Titanium-Mediated Reduction of Carboxamides to Amines with Borane–Ammonia
Abstract
1. Introduction
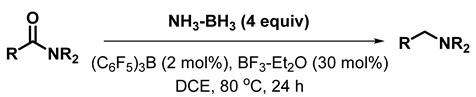



2. Results and Discussion
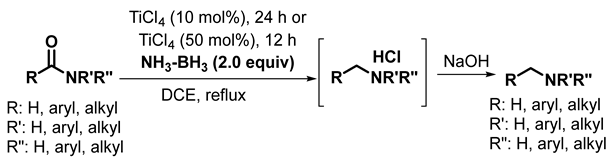
3. Materials and Methods
3.1. General Information
3.2. Experimental
Characterization of Product Amines
- Dibenzylamine (3a); The compound was prepared as described in the general procedure (yellow oil, mass = 189 mg, 96% yield); 1H NMR (400 MHz, CDCl3) δ 7.4–7.3 (m, 8H), 7.3–7.2 (m, 2H), 3.8 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 140.2, 128.3, 128.1, 126.9, 53.1. The compound characterization is in accordance with previous reports [43].
- N-benzyl-1-(4-methoxyphenyl)methanamine (3b); The compound was prepared as described in the general procedure (yellow oil, mass = 215 mg, 95% yield); 1H NMR (400 MHz, CDCl3) δ 7.4–7.3 (m, 4H), 7.3–7.2 (m, 3H), 6.9–6.8 (m, 2H), 3.8 (s, 5H), 3.8 (s, 2H), 2.3 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 158.6, 139.9, 131.9, 129.4, 128.3, 128.2, 126.9, 113.7, 55.2, 52.8, 52.3. The compound characterization is in accordance with previous reports [43].
- N-benzyl-1-(p-tolyl)methanamine (3c); The compound was prepared as described in the general procedure (yellow oil, mass = 200 mg, 95% yield); 1H NMR (400 MHz, CDCl3) δ 7.4–7.3 (m, 4H), 7.3–7.2 (m, 3H), 7.2 (d, J = 7.9 Hz, 2H), 3.8 (s, 2H), 3.8 (s, 2H), 2.4 (s, 3H), 1.8 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 140.3, 137.2, 136.4, 129.0, 128.3, 128.1, 128.0, 126.8, 53.0, 52.8, 21.0. The compound characterization is in accordance with previous reports [43].
- N-benzyl-1-(4-(trifluoromethyl)phenyl)methanamine (3d); The compound was prepared as described in the general procedure (colorless oil, mass = 172 mg, 65% yield); 1H NMR (400 MHz, CDCl3) δ 7.6 (d, J = 8.0 Hz, 2H), 7.5 (d, J = 8.0 Hz, 2H), 7.4–7.2 (m, 5H), 3.9 (s, 2H), 3.8 (s, 2H), 1.9 (s, 1H).13C NMR (101 MHz, CDCl3) δ 144.3, 139.8, 129.3, 129.0, 128.4, 128.2, 128.1, 127.0, 125.23, 125.19, 53.1, 52.4. 19F NMR (376 MHz, CDCl3) δ −63.8. The compound characterization is in accordance with previous reports [44].
- N-benzylaniline (3e); The compound was prepared as described in the general procedure (colorless oil, mass = 172 mg, 94% yield); 1H NMR (400 MHz, CDCl3) δ 7.5–7.3 (m, 4H), 7.3–7.3 (m, 1H), 7.2 (dd, J = 8.6, 7.3 Hz, 2H), 6.7 (tt, J = 7.3, 1.1 Hz, 1H), 6.7 (dd, J = 8.7, 1.1 Hz, 2H), 4.3 (s, 2H), 4.1 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 148.0, 139.3, 129.2, 128.5, 127.4, 127.1, 117.5, 112.77, 48.3. The compound characterization is in accordance with previous reports [45].
- N-benzylcyclohexanamine (3f); The compound was prepared as described in the general procedure (yellow oil, mass = 182 mg, 97% yield); 1H NMR (400 MHz, CDCl3) δ 7.3 (d, J = 4.4 Hz, 4H), 7.3–7.2 (m, 1H), 3.8 (s, 2H), 2.6–2.4 (m,1), 1.9 (d, J = 12.0 Hz, 2H), 1.8–1.7 (m, 2H), 1.6–1.6 (m, 1H), 1.3–1.1 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 140.9, 128.3, 128.0, 126.7, 56.1, 51.0, 33.5, 26.1, 24.9. The compound characterization is in accordance with previous reports [46].
