Structural and Spectroscopic Study of New Copper(II) and Zinc(II) Complexes of Coumarin Oxyacetate Ligands and Determination of Their Antimicrobial Activity
Abstract
1. Introduction
2. Results and Discussion
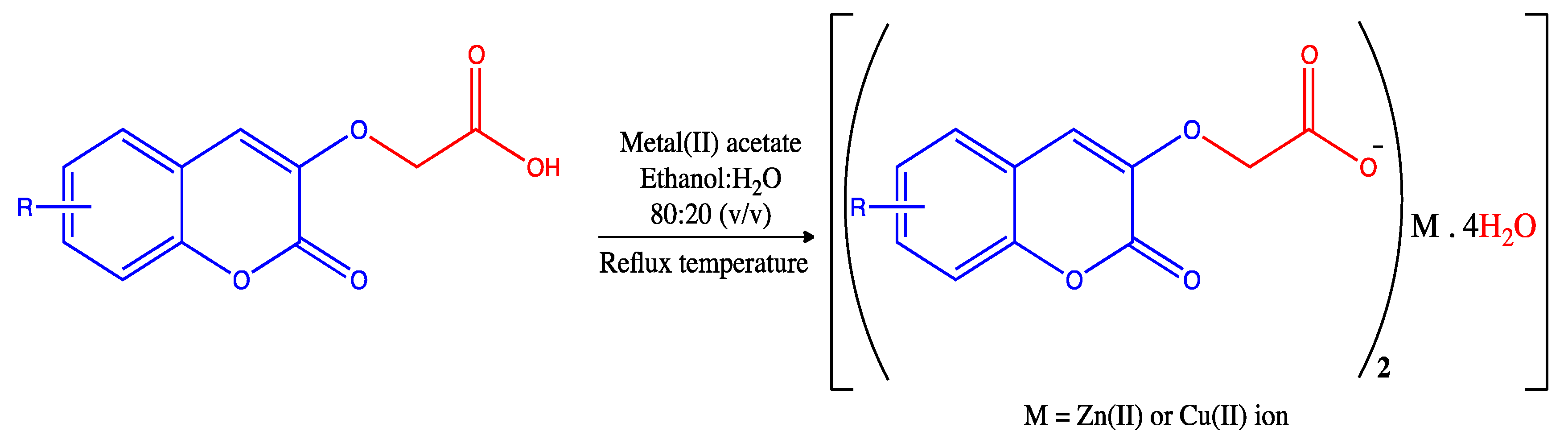
2.1. Synthesis
2.1.1. Synthesis of Ligands
2.1.2. Synthesis of Zn(II) (11–20) and Cu(II) (21–30) Complexes of 2-(2-oxo-2H-chromen-substituted-yl)oxy Acetate
2.2. Characterisation of Zn(II) (11–20) and Cu(II) (21–30) Complexes
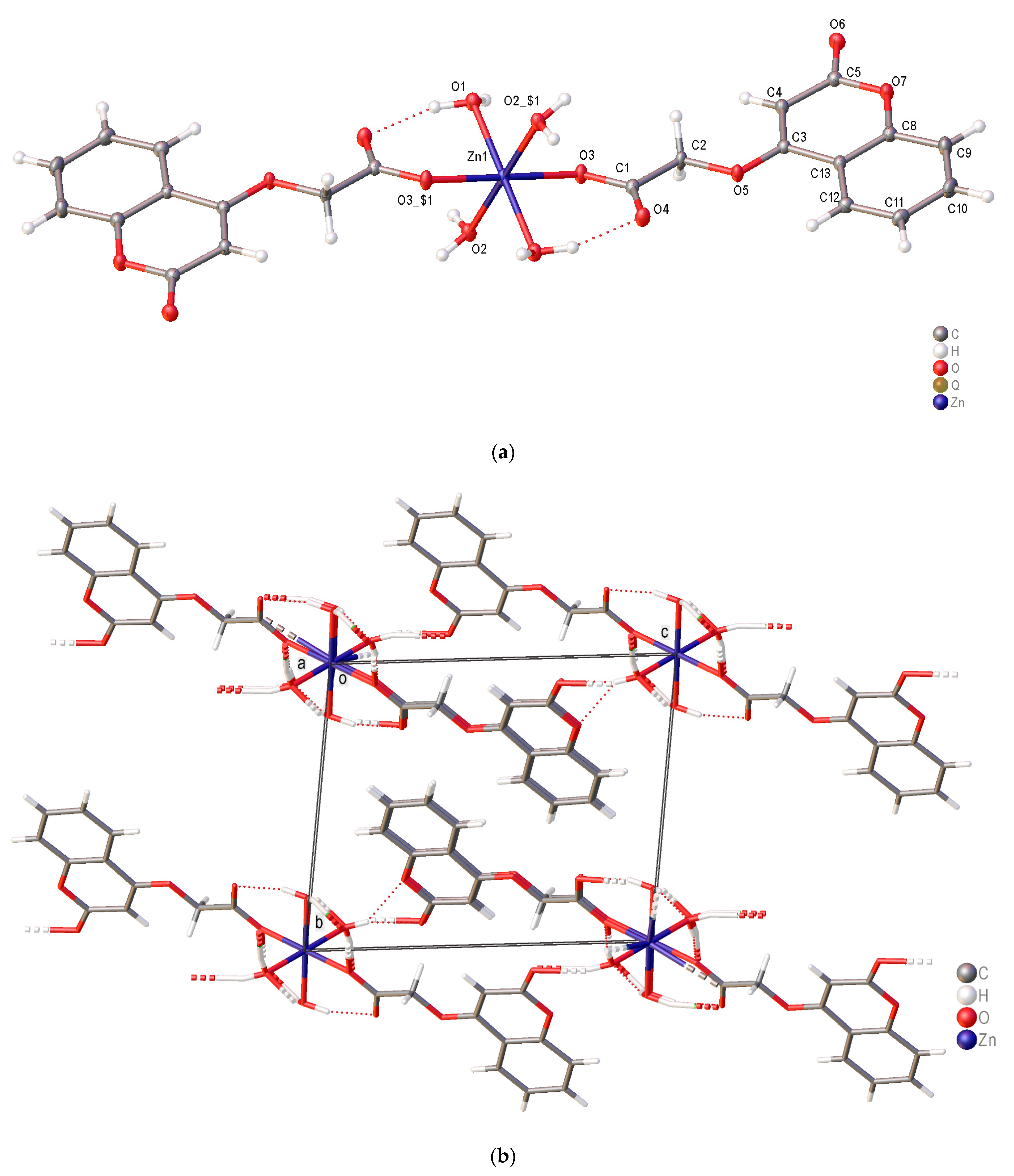
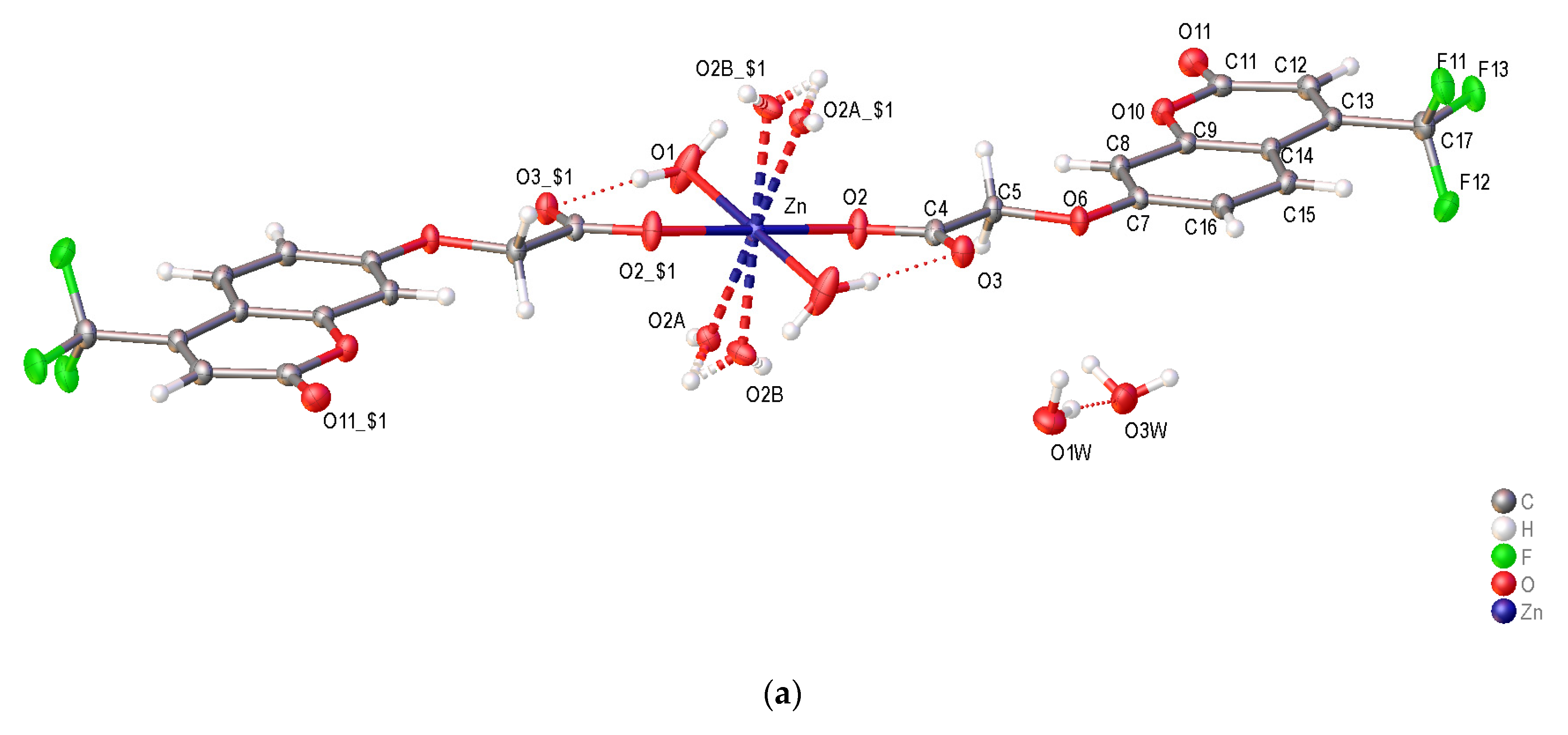
2.3. Crystal structure of [Zn(C-4oxy-acet)2(H2O)4] (12) and [Zn(4CF3-C-7oxy-acet)2(H2O)4] (19)
