The Transport and Uptake of Resveratrol Mediated via Glucose Transporter 1 and Its Antioxidant Effect in Caco-2 Cells
Abstract
1. Introduction
2. Results
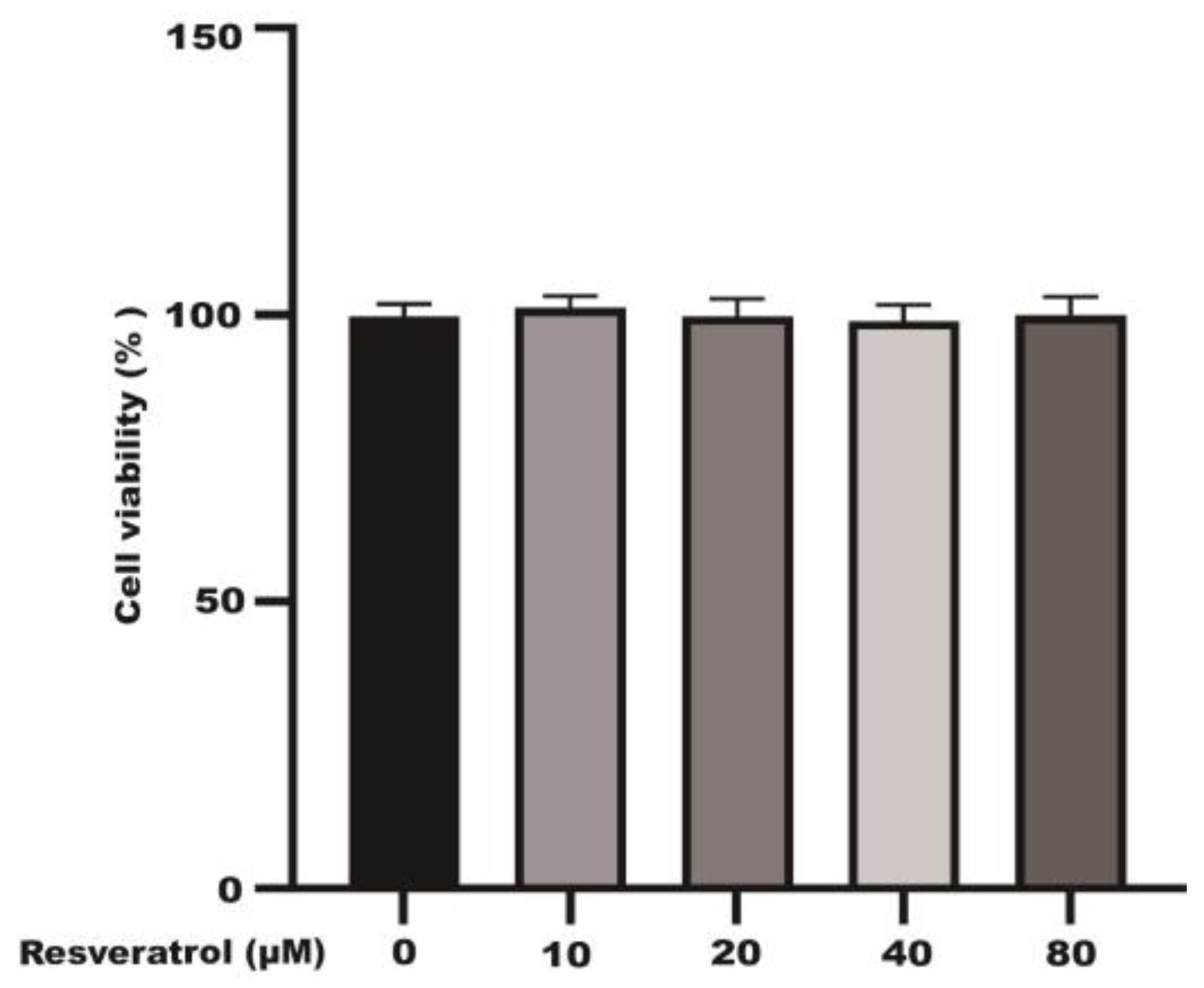
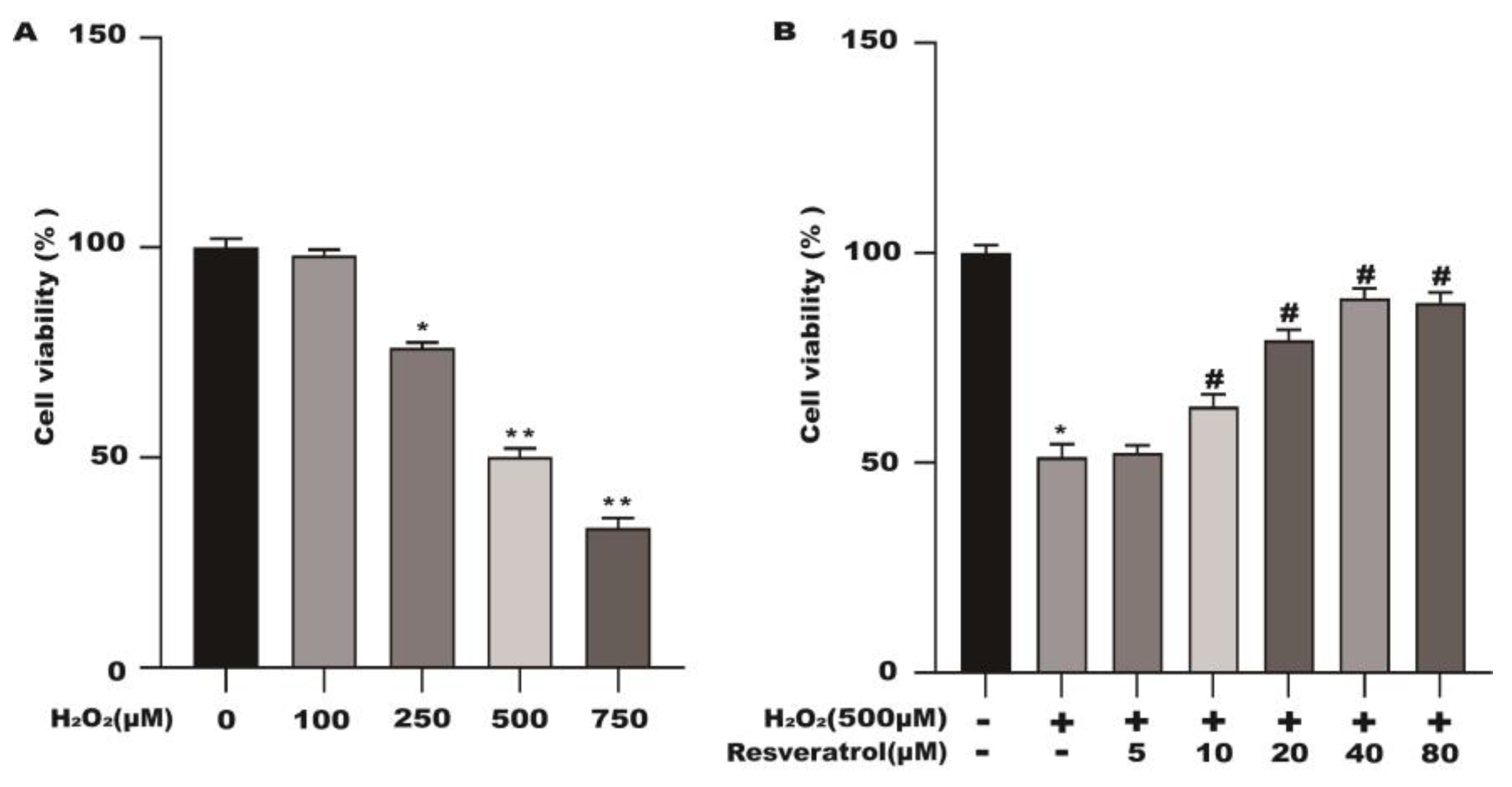
2.1. Effects of Different Concentrations of Resveratrol on the Viability of Caco-2 Cells
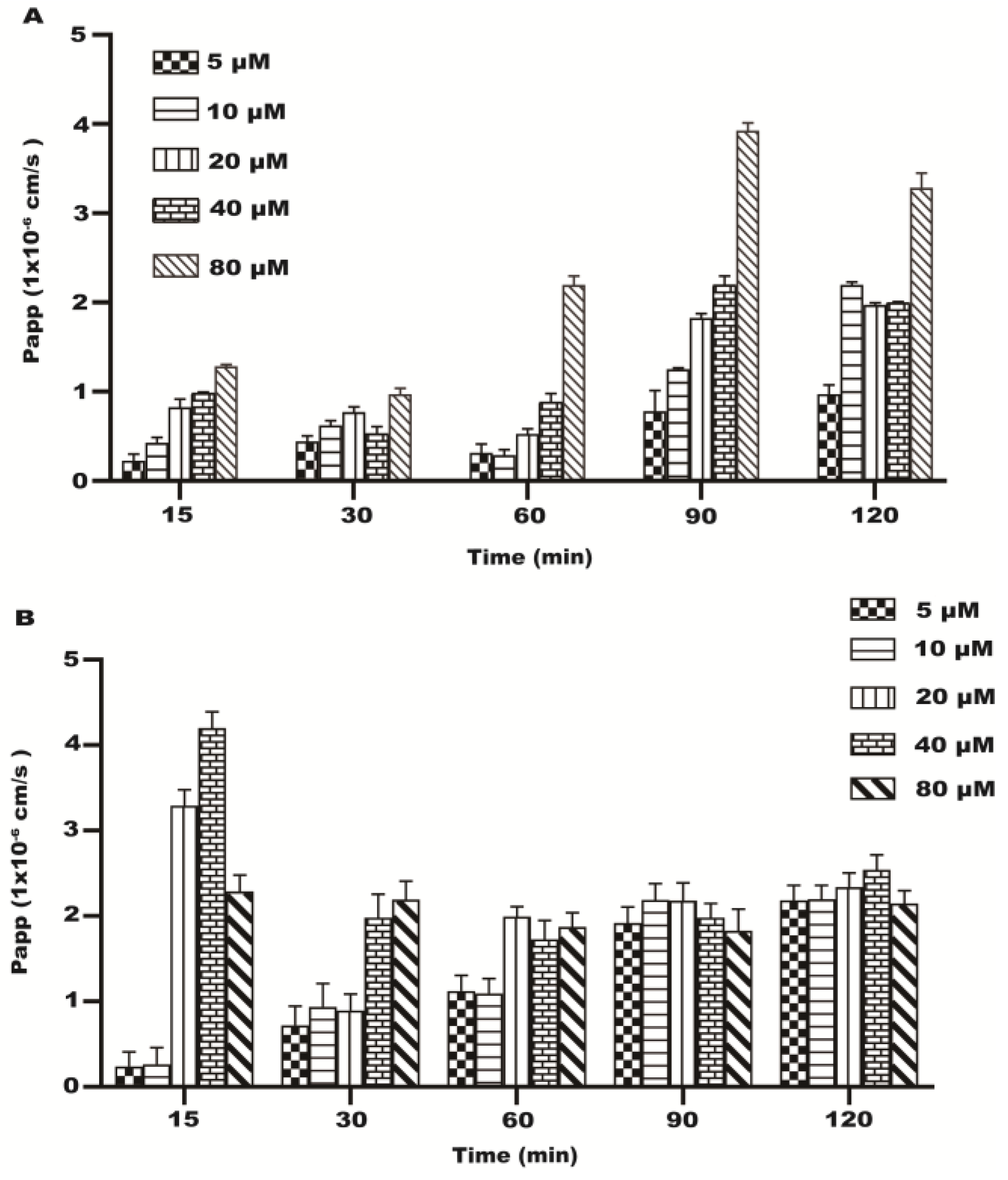
2.2. Transport Experiment Results
2.3. Papp of the Transport of Resveratrol
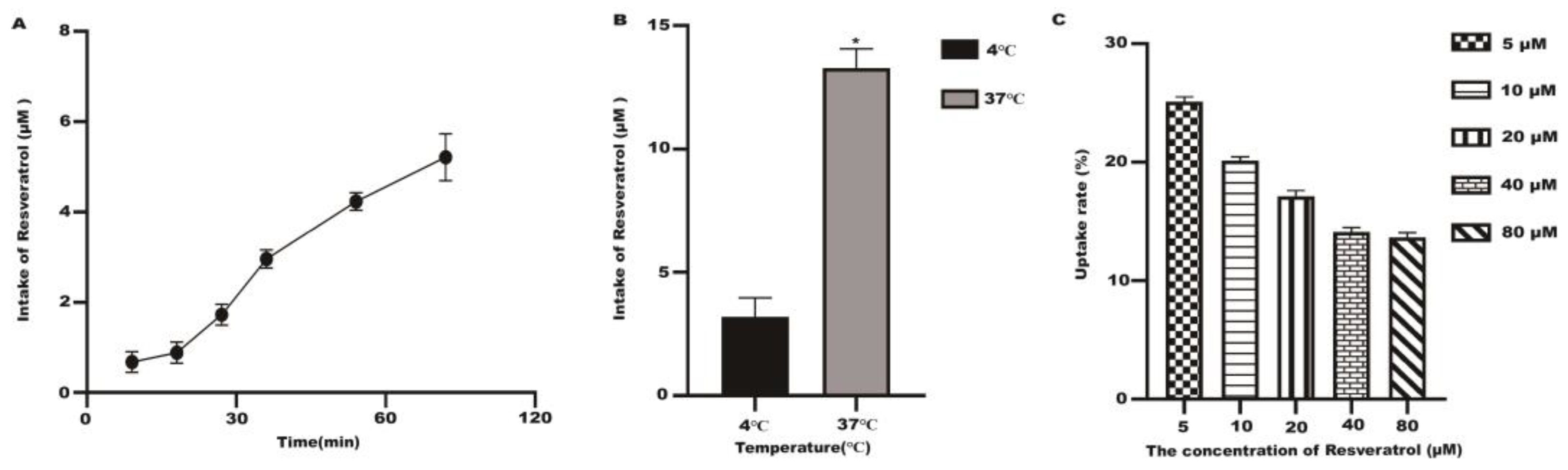
2.4. Uptake Test Results
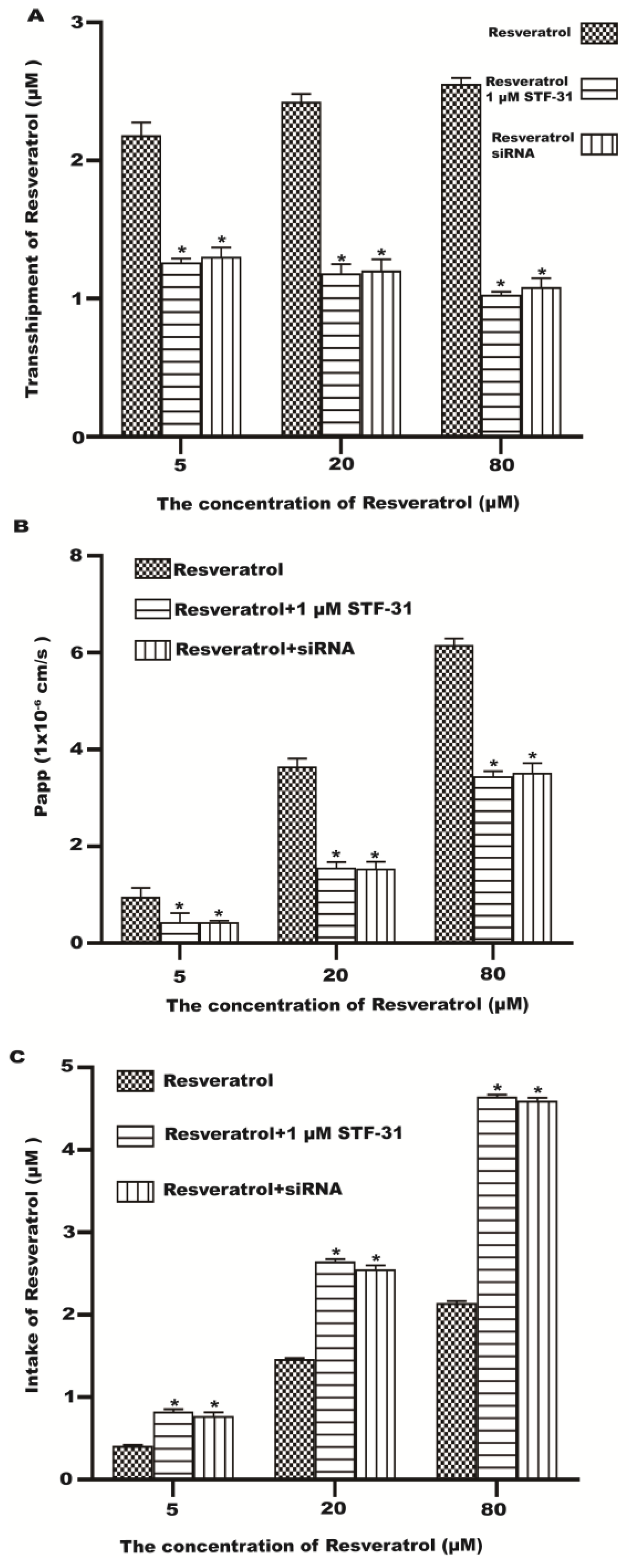
2.5. Effect of GLUT1 Inhibition on Resveratrol Transport and Uptake
2.6. Resveratrol Protects the Cell Viability of H2O2-Stimulated Caco-2 Cells
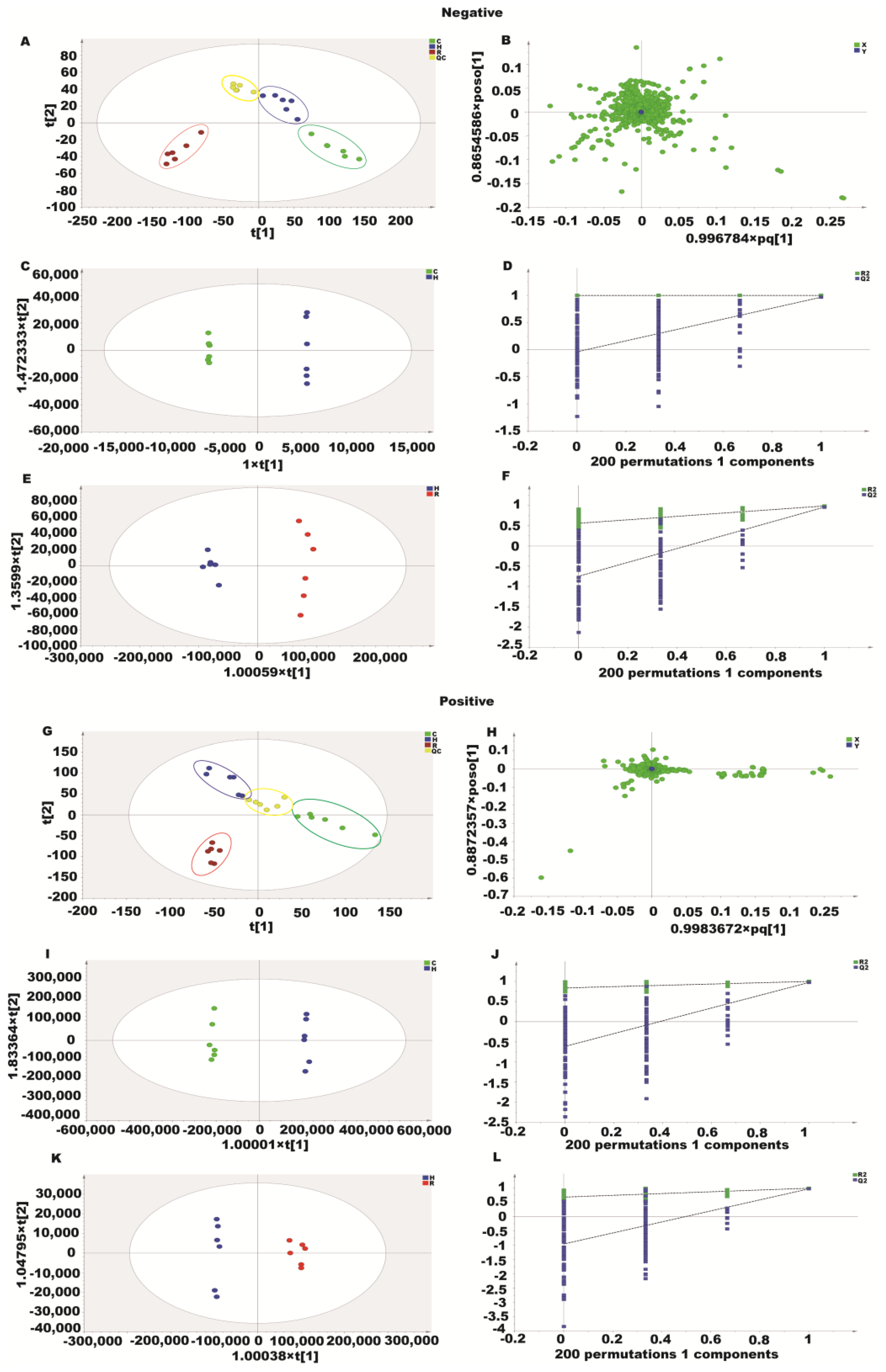
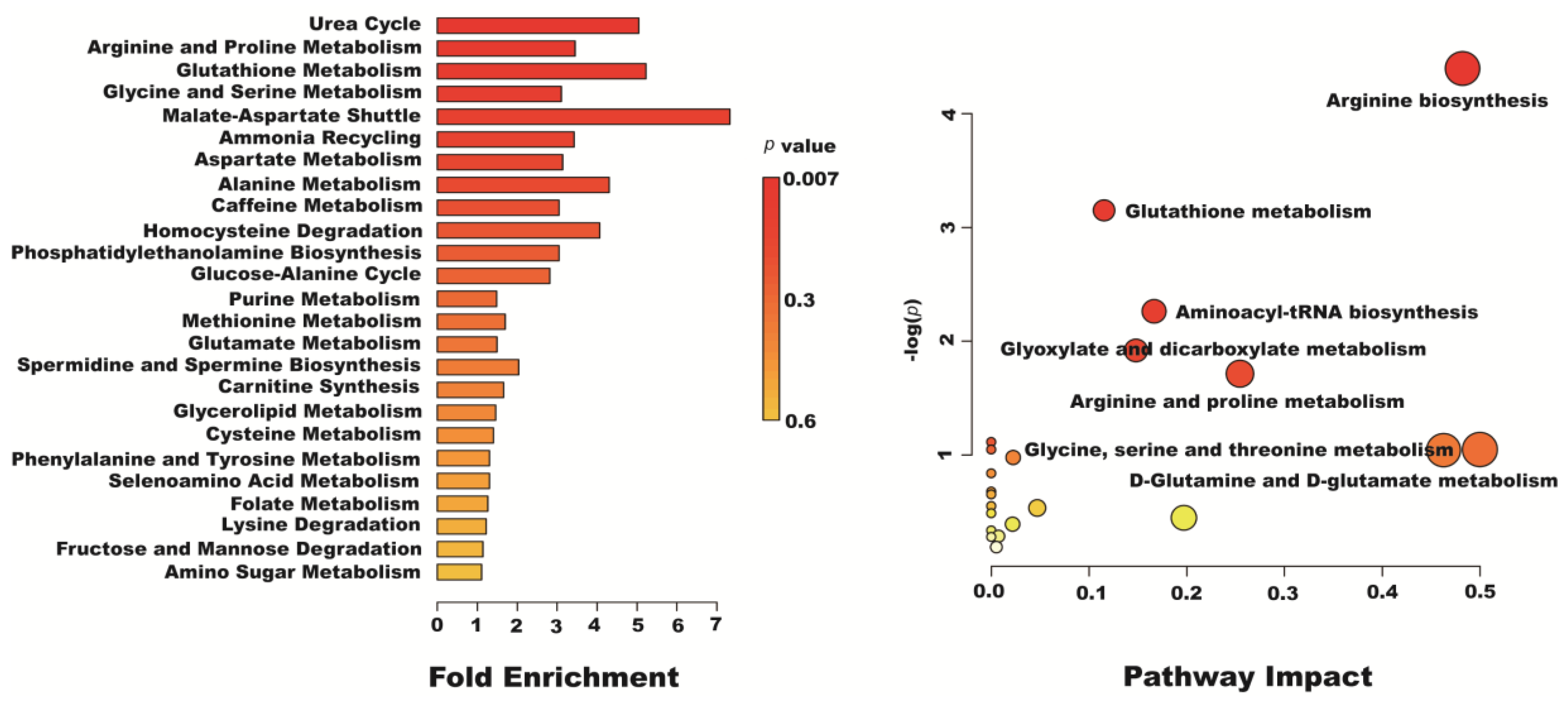
2.7. Metabolomics Analysis of Resveratrol Effect on H2O2-Induced Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cell Viability
4.4. Establishment and Evaluation of the Caco-2 Cell Uptake Model
4.5. Analytical Methods
4.5.1. HBSS Buffer Sample Extraction
4.5.2. Liquid Chromatography Analysis
4.6. Transport Assay of Caco-2 Cells
4.7. Caco-2 Cells Uptake Experiment
4.8. Metabonomic Analysis
4.8.1. Cellular Metabolite Extraction
4.8.2. UHPLC–QE Orbitrap/MS/MS Conditions
4.8.3. Qualitative Analysis of Metabolites
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The intestinal neuro-immune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021, 14, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liang, Z.; Lv, N.; Shan, J.; Zhou, H.; Zhang, J.; Shi, L. Exploring the total flavones of Abelmoschus manihot against IAV-induced lung inflammation by network pharmacology. BMC Complement. Med. Ther. 2022, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Kriseldi, R.; Johnson, J.A.; Walk, C.L.; Bedford, M.R.; Dozier, W.A., 3rd. Influence of exogenous phytase supplementation on phytate degradation, plasma inositol, alkaline phosphatase, and glucose concentrations of broilers at 28 days of age. Poult. Sci. 2021, 100, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Fierabracci, A. Insights on the Effects of Resveratrol and Some of Its Derivatives in Cancer and Autoimmunity: A Molecule with a Dual Activity. Antioxidants 2020, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Nowak, A. Cytotoxic and DNA-Damaging Effects of Aronia melanocarpa, Cornus mas, and Chaenomeles superba Leaf Extracts on the Human Colon Adenocarcinoma Cell Line Caco-2. Antioxidants 2020, 9, 1030. [Google Scholar] [CrossRef]
- Aleksiejczuk, M.; Gromotowicz-Poplawska, A.; Marcinczyk, N.; Stelmaszewska, J.; Dzieciol, J.; Chabielska, E. Aldosterone Increases Vascular Permeability in Rat Skin. Cells 2022, 11, 2707. [Google Scholar] [CrossRef]
- Cui, Y.; Claus, S.; Schnell, D.; Runge, F.; MacLean, C. In-Depth Characterization of EpiIntestinal Microtissue as a Model for Intestinal Drug Absorption and Metabolism in Human. Pharmaceutics 2020, 12, 405. [Google Scholar] [CrossRef]
- Morresi, C.; Vasarri, M.; Bellachioma, L.; Ferretti, G.; Degl Innocenti, D.; Bacchetti, T. Glucose Uptake and Oxidative Stress in Caco-2 Cells: Health Benefits from Posidonia oceanica (L.) Delile. Mar. Drugs 2022, 20, 457. [Google Scholar] [CrossRef]
- Arena, A.; Romeo, M.A.; Benedetti, R.; Masuelli, L.; Bei, R.; Gilardini Montani, M.S.; Cirone, M. New Insights into Curcumin- and Resveratrol-Mediated Anti-Cancer Effects. Pharmaceuticals 2021, 14, 1068. [Google Scholar] [CrossRef]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.M.; Resurreccion, A.V. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta 2016, 1860, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, Y.; Zhang, T.; Bo, S.; Bai, X.; Liu, H.; Li, T.; Liu, S.; Zhou, Y.; Cong, X.; et al. Resveratrol reverses chronic restraint stress-induced depression-like behaviour: Involvement of BDNF level, ERK phosphorylation and expression of Bcl-2 and Bax in rats. Brain Res. Bull. 2016, 125, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Das, D.K. Physiological effects of resveratrol. Biofactors 2010, 36, 401–406. [Google Scholar] [CrossRef]