- 4-benzylmorpholine (3g); The compound was prepared as described in the general procedure (yellow oil, mass = 168 mg, 95% yield); 1H NMR (400 MHz, CDCl3) δ 7.4–7.3 (m, 4H), 7.3–7.2 (m, 1H), 3.7–3.7 (m, 4H), 3.5 (s, 2H), 2.5–2.4 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 137.6, 129.1, 128.2, 127.1, 66.9, 63.4, 53.5. The compound characterization is in accordance with previous reports [46].
- Tribenzylamine (3h); The compound was prepared as described in the general procedure (yellow solid, mass = 243 mg, 85% yield); 1H NMR (400 MHz, CDCl3) δ 7.5–7.4 (m, 6H), 7.4–7.3 (m, 6H), 7.3–7.2 (m, 3H), 3.6 (d, J = 3.2 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 139.6, 128.7, 128.1, 126.8, 57.9. The compound characterization is in accordance with previous reports [46].
- Hexan-1-amine hydrochloride (3i); The compound was prepared as described in the general procedure (white solid, mass = 109 mg, 80% yield); 1H NMR (400 MHz, CDCl3) δ 6.0 (s, 2H), 2.7 (p, J = 7.5 Hz, 2H), 1.7 (p, J = 7.5 Hz, 2H), 1.4–1.2 (m, 3H), 0.9–0.8 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 44.5, 31.2, 28.7, 26.4, 22.4, 13.8. The compound characterization is in accordance with previous reports [47].
- N-methyl-1-phenylmethanamine (3j); The compound was prepared as described in the general procedure (yellow oil, mass = 109 mg, 90% yield); 1H NMR (400 MHz, CDCl3) δ 7.4–7.2 (m, 5H), 3.7 (s, 2H), 2.5 (s, 3H), 1.5 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 140.1, 128.3, 128.1, 126.8, 56.0, 36.0. The compound characterization is in accordance with previous reports [46].
- N-ethylaniline (3k); The compound was prepared as described in the general procedure (yellow oil, mass = 115 mg, 95% yield); 1H NMR (400 MHz, CDCl3) δ 7.2 (dd, J = 8.6, 7.3 Hz, 2H), 6.7 (tt, J = 7.3, 1.1 Hz, 1H), 6.6 (d, J = 7.6 Hz, 2H), 3.6 (s, 1H), 3.2 (q, J = 7.1 Hz, 2H), 1.3 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 148.3, 129.1, 117.1, 112.7, 38.4, 14.8. The compound characterization is in accordance with previous reports [48].
- N-benzylhexan-1-amine (3l); The compound was prepared as described in the general procedure (yellow oil, mass = 172 mg, 90% yield); 1H NMR (400 MHz, CDCl3) δ 7.3 (d, J = 4.7 Hz, 4H), 7.3–7.2 (m, 1H), 3.8 (s, 2H), 2.7–2.6 (m, 2H), 1.5 (q, J = 7.9 Hz, 2H), 1.3–1.2 (m, 6H), 0.9 (t, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 140.5, 128.3, 128.0, 126.7, 54.0, 49.5, 31.7, 30.0, 27.0, 22.5, 14.0. The compound characterization is in accordance with previous reports [49].
- N-(cyclohexylmethyl)hexan-1-amine (3m); The compound was prepared as described in the general procedure (yellow oil, mass = 183 mg, 93% yield); 1H NMR (400 MHz, CDCl3) δ 2.55 (t, J = 7.2 Hz, 2H), 2.4 (d, J = 6.7 Hz, 2H), 1.7–1.6 (m, 5H), 1.5–1.4 (m, 3H), 1.3–1.2 (m, 9H), 0.9–0.9 (m, 5H). 13C NMR (101 MHz, CDCl3) δ 56.9, 50.2, 37.9, 31.7, 31.4, 30.1, 27.0, 26.6, 26.0, 22.5, 14.0. The compound characterization is in accordance with previous reports [50].