2.4. Experimental and Theoretical IR Spectral Analysis of Zn(II) and Cu(II) Complexes of 2-(2-oxo-2H-chromen-Substituted-yl)oxy Acetic Acid Ligands. Magnetic Moment Data of Cu(II) Complexes
2.5. Experimental and Theoretical UV-Vis Spectral Analysis of Zn(II) and Cu(II) Complexes of 2-(2-oxo-2H-chromen-Substituted-yl)oxy Acetic Acid Ligands
2.6. Experimental and Theoretical 1H, 13C NMR Spectral Analysis of Zn(II) Complexes of 2-(2-oxo-2H-chromen-Substituted-yl)oxy Acetic Acid Ligands
2.7. Antimicrobial Studies
3. Materials and Methods
3.1. Experimental Methods
3.2. Computational Details
3.3. Antimicrobial Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakhy, H. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/health-topics/antimicrobial-resistance (accessed on 30 May 2023).
- CDC. Infographic: Antibiotic Resistance the Global Threat; CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- European Centre for Disease Prevention and Control; World Health Organization. Antimicrobial Resistance Surveillance in Europe 2022–2020 Data; European Centre for Disease Prevention and Control: Solna, Sweden; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Antimicrobial Consumption in "Annual Epidemiological Report for 2019"; European Centre for Disease Prevention and Control: Solna, Sweden, 2020.
