| T9 | 9.58 kl | 0.070 | 4.75 jk | 0.025 | 15.16 l | 0.061 | 10.11 l | 0.120 |
| T10 | 11.67 f | 0.121 | 5.59 f | 0.085 | 20.42 f | 0.103 | 16.43 f | 0.086 |
| T11 | 11.96 e | 0.047 | 6.12 e | 0.115 | 21.48 e | 0.135 | 17.16 e | 0.050 |
| T12 | 12.42 d | 0.163 | 6.52 d | 0.070 | 23.54 d | 0.081 | 18.11 d | 0.105 |
| T13 | 9.71 j | 0.050 | 4.84 jk | 0.046 | 16.07 k | 0.115 | 11.04 k | 0.072 |
| T14 | 13.34 c | 0.110 | 6.80 c | 0.075 | 25.58 c | 0.108 | 18.43 c | 0.086 |
| T15 | 14.29 b | 0.052 | 7.12 b | 0.116 | 27.15 b | 0.078 | 19.39 b | 0.046 |
| T16 | 15.07 a | 0.062 | 7.46 a | 0.060 | 28.86 a | 0.146 | 20.17 a | 0.074 |
| LSD = 0.05 | 0.119 | 0.104 | 0.165 | 0.152 | ||||
| Treatments | PAL (U/g FW) | SOD (U/g FW) | POD (U/g FW) | CAT (U/g FW) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | ±S.D. | Mean | ±S.D. | Mean | ±S.D. | Mean | ±S.D. | |
| T1 | 1.83 k | 0.023 | 0.70 j | 0.006 | 0.67 l | 0.006 | 0.19 j | 0.005 |
| T2 | 1.99 ij | 0.016 | 0.71 j | 0.003 | 0.70 kl | 0.002 | 0.21 ij | 0.004 |
| T3 | 2.09 i | 0.069 | 0.73 j | 0.004 | 0.71 jkl | 0.004 | 0.23 ij | 0.006 |
| T4 | 2.19 h | 0.022 | 0.77 ij | 0.006 | 0.72 jkl | 0.003 | 0.26 hij | 0.004 |
| T5 | 2.08 i | 0.025 | 0.82 i | 0.009 | 0.76 ijk | 0.004 | 0.30 hi | 0.003 |
| T6 | 2.23 h | 0.085 | 1.03 h | 0.091 | 0.80 i | 0.010 | 0.33 h | 0.008 |
| T7 | 3.10 g | 0.086 | 1.49 g | 0.076 | 1.81 g | 0.037 | 0.67 g | 0.016 |
| T8 | 3.37 f | 0.072 | 3.28 d | 0.056 | 1.95 f | 0.057 | 1.99 e | 0.091 |
| T9 | 1.90 jk | 0.031 | 0.76 ij | 0.023 | 0.71 jkl | 0.004 | 0.24 ij | 0.003 |
| T10 | 3.44 f | 0.111 | 1.75 f | 0.071 | 1.30 h | 0.066 | 0.81 f | 0.022 |
| T11 | 3.94 e | 0.051 | 4.07 b | 0.061 | 2.96 d | 0.047 | 2.17 d | 0.166 |
| T12 | 4.14 d | 0.078 | 2.15 e | 0.061 | 2.53 e | 0.081 | 1.99 e | 0.025 |
| T13 | 2.07 i | 0.053 | 0.78 ij | 0.003 | 0.77 ij | 0.006 | 0.26 ij | 0.008 |
| T14 | 5.16 c | 0.045 | 3.96 c | 0.057 | 3.21 c | 0.075 | 2.45 c | 0.056 |
| T15 | 5.37 b | 0.026 | 4.12 b | 0.040 | 3.40 b | 0.031 | 2.69 b | 0.034 |
| T16 | 5.53 a | 0.021 | 4.34 a | 0.098 | 3.68 a | 0.031 | 2.84 a | 0.022 |
| LSD = 0.05 | 0.096 | 0.088 | 0.066 | 0.085 | ||||
| Variables | PAL | SOD | POD | CAT | CMC | Mang | Amaro | TF | TP | Antho | DPPH | SL | RL | Nos/e | Nor/s |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAL | 1 | ||||||||||||||
| SOD | 0.942 * | ||||||||||||||
| POD | 0.954 * | 0.984 * | |||||||||||||
| CAT | 0.961 * | 0.984 * | 0.987 * | ||||||||||||
| CMC | 0.950 * | 0.926 * | 0.956 * | 0.937 * | |||||||||||
| Mang | 0.966 * | 0.943 * | 0.965 * | 0.952 * | 0.992 * | ||||||||||
| Amaro | 0.964 * | 0.930 * | 0.952 * | 0.937 * | 0.990 * | 0.995 * | |||||||||
| TF | 0.963 * | 0.928 * | 0.950 * | 0.941 * | 0.989 * | 0.992 * | 0.996 * | ||||||||
| TP | 0.964 * | 0.928 * | 0.950 * | 0.941 * | 0.987 * | 0.993 * | 0.996 * | 0.998 * | |||||||
| Antho | 0.961 * | 0.941 * | 0.962 * | 0.952 * | 0.989 * | 0.995 * | 0.993 * | 0.991 * | 0.991 * | ||||||
| DPPH | 0.945 * | 0.934 * | 0.955 * | 0.941 * | 0.987 * | 0.991 * | 0.992 * | 0.989 * | 0.989 * | 0.993 * | |||||
| SL | 0.967 * | 0.938 * | 0.959 * | 0.946 * | 0.982 * | 0.991 * | 0.993 * | 0.991 * | 0.992 * | 0.988 * | 0.986 * | ||||
| RL | 0.964 * | 0.947 * | 0.967 * | 0.953 * | 0.992 * | 0.998 * | 0.995 * | 0.993 * | 0.992 * | 0.996 * | 0.992 * | 0.990 * | |||
| Nos/e | 0.958 * | 0.922 * | 0.948 * | 0.933 * | 0.990 * | 0.990 * | 0.993 * | 0.992 * | 0.993 * | 0.989 * | 0.988 * | 0.990 * | 0.989 * | ||
| Nor/s | 0.947 * | 0.921 * | 0.949 * | 0.930 * | 0.992 * | 0.991 * | 0.994 * | 0.989 * | 0.988 * | 0.990 * | 0.989 * | 0.984 * | 0.989 * | 0.987 * | |