- Frazzi, R.; Tigano, M. The multiple mechanisms of cell death triggered by resveratrol in lymphoma and leukemia. Int. J. Mol. Sci. 2014, 15, 4977–4993. [Google Scholar] [CrossRef]
- Ling, L.; Gu, S.; Cheng, Y. Resveratrol activates endogenous cardiac stem cells and improves myocardial regeneration following acute myocardial infarction. Mol. Med. Rep. 2017, 15, 1188–1194. [Google Scholar] [CrossRef]
- Liu, M.H.; Yuan, C.; He, J.; Tan, T.P.; Wu, S.J.; Fu, H.Y.; Liu, J.; Yu, S.; Chen, Y.D.; Le, Q.F.; et al. Resveratrol Protects PC12 Cells from High Glucose-Induced Neurotoxicity Via PI3K/Akt/FoxO3a Pathway. Cell. Mol. Neurobiol. 2015, 35, 513–522. [Google Scholar] [CrossRef]
- Zeng, Y.H.; Zhou, L.Y.; Chen, Q.Z.; Li, Y.; Shao, Y.; Ren, W.Y.; Liao, Y.P.; Wang, H.; Zhu, J.H.; Huang, M.; et al. Resveratrol inactivates PI3K/Akt signaling through upregulating BMP7 in human colon cancer cells. Oncol. Rep. 2017, 38, 456–464. [Google Scholar] [CrossRef]
- JanssenDuijghuijsen, L.M.; Grefte, S.; de Boer, V.C.J.; Zeper, L.; van Dartel, D.A.M.; van der Stelt, I.; Bekkenkamp-Grovenstein, M.; van Norren, K.; Wichers, H.J.; Keijer, J. Mitochondrial ATP Depletion Disrupts Caco-2 Monolayer Integrity and Internalizes Claudin 7. Front. Physiol. 2017, 8, 794. [Google Scholar] [CrossRef]
- Mena, J.; Elgueta, E.; Espinola-Gonzales, F.; Cardenas, H.; Orihuela, P.A. Hydroethanolic Extracts of the Aristotelia Chilensis (Maqui) Berry Reduces Cellular Viability and Invasiveness in the Endometrial Cancer Cell Line Ishikawa. Integr. Cancer Ther. 2021, 20, 15347354211007560. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols From Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Krieg, R.; Massey, H.D.; Carl, D.; Ghosh, S.; Gehr, T.W.B.; Ghosh, S.S. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrol. Dial. Transpl. 2019, 34, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Gao, J.; Hou, R.; Xu, X.; Yang, N.; Huang, S. Grape Seed Proanthocyanidins Inhibit Migration and Invasion of Bladder Cancer Cells by Reversing EMT through Suppression of TGF-beta Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5564312. [Google Scholar] [CrossRef]