- Gur'eva, Y.A.; Zalevskaya, O.A.; Shevchenko, O.G.; Slepukhin, P.A.; Makarov, V.A.; Kuchin, A.V. Copper(II) complexes with terpene derivatives of ethylenediamine: Synthesis, and antibacterial, antifungal and antioxidant activity. RSC Adv. 2022, 12, 8841–8851. [Google Scholar] [CrossRef] [PubMed]
- Damena, T.; Alem, M.B.; Zeleke, D.; Desalegn, T.; Eswaramoorthy, R.; Demissie, T.B. Synthesis, characterization, and biological activities of zinc(II), copper(II) and nickel(II) complexes of an aminoquinoline derivative. Front. Chem. 2022, 10, 1053532. [Google Scholar] [CrossRef] [PubMed]
- Mujahid, M.; Trendafilova, N.; Arfa-Kia, A.F.; Rosair, G.; Kavanagh, K.; Devereux, M.; Walsh, M.; McClean, S.; Creaven, B.S.; Georgieva, I. Novel silver(I) complexes of coumarin oxyacetate ligands and their phenanthroline adducts: Biological activity, structural and spectroscopic characterisation. J. Inorg. Biochem. 2016, 163, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterization and antimicrobial activity of a series of substituted coumarin-3-carboxylatosilver(I) complexes. Inorg. Chim. Acta 2006, 359, 3976–3984. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis and antimicrobial activity of copper(II) and silver(I) complexes of hydroxynitrocoumarins: X-ray crystal structures of [Cu(hnc)(2)(H2O)(2)] center dot 2H(2)O and [Ag(hnc)] (hncH=4-hydroxy-3-nitro-2H-chromen-2-one). Polyhedron 2005, 24, 949–957. [Google Scholar] [CrossRef]
- Thornton, L.; Dixit, V.; Assad, L.O.N.; Ribeiro, T.P.; Queiroz, D.D.; Kellett, A.; Casey, A.; Colleran, J.; Pereira, M.D.; Rochford, G.; et al. Water-soluble and photo-stable silver(I) dicarboxylate complexes containing 1,10-phenanthroline ligands: Antimicrobial and anticancer chemotherapeutic potential, DNA interactions and antioxidant activity. J. Inorg. Biochem. 2016, 159, 120–132. [Google Scholar] [CrossRef]
- Thati, B.; Noble, A.; Rowan, R.; Creaven, B.S.; Walsh, M.; McCann, M.; Egan, D.; Kavanagh, K. Mechanism of action of coumarin and silver(I)-coumarin complexes against the pathogenic yeast Candida albicans. Toxicol. Vitr. 2007, 21, 801–808. [Google Scholar] [CrossRef]
- Mujahid, M.; Kia, A.F.A.; Duff, B.; Egan, D.A.; Devereux, M.; McClean, S.; Walsh, M.; Trendafilova, N.; Georgieva, I.; Creaven, B.S. Spectroscopic studies, DFT calculations, and cytotoxic activity of novel silver(I) complexes of hydroxy ortho-substituted-nitro-2 H-chromen-2-one ligands and a phenanthroline adduct. J. Inorg. Biochem. 2015, 153, 103–113. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Pisani, L.; Catto, M.; Muncipinto, G.; Nicolotti, O.; Carrieri, A.; Rullo, M.; Stefanachi, A.; Leonetti, F.; Altomare, C. A twenty-year journey exploring coumarin-based derivatives as bioactive molecules. Front. Chem. 2022, 10, 1002547. [Google Scholar] [CrossRef]
- Sahoo, C.R.; Sahoo, J.; Mahapatra, M.; Lenka, D.; Sahu, P.K.; Dehury, B.; Padhy, R.N.; Paidesetty, S.K. Coumarin derivatives as promising antibacterial agent(s). Arab. J. Chem. 2021, 14, 102922. [Google Scholar] [CrossRef]
- Patonay, T.; Litkei, G.; Bognar, R.; Erdei, J.; Miszti, C. Synthesis, Antibacterial and Antifungal Activity of 4-Hydroxycoumarin Derivatives, Analogs of Novobiocin. Pharmazie 1984, 39, 86–91. [Google Scholar]
- Gnerre, C.; Catto, M.; Leonetti, F.; Weber, P.; Carrupt, P.A.; Altomare, C.; Carotti, A.; Testa, B. Inhibition of monoamine oxidases by functionalized coumarin derivatives: Biological activities, QSARs, and 3D-QSARs. J. Med. Chem. 2000, 43, 4747–4758. [Google Scholar] [CrossRef]
- Shaker, R.M. Synthesis and reactions of some new 4H-pyrano[3,2-c]benzopyran-5-one derivatives and their potential biological activities. Pharmazie 1996, 51, 148–151. [Google Scholar] [CrossRef]
