Progress in the Mechanism of the Effect of Fe3O4 Nanomaterials on Ferroptosis in Tumor Cells
Abstract
1. Introduction
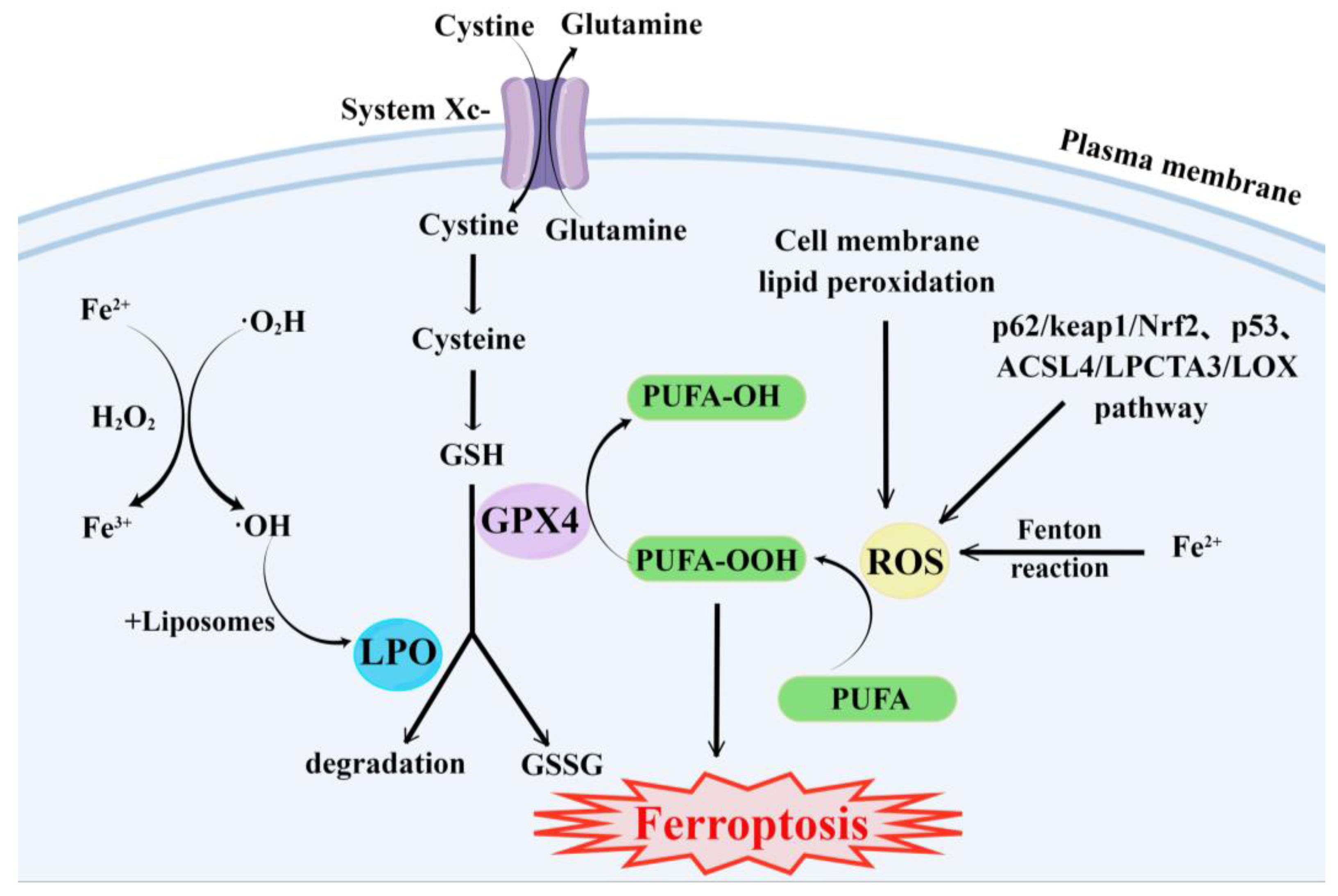
2. Ferroptosis
- (1)
- GSH synthesis pathway is inhibited and LPO accumulates, thus inducing ferroptosis in cells [55].
- (2)
- Iron metabolism is altered. Iron is a redox-active metal involved in ROS formation and LPO diffusion, and a rising iron level could increase cellular susceptibility to iron-dependent cell death [56]. The accepted explanation today is that Fe2+ can transfer electrons to intracellular oxygen, and then react with intracellular lipids to form LPO, which further induces ferroptosis [57,58]. Iron metabolism genes and iron metabolism regulation genes play a key role in intracellular system iron homeostasis. For example, the gene of Iron Responsive Element Binding Protein 2 (IREB2) is a key player in the Erastin-induced ferroptosis of HT-1080 fibrosarcoma cells and Calu-1 lung cancer cells [59]. Thus, intracellular iron overload is critical for ferroptosis [60].
- (3)
- ROS metabolic pathway effects. This pathway also plays an important role in ferroptosis. Cytosolic cystine/glutamate transport receptor (System Xc-) and voltage-dependent anion channels (VDACs) [18] in the outer mitochondrial membrane, GPX4 and ferroptosis suppressor protein 1 (FSP1) ferroptosis-related proteins [61,62], and p62/keap1/Nrf2 [63], p53-related pathway [64,65], and ACSL4/LPCTA3/LOX [66] ferroptosis-related pathways play their roles in regulating ferroptosis by affecting ROS metabolism pathways [67].