- 4-(cyclohexylmethyl)morpholine (3n); The compound was prepared as described in the general procedure (colorless oil, mass = 180 mg, 98% yield); 1H NMR (400 MHz, CDCl3) δ 3.7 (t, J = 4.5 Hz, 4H), 2.5–2.3 (m, 4H), 2.1 (d, J = 7.2 Hz, 2H), 1.8 (d, J = 12.7 Hz, 2H), 1.7–1.6 (m, 3H), 1.6–1.4 (m, 1H), 1.3–1.1 (m, 3H), 0.9–0.8 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 66.7, 65.9, 54.0, 34.4, 31.7, 26.6, 26.0. The compound characterization is in accordance with previous reports [51].
- 4-phenethylmorpholine (3o); The compound was prepared as described in the general procedure (yellow oil, mass = 187 mg, 98% yield); 1H NMR (400 MHz, CDCl3) δ 7.3–7.2 (m, 2H), 7.2–7.2 (m, 3H), 3.8–3.7 (m, 4H), 2.9–2.8 (m, 2H), 2.6–2.6 (m, 2H), 2.6–2.5 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 140.1, 128.6, 128.3, 126.0, 66.9, 60.8, 53.6, 33.2. The compound characterization is in accordance with previous reports [52].
- Azepane hydrochloride (3p); The compound was prepared as described in the general procedure (yellow solid, mass = 114 mg, 85% yield); 1H NMR (400 MHz, CDCl3) δ 9.6 (s, 2H), 3.2 (p, J = 5.1 Hz, 4H), 2.0–1.9 (m, 4H), 1.8–1.7 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 45.6, 26.6, 25.1. The compound characterization is in accordance with previous reports [53].
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brown, H.C.; Ramachandran, P.V. Sixty years of hydride reductions. In Reductions in Organic Synthesis: Recent Advances and Practical Applications; American Chemical Society: Washington, DC, USA, 1996; Volume 641, pp. 1–30. [Google Scholar]
- Lee, C.W.; Ko, H.M. Metal-Free Catalytic Reduction of Amides: Recent Progress. Asian J. Org. Chem. 2023, 12, e202300098. [Google Scholar] [CrossRef]
- Chardon, A.; Morisset, E.; Rouden, J.; Blanchet, J. Recent Advances in Amide Reductions. Synthesis 2018, 50, 984–997. [Google Scholar] [CrossRef]
- Ricci, A. Amino Group Chemistry—From Synthesis to the Life Sciences; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.P.; Boutevin, B. Biobased amines: From synthesis to polymers; present and future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef]
- Roose, P.E.K.; Henkes, E.; Rossbacher, R.; Höke, H. Amines, Aliphatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Hoboken, NJ, USA, 2015. [Google Scholar]
- Morrison, A.L.; Long, R.F.; Konigstein, M. The reduction of acid amides with lithium aluminum hydride. J. Chem. Soc. 1951, 952–955. [Google Scholar] [CrossRef]
- Satoh, T.; Suzuki, S.; Suzuki, Y.; Miyaji, Y.; Imai, Z. Reduction of organic compounds with sodium borohydride-transition metal salt systems.1. Reduction of organic nitrile, nitro and amide compounds to primary amines. Tetrahedron Lett. 1969, 10, 4555–4558. [Google Scholar] [CrossRef]
- Akabori, S.; Takanohashi, Y. A novel highly selective reduction of tertiary amides to amines with sodium borohydride-bis(2-bromoethyl)selenium dibromide. Chem. Lett. 1990, 19, 251–252. [Google Scholar] [CrossRef]
- Xiang, S.H.; Xu, J.A.; Yuan, H.Q.; Huang, P.Q. Amide Activation by Tf2O: Reduction of Amides to Amines by NaBH4 under Mild Conditions. Synlett 2010, 2010, 1829–1832. [Google Scholar] [CrossRef]
