Effects of Graphene Oxide Nanosheets in Freshwater Biofilms
Abstract
1. Introduction
2. Results
2.1. Morphology and Elements Abundance Analysis by Scanning Electron Microscopy (SEM-EDX)
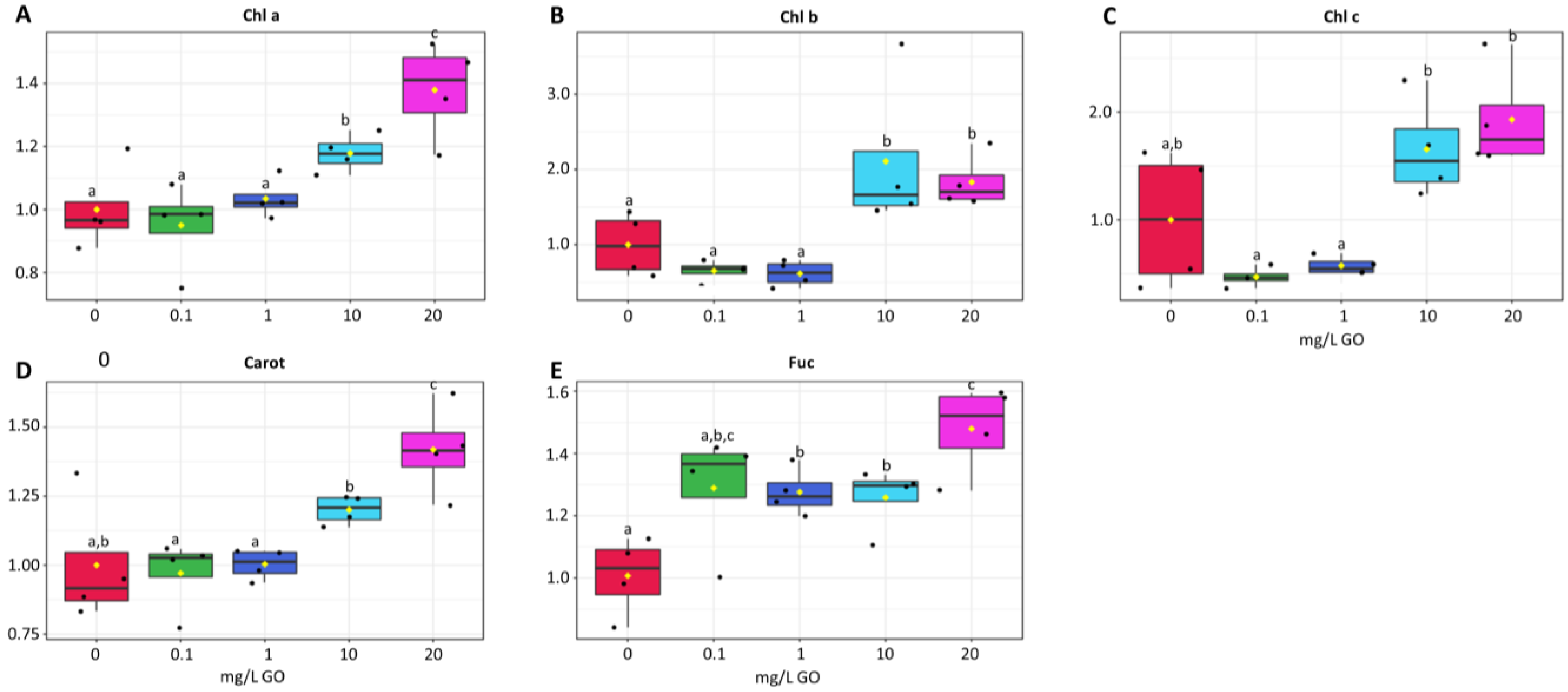
2.2. Photosynthetic Pigments
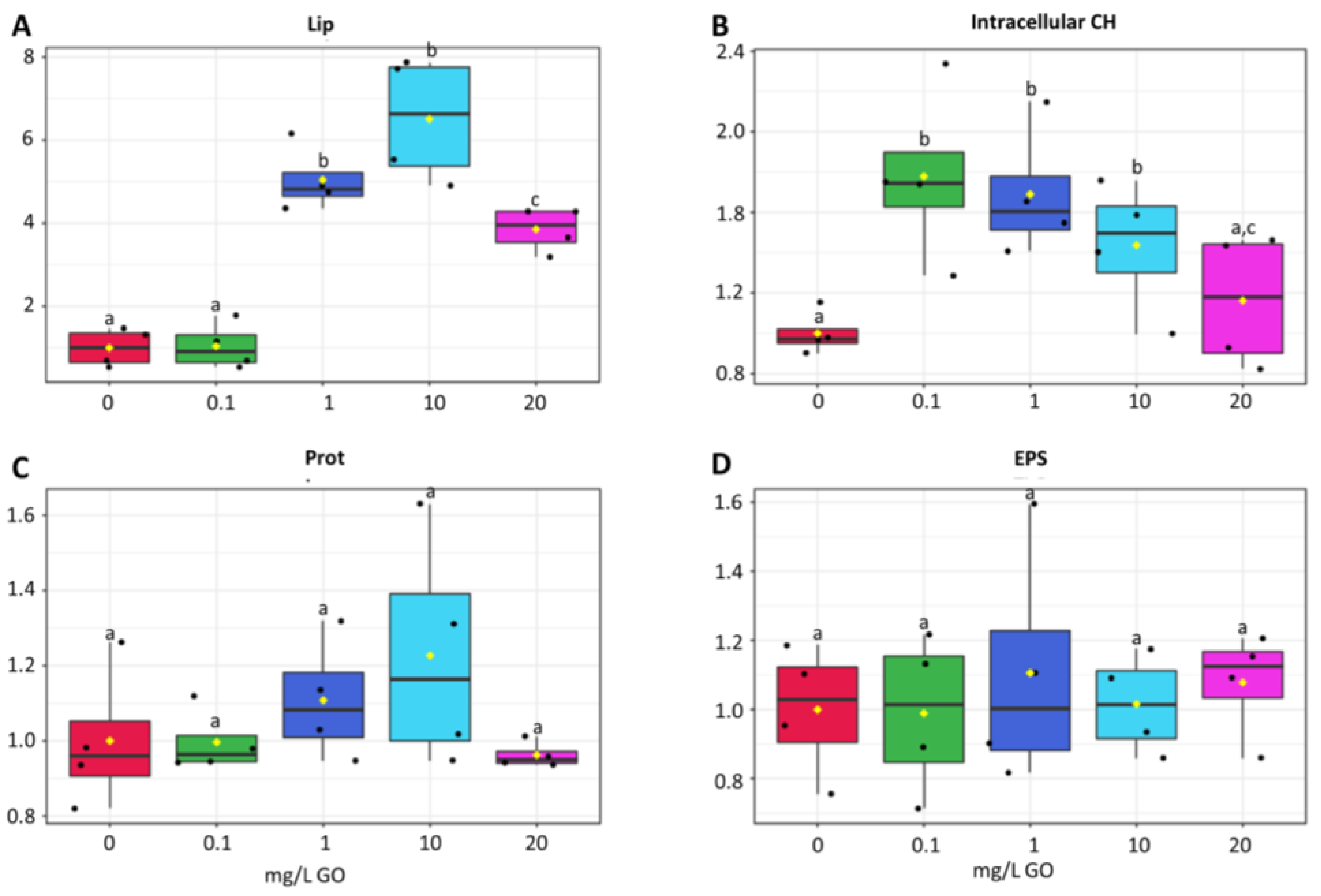
2.3. Cell Metabolism
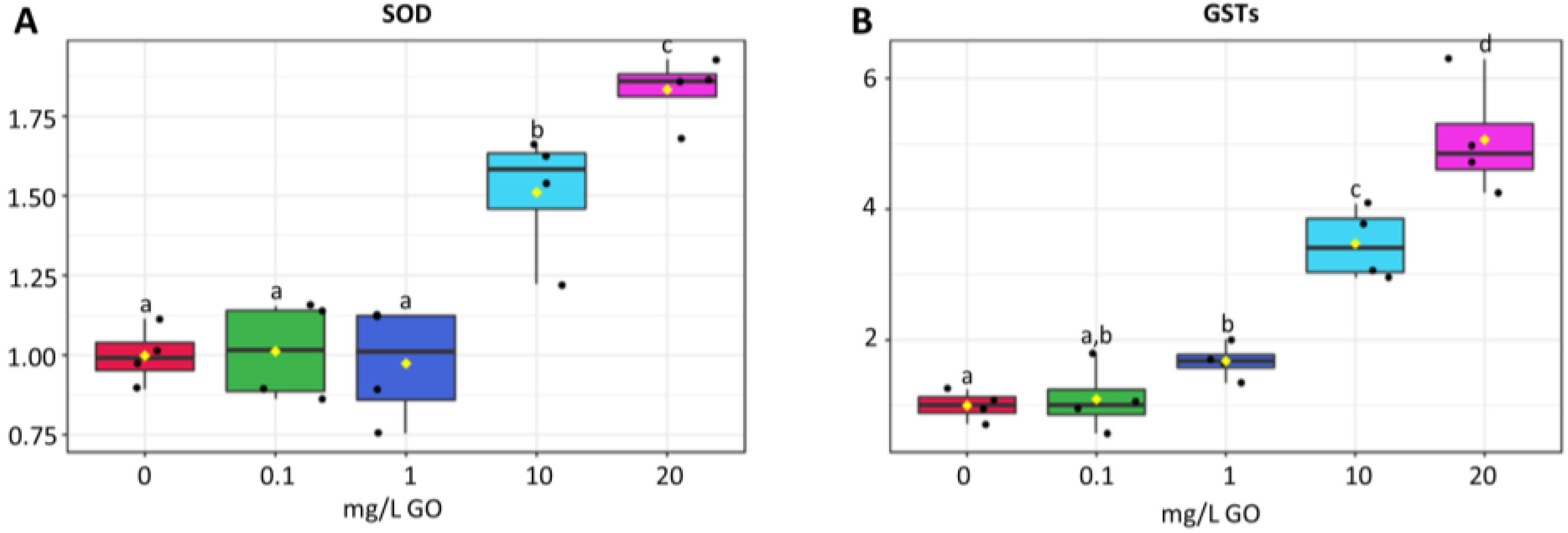
2.4. Antioxidant and Biotransformation Response
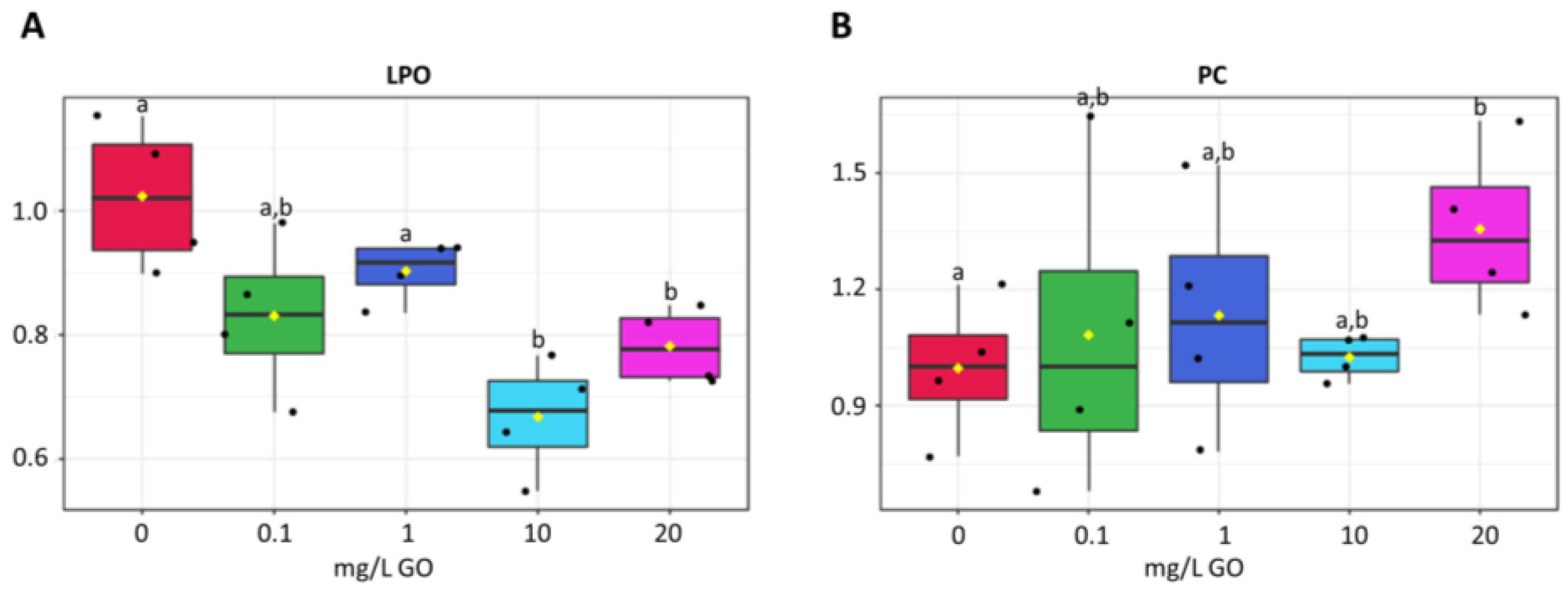
2.5. Cell Damage
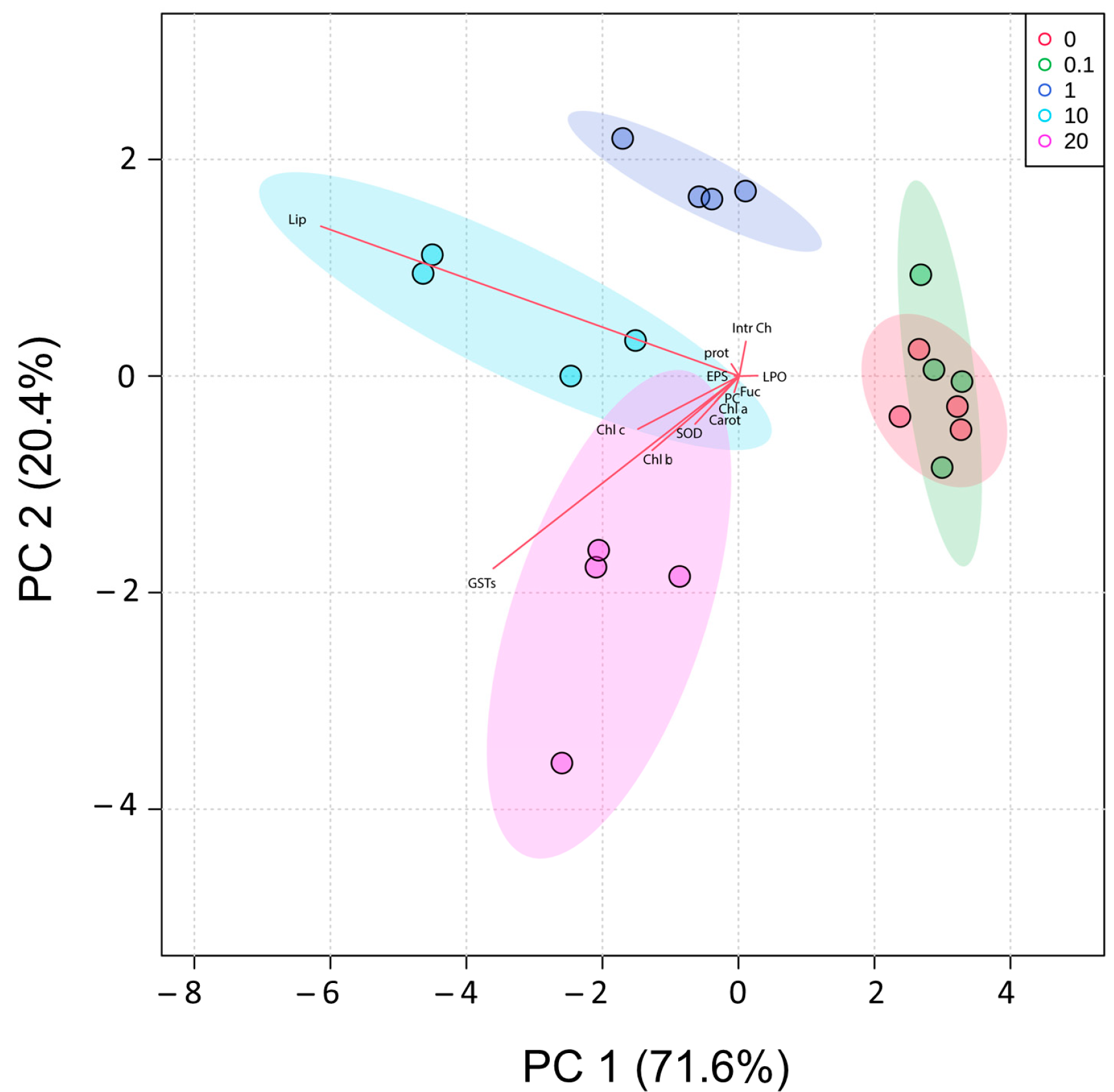
2.6. PCA
3. Discussion
4. Materials and Methods
4.1. Graphene Oxide Nanosheets
4.2. Biological Material and Experimental Setup
4.3. SEM-EDX Analysis
4.4. Photosynthetic Pigments
4.5. Exopolysaccharides (EPSs)
4.6. Energy Related Parameters
4.7. Antioxidant Enzyme Response
4.8. Oxidative Damage
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pan, B.; Xing, B. Applications and Implications of Manufactured Nanoparticles in Soils: A Review. Eur. J. Soil Sci. 2012, 63, 437–456. [Google Scholar] [CrossRef]
- Armano, A.; Agnello, S. Two-Dimensional Carbon: A Review of Synthesis Methods, and Electronic, Optical, and Vibrational Properties of Single-Layer Graphene. C 2019, 5, 67. [Google Scholar] [CrossRef]
- Perreault, F.; De Faria, A.F.; Elimelech, M. Environmental Applications of Graphene-Based Nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.; Cruz, C.; Cardoso, P.; Pinto, R.; Marques, P.A.A.P.; Figueira, E. A Multifactorial Approach to Untangle Graphene Oxide (GO) Nanosheets Effects on Plants: Plant Growth-Promoting Bacteria Inoculation, Bacterial Survival, and Drought. Nanomaterials 2021, 11, 771. [Google Scholar] [CrossRef]