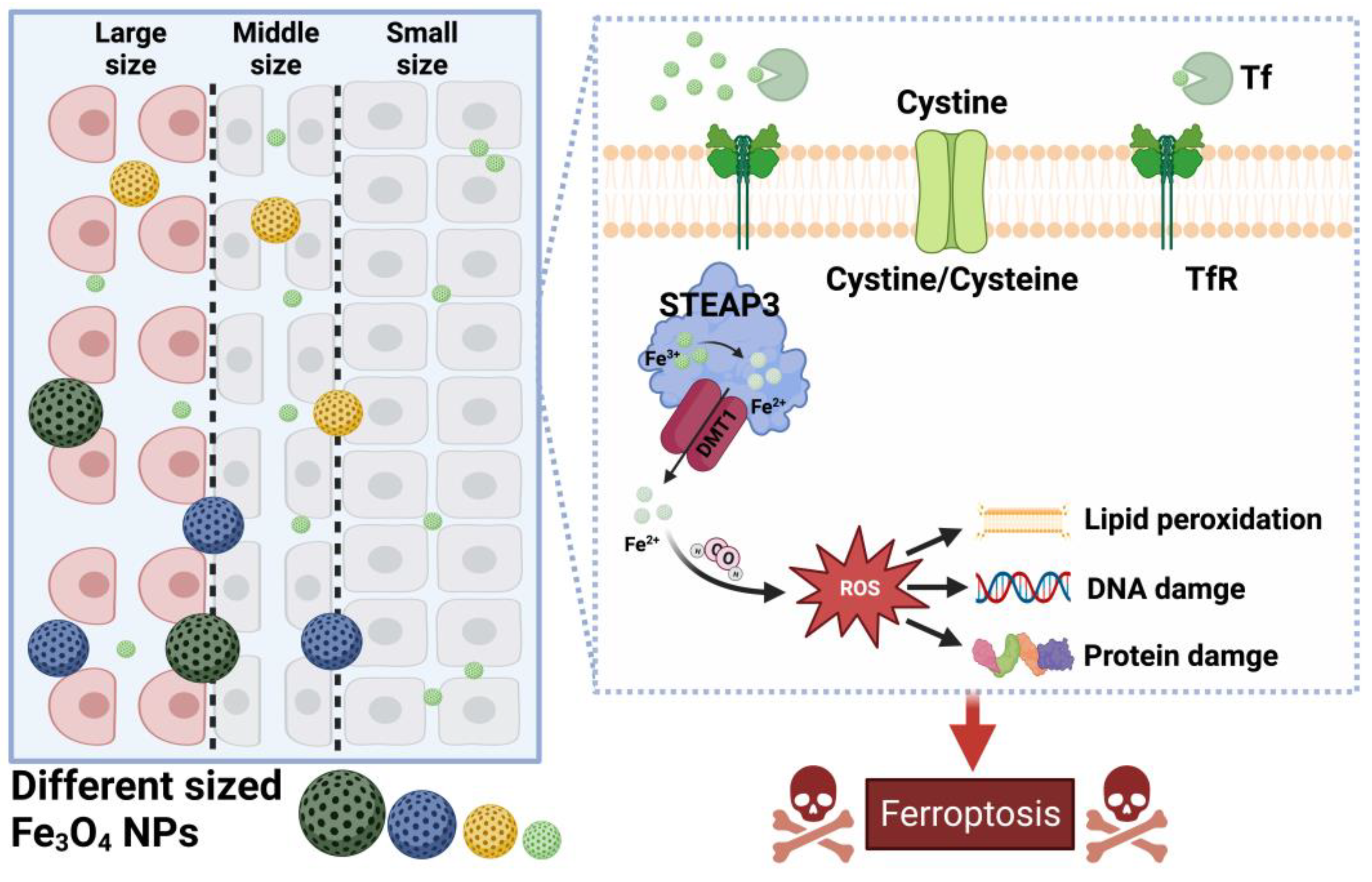
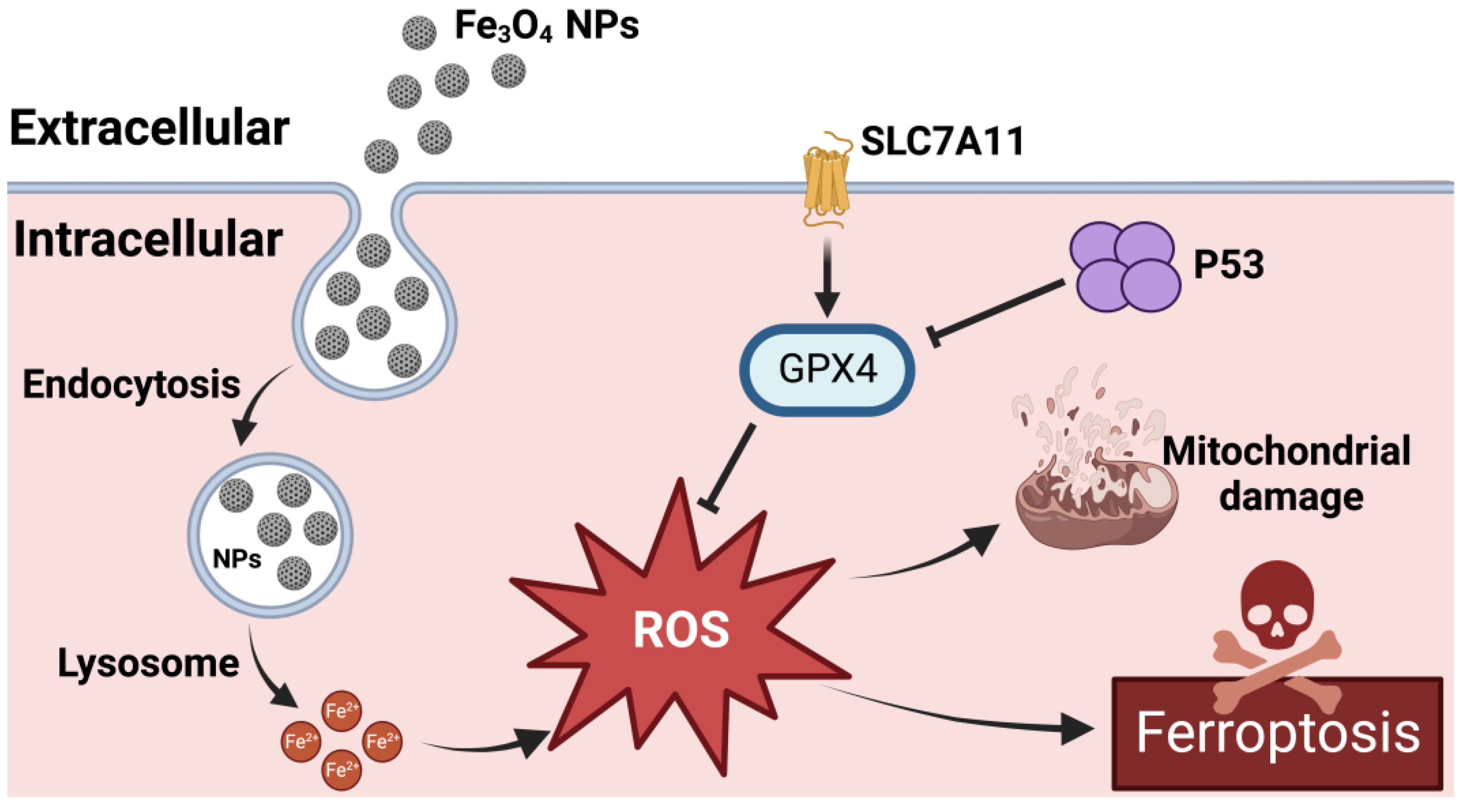
3. Mechanism of the Effect of Fe3O4-NPs on Ferroptosis in Tumor Cells
3.1. Effect of Fe3O4-NPs on the Expression of Ferroptosis-Related Genes
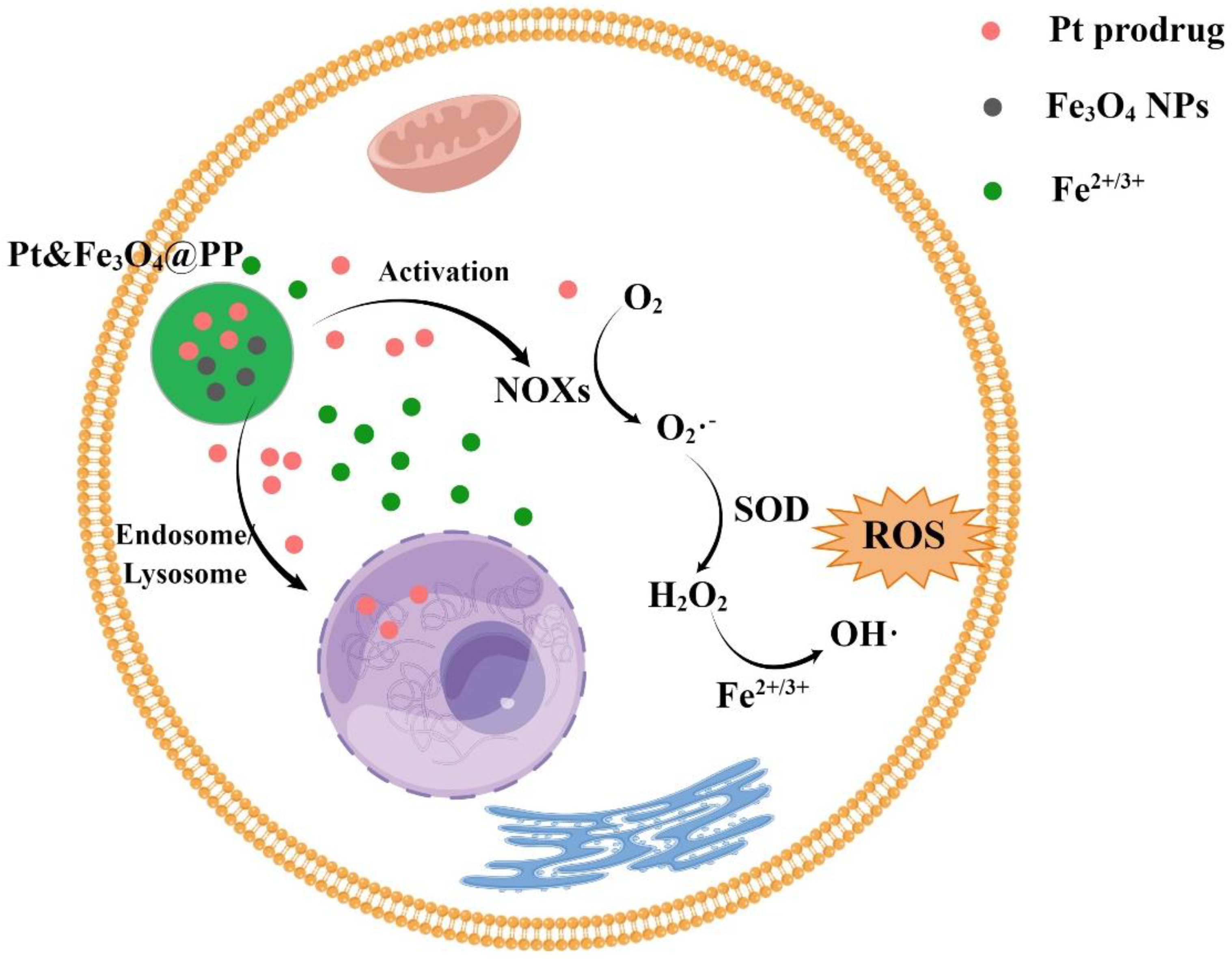
3.2. Fe3O4-NPs Enhance the Sensitivity of Tumor Cells to Anticancer Drugs
3.3. Fe3O4-NPs Can Enhance the Efficacy of Drugs or Synergize with Them to Promote Ferroptosis
4. Fe3O4-NPs in Combination with PDT, Heat Stress, and SDT Further Induced Ferroptosis in Tumor Cells
4.1. Synergy of Photodynamic Therapy (PDT)
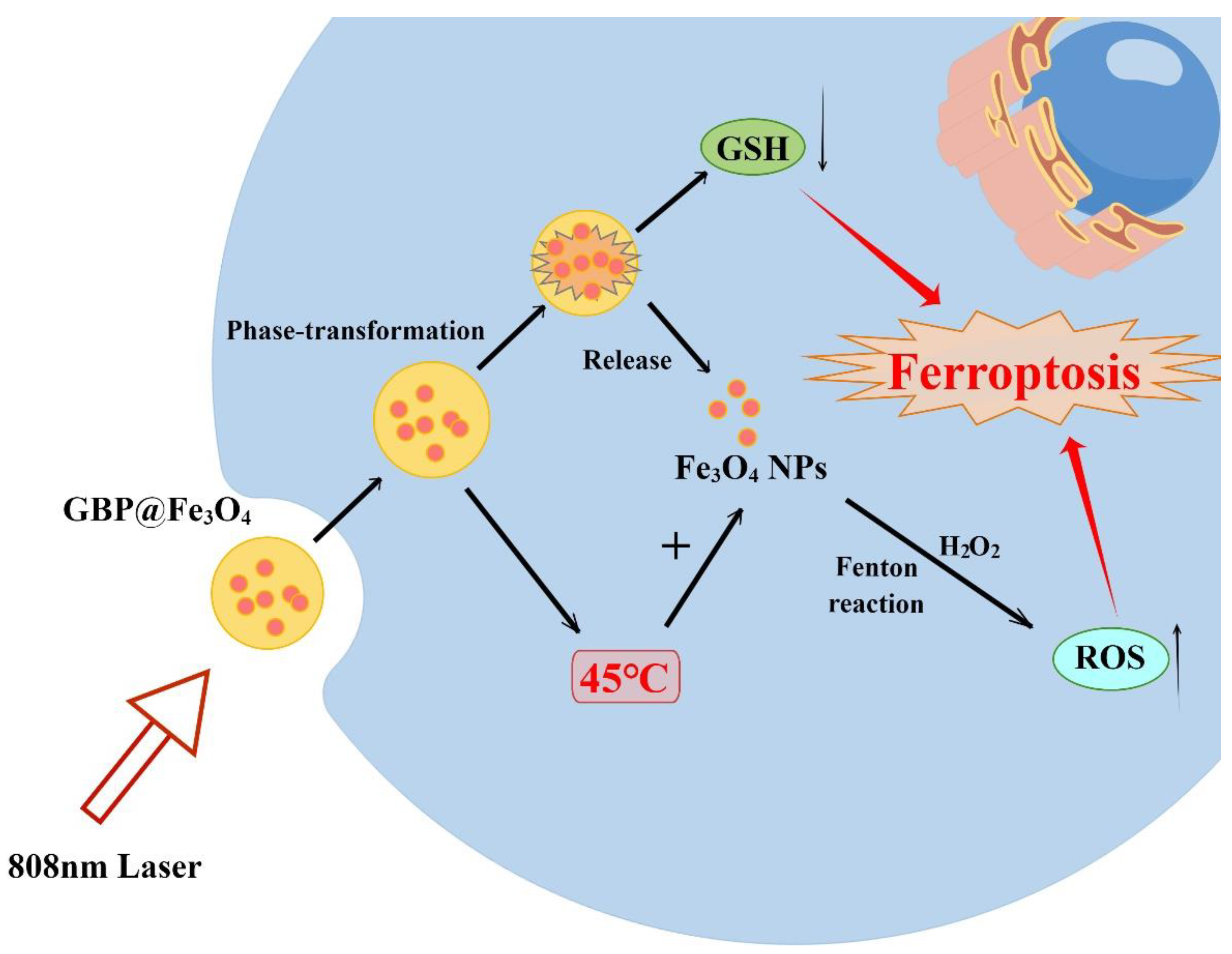
4.2. Metabolism Modulation by Heat Stress
4.3. Promotion of Sonodynamic Therapy (SDT)
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Z.; Huang, Y.; Xu, H.; He, L.; Deng, Y.; Zeng, X.; He, N. Delivery of PUMA Apoptosis Gene Using Polyethyleneimine-SMCC-TAT/DNA Nanoparticles: Biophysical Characterization and In Vitro Transfection Into Malignant Melanoma Cells. J. Biomed. Nanotechnol. 2015, 11, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Dang, Z.; Deng, Y.; Lu, G. Regulation of c-Myc and Bcl-2 induced apoptosis of human bronchial epithelial cells by zinc oxide nanoparticles. J. Biomed. Nanotechnol. 2012, 8, 669–675. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Li, Z.; Shi, Y.; Deng, J.; Bai, J.; Ma, L.; Zeng, X.; Feng, S.; Ren, J.; et al. Unravelling the Role of LncRNA WT1-AS/miR-206/NAMPT Axis as Prognostic Biomarkers in Lung Adenocarcinoma. Biomolecules 2021, 11, 203. [Google Scholar] [CrossRef]
- Li, W.; Jia, M.; Deng, J.; Wang, J.; Lin, Q.; Tang, J.; Zeng, X.; Cai, F.; Ma, L.; Su, W.; et al. Down-regulation of microRNA-200b is a potential prognostic marker of lung cancer in southern-central Chinese population. Saudi J. Biol. Sci. 2019, 26, 173–177. [Google Scholar] [CrossRef]
- Li, W.; Jia, M.; Wang, J.; Lu, J.; Deng, J.; Tang, J.; Liu, C. Association of MMP9-1562C/T and MMP13-77A/G Polymorphisms with Non-Small Cell Lung Cancer in Southern Chinese Population. Biomolecules 2019, 9, 107. [Google Scholar] [CrossRef]
- Yang, S.; Guo, H.; Wei, B.; Zhu, S.; Cai, Y.; Jiang, P.; Tang, J. Association of miR-502-binding site single nucleotide polymorphism in the 3′-untranslated region of SET8 and TP53 codon 72 polymorphism with non-small cell lung cancer in Chinese population. Acta Biochim. Biophys. Sin. 2014, 46, 149–154. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, J.; Ge, F.J.; Yu, X.H.; Bian, H.M.; Zhang, F.L.; Fang, J. Analysis of the Efficacy of Irinotecan in the Second-line Treatment of Refractory and Relapsed Small Cell Lung Cancer. Chin. J. Lung Cancer 2021, 24, 167–172. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Eggert, T.; Greten, T.F. Current Standard and Future Perspectives on Non-Surgical Therapy for Hepatocellular Carcinoma. Digestion 2017, 96, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, S.; Wang, Y.; Xie, R.; Liu, L.; Deng, Y. In Vivo Self-Assembly Based Cancer Therapy Strategy. J. Biomed. Nanotechnol. 2020, 16, 997–1017. [Google Scholar] [CrossRef]
- Liu, M.; Yu, X.C.; Chen, Z.; Yang, T.; Yang, D.D.; Liu, Q.Q.; Du, K.K.; Li, B.; Wang, Z.F.; Li, S.; et al. Aptamer selection and applications for breast cancer diagnostics and therapy. J. Nanobiotechnol. 2017, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xi, L.; Tan, T.; Jin, L.; Wang, Z.F.; He, N.Y. A novel aptamer-based histochemistry assay for specific diagnosis of clinical breast cancer tissues. Chin. Chem. Lett. 2021, 32, 1726–1730. [Google Scholar] [CrossRef]
- Xie, H.; Di, K.L.; Huang, R.R.; Khan, A.; Xia, Y.Y.; Xu, H.P.; Liu, C.; Tan, T.T.; Tian, X.Y.; Shen, H.; et al. Extracellular vesicles based electrochemical biosensors for detection of cancer cells: A review. Chin. Chem. Lett. 2020, 31, 1737–1745. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, J.H.; Yang, H.W.; Chen, S.Q.; Ma, G.J.; Zhang, X.M.; Zhu, M.; Yu, J.; Singh, R.; Zhang, Y.Y.; et al. Ultrasensitive Detection of Gastric Cancer Plasma MicroRNAs via Magnetic Beads-Based Chemiluminescent Assay. J. Biomed. Nanotechnol. 2017, 13, 1272–1280. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Bannai, S.; Tsukeda, H.; Okumura, H. Effect of antioxidants on cultured human diploid fibroblasts exposed to cystine-free medium. Biochem. Biophys. Res. Commun. 1977, 74, 1582–1588. [Google Scholar] [CrossRef]