- Nofal, Z.M.; Mandour, A.H.; Nassar, M.I. Synthesis of 4-hydroxycoumarin derivatives with anticipated biological activity. Egypt. J. Chem. 1990, 33, 509–517. [Google Scholar]
- Kodukulla, R.P.K.; Hariharan, S.; Trivedi, G.K. Stereochemical Investigation in the 1,3-Dipolar Cycloadditions of 3-Nitro-2-Phenyl-2h-1-Benzopyrans to Diazoalkanes—Synthesis and Antimicrobial Activity of Novel Benzopyranopyrazole Derivatives. Tetrahedron 1994, 50, 4623–4634. [Google Scholar] [CrossRef]
- Sharma, M.; Vyas, V.K.; Bhatt, S.; Ghate, M.D. Therapeutic potential of 4-substituted coumarins: A conspectus. Eur. J. Med. Chem. Rep. 2022, 6, 100086. [Google Scholar] [CrossRef]
- Sullivan, M.; Kia, A.F.A.; Long, M.; Walsh, M.; Kavanagh, K.; McClean, S.; Creaven, B.S. Isolation and characterisation of silver(I) complexes of substituted coumarin-4-carboxylates which are effective against Pseudomonas aeruginosa biofilms. Polyhedron 2014, 67, 549–559. [Google Scholar] [CrossRef]
- Satheesh, C.E.; Kumar, P.R.; Shivakumar, N.; Lingaraju, K.; Krishna, P.M.; Rajanaika, H.; Hosamani, A. Synthesis, structural characterization, antimicrobial and DNA binding studies of homoleptic zinc and copper complexes of NO Schiff bases derived from homoveratrylamine. Inorg. Chim. Acta 2019, 495, 118929. [Google Scholar] [CrossRef]
- Kalinowska, M.; Sienkiewicz-Gromiuk, J.; Swiderski, G.; Pietryczuk, A.; Cudowski, A.; Lewandowski, W. Zn(II) Complex of Plant Phenolic Chlorogenic Acid: Antioxidant, Antimicrobial and Structural Studies. Materials 2020, 13, 3745. [Google Scholar] [CrossRef]
- Andrejevic, T.P.; Warzajtis, B.; Glisic, B.D.; Vojnovic, S.; Mojicevic, M.; Stevanovic, N.L.J.; Nikodinovic-Runic, J.; Rychlewska, U.; Djuran, M.I. Zinc(II) complexes with aromatic nitrogen-containing heterocycles as antifungal agents: Synergistic activity with clinically used drug nystatin. J. Inorg. Biochem. 2020, 208, 111089. [Google Scholar] [CrossRef] [PubMed]
- Abu Ali, H.; Omar, S.N.; Darawsheh, M.D.; Fares, H. Synthesis, characterization and antimicrobial activity of zinc(II) ibuprofen complexes with nitrogen-based ligands. J. Coord. Chem. 2016, 69, 1110–1122. [Google Scholar] [CrossRef]
- Gudasi, K.B.; Patil, M.S.; Vadavi, R.S. Synthesis, characterization of copper(II), cobalt(II), nickel(II), zinc(II) and cadmium(II) complexes of [7-hydroxy-4-methyl-8-coumarinyl]glycine and a comparitive study of their microbial activities. Eur. J. Med. Chem. 2008, 43, 2436–2441. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, N.L.; Aleksic, I.; Kljun, J.; Bogojevic, S.S.; Veselinovic, A.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; Glisic, B.D. Copper(II) and Zinc(II) Complexes with the Clinically Used Fluconazole: Comparison of Antifungal Activity and Therapeutic Potential. Pharmaceuticals 2021, 14, 24. [Google Scholar] [CrossRef]
- Creaven, B.S.; Devereux, M.; Georgieva, I.; Karcz, D.; McCann, M.; Trendafilova, N.; Walsh, M. Molecular structure and spectroscopic studies on novel complexes of coumarin-3-carboxylic acid with Ni(II), Co(II), Zn(II) and Mn(II) ions based on density functional theory. Spectrochim. Acta A 2011, 84, 275–285. [Google Scholar] [CrossRef]
- Creaven, B.S.; Devereux, M.; Karcz, D.; Kellett, A.; McCann, M.; Noble, A.; Walsh, M. Copper(II) complexes of coumarin-derived Schiff bases and their anti-Candida activity. J. Inorg. Biochem. 2009, 103, 1196–1203. [Google Scholar] [CrossRef]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kaminski, D.; Creaven, B.; Jenkins, H.; Radomski, P.; Milos, A.; Slusarczyk, L.; et al. Novel Coumarin-Thiadiazole Hybrids and Their Cu(II) and Zn(II) Complexes as Potential Antimicrobial Agents and Acetylcholinesterase Inhibitors. Int. J. Mol. Sci. 2021, 22, 9709. [Google Scholar] [CrossRef]