- Sengupta, J. Application of Carbon Nanomaterials in the Electronic Industry. In Micro and Nano Technologies, Handbook of Nanomaterials for Manufacturing Applications; Elsevier: New York, NY, USA, 2020; ISBN 9780128213810. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. [Google Scholar] [CrossRef]
- Amirov, R.R.; Shayimova, J.; Nasirova, Z.; Solodov, A.; Dimiev, A.M. Analysis of Competitive Binding of Several Metal Cations by Graphene Oxide Reveals the Quantity and Spatial Distribution of Carboxyl Groups on Its Surface. Phys. Chem. Chem. Phys. 2018, 20, 2320–2329. [Google Scholar] [CrossRef]
- Yang, K.; Chen, B.; Zhu, X.; Xing, B. Aggregation, Adsorption, and Morphological Transformation of Graphene Oxide in Aqueous Solutions Containing Different Metal Cations. Environ. Sci. Technol. 2016, 50, 11066–11075. [Google Scholar] [CrossRef]
- Kiiski, H.; Dittmar, H.; Drach, M.; Vosskamp, R.; Trenkel, M.E.; Gutser, R.; Steffens, G. Fertilizers, 2. Types. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2016; pp. 1–53. [Google Scholar]
- Etesami, H.; Mirsyed Hosseini, H.; Alikhani, H. In Planta Selection of Plant Growth Promoting Endophytic Bacteria for Rice (Oryza Sativa L.). J. Soil Sci. Plant Nutr. 2014, 14. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal Natural Products—Status and Future Potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Zhang, M.M.; Li, H.X.; Andrade, M.; Xuan, W.; Correa, A. Feasible Bioprocessing Technologies for Low-Grade Iron Ores. Min. Metall. Explor. 2015, 32, 78–87. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Chen, J.; Li, Y.; Creamer, A.E.; Chen, H. Slow-Release Fertilizer Encapsulated by Graphene Oxide Films. Chem. Eng. J. 2014, 255, 107–113. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, L.; Cheng, S.; Zhu, L.; Liu, L.; Wen, P.; Zhou, L.; Xue, W.; Lu, S.; Zhang, W.; et al. An Overview of Light-Mediated Impact of Graphene Oxide on Algae: Photo-Transform, Toxicity and Mechanism. Water 2022, 14, 2997. [Google Scholar] [CrossRef]