- Magesa, F.; Wu, Y.Y.; Dong, S.; Tian, Y.L.; Li, G.L.; Vianney, J.M.; Buza, J.; Liu, J.; He, Q.G. Electrochemical Sensing Fabricated with Ta2O5 Nanoparticle-Electrochemically Reduced Graphene Oxide Nanocomposite for the Detection of Oxytetracycline. Biomolecules 2020, 10, 110. [Google Scholar] [CrossRef]
- Yang, G.J.; Huang, H.; Xiao, Z.Q.; Zhang, C.X.; Guo, W.F.; Ma, T.T.; Ma, L.; Chen, Z.; Deng, Y. A Novel Strategy for Liquid Exfoliation of Ultrathin Black Phosphorus Nanosheets. J. Biomed. Nanotechnol. 2020, 16, 548–552. [Google Scholar] [CrossRef] [PubMed]
- He, Q.G.; Tian, Y.L.; Wu, Y.Y.; Liu, J.; Li, G.L.; Deng, P.H.; Chen, D.C. Electrochemical Sensor for Rapid and Sensitive Detection of Tryptophan by a Cu2O Nanoparticles-Coated Reduced Graphene Oxide Nanocomposite. Biomolecules 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- He, Q.G.; Liu, J.; Liu, X.P.; Li, G.L.; Deng, P.H.; Liang, J. Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloids Surf. B-Biointerfaces 2018, 172, 565–572. [Google Scholar] [CrossRef]
- Liu, J.; Dong, S.; He, Q.G.; Yang, S.C.; Xie, M.; Deng, P.H.; Xia, Y.H.; Li, G.L. Facile Preparation of Fe3O4/C Nanocomposite and Its Application for Cost-Effective and Sensitive Detection of Tryptophan. Biomolecules 2019, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.R.; Zeng, X.; Xi, Z.J.; Liu, M.; Li, C.Y.; Li, Z.Y.; Jin, L.; Wang, Z.F.; Deng, Y.; He, N.Y. Improvement on Controllable Fabrication of Streptavidin-Modified Three-Layer Core-Shell Fe3O4@SiO2@Au Magnetic Nanocomposites with Low Fluorescence Background. J. Biomed. Nanotechnol. 2013, 9, 674–684. [Google Scholar] [CrossRef]
- Ma, C.; Li, C.Y.; He, N.Y.; Wang, F.; Ma, N.N.; Zhang, L.M.; Lu, Z.X.; Ali, Z.; Xi, Z.J.; Li, X.L.; et al. Preparation and Characterization of Monodisperse Core-Shell Fe3O4@SiO2 Microspheres and Its Application for Magnetic Separation of Nucleic Acids from E. coli BL21. J. Biomed. Nanotechnol. 2012, 8, 1000–1005. [Google Scholar] [CrossRef]
- Fan, L.; Liu, H.; Gao, C.X.; Zhu, P.Z. Facile synthesis and characterization of magnetic hydroxyapatite/Fe3O4 microspheres. Mater. Lett. 2022, 313, 131648. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, H.; Chang, L.; Yu, B.Z.; Liu, Q.Y.; Wu, J.P.; Miao, Y.Q.; Ma, P.; Fan, D.D.; Fan, H.M. Human-like collagen protein-coated magnetic nanoparticles with high magnetic hyperthermia performance and improved biocompatibility. Nanoscale Res. Lett. 2015, 10, 28. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.T.; Chen, J.D.; Su, J.C.; Zhi, X.; Pan, P.P.; Zou, L.; Zhang, Q.Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B-Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef]
- He, L.; Yang, H.W.; Xiao, P.F.; Singh, R.; He, N.Y.; Liu, B.; Li, Z.Y. Highly Selective, Sensitive and Rapid Detection of Escherichia coli O157: H7 Using Duplex PCR and Magnetic Nanoparticle-Based Chemiluminescence Assay. J. Biomed. Nanotechnol. 2017, 13, 1243–1252. [Google Scholar] [CrossRef]
- Ling, Y.Z.; Zhu, Y.F.; Fan, H.H.; Zha, H.L.; Yang, M.; Wu, L.; Chen, H.; Li, W.; Wu, Y.P.; Chen, H.B. Rapid Method for Detection of Staphylococcus aureus in Feces. J. Biomed. Nanotechnol. 2019, 15, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.N.; Dong, H.M.; Chen, Z.; Lin, L.; Chen, H.; Li, S.; Deng, Y. Magnetic Nanoparticles Enhanced Microarray Detection of Multiple Foodborne Pathogens. J. Biomed. Nanotechnol. 2017, 13, 1333–1343. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.N.; Deng, Y.; Lin, L.; He, N.Y. Development of a Magnetic Nanoparticles Microarray for Simultaneous and Simple Detection of Foodborne Pathogens. J. Biomed. Nanotechnol. 2013, 9, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.B.; Chen, Z.; Li, T.T.; Liu, M.; Liu, Y.; Ali, Z.; Li, S.; Zhu, Y.B.; Li, Z.Y.; Deng, Y. A Highly Sensitive Strategy for Low-Abundance Hepatitis B Virus Detection via One-Step Nested Polymerase Chain Reaction, Chemiluminescence Technology and Magnetic Separation. J. Biomed. Nanotechnol. 2019, 15, 1832–1838. [Google Scholar] [CrossRef]