- Bullon-Vela, V.; Abete, I.; Zulet, M.A.; Xu, Y.; Martinez-Gonzalez, M.A.; Sayon-Orea, C.; Ruiz-Canela, M.; Toledo, E.; Sanchez, V.M.; Estruch, R.; et al. Urinary Resveratrol Metabolites Output: Differential Associations with Cardiometabolic Markers and Liver Enzymes in House-Dwelling Subjects Featuring Metabolic Syndrome. Molecules 2020, 25, 4340. [Google Scholar] [CrossRef]
- Zortea, K.; Franco, V.C.; Guimarães, P.; Belmonte-de-Abreu, P.S. Resveratrol Supplementation Did Not Improve Cognition in Patients with Schizophrenia: Results from a Randomized Clinical Trial. Front. Psychiatry 2016, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, C.; Li, H.; Li, M.; Shu, X. Resveratrol-mediated reversal of tumor multi-drug resistance. Curr. Drug Metab. 2014, 15, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Tomassen, M.M.M.; Govers, C.; Vos, A.P.; de Wit, N.J.W. Dietary fat induced chylomicron-mediated LPS translocation in a bicameral Caco-2cell model. Lipids Health Dis. 2023, 22, 4. [Google Scholar] [CrossRef]
- Bilotta, S.; Arbogast, J.; Schart, N.; Frei, M.; Lorentz, A. Resveratrol Treatment Prevents Increase of Mast Cells in Both Murine OVA Enteritis and IL-10(-/-) Colitis. Int. J. Mol. Sci. 2022, 23, 1213. [Google Scholar] [CrossRef]
- Henry, C.; Vitrac, X.; Decendit, A.; Ennamany, R.; Krisa, S.; Merillon, J.M. Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J. Agric. Food Chem. 2005, 53, 798–803. [Google Scholar] [CrossRef]
- Subramanian, P.; Oh, B.J.; Mani, V.; Lee, J.K.; Lee, C.M.; Sim, J.S.; Koo, J.C.; Hahn, B.S. Differential Metabolic Profiles during the Developmental Stages of Plant-Parasitic Nematode Meloidogyne incognita. Int. J. Mol. Sci. 2017, 18, 1351. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; He, J.; Deng, J.; Yang, X.; Cui, L.; Ran, R.; Du, G.; Jiang, X. Small molecule metabolite biomarkers for hepatocellular carcinoma with bile duct tumor thrombus diagnosis. Sci. Rep. 2018, 8, 3309. [Google Scholar] [CrossRef]