- Prasad, A.S.B.; Kanth, J.V.B.; Periasamy, M. Convenient methods for the reduction of amides, nitriles, carboxylic esters, acids and hydroboration of alkenes using nabh4/i2 system. Tetrahedron 1992, 48, 4623–4628. [Google Scholar] [CrossRef]
- Sengupta, S.; Sahu, D.P.; Chatterjee, S.K. Sodium borohydride-boron trifluoride ethereate, a convenient and efficient reagent for the reduction of amides. Ind. J. Chem. B 1994, 33, 285–287. [Google Scholar] [CrossRef]
- Huang, P.Q.; Geng, H. Simple, versatile, and chemoselective reduction of secondary amides and lactams to amines with the Tf2O-NaBH4 or Cp2ZrHCl-NaBH4 system. Org. Chem. Front. 2015, 2, 150–158. [Google Scholar] [CrossRef]
- Zhu, H.J.; Lu, K.T.; Sun, G.R.; He, J.B.; Li, H.Q.; Pittman, C.U. Reduction of amides with NaBH4 in diglyme at 162 degrees C. New J. Chem. 2003, 27, 409–413. [Google Scholar] [CrossRef]
- Yamada, S.I.; Kikugawa, Y.; Ikegami, S. Reduction of acid amides to amines with sodium borohydride. Chem. Pharm. Bull. 1965, 13, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Madhavan, S.; Balakumar, R.; Swarnalakshmi, S. Unusual reactivity of zinc borohydride—Reduction of amides to amines. Synth. Commun. 1997, 27, 391–394. [Google Scholar] [CrossRef]
- Davis, M. Reduction of amides by lithium borohydride. J. Chem. Soc. 1956, 3981–3982. [Google Scholar]
- Brown, H.C.; Heim, P. Diborane as mild reducing agent for conversion of primary, secondary and tertiary amides into corresponding amines. J. Am. Chem. Soc. 1964, 86, 3566–3567. [Google Scholar] [CrossRef]
- Brown, H.C.; Heim, P. Selective reductions.18. Fast reaction of primary, secondary, and tertiary amides with diborane—Simple, convenient procedure for conversion of amides to corresponding amines. J. Org. Chem. 1973, 38, 912–916. [Google Scholar] [CrossRef]
- Brown, H.C.; Narasimhan, S.; Choi, Y.M. Improved procedure for borane-dimethyl sulfide reduction of tertiary and secondary amides in the presence of boron-trifluoride etherate. Synthesis 1981, 12, 996–997. [Google Scholar] [CrossRef]
- Bonnat, M.; Hercouet, A.; Lecorre, M. Effect of the temperature on the stoichiometry of borane dimethyl sulfide reduction of secondary and tertiary amides. Synth. Commun. 1991, 21, 1579–1582. [Google Scholar] [CrossRef]
- Barger, C.J.; Dicken, R.D.; Weidner, V.L.; Motta, A.; Lohr, T.L.; Marks, T.J. La N(SiMe3)(2) (3)-Catalyzed Deoxygenative Reduction of Amides with Pinacolborane. Scope and Mechanism. J. Am. Chem. Soc. 2020, 142, 8019–8028. [Google Scholar] [CrossRef]
- Yao, W.B.; Wang, J.L.; Zhong, A.G.; Wang, S.L.; Shao, Y.L. Transition-metal-free catalytic hydroboration reduction of amides to amines. Org. Chem. Front. 2020, 7, 3515–3520. [Google Scholar] [CrossRef]
- Zhang, F.C.; Guo, C.J.; Gong, M.L.; Xie, H.Z.; Luo, Y.J. Hydroborative reduction of amides to amines mediated by La(CH2C6H4NMe2-o)(3). New J. Chem. 2022, 46, 779–791. [Google Scholar] [CrossRef]
- Yu, C.; Guo, C.J.; Jiang, L.H.; Gong, M.L.; Luo, Y.J. Deoxygenation of Primary Amides to Amines with Pinacolborane Catalyzed by Ca N(SiMe3)(2) (2)(THF)(2). Organometallics 2021, 40, 1201–1206. [Google Scholar] [CrossRef]
- Kumar, G.S.; Bhattacharjee, J.; Kumari, K.; Moorthy, S.; Bandyopadhyay, A.; Singh, S.K.; Panda, T.K. Hydroboration of nitriles, esters, and amides catalyzed by simple neosilyllithium. Polyhedron 2022, 219, 115784. [Google Scholar] [CrossRef]