- Hao, H.Q.; Ma, Q.M.; He, F.; Yao, P. Doxorubicin and Fe3O4 loaded albumin nanoparticles with folic acid modified dextran surface for tumor diagnosis and therapy. J. Mater. Chem. B 2014, 2, 7978–7987. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.M.; Yan, H.; Kang, F.Y.; Li, Z.F.; Qiao, Y.Y.; Li, D. A cross-talk EGFR/VEGFR-targeted bispecific nanoprobe for magnetic resonance/near-infrared fluorescence imaging of colorectal cancer. MRS Commun. 2018, 8, 1008–1017. [Google Scholar] [CrossRef]
- Wang, M.L.; Yang, Q.M.; Li, M.; Zou, H.M.; Wang, Z.G.; Ran, H.T.; Zheng, Y.Y.; Jian, J.; Zhou, Y.; Luo, Y.D.; et al. Multifunctional Nanoparticles for Multimodal Imaging-Guided Low-Intensity Focused Ultrasound/Immunosynergistic Retinoblastoma Therapy. ACS Appl. Mater. Interfaces 2020, 12, 5642–5657. [Google Scholar] [CrossRef]
- Tang, Y.J.; Ali, Z.; Dai, J.G.; Liu, X.L.; Wu, Y.Q.; Chen, Z.; He, N.Y.; Li, S.; Wang, L.J. Single-Nucleotide Polymorphism Genotyping of exoS in Pseudomonas aeruginosa Using Dual-Color Fluorescence Hybridization and Magnetic Separation. J. Biomed. Nanotechnol. 2018, 14, 206–214. [Google Scholar] [CrossRef]
- Mou, X.B.; Sheng, D.N.; Chen, Z.; Liu, M.; Liu, Y.; Deng, Y.; Xu, K.; Hou, R.X.; Zhao, J.Y.; Zhu, Y.B.; et al. In-Situ Mutation Detection by Magnetic Beads-Probe Based on Single Base Extension and Its Application in Genotyping of Hepatitis B Virus Pre-C Region 1896nt Locus Single Nucleotide Polymorphisms. J. Biomed. Nanotechnol. 2019, 15, 2393–2400. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.Y.; Ma, M.; Li, Z.Y.; Liu, H.N.; Li, S.; Deng, Y.; Zhang, L.M.; Lu, Z.X.; Wang, W.; et al. High Throughput SNP Detection System Based on Magnetic Nanoparticles Separation. J. Biomed. Nanotechnol. 2013, 9, 247–256. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.N.; Jia, Y.Y.; Mou, X.B.; Deng, Y.; Lin, L.; Liu, B.; He, N.Y. An Automatic High-Throughput Single Nucleotide Polymorphism Genotyping Approach Based on Universal Tagged Arrays and Magnetic Nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 689–698. [Google Scholar] [CrossRef]
- Tang, C.L.; He, Z.Y.; Liu, H.M.; Xu, Y.Y.; Huang, H.; Yang, G.J.; Xiao, Z.Q.; Li, S.; Liu, H.N.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Shi, Z.; Sun, Y.; Wu, X.Y.; Li, S.; Dong, S.B. Preparation of amphiphilic magnetic polyvinyl alcohol targeted drug carrier and drug delivery research. Des. Monomers Polym. 2020, 23, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.Z.; Li, B.; Qiao, Y.S. Fe3O4 Nanoparticles in Targeted Drug/Gene Delivery Systems. Materials 2018, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Hu, Y.; Mignani, S.; Majoral, J.P.; Shen, M.W.; Shi, X.Y. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem. Soc. Rev. 2018, 47, 1874–1900. [Google Scholar] [CrossRef]
- Wang, K.; Xu, X.G.; Ma, Y.L.; Sheng, C.R.; Li, L.N.; Lu, L.Y.; Wang, J.; Wang, Y.N.; Jiang, Y. Fe3O4@Angelica sinensis polysaccharide nanoparticles as an ultralow-toxicity contrast agent for magnetic resonance imaging. Rare Met. 2021, 40, 2486–2493. [Google Scholar] [CrossRef]
- Li, J.J.; Wang, J.H.; Li, J.Y.; Yang, X.; Wan, J.L.; Zheng, C.S.; Du, Q.; Zhou, G.F.; Yang, X.L. Fabrication of Fe3O4@PVA microspheres by one-step electrospray for magnetic resonance imaging during transcatheter arterial embolization. Acta Biomater. 2021, 131, 532–543. [Google Scholar] [CrossRef]
- Wu, C.H.; Zhao, W.W.; Yu, J.; Li, S.J.; Lin, L.G.; Chen, X.P. Induction of ferroptosis and mitochondrial dysfunction by oxidative stress in PC12 cells. Sci. Rep. 2018, 8, 574. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Lan, T.; Qin, W.; Zhu, Y.T.; Qin, K.; Gao, J.J.; Wang, H.B.; Hou, X.M.; Chen, N.; et al. Quantitative Profiling of Protein Carbonylations in Ferroptosis by an Aniline-Derived Probe. J. Am. Chem. Soc. 2018, 140, 4712–4720. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.Y.; Meng, D.N.; Wang, X.; Chen, X.R. Ferroptosis-Driven Nanotherapeutics to Reverse Drug Resistance in Tumor Microenvironment. ACS Appl. Bio Mater. 2022, 5, 2481–2506. [Google Scholar] [CrossRef]
- Dixon, S.J. Ferroptosis: Bug or feature? Immunol. Rev. 2017, 277, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.Y.; Hu, R.L.; Yu, R.X.; Tang, Y.W.; Li, J.R. A narrative review of mechanisms of ferroptosis in cancer: New challenges and opportunities. Ann. Transl. Med. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Ma, L.F.; Wang, J.Y.; Qiao, Y.X. Mechanism of Ferroptosis and Its Research Progress in Lung Cancer. Chin. J. Lung Cancer 2020, 23, 811–817. [Google Scholar]