- Lanphere, J.D.; Rogers, B.; Luth, C.; Bolster, C.H.; Walker, S.L. Stability and Transport of Graphene Oxide Nanoparticles in Groundwater and Surface Water. Environ. Eng. Sci. 2014, 31, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Tang, Y.; Liu, S.; Yang, X.; Xia, S.; Yin, D.; Yu, S. Impact of Graphene Oxide on Algal Organic Matter of Microcystis Aeruginosa. ACS Omega 2018, 3, 16969–16975. [Google Scholar] [CrossRef]
- Mottier, A.; Mouchet, F.; Gauthier, L.; Flahaut, E. Environmental Impact of Engineered Carbon Nanoparticles: From Releases to Effects on the Aquatic Biota. Curr. Opin. Biotechnol. 2017, 46, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Sun, A.; Mu, L.; Zhou, Q. Trends in Analytical Chemistry Separation and Analysis of Carbon Nanomaterials in Complex Matrix. Trends Anal. Chem. 2016, 80, 416–428. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Zou, W.; Hu, X. Molecular Mechanisms of Developmental Toxicity Induced by Graphene Oxide at Predicted Environmental Concentrations. Environ. Sci. Technol. 2017, 51, 7861–7871. [Google Scholar] [CrossRef]
- Hu, C.; Hu, N.; Li, X.; Zhao, Y. Graphene Oxide Alleviates the Ecotoxicity of Copper on the Freshwater Microalga Scenedesmus Obliquus. Ecotoxicol. Environ. Saf. 2016, 132, 360–365. [Google Scholar] [CrossRef]
- B-Béres, V.; Stenger-Kovács, C.; Buczkó, K.; Padisák, J.; Selmeczy, G.B.; Lengyel, E.; Tapolczai, K. Ecosystem Services Provided by Freshwater and Marine Diatoms. Hydrobiologia 2022. [Google Scholar] [CrossRef]
- Alejo, J.A. The Role of Fungi and Bacteria on the Organic Matter Decomposition Process in Streams: Interaction and Relevance in Matter Decomposition Process in Biofilms. Ph.D. Thesis, Universitat de Girona, Girona, Spain, 2009; pp. 5–199. [Google Scholar]