- Kumar, R.; Bisai, M.K.; Jain, S.; Vanka, K.; Sen, S.S. Deoxygenative hydroboration of primary and secondary amides: A catalyst-free and solvent-free approach. Chem. Commun. 2021, 57, 10596–10599. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.L.; Joh, A.Y.; Hurley, Z.Q.; Anderson, C.L.; Singaram, B. Controlled Reduction of Tertiary Amides to the Corresponding Alcohols, Aldehydes, or Amines Using Dialkylboranes and Aminoborohydride Reagents. J. Org. Chem. 2016, 81, 3619–3628, Article. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, A.M.; Burkhardt, E.R. N,N-diethylaniline-borane, an efficient reducing agent for reduction of representative functional groups. Tetrahedron Lett. 1997, 38, 1519–1522. [Google Scholar] [CrossRef]
- Coleridge, B.M.; Angert, T.P.; Marks, L.R.; Hamilton, P.N.; Sutton, C.P.; Matos, K.; Burkhardt, E.R. Spiroborate catalyzed reductions with N,N-diethylaniline borane. Tetrahedron Lett. 2010, 51, 5973–5976. [Google Scholar] [CrossRef]
- Flaniken, J.M.; Collins, C.J.; Lanz, M.; Singaram, B. Aminoborohydrides. 11. Facile reduction of N-alkyl lactams to the corresponding amines using lithium aminoborohydrides. Org. Lett. 1999, 1, 799–801. [Google Scholar] [CrossRef]
- Nair, A.; Tiwari, V.; Verma, A.; Saini, P.; Elias, A.J. In situ generated aminodiborane as a reagent for deoxygenative reduction of carboxamides to amines. Org. Chem. Front. 2023, 10, 327–334. [Google Scholar] [CrossRef]
- Pan, Y.X.; Luo, Z.L.; Han, J.H.; Xu, X.; Chen, C.J.; Zhao, H.Q.; Xu, L.J.; Fan, Q.H.; Xiao, J.L. B(C6F5)(3)-Catalyzed Deoxygenative Reduction of Amides to Amines with Ammonia Borane. Adv. Synth. Catal. 2019, 361, 2301–2308. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Hamann, H.J.; Mishra, S. Aminoboranes via Tandem Iodination/Dehydroiodination for One-Pot Borylation. ACS Omega 2022, 7, 14377–14389. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.V.; Mistry, H.; Kulkarni, A.S.; Gagare, P.D. Ammonia-mediated, large-scale synthesis of ammonia borane. Dalton Trans. 2014, 43, 16580–16583. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.V.; Kulkarni, A.S. Water-promoted, safe and, scalable preparation of ammonia borane. Int. J. Hydrog. Energy 2017, 42, 1451–1455. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Hamann, H.J. Ammonia-borane as a Catalyst for the Direct Amidation of Carboxylic Acids. Org. Lett. 2021, 23, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.V.; Alawaed, A.A.; Hamann, H.J. TiCl4-catalyzed hydroboration of ketones with ammonia borane. J. Org. Chem. 2022, 87, 13259–13269. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Alawaed, A.A.; Hamann, H.J. A Safer Reduction of Carboxylic Acids with Titanium Catalysis. Org. Lett. 2022, 24, 8481–8486. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Alawaed, A.A. Room Temperature Reduction of Titanium Tetrachloride-Activated Nitriles to Primary Amines with Ammonia-Borane. Molecules 2023, 28, 60. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Gagare, P.D. Preparation of ammonia borane in high yield and purity, methanolysis, and regeneration. Inorg. Chem. 2007, 46, 7810–7817. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Hamann, H.J.; Choudhary, S. Amine-boranes as Dual-Purpose Reagents for Direct Amidation of Carboxylic Acids. Org. Lett. 2020, 22, 8593–8597. [Google Scholar] [CrossRef]