- Stoessel, D.; Stellmann, J.P.; Willing, A.; Behrens, B.; Rosenkranz, S.C.; Hodecker, S.C.; Sturner, K.H.; Reinhardt, S.; Fleischer, S.; Deuschle, C.; et al. Metabolomic Profiles for Primary Progressive Multiple Sclerosis Stratification and Disease Course Monitoring. Front. Hum. Neurosci. 2018, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.; Zhong, J.; Zhang, M.; Chen, Z.H.; Wang, J.Y.; Chen, H.Y.; Wang, X.Q.; Zhang, B. The Alexipharmic Mechanisms of Five Licorice Ingredients Involved in CYP450 and Nrf2 Pathways in Paraquat-Induced Mice Acute Lung Injury. Oxid. Med. Cell. Longev. 2019, 2019, 7283104. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Yang, H.W.; Tain, Y.L. Perinatal Resveratrol Therapy Prevents Hypertension Programmed by Maternal Chronic Kidney Disease in Adult Male Offspring: Implications of the Gut Microbiome and Their Metabolites. Biomedicines 2020, 8, 567. [Google Scholar] [CrossRef]
- O’Brien, W.G., 3rd; Berka, V.; Tsai, A.L.; Zhao, Z.; Lee, C.C. CD73 and AMPD3 deficiency enhance metabolic performance via erythrocyte ATP that decreases hemoglobin oxygen affinity. Sci. Rep. 2015, 5, 13147. [Google Scholar] [CrossRef]
- Chak, C.M.; Lacruz, M.E.; Adam, J.; Brandmaier, S.; Covic, M.; Huang, J.; Meisinger, C.; Tiller, D.; Prehn, C.; Adamski, J.; et al. Ageing Investigation Using Two-Time-Point Metabolomics Data from KORA and CARLA Studies. Metabolites 2019, 9, 44. [Google Scholar] [CrossRef]
- De Sa Hyacienth, B.M.; Tavares Picanco, K.R.; Sanchez-Ortiz, B.L.; Barros Silva, L.; Matias Pereira, A.C.; Machado Goes, L.D.; Sousa Borges, R.; Cardoso Ataide, R.; Dos Santos, C.B.R.; de Oliveira Carvalho, H.; et al. Hydroethanolic extract from Endopleura uchi (Huber) Cuatrecasas and its marker bergenin: Toxicological and pharmacokinetic studies in silico and in vivo on zebrafish. Toxicol. Rep. 2020, 7, 217–232. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, T.; Zhao, F.; Xiong, D.; He, B.; Hua, Q.; Lin, M.; Deng, L.; Sang, X.; Xie, W.; et al. p62-Nrf2 Regulatory Loop Mediates the anti-Pulmonary Fibrosis Effect of Bergenin. Antioxidants 2022, 11, 307. [Google Scholar] [CrossRef]