- Hazeem, L.J.; Bououdina, M.; Dewailly, E.; Slomianny, C.; Barras, A.; Coffinier, Y.; Szunerits, S.; Boukherroub, R. Toxicity Effect of Graphene Oxide on Growth and Photosynthetic Pigment of the Marine Alga Picochlorum sp. during Different Growth Stages. Environ. Sci. Pollut. Res. 2017, 24, 4144–4152. [Google Scholar] [CrossRef]
- Yin, J.; Fan, W.; Du, J.; Feng, W.; Dong, Z.; Liu, Y.; Zhou, T. The Toxicity of Graphene Oxide Affected by Algal Physiological Characteristics: A Comparative Study in Cyanobacterial, Green Algae, Diatom. Environ. Pollut. 2020, 260, 113847. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tian, J.; Li, S.; Xue, C.; Xue, Z.; Yin, D.; Yu, S. Combined Effects of Graphene Oxide and Cd on the Photosynthetic Capacity and Survival of Microcystis Aeruginosa. Sci. Total Environ. 2015, 532, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Hu, X.; Zhou, Q. Envelopment–Internalization Synergistic Effects and Metabolic Mechanisms of Graphene Oxide on Single-Cell Chlorella Vulgaris Are Dependent on the Nanomaterial Particle Size. ACS Appl. Mater. Interfaces 2015, 7, 18104–18112. [Google Scholar] [CrossRef]
- Garacci, M.; Barret, M.; Mouchet, F.; Sarrieu, C.; Lonchambon, P.; Flahaut, E.; Gauthier, L.; Silvestre, J.; Pinelli, E. Few Layer Graphene Sticking by Biofilm of Freshwater Diatom Nitzschia Palea as a Mitigation to Its Ecotoxicity. Carbon N. Y. 2017, 113, 139–150. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Xie, Y.; Cao, H.; Zhao, H.; Zhang, Y. The Evolution of Surface Charge on Graphene Oxide during the Reduction and Its Application in Electroanalysis. Carbon N. Y. 2014, 66, 302–311. [Google Scholar] [CrossRef]