- Gao, M.H.; Monian, P.; Pan, Q.H.; Zhang, W.; Xiang, J.; Jiang, X.J. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef]
- He, Y.J.; Liu, X.Y.; Xing, L.; Wan, X.; Chang, X.; Jiang, H.L. Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials 2020, 241, 119911. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.L.; Zhang, M.K.; Yu, W.Y.; Gao, F.; Li, C.X.; Wang, S.B.; Feng, J.; Zhang, X.Z. Ferrous-Supply-Regeneration Nanoengineering for Cancer-Cell-Specific Ferroptosis in Combination with Imaging-Guided Photodynamic Therapy. ACS Nano 2018, 12, 12181–12192. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem. Sci. 2016, 41, 274–286. [Google Scholar] [CrossRef]
- Angeli, J.P.F.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid Peroxidation-Dependent Cell Death Regulated by GPx4 and Ferroptosis. In Apoptotic and Non-Apoptotic Cell Death; Nagata, S., Nakano, H., Eds.; Springer: Cham, Switzerland, 2017; pp. 143–170. [Google Scholar]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; Dallaglio, K.; Torquato, P.; Piroddi, M.; Galli, F. Nrf2-p62 autophagy pathway and its response to oxidative stress in hepatocellular carcinoma. Transl. Res. 2018, 193, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Kroemer, G.; Tang, D.L. The tumor suppressor protein p53 and the ferroptosis network. Free Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef]
- Chu, B.; Kon, N.; Chen, D.L.; Li, T.Y.; Liu, T.; Jiang, L.; Song, S.J.; Tavana, O.; Gu, W. ALOX12 is required for p53-mediated tumour suppression through a distinct ferroptosis pathway. Nat. Cell Biol. 2019, 21, 579–591. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; IngoId, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Shen, Z.; Song, J.; Yung, B.C.; Zhou, Z.; Wu, A.; Chen, X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv. Mater. 2018, 30, e1704007. [Google Scholar] [CrossRef]
- Lai, Y.X.; Deng, Y.; Yang, G.J.; Li, S.; Zhang, C.X.; Liu, X.Y. Molecular Imprinting Polymers Electrochemical Sensor Based on AuNPs/PTh Modified GCE for Highly Sensitive Detection of Carcinomaembryonic Antigen. J. Biomed. Nanotechnol. 2018, 14, 1688–1694. [Google Scholar] [CrossRef]
- Lai, Y.X.; Wang, L.J.; Liu, Y.; Yang, G.J.; Tang, C.L.; Deng, Y.; Li, S. Immunosensors Based on Nanomaterials for Detection of Tumor Markers. J. Biomed. Nanotechnol. 2018, 14, 44–65. [Google Scholar] [CrossRef]
- Su, W.; Ma, L.; Wu, S.H.; Li, W.; Tang, J.X.; Deng, J.; Liu, J.X. Effect of Surface Modification of Silver Nanoparticles on the Proliferation of Human Lung Squamous Cell Carcinoma (HTB182) and Bronchial Epithelial (HBE) Cells In Vitro. J. Biomed. Nanotechnol. 2017, 13, 1281–1291. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Deng, P.H.; Tian, Y.L.; Ding, Z.Y.; Li, G.L.; Liu, J.; Zuberi, Z.; He, Q.G. Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 2020, 131, 107393. [Google Scholar] [CrossRef]
- Gong, L.; Zhao, L.; Tan, M.D.; Pan, T.; He, H.; Wang, Y.L.; He, X.L.; Li, W.J.; Tang, L.; Nie, L.B. Two-Photon Fluorescent Nanomaterials and Their Applications in Biomedicine. J. Biomed. Nanotechnol. 2021, 17, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Su, E.B.; Liu, Y.; He, N.Y.; Deng, Y.; Jin, L.; Chen, Z.; Li, S. A microfluidic device for accurate detection of hs-cTnI. Chin. Chem. Lett. 2021, 32, 1555–1558. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Yang, H.C.; Jiang, P.F.; Chen, Z.; Ji, C.Y.; Nie, L.B. Multi-Functional Fe3O4@mSiO(2)-AuNCs Composite Nanoparticles Used as Drug Delivery System. J. Biomed. Nanotechnol. 2017, 13, 1292–1299. [Google Scholar] [CrossRef]
- Zhao, H.; Su, E.B.; Huang, L.; Zai, Y.F.; Liu, Y.; Chen, Z.; Li, S.; Jin, L.; Deng, Y.; He, N.Y. Washing-free chemiluminescence immunoassay for rapid detection of cardiac troponin I in whole blood samples. Chin. Chem. Lett. 2022, 33, 743–746. [Google Scholar] [CrossRef]
- Rezaei, S.J.T.; Malekzadeh, A.M.; Ramazani, A.; Niknejad, H. pH-Sensitive Magnetite Nanoparticles Modified with Hyperbranched Polymers and Folic Acid for Targeted Imaging and Therapy. Curr. Drug Deliv. 2019, 16, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.Z.; Yu, X.J.; Qian, Y.N.; Chen, W.; Shen, J.L. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef]