- Qiao, S.; Liu, R.; Lv, C.; Miao, Y.; Yue, M.; Tao, Y.; Wei, Z.; Xia, Y.; Dai, Y. Bergenin impedes the generation of extracellular matrix in glomerular mesangial cells and ameliorates diabetic nephropathy in mice by inhibiting oxidative stress via the mTOR/beta-TrcP/Nrf2 pathway. Free Radic. Biol. Med. 2019, 145, 118–135. [Google Scholar] [CrossRef]
- Qi, Q.; Dong, Z.; Sun, Y.; Li, S.; Zhao, Z. Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules 2018, 23, 2668. [Google Scholar] [CrossRef]
- Gao, X.J.; Guo, M.Y.; Zhang, Z.C.; Wang, T.C.; Cao, Y.G.; Zhang, N.S. Bergenin Plays an Anti-Inflammatory Role via the Modulation of MAPK and NF-kappaB Signaling Pathways in a Mouse Model of LPS-Induced Mastitis. Inflammation 2015, 38, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, B.; Zheng, N.; Wu, G.; Ma, J.; Tao, X.; Chen, L.; Zhong, J.; Sheng, L.; Li, H. Integrated Metagenomic and Metabolomic Analyses of the Effect of Astragalus Polysaccharides on Alleviating High-Fat Diet-Induced Metabolic Disorders. Front. Pharmacol. 2020, 11, 833. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lu, X.; Chen, R.; Chen, Y. Comparative study of the effects of gold and silver nanoparticles on the metabolism of human dermal fibroblasts. Regen. Biomater. 2020, 7, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, F.; Tan, K.S.; Ser, H.L.; Tan, L.T.; Lee, L.H.; Tan, W. Effect of (R)-salbutamol on the switch of phenotype and metabolic pattern in LPS-induced macrophage cells. J. Cell. Mol. Med. 2020, 24, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Wang, H.L.; Cheng, X.L.; Wei, F.; Bai, X.; Lin, R.C.; Vaziri, N.D. Metabolomics analysis reveals the association between lipid abnormalities and oxidative stress, inflammation, fibrosis, and Nrf2 dysfunction in aristolochic acid-induced nephropathy. Sci. Rep. 2015, 5, 12936. [Google Scholar] [CrossRef]