- Karaliota, A.; Kretsi, O.; Tzougraki, C. Synthesis and characterization of a binuclear coumarin-3-carboxylate copper(II) complex. J. Inorg. Biochem. 2001, 84, 33–37. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Alagha, A.; Brown, D.A.; Elawad, M.; Muller-Bunz, H.; Nimir, H.; Yanovsky, A.I.; Nolan, K.B. The preparation and crystal structure of acetatobis(L-arginine)zinc(II) acetate trihydrate, the first reported X-ray structure of a zinc(II)-arginine complex. Inorg. Chim. Acta 2011, 377, 185–187. [Google Scholar] [CrossRef]
- Chen, J.X.; Lin, W.E.; Zhou, C.Q.; Yau, L.F.; Wang, J.R.; Wang, B.; Chen, W.H.; Jiang, Z.H. Synthesis, crystal structures and biological evaluation of water-soluble zinc complexes of zwitterionic carboxylates. Inorg. Chim. Acta 2011, 376, 389–395. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Patel, K.S.; Patel, J.C.; Dholariya, H.R.; Patel, K.D. Multiple heating rate kinetic parameters, thermal, X-ray diffraction studies of newly synthesized octahedral copper complexes based on bromo-coumarins along with their antioxidant, anti-tubercular and antimicrobial activity evaluation. Spectrochim. Acta A 2012, 96, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.H.; Xie, X.; Zhao, J.; Huang, L.M.; Liu, X.M. A novel hexadentate ligand and its complexes with divalent metal ions (Zinc, Copper, and Cobalt): Synthesis, characterization, and electrochemical investigation. Inorg. Chim. Acta 2012, 387, 277–282. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier Science Publishers B.V: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Mette, M.; Connolly, L.; Vishwanath, N.; Allu, S.; Whitaker, C.; Stone, B.K.; Antoci, V.; Born, C.T.; Garcia, D.R. Silver Carboxylate as an Antibiotic-Independent Antimicrobial: A Review of Current Formulations, in vitro Efficacy, and Clinical Relevance. Med. Res. Arch. 2022, 10, 3388. [Google Scholar] [CrossRef] [PubMed]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Lee, I.C.; Ko, J.W.; Park, S.H.; Lim, J.O.; Shin, I.S.; Moon, C.; Kim, S.H.; Heo, J.D.; Kim, J.C. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int. J. Nanomed. 2016, 11, 2883–2900. [Google Scholar] [CrossRef]
- Almoudi, M.M.; Hussein, A.S.; Abu Hassan, M.I.; Zain, N.M. A systematic review on antibacterial activity of zinc against Streptococcus mutans. Saudi Dent. J. 2018, 30, 283–291. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Karcz, D.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterisation and antimicrobial activity of copper(II) and manganese(II) complexes of coumarin-6,7-dioxyacetic acid (cdoaH(2)) and 4-methylcoumarin-6,7-dioxyacetic acid (4-MecdoaH(2)): X-ray crystal structures of [Cu(cdoa)(phen)(2)] center dot 8.8H(2)O and [Cu(4-Mecdoa)(phen)(2)] center dot 13H(2)O (phen=1,10-phenanthroline). J. Inorg. Biochem. 2007, 101, 1108–1119. [Google Scholar] [CrossRef]
- Stepanenko, I.; Babak, M.V.; Spengler, G.; Hammerstad, M.; Popovic-Bijelic, A.; Shova, S.; Buchel, G.E.; Darvasiova, D.; Rapta, P.; Arion, V.B. Coumarin-Based Triapine Derivatives and Their Copper(II) Complexes: Synthesis, Cytotoxicity and mR2 RNR Inhibition Activity. Biomolecules 2021, 11, 862. [Google Scholar] [CrossRef]
- Creaven, B.S.; Devereux, M.; Foltyn, A.; McClean, S.; Rosair, G.; Thangella, V.R.; Walsh, M. Quinolin-2(1H)-one-triazole derived Schiff bases and their Cu(II) and Zn(II) complexes: Possible new therapeutic agents. Polyhedron 2010, 29, 813–822. [Google Scholar] [CrossRef]
- Sheldrick, G.M. TWINABS; University of Göttingen: Göttingen, Germany, 2009. [Google Scholar]
- Bruker. APEX2 Software Suite, APEX2 V2011.4-1; M. Bruker: Billerica, MA, USA, 2011. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations, Version 1.8, Build 648. Available online: https://www.chemcraftprog.com (accessed on 30 May 2023).