- Hu, X.; Lu, K.; Mu, L.; Kang, J.; Zhou, Q. Interactions between Graphene Oxide and Plant Cells: Regulation of Cell Morphology, Uptake, Organelle Damage, Oxidative Effects and Metabolic Disorders. Carbon N. Y. 2014, 80, 665–676. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Vijver, M.G.; Peijnenburg, W.J.G.M. Graphene Nanoplatelets and Reduced Graphene Oxide Elevate the Microalgal Cytotoxicity of Nano-Zirconium Oxide. Chemosphere 2021, 276, 130015. [Google Scholar] [CrossRef]
- Thapa, S.; Bharti, A.; Prasanna, R. Chapter 14—Algal Biofilms and Their Biotechnological Significance. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A.B.T.-A.G.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–303. ISBN 978-0-444-63784-0. [Google Scholar]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, S.-X.; Wei, C.-L.; Yu, S.-Y.; Shi, J.-P.; Zhang, B.-G. Effect of magnesium deficiency on photosynthetic physiology and triacylglyceride (TAG) accumulation of Chlorella vulgaris. Huan Jing Ke Xue = Huanjing Kexue 2014, 35, 1462–1467. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.; Nahar, K.; Hossain, M.; Mahmud, J.; Hossen, M.; Masud, A.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Król, E.; Dziubinska, H.; Trebacz, K. What Do Plants Need Action Potentials For ? In Action Potential: Biophysical and Cellular Context, Initiation, Phases and Propagation; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 1–26. [Google Scholar]
- Su, Y.; Yang, G.; Lu, K.; Petersen, E.J.; Mao, L. Colloidal Properties and Stability of Aqueous Suspensions of Few-Layer Graphene: Importance of Graphene Concentration. 2017, 220, 469–477. Environ. Pollut. 