- Kranawetter, C.; Zeng, S.; Joshi, T.; Sumner, L.W. A Medicago truncatula Metabolite Atlas Enables the Visualization of Differential Accumulation of Metabolites in Root Tissues. Metabolites 2021, 11, 238. [Google Scholar] [CrossRef]
- Mihasan, M.; Babii, C.; Aslebagh, R.; Channaveerappa, D.; Dupree, E.; Darie, C.C. Proteomics based analysis of the nicotine catabolism in Paenarthrobacter nicotinovorans pAO1. Sci. Rep. 2018, 8, 16239. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Tao, Q.; Qin, Z.; Liu, X.W.; Li, S.H.; Bai, L.X.; Yang, Y.J.; Li, J.Y. Uptake and Transport of Naringenin and Its Antioxidant Effects in Human Intestinal Epithelial Caco-2 Cells. Front. Nutr. 2022, 9, 894117. [Google Scholar] [CrossRef]
- Huang, M.Z.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, J.Y. Aspirin Eugenol Ester Reduces H2O2-Induced Oxidative Stress of HUVECs via Mitochondria-Lysosome Axis. Oxid. Med. Cell. Longev. 2019, 2019, 8098135. [Google Scholar] [CrossRef]



| No. | RT | VIP | Formula | Metabolites | SM | m/z | Fold Change | |
|---|---|---|---|---|---|---|---|---|
| C/H | H/R | |||||||
| 1 | 1.072532 | 3.38 | C6H11NO2 | Pipecolinic acid | ESI+ | 129.157 | 0.42 | 2.91 |
| 2 | 7.066492 | 1.31 | C5H5N5 | Adenine | ESI+ | 680.344 | 1.38 | 2.42 |
| 3 | 1.835533 | 2.55 | C9H12N2O6 | Uridine | ESI+ | 245.0759 | 1.21 | 2.95 |
| 4 | 4.238871 | 3.65 | C14H16O9 | Bergenin | ESI+ | 328.27 | 0.68 | 2.56 |
| 5 | 0.913932 | 4.42 | C22H22O10 | Glycitin | ESI+ | 485.015 | 0.63 | 4.27 |
| 6 | 0.947492 | 1.86 | C6H14N4O2 | Arginine | ESI+ | 174.20 | 0.22 | 3.83 |
| 7 | 0.918416 | 5.23 | C6H13N4O3 | Citrulline | ESI+ | 193.131 | 0.41 | 2.36 |
| 8 | 8.736221 | 2.23 | C40H80NO8P | Phosphatidylethanolamine 18 | ESI+ | 734.039 | 1.58 | 0.84 |
| 9 | 1.436667 | 1.38 | C2H5NO2 | Glycine | ESI+ | 76.03984 | 1.85 | 4.57 |
| 10 | 1.576442 | 1.51 | C10H20N4O6 | Glutamine | ESI+ | 185.029 | 0.84 | 2.48 |
| 11 | 3.923614 | 2.31 | C41H78NO8P | Phosphatidylethanolamine 15 | ESI+ | 744 | 1.38 | 0.14 |