- Lee, O.Y.; Law, K.L.; Yang, D. Secondary Amine Formation from Reductive Amination of Carbonyl Compounds Promoted by Lewis Acid Using the InCl3/Et3SiH System. Org. Lett. 2009, 11, 3302–3305. [Google Scholar] [CrossRef]
- Zheng, J.X.; Roisnel, T.; Darcel, C.; Sortais, J.B. Nickel-Catalysed Reductive Amination with Hydrosilanes. Chemcatchem 2013, 5, 2861–2864. [Google Scholar] [CrossRef]
- Guo, X.W.; Wenger, O.S. Reductive Amination by Photoredox Catalysis and Polarity-Matched Hydrogen Atom Transfer. Angew. Chem. Int. Ed. Engl. 2018, 57, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Corre, Y.; Trivelli, X.; Capet, F.; Djukic, J.P.; Agbossou-Niedercorn, F.; Michon, C. Efficient and Selective Hydrosilylation of Secondary and Tertiary Amides Catalyzed by an Iridium(III) Metallacycle: Development and Mechanistic Investigation. Chemcatchem 2017, 9, 2009–2017. [Google Scholar] [CrossRef]
- Alshakova, I.D.; Gabidullin, B.; Nikonov, G.I. Ru-Catalyzed Transfer Hydrogenation of Nitriles, Aromatics, Olefins, Alkynes and Esters. Chemcatchem 2018, 10, 4874–4883. [Google Scholar] [CrossRef]
- Benitez-Medina, G.E.; Garcia, J.J. Hydrogenation and N-alkylation of anilines and imines via transfer hydrogenation with homogeneous nickel compounds. Dalton Trans. 2019, 48, 17579–17587. [Google Scholar] [CrossRef] [PubMed]
- Guyon, C.; Da Silva, E.; Lafon, R.; Metay, E.; Lemaire, M. Reductive amination using a combination of CaH2 and noble metal. RSC Adv. 2015, 5, 2292–2298. [Google Scholar] [CrossRef]
- Savela, R.; Vogt, D.; Leino, R. Ruthenium Catalyzed N-Alkylation of Cyclic Amines with Primary Alcohols. Eur. J. Org. Chem. 2020, 2020, 3030–3040. [Google Scholar] [CrossRef]
- Mitsudome, T.; Miyagawa, K.; Maeno, Z.; Mizugaki, T.; Jitsukawa, K.; Yamasaki, J.; Kitagawa, Y.; Kaneda, K. Mild Hydrogenation of Amides to Amines over a Platinum-Vanadium Bimetallic Catalyst. Angew. Chem. Int. Ed. Engl. 2017, 56, 9381–9385. [Google Scholar] [CrossRef]
- Dong, S.T.; Zong, Z.J.; Sun, N.; Hu, B.X.; Shen, Z.L.; Hu, X.Q.; Jin, L.Q. Hydrosilylative reduction of secondary amides to amines catalyzed by geometry-constrained NNN-cobalt complexes. New J. Chem. 2023, 47, 5603–5610. [Google Scholar] [CrossRef]
- Reeves, J.T.; Tan, Z.L.; Marsini, M.A.; Han, Z.X.S.; Xu, Y.B.; Reeves, D.C.; Lee, H.; Lu, B.Z.; Senanayakea, C.H. A Practical Procedure for Reduction of Primary, Secondary and Tertiary Amides to Amines. Adv. Synth. Catal. 2013, 355, 47–52. [Google Scholar] [CrossRef]
| Entry | LA | LA (mol%) | Solvent | Temp. | Time (h) | Product Conversion (%) b |
|---|---|---|---|---|---|---|
| 1 | TiCl4 | 10 | Et2O | RT | 4 | 13 |
| 2 | TiCl4 | 10 | Et2O | RT | 12 | 20 |
| 3 | TiCl4 | 10 | THF | RT | 12 | NR |
| 4 | TiCl4 | 50 | THF | RT | 12 | NR |
| 5 | TiCl4 | 50 | THF | reflux | 12 | 73 |
| 6 | TiCl4 | 50 | Toluene | reflux | 12 | 75 |
| 7 | TiCl4 | 50 | CHCl3 | reflux | 12 | 82 |
| 8 | TiCl4 | 10 | DCE | reflux | 24 | 98 c |
| 9 | TiCl4 | 20 | DCE | reflux | 18 | 94 |
| 10 | TiCl4 | 20 | DCE | reflux | 24 | 99 c |
| 11 | TiCl4 | 50 | DCE | reflux | 12 | 96 c |