2017, 220, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, X.; Wang, Z.; Dai, Y.; Xing, B. Mechanistic Understanding toward the Toxicity of Graphene-Family Materials to Freshwater Algae. Water Res. 2017, 111, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Ji, J.; Yang, K.; Lin, D.; Wu, F. Systematic and Quantitative Investigation of the Mechanism of Carbon Nanotubes’ Toxicity toward Algae. Environ. Sci. Technol. 2012, 46, 8458–8466. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U. New Emitter-Detector-Cuvette Assembly for Measuring Modulated Chlorophyll Fluorescence of Highly Diluted Suspensions in Conjunction with the Standard PAM Fluorometer. Z. Naturforsch. C 1994, 49, 646–656. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Carvalho, C.F.M.; Pereira, H.; Gangadhar, K.N.; Schüler, L.M.; Santos, T.F.; Varela, J.C.S.; Barreira, L. Urban Wastewater Treatment by Tetraselmis Sp. CTP4 (Chlorophyta). Bioresour. Technol. 2017, 223, 175–183. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Pinto, R.F.; Sant’Anna, C. Low Light Intensity and Nitrogen Starvation Modulate the Chlorophyll Content of Scenedesmus Dimorphus. J. Appl. Microbiol. 2016, 120, 661–670. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In Vitro Toxicity Evaluation of Graphene Oxide on A549 Cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Nogueira, P.F.M.; Nakabayashi, D.; Zucolotto, V. The Effects of Graphene Oxide on Green Algae Raphidocelis Subcapitata. Aquat. Toxicol. 2015, 166, 29–35. [Google Scholar] [CrossRef]
- Shi, T.; Wang, L.; Zhang, Z.; Sun, X.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef]
- Arif, M.; Bai, Y.; Usman, M.; Jalalah, M. Highest Accumulated Microalgal Lipids (Polar and Non-Polar) for Biodiesel Production with Advanced Wastewater Treatment: Role of Lipidomics. Bioresour. Technol. 2020, 298, 122299. [Google Scholar] [CrossRef]