| 12 | 4.836229 | 1.33 | C8H14CaO10 | Threonic acid | ESI+ | 310.27 | 0.59 | 2.84 |
| 13 | 1.880109 | 2.41 | C6H6N4O2 | 7-Methylxanthine | ESI+ | 167.0549 | 2.78 | 0.89 |
| 14 | 5.302783 | 3.02 | C9H3Cl6NO2 | Coumaroyl Hexoside | ESI− | 369.83 | 0.52 | 1.83 |
| 15 | 1.376172 | 3.06 | C4H6O5 | Malic acid | ESI− | 135.0273 | 0.72 | 2.88 |
| 16 | 4.237209 | 3.17 | C17H20O9 | Feruloyl quinic acid | ESI− | 368.3353 | 0.27 | 2.98 |
| 17 | 0.994167 | 2.44 | C5H7NO3 | Pyroglutamic acid | ESI− | 130.0497 | 2.67 | 0.66 |
| 18 | 0.582613 | 2.82 | C5H13N2O2 | L-ornithine | ESI− | 132.16 | 0.78 | 2.86 |
| 19 | 0.996538 | 4.25 | C3H7NO3 | L-serine | ESI− | 106.05 | 0.97 | 3.75 |
| 20 | 1.862335 | 2.99 | C6H11O7 | Gluconic acid | ESI− | 195.148 | 0.42 | 1.56 |
| 21 | 1.641869 | 1.72 | C5H9NO4 | L-Glutamic acid | ESI− | 148.0603 | 0.86 | 2.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-D.; Tao, Q.; Bai, L.-X.; Qin, Z.; Liu, X.-W.; Li, S.-H.; Yang, Y.-J.; Ge, W.-B.; Li, J.-Y. The Transport and Uptake of Resveratrol Mediated via Glucose Transporter 1 and Its Antioxidant Effect in Caco-2 Cells. Molecules 2023, 28, 4569. https://doi.org/10.3390/molecules28124569
Zhang Z-D, Tao Q, Bai L-X, Qin Z, Liu X-W, Li S-H, Yang Y-J, Ge W-B, Li J-Y. The Transport and Uptake of Resveratrol Mediated via Glucose Transporter 1 and Its Antioxidant Effect in Caco-2 Cells. Molecules. 2023; 28(12):4569. https://doi.org/10.3390/molecules28124569
Chicago/Turabian StyleZhang, Zhen-Dong, Qi Tao, Li-Xia Bai, Zhe Qin, Xi-Wang Liu, Shi-Hong Li, Ya-Jun Yang, Wen-Bo Ge, and Jian-Yong Li. 2023. "The Transport and Uptake of Resveratrol Mediated via Glucose Transporter 1 and Its Antioxidant Effect in Caco-2 Cells" Molecules 28, no. 12: 4569. https://doi.org/10.3390/molecules28124569
APA StyleZhang, Z.-D., Tao, Q., Bai, L.-X., Qin, Z., Liu, X.-W., Li, S.-H., Yang, Y.-J., Ge, W.-B., & Li, J.-Y. (2023). The Transport and Uptake of Resveratrol Mediated via Glucose Transporter 1 and Its Antioxidant Effect in Caco-2 Cells. Molecules, 28(12), 4569. https://doi.org/10.3390/molecules28124569