| 12 | ZrCl4 | 50 | DCE | reflux | 12 | 25 |
| 13 | HfCl4 | 50 | DCE | reflux | 12 | 31 |
| 14 | FeCl3 | 50 | DCE | reflux | 12 | 30 |
| Entry | Amide | LA (mol%) | React. Time (h) | Product Amine | |||
|---|---|---|---|---|---|---|---|
| # | Structure | # | Structure | Yield (%) b | |||
| 1 | 2a |  | 10 | 24 | 3a | 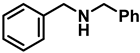 | 98 |
| 2 | 2a | 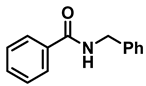 | 50 | 12 | 3a | 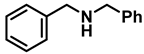 | 96 |
| 3 | 2b | 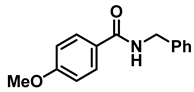 | 50 | 12 | 3b | 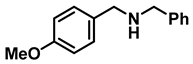 | 95 |
| 4 | 2c | 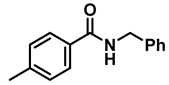 | 50 | 12 | 3c | 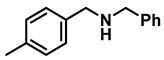 | 95 |
| 5 | 2d | 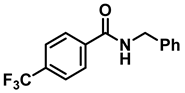 | 50 | 12 | 3d | 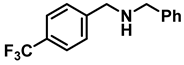 | 65 c |
| 6 | 2e | 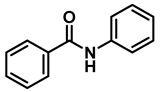 | 10 | 24 | 3e | 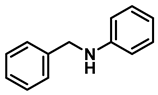 | 88 |
| 7 | 2e | 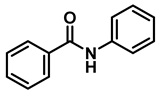 | 50 | 12 | 3e | 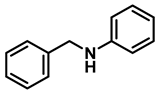 | 94 |
| 8 | 2f | 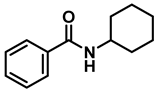 | 10 | 24 | 3f | 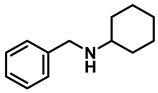 | 91 |
| 9 | 2f | 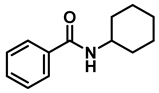 | 50 | 12 | 3f | 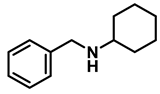 | 97 |
| 10 | 2g | 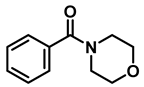 | 50 | 12 | 3g |  | 95 |
| 11 | 2h | 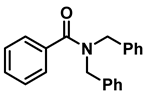 | 50 | 12 | 3h | 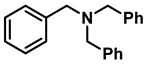 | 85 |
| Entry | Amide | LA (mol%) | React. Time (h) | Product Amine | |||
|---|---|---|---|---|---|---|---|
| # | Structure | # | Structure | Yield (%) b | |||
| 1 | 2i | 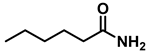 | 50 | 12 | 3i |  | 80 |
| 2 | 2j |  | 50 | 12 | 3j |  | 95 |
| 3 | 2k | 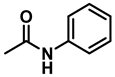 | 50 | 12 | 3k | 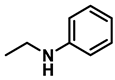 | 95 |
| 4 | 2l |  | 50 | 12 | 3l | 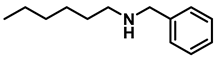 | 90 |
| 5 | 2m | 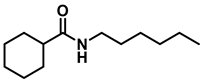 | 50 | 12 | 3m | 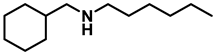 | 93 |
| 6 | 2n | 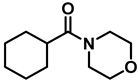 | 10 | 24 | 3n | 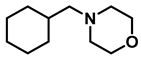 | 98 |
| 7 | 2o | 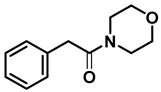 | 50 | 12 | 3o | 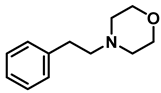 | 98 |
| 8 | 2p |  | 50 | 12 | 3p | 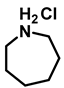 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramachandran, P.V.; Alawaed, A.A.; Singh, A. Titanium-Mediated Reduction of Carboxamides to Amines with Borane–Ammonia. Molecules 2023, 28, 4575. https://doi.org/10.3390/molecules28124575
Ramachandran PV, Alawaed AA, Singh A. Titanium-Mediated Reduction of Carboxamides to Amines with Borane–Ammonia. Molecules. 2023; 28(12):4575. https://doi.org/10.3390/molecules28124575
Chicago/Turabian StyleRamachandran, P. Veeraraghavan, Abdulkhaliq A. Alawaed, and Aman Singh. 2023. "Titanium-Mediated Reduction of Carboxamides to Amines with Borane–Ammonia" Molecules 28, no. 12: 4575. https://doi.org/10.3390/molecules28124575
APA StyleRamachandran, P. V., Alawaed, A. A., & Singh, A. (2023). Titanium-Mediated Reduction of Carboxamides to Amines with Borane–Ammonia. Molecules, 28(12), 4575. https://doi.org/10.3390/molecules28124575