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6-31G* basis set for atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry.3. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Georgieva, I.; Trendafilova, N.; Creaven, B.S.; Walsh, M.; Noble, A.; McCann, M. Is the C=O frequency shift a reliable indicator of coumarin binding to metal ions through the carbonyl oxygen? Chem. Phys. 2009, 365, 69–79. [Google Scholar] [CrossRef]
- Mihaylov, T.; Trendafilova, N.; Kostova, I.; Georgieva, I.; Bauer, G. DFT modeling and spectroscopic study of metal-ligand bonding in La(III) complex of coumarin-3-carboxylic acid. Chem. Phys. 2006, 327, 209–219. [Google Scholar] [CrossRef]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Mennucci, B.; Tomasi, J. Ab initio study of ionic solutions by a polarizable continuum dielectric model. Chem. Phys. Lett. 1998, 286, 253–260. [Google Scholar] [CrossRef]
- Mennucci, B.; Tomasi, J. Continuum solvation models: A new approach to the problem of solute's charge distribution and cavity boundaries. J. Chem. Phys. 1997, 106, 5151–5158. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Baroni, S.; de Gironcoli, S.; Dal Corso, A.; Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef]
- Wu, X.; Vanderbilt, D.; Hamann, D.R. Systematic treatment of displacements, strains, and electric fields in density-functional perturbation theory. Phys. Rev. B 2005, 72, 035105. [Google Scholar] [CrossRef]
- Gajdos, M.; Hummer, K.; Kresse, G.; Furthmuller, J.; Bechstedt, F. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 2006, 73, 045112. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Cuenca-Estrella, M.; Diaz-Guerra, T.M.; Mellado, E. Standardization of antifungal susceptibility variables for a semiautomated methodology. J. Clin. Microbiol. 2001, 39, 2513–2517. [Google Scholar] [CrossRef] [PubMed]



| Complex/Molecular Formula | Yield (%) | MP (°C) a | Calc./Found | ||
|---|---|---|---|---|---|
| %C | %H | %Zn/Cu | |||
| [Zn(C-3oxy-acet)2(H2O)4] (11)/C22H22ZnO14 | 68 | 275 | 45.89/45.56 | 3.85/3.28 | 11.36/11.60 |
| [Zn(C-4oxy-acet)2(H2O)4] (12)/C22H22ZnO14 | 65 | 260 | 45.89/45.90 | 3.85/3.49 | 11.36/11.13 |
| [Zn(6Cl-C-4oxy-acet)2(H2O)4] (13)/C22H20Cl2ZnO14 | 72 | 270 | 40.99/40.73 | 3.13/3.03 | 10.15/10.07 |