- Girão, A.F.; Sousa, J.; Domínguez-Bajo, A.; González-Mayorga, A.; Bdikin, I.; Pujades-Otero, E.; Casañ-Pastor, N.; Hortigüela, M.J.; Otero-Irurueta, G.; Completo, A.; et al. 3D Reduced Graphene Oxide Scaffolds with a Combinatorial Fibrous-Porous Architecture for Neural Tissue Engineering. ACS Appl. Mater. Interfaces 2020, 12, 38962–38975. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.P. The Influence of the Mineral Composition of the Medium on the Growth of Planktonic Algae: Part I. Methods and Culture Media. J. Ecol. 1942, 30, 284–325. [Google Scholar] [CrossRef]
- Pires, A.; Figueira, E.; Silva, M.S.S.; Carina, S. Effects of Graphene Oxide Nanosheets in the Polychaete Hediste Diversicolor: Behavioural, Physiological and Biochemical Responses. Environ. Pollut. 2022, 299, 118869. [Google Scholar] [CrossRef] [PubMed]
- Domingues, E.M.; Gonçalves, G.; Henriques, B.; Pereira, E.; Marques, P.A.A.P. High Affinity of 3D Spongin Scaffold towards Hg(II) in Real Waters. J. Hazard. Mater. 2021, 407, 124807. [Google Scholar] [CrossRef] [PubMed]
- Nayek, S.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Li, F.L.; Wang, L.J.; Fan, Y.; Parsons, R.L.; Hu, G.R.; Zhang, P.Y. A Rapid Method for the Determination of Fucoxanthin in Diatom. Mar. Drugs 2018, 16, 33. [Google Scholar] [CrossRef]
- Staats, N.; de Winder, B.; Stal, L.J.; Mur, L.R. Isolation and Characterization of Extracellular Polysaccharides from the Epipelic Diatoms Cylindrotheca Closterium and Navicula Salinarum. Eur. J. Phycol. 1999, 34, 161–169. [Google Scholar] [CrossRef]
- Santos, J.; Almeida, S.F.P.; Figueira, E. Cadmium Chelation by Frustulins: A Novel Metal Tolerance Mechanism in Nitzschia Palea (Kutzing) W. Smith. Ecotoxicology 2013, 22, 166–173. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Robinson, H.W.; Hogden, C.G. The biuret reaction in the determination of serum proteins. J. Biol. Chem. 1940, 135, 707–725. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1953, 226, 497–509. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Zheng, Y.; VanderGheynst, J.S. Rapid Quantitative Analysis of Lipids Using a Colorimetric Method in a Microplate Format. Lipids 2011, 46, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-Dinitrophenylhydrazine Spectrophotometric Assay for Quantification of Carbonyls in Oxidized Proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Udechukwu, M.C.; Yiridoe, C.; Gibson, A.; Gong, M. Antioxidant Mechanism of Potato Protein Hydrolysates against in Vitro Oxidation of Reduced Glutathione. J. Funct. Foods 2016, 20, 195–203. [Google Scholar] [CrossRef]
- Anderson, M.; Gorley, R.N.; Clarke, K. PERMANOVA+ for Primer: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); PRIMER-E Ltd.: Plymouth, UK, 2006. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, D.; Almeida, S.F.P.; Marques, P.A.A.P.; Pinto, S.; Figueira, E. Effects of Graphene Oxide Nanosheets in Freshwater Biofilms. Molecules 2023, 28, 4577. https://doi.org/10.3390/molecules28124577
Matos D, Almeida SFP, Marques PAAP, Pinto S, Figueira E. Effects of Graphene Oxide Nanosheets in Freshwater Biofilms. Molecules. 2023; 28(12):4577. https://doi.org/10.3390/molecules28124577
Chicago/Turabian StyleMatos, Diana, Salomé F. P. Almeida, Paula A. A. P. Marques, Sofia Pinto, and Etelvina Figueira. 2023. "Effects of Graphene Oxide Nanosheets in Freshwater Biofilms" Molecules 28, no. 12: 4577. https://doi.org/10.3390/molecules28124577
APA StyleMatos, D., Almeida, S. F. P., Marques, P. A. A. P., Pinto, S., & Figueira, E. (2023). Effects of Graphene Oxide Nanosheets in Freshwater Biofilms. Molecules, 28(12), 4577. https://doi.org/10.3390/molecules28124577