- Guo, L.L.; Chen, H.; He, N.Y.; Deng, Y. Effects of surface modifications on the physicochemical properties of iron oxide nanoparticles and their performance as anticancer drug carriers. Chin. Chem. Lett. 2018, 29, 1829–1833. [Google Scholar] [CrossRef]
- Shah, M.A.A.; He, N.Y.; Li, Z.Y.; Ali, Z.S.; Zhang, L.M. Nanoparticles for DNA Vaccine Delivery. J. Biomed. Nanotechnol. 2014, 10, 2332–2349. [Google Scholar] [CrossRef]
- Song, J.; Lin, L.; Yang, Z.; Zhu, R.; Zhou, Z.; Li, Z.-W.; Wang, F.; Chen, J.; Yang, H.; Chen, X. Self-Assembled Responsive Bilayered Vesicles with Adjustable Oxidative Stress for Enhanced Cancer Imaging and Therapy. J. Am. Chem. Soc. 2019, 141, 8158–8170. [Google Scholar] [CrossRef]
- Tian, X.; Ruan, L.; Zhou, S.; Wu, L.; Cao, J.; Qi, X.; Zhang, X.; Shen, S. Appropriate Size of Fe3O4 Nanoparticles for Cancer Therapy by Ferroptosis. ACS Appl. Bio Mater. 2022, 5, 1692–1699. [Google Scholar] [CrossRef]
- Trujillo-Alonso, V.; Pratt, E.C.; Zong, H.L.; Lara-Martinez, A.; Kaittanis, C.; Rabie, M.O.; Longo, V.; Becker, M.W.; Roboz, G.J.; Grimm, J.; et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 2019, 14, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Luo, T.; Wang, J. Gene interfered-ferroptosis therapy for cancers. Nat. Commun. 2021, 12, 5311. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Peng, Y.B.; Wang, Q.Q.; Lin, Q.L. An ESIPT-based two-photon fluorescent probe detection of hydrogen peroxide in live cells and tissues. J. Photochem. Photobiol. B-Biol. 2017, 167, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, D.; Yuan, Y. Long-term moderate intensity exercise alleviates myocardial fibrosis in type 2 diabetic rats via inhibitions of oxidative stress and TGF-β1/Smad pathway. J. Physiol. Sci. JPS 2019, 69, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; He, N.Y.; Xu, L.J.; Li, X.L.; Li, S.; Li, Z.Y.; Liu, H.N. A Rapid Scavenger of the Lipid Peroxidation Product Malondialdehyde: New Perspective of Taurine. Adv. Sci. Lett. 2011, 4, 442–448. [Google Scholar] [CrossRef]
- Jiang, L.; Kon, N.; Li, T.Y.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Xie, Y.C.; Zhu, S.; Song, X.X.; Sun, X.F.; Fan, Y.; Liu, J.B.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, X.; Lv, X.; Song, E.; Song, Y. Iron-bearing nanoparticles trigger human umbilical vein endothelial cells ferroptotic responses by promoting intracellular iron level. Environ. Pollut. 2021, 287, 117345. [Google Scholar] [CrossRef]
- Wu, C.; Shen, Z.; Lu, Y.; Sun, F.; Shi, H. p53 Promotes Ferroptosis in Macrophages Treated with Fe3O4 Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 42791–42803. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, X.; Zhang, Y.; Sun, W.; Xu, E.; Chen, X. Mdm2 is a target and mediator of IRP2 in cell growth control. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Que, K.; Zhang, Z.; Yi, Z.; Zhao, P.; You, Y.; Gong, J.; Liu, Z. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med. 2018, 7, 4012–4022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Koppula, P.; Gan, B.Y. Regulation of H2A ubiquitination and SLC7A11 expression by BAP1 and PRC1. Cell Cycle 2019, 18, 773–783. [Google Scholar] [CrossRef]
- Wen, J.; Chen, H.; Ren, Z.; Zhang, P.; Chen, J.; Jiang, S. Ultrasmall iron oxide nanoparticles induced ferroptosis via Beclin1/ATG5-dependent autophagy pathway. Nano Converg. 2021, 8, 10. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D.L. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Yu, H.T.; Guo, P.Y.; Xie, X.Z.; Wang, Y.; Chen, G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 2017, 21, 648–657. [Google Scholar] [CrossRef]
- Wu, Y.N.; Yu, C.C.; Luo, M.; Cen, C.; Qiu, J.L.; Zhang, S.Z.; Hu, K.M. Ferroptosis in Cancer Treatment: Another Way to Rome. Front. Oncol. 2020, 10, 571127. [Google Scholar] [CrossRef]
- Yang, G.J.; Lai, Y.X.; Xiao, Z.Q.; Tang, C.L.; Deng, Y. Ultrasensitive electrochemical immunosensor of carcinoembryonic antigen based on gold-label silver-stain signal amplification. Chin. Chem. Lett. 2018, 29, 1857–1860. [Google Scholar] [CrossRef]