| Toco | 0.939 * | 0.965 * | 0.979 * | 0.967 * | 0.968 * | 0.970 * | 0.956 * | 0.956 * | 0.954 * | 0.965 * | 0.966 * | 0.954 * | 0.975 * | 0.955 * | 0.954 * |
| Variation Source | a df | Shoot Length | Root Length | No. of Shoots/Explant | No. of Roots/Shoots | Callus Moisture | DPPH Scavenging % |
|---|---|---|---|---|---|---|---|
| Treatment effect in rows (F1) | 3 | 2.997 b *** (0.000) | 1.623 *** (0.000) | 8.781 *** (0.000) | 0.234 *** (0.000) | 1317.90 *** (0.000) | 1849.13 *** (0.000) |
| Treatment effect in columns (F2) | 3 | 5.197 *** (0.000) | 2.857 *** (0.000) | 12.848 *** (0.000) | 0.401 *** (0.000) | 2570.40 *** (0.000) | 3156.54 *** (0.000) |
| Interaction (F1 × F2) | 9 | 0.378 *** (0.000) | 0.121 *** (0.000) | 0.305 *** (0.000) | 0.014 *** (0.000) | 56.61 *** (0.000) | 71.48 *** (0.000) |
| Error | 32 | 0.002 | 0.001 | 0.017 | 0.00004 | 3.125 | 3.312 |
| Variation Source | df | Anthocyanin | Tocopherol | Superoxide dismutase | Catalase | Peroxidase | Phenylalanine ammonia lyase |
| Treatment effect in rows (F1) | 3 | 3.691 *** (0.000) | 0.757 *** (0.000) | 9.233 *** (0.000) | 5.254 *** (0.000) | 6.071 *** (0.000) | 8.190 *** (0.000) |
| Treatment effect in columns (F2) | 3 | 8.312 *** (0.000) | 1.896 *** (0.000) | 13.823 *** (0.000) | 7.203 *** (0.000) | 9.175 *** (0.000) | 13.716 *** (0.000) |
| Interaction among the factors (F1 × F2) | 9 | 0.565 *** (0.000) | 0.084 *** (0.000) | 2.948 *** (0.000) | 1.213 *** (0.000) | 1.281 *** (0.000) | 1.441 *** (0.000) |
| Error | 32 | 0.003 | 0.001 | 0.002 | 0.002 | 0.001 | 0.003 |
| Variation Source | df | Amarogentin | Mangiferin | Flavonoids | Phenolics | ||
| Treatment effect in rows (F1) | 3 | 4.810 *** (0.000) | 16.942 *** (0.000) | 136.33 *** (0.000) | 98.598 *** (0.000) | ||
| Treatment effect in columns (F2) | 3 | 8.650 *** (0.000) | 29.668 *** (0.000) | 209.283 *** (0.000) | 138.373 *** (0.000) | ||
| Interaction among the factors (F1 × F2) | 9 | 0.57 *** (0.000) | 2.5001 *** (0.000) | 10.783 *** (0.000) | 5.966 *** (0.000) | ||
| Error | 32 | 0.003 | 0.005 | 0.009 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sardar, T.; Maqbool, M.; Ishtiaq, M.; Mazhar, M.W.; El-Sheikh, M.A.; Casini, R.; Mahmoud, E.A.; Elansary, H.O. Synergistic Influence of Yeast Extract and Calcium Oxide Nanoparticles on the Synthesis of Bioactive Antioxidants and Metabolites in Swertia chirata In Vitro Callus Cultures. Molecules 2023, 28, 4607. https://doi.org/10.3390/molecules28124607
Sardar T, Maqbool M, Ishtiaq M, Mazhar MW, El-Sheikh MA, Casini R, Mahmoud EA, Elansary HO. Synergistic Influence of Yeast Extract and Calcium Oxide Nanoparticles on the Synthesis of Bioactive Antioxidants and Metabolites in Swertia chirata In Vitro Callus Cultures. Molecules. 2023; 28(12):4607. https://doi.org/10.3390/molecules28124607
Chicago/Turabian StyleSardar, Tauqeer, Mehwish Maqbool, Muhammad Ishtiaq, Muhammad Waqas Mazhar, Mohamed A. El-Sheikh, Ryan Casini, Eman A. Mahmoud, and Hosam O. Elansary. 2023. "Synergistic Influence of Yeast Extract and Calcium Oxide Nanoparticles on the Synthesis of Bioactive Antioxidants and Metabolites in Swertia chirata In Vitro Callus Cultures" Molecules 28, no. 12: 4607. https://doi.org/10.3390/molecules28124607
APA StyleSardar, T., Maqbool, M., Ishtiaq, M., Mazhar, M. W., El-Sheikh, M. A., Casini, R., Mahmoud, E. A., & Elansary, H. O. (2023). Synergistic Influence of Yeast Extract and Calcium Oxide Nanoparticles on the Synthesis of Bioactive Antioxidants and Metabolites in Swertia chirata In Vitro Callus Cultures. Molecules, 28(12), 4607. https://doi.org/10.3390/molecules28124607