- Mou, X.B.; Ali, Z.; Li, B.; Li, T.T.; Yi, H.; Dong, H.M.; He, N.Y.; Deng, Y.; Zeng, X. Multiple genotyping based on multiplex PCR and microarray. Chin. Chem. Lett. 2016, 27, 1661–1665. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Deng, P.H.; Tian, Y.L.; Feng, J.X.; Xiao, J.Y.; Li, J.H.; Liu, J.; Li, G.L.; He, Q.G. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef]
- Gao, Z.; He, T.; Zhang, P.; Li, X.; Zhang, Y.; Lin, J.; Hao, J.; Huang, P.; Cui, J. Polypeptide-Based Theranostics with Tumor-Microenvironment-Activatable Cascade Reaction for Chemo-ferroptosis Combination Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20271–20280. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.P.; Xu, B.F.; Han, Q.; Zhou, H.X.; Xia, Y.; Gong, C.W.; Dai, X.F.; Li, Z.Y.; Wu, G. Ferroptosis: A Novel Anti-tumor Action for Cisplatin. Cancer Res. Treat. 2018, 50, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ji, Q.; Cheng, Y.; Liu, M.; Zhang, B.; Mei, Q.; Liu, D.; Zhou, S. Biomimetic GBM-targeted drug delivery system boosting ferroptosis for immunotherapy of orthotopic drug-resistant GBM. J. Nanobiotechnol. 2022, 20, 161. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.; Mochizuki, A.; Orpilla, J.; Hugo, W.; Lee, A.; Davidson, T.; Wang, A.; Ellingson, B.; Rytlewski, J.; Sanders, C.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Shu, G.; Shen, L.; Qiao, E.; Zhang, N.; Fang, S.; Chen, X.; Zhao, Z.; Tu, J.; et al. Homogenous multifunctional microspheres induce ferroptosis to promote the anti-hepatocarcinoma effect of chemoembolization. J. Nanobiotechnol. 2022, 20, 179. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Yao, X.; Xie, R.; Cao, Y.; Tang, J.; Men, Y.; Peng, H.; Yang, W. Simvastatin induced ferroptosis for triple-negative breast cancer therapy. J. Nanobiotechnol. 2021, 19, 311. [Google Scholar] [CrossRef]
- Wu, F.; Du, Y.; Yang, J.; Shao, B.; Mi, Z.; Yao, Y.; Cui, Y.; He, F.; Zhang, Y.; Yang, P. Peroxidase-like Active Nanomedicine with Dual Glutathione Depletion Property to Restore Oxaliplatin Chemosensitivity and Promote Programmed Cell Death. ACS Nano 2022, 16, 3647–3663. [Google Scholar] [CrossRef]
- Wang, X.; Li, P.; Jing, X.; Zhou, Y.; Shao, Y.; Zheng, M.; Wang, J.; Ran, H.; Tang, H. Folate-modified erythrocyte membrane nanoparticles loaded with Fe3O4 and artemisinin enhance ferroptosis of tumors by low-intensity focused ultrasound. Front. Oncol. 2022, 12, 864444. [Google Scholar] [CrossRef]
- Yang, H.W.; Liang, W.B.; Si, J.; Li, Z.Y.; He, N.Y. Long Spacer Arm-Functionalized Magnetic Nanoparticle Platform for Enhanced Chemiluminescent Detection of Hepatitis B Virus. J. Biomed. Nanotechnol. 2014, 10, 3610–3619. [Google Scholar] [CrossRef]
- Fang, Y.L.; Liu, H.R.; Wang, Y.; Su, X.Y.; Jin, L.; Wu, Y.Q.; Deng, Y.; Li, S.; Chen, Z.; Chen, H.; et al. Fast and Accurate Control Strategy for Portable Nucleic Acid Detection (PNAD) System Based on Magnetic Nanoparticles. J. Biomed. Nanotechnol. 2021, 17, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, Y.Q.; Chen, Z.; Hu, Z.L.; Fang, Y.L.; Liao, P.; Deng, Y.; He, N.Y. Performance Evaluation of a Novel Sample In-Answer Out (SIAO) System Based on Magnetic Nanoparticles. J. Biomed. Nanotechnol. 2017, 13, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, C.Y.; Wang, F.; Ma, N.N.; Li, X.L.; Li, Z.Y.; Deng, Y.; Wang, Z.F.; Xi, Z.J.; Tang, Y.J.; et al. Magnetic Nanoparticles-Based Extraction and Verification of Nucleic Acids from Different Sources. J. Biomed. Nanotechnol. 2013, 9, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.W.; Liu, M.; Jiang, H.R.; Zeng, Y.; Jin, L.; Luan, T.; Deng, Y.; He, N.Y.; Zhang, G.; Zeng, X. Copy Number Variation Analysis Based on Gold Magnetic Nanoparticles and Fluorescence Multiplex Ligation-Dependent Probe Amplification. J. Biomed. Nanotechnol. 2017, 13, 655–664. [Google Scholar] [CrossRef]
- Guo, L.L.; Wang, T.; Chen, Z.; He, N.Y.; Chen, Y.Z.; Yuan, T. Light scattering based analyses of the effects of bovine serum proteins on interactions of magnetite spherical particles with cells. Chin. Chem. Lett. 2018, 29, 1291–1295. [Google Scholar] [CrossRef]
- Liang, H.; Wu, X.; Zhao, G.; Feng, K.; Ni, K.; Sun, X. Renal Clearable Ultrasmall Single-Crystal Fe Nanoparticles for Highly Selective and Effective Ferroptosis Therapy and Immunotherapy. J. Am. Chem. Soc. 2021, 143, 15812–15823. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.M.; Liu, C.Q.; Wang, Z.Z.; Kang, L.H.; Huang, Y.Y.; Dong, K.; Ren, J.S.; Qu, X.G. Nanozyme Decorated Metal-Organic Frameworks for Enhanced Photodynamic Therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Liu, T.; Li, Y.; Lau, J.; Yang, Z.; Fan, W.P.; Zhou, Z.J.; Shi, C.R.; Ke, C.M.; Bregadze, V.I.; et al. Fenton-Reaction-Acceleratable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. ACS Nano 2018, 12, 11355–11365. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. Engl. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Xie, L.; Chen, W.; Xu, Z.; Song, E.; Zhu, X.; Song, Y. Iron-based nanoparticles for MR imaging-guided ferroptosis in combination with photodynamic therapy to enhance cancer treatment. Nanoscale 2021, 13, 4855–4870. [Google Scholar] [CrossRef]
- Liang, X.; Chen, M.; Bhattarai, P.; Hameed, S.; Tang, Y.; Dai, Z. Complementing Cancer Photodynamic Therapy with Ferroptosis through Iron Oxide Loaded Porphyrin-Grafted Lipid Nanoparticles. ACS Nano 2021, 15, 20164–20180. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Zhao, X.H.; Huang, J.G.; Li, J.C.; Upputuri, P.K.; Sun, H.; Han, X.; Pramanik, M.; Miao, Y.S.; Duan, H.W.; et al. Transformable hybrid semiconducting polymer nanozyme for second near-infrared photothermal ferrotherapy. Nat. Commun. 2020, 11, 1857. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Xie, S.; Sun, W.; Zhang, C.; Dong, B.; Yang, J.; Hou, M.; Xiong, L.; Cai, B.; Liu, X.; Xue, W. Metabolic Control by Heat Stress Determining Cell Fate to Ferroptosis for Effective Cancer Therapy. ACS Nano 2021, 15, 7179–7194. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, J.; Gu, Z.; Chen, Y. Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials 2019, 211, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.X.; Chen, H.R.; Liu, R.Q.; Suo, Y.K.; Li, Q.Q.; Zhang, Y.L.; Liu, H.G.; Cheng, Z.; Chang, Y.L. An active-passive strategy for enhanced synergistic photothermal-ferroptosis therapy in the NIR-I/II biowindows. Biomater. Sci. 2022, 10, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, M.A.; Pazik, R.; Bazylinska, U.; Wiwatowski, K.; Tomaszewska, A.; Kulpa-Greszta, M.; Adamczyk-Grochala, J.; Wnuk, M.; Mackowski, S.; Lewinska, A.; et al. Multimodal polymer encapsulated CdSe/Fe3O4 nanoplatform with improved biocompatibility for two-photon and temperature stimulated bioapplications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112224. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Li, B.B.; Xu, Q.N.; Li, X.F.; Zhang, P.; Zhao, X.; Wang, Y.X. Redox-responsive hyaluronic acid nanogels for hyperthermia-assisted chemotherapy to overcome multidrug resistance. Carbohydr. Polym. 2019, 203, 378–385. [Google Scholar] [CrossRef]
- Chen, M.J.; Zhang, F.; Song, J.J.; Weng, Q.Y.; Li, P.C.; Li, Q.; Qian, K.; Ji, H.X.; Pietrini, S.; Ji, J.S.; et al. Image-Guided Peri-Tumoral Radiofrequency Hyperthermia-Enhanced Direct Chemo-Destruction of Hepatic Tumor Margins. Front. Oncol. 2021, 11, 593996. [Google Scholar] [CrossRef]
- Zhu, M.T.; Wu, P.Y.; Li, Y.; Zhang, L.; Zong, Y.J.; Wan, M.X. Synergistic therapy for orthotopic gliomas via biomimetic nanosonosensitizer-mediated sonodynamic therapy and ferroptosis. Biomater. Sci. 2022, 10, 3911–3923. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, X.L.; Yang, M.S.; Dong, X.C. Recent Progress in Ferroptosis Inducers for Cancer Therapy. Adv. Mater. 2019, 31, 1904197. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, X.; Bao, X.; Mou, X. Progress in the Mechanism of the Effect of Fe3O4 Nanomaterials on Ferroptosis in Tumor Cells. Molecules 2023, 28, 4562. https://doi.org/10.3390/molecules28114562
Wang Y, Wu X, Bao X, Mou X. Progress in the Mechanism of the Effect of Fe3O4 Nanomaterials on Ferroptosis in Tumor Cells. Molecules. 2023; 28(11):4562. https://doi.org/10.3390/molecules28114562
Chicago/Turabian StyleWang, Yaxuan, Xiao Wu, Xiaoying Bao, and Xianbo Mou. 2023. "Progress in the Mechanism of the Effect of Fe3O4 Nanomaterials on Ferroptosis in Tumor Cells" Molecules 28, no. 11: 4562. https://doi.org/10.3390/molecules28114562
APA StyleWang, Y., Wu, X., Bao, X., & Mou, X. (2023). Progress in the Mechanism of the Effect of Fe3O4 Nanomaterials on Ferroptosis in Tumor Cells. Molecules, 28(11), 4562. https://doi.org/10.3390/molecules28114562