| [Zn(4Me-C-6oxy-acet)2(H2O)4] (14)/C24H26ZnO14 | 64 | 280 | 47.47/47.75 | 4.34/4.69 | 10.83/10.73 |
| [Zn(C-7oxy-acet)2(H2O)4] (15)/C22H22ZnO14 | 66 | 280 | 45.89/45.52 | 3.85/3.54 | 11.36/11.03 |
| [Zn(4Me-C-7oxy-acet)2(H2O)4] (16)/C24H26ZnO14 | 68 | 290 | 47.74/47.53 | 4.34/4.29 | 10.83/10.44 |
| [Zn(3,4,8-triMe-C-7oxy-acet)2(H2O)4] (17)/C28H34ZnO14 | 65 | 285 | 50.96/50.62 | 5.91/5.73 | 9.91/10.78 |
| [Zn(3Cl-4Me-C-7oxy-acet)2(H2O)4] (18)/C24H24Cl2ZnO14 | 68 | 275 | 42.85/42.33 | 3.60/3.65 | 9.72/9.93 |
| [Zn(4CF3-C-7oxy-acet)2(H2O)4] (19)/C24H20F6ZnO14 | 62 | 300 | 40.50/40.86 | 2.83/2.73 | 9.19/9.32 |
| [Zn(8acetyl-C-7oxy-acet)2(H2O)4] (20)/C26H26ZnO16 | 60 | 289 | 47.32/47.06 | 3.97/3.91 | 9.91/9.97 |
| [Cu(C-3oxy-acet)2(H2O)4] (21)/C22H22CuO14 | 69 | 230 | 46.04/45.97 | 3.86/3.63 | 11.07/11.17 |
| [Cu(C-4oxy-acet)2(H2O)4] (22)/C22H22CuO14 | 63 | 290 | 46.04/46.14 | 3.86/3.81 | 11.07/11.21 |
| [Cu(6Cl-C-4oxy-acet)2(H2O)4] (23)/C22H20Cl2CuO14 | 66 | >300 | 41.10/41.14 | 3.14/3.27 | 9.89/10.03 |
| [Cu(4Me-C-6oxy-acet)2(H2O)4] (24)/C24H26CuO14 | 65 | 260 | 47.88/47.54 | 4.35/4.17 | 10.56/10.37 |
| [Cu(C-7oxy-acet)2(H2O)4] (25)/C22H22CuO14 | 65 | 255 | 46.04/46.14 | 3.86/3.71 | 11.07/11.18 |
| [Cu(4Me-C-7oxy-acet)2(H2O)4] (26)/C24H26CuO14 | 67 | 290 | 47.88/47.92 | 4.35/4.20 | 10.56/10.80 |
| [Cu(3,4,8-triMe-C-7oxy-acet)2(H2O)4] (27)/C28H34CuO14 | 63 | 270 | 51.10/51.10 | 5.21/5.26 | 9.66/9.41 |
| [Cu(3Cl-4Me-C-7oxy-acet)2(H2O)4] (28)/C24H24Cl2CuO14 | 62 | 285 | 42.97/42.74 | 3.61/3.38 | 9.47/9.60 |
| [Cu(4CF3-C-7oxy-acet)2(H2O)4] (29)/C24H20CuF6O14 | 67 | 265 | 40.70/40.70 | 2.84/2.89 | 8.95/8.80 |
| [Cu(8acetyl-C-7oxy-acet)2(H2O)4] (30)/C26H26CuO16 | 65 | 255 | 47.46/47.33 | 3.98/3.75 | 9.66/9.62 |
| Complex 12 a | Complex 19 b | ||
|---|---|---|---|
| Bond lengths (Å) | |||
| Zn1-O3 | 2.0732 (9) | Zn-O1 | 2.1906 (17) |
| Zn1-O3 i | 2.0732 (9) | Zn-O1 i | 2.1906 (17) |
| Zn1-O2 i | 2.1205 (10) | Zn-O2 i | 2.0486 (15) |
| Zn1-O2 | 2.1205 (10) | Zn-O2 | 2.0486 (15) |
| Zn1-O1 | 2.1227 (10) | Zn-O2A | 2.086 (5) |
| Zn1-O1 i | 2.1227 (10) | Zn-O2A i | 2.086 (5) |
| C1-O4 | 1.2363 (15) | O2-C4 | 1.270 (3) |
| C1-O3 | 1.2900 (15) | O3-C4 | 1.244 (2) |
| C1-C2 | 1.5156 (18) | O6-C5 | 1.437 (3) |
| C2-O5 | 1.4361 (15) | C4-C5 | 1.511 (3) |
| Bond angles (deg) | |||
| O3-Zn1-O3 i | 180.00 (7) | O1 i-Zn-O1 | 180.00 (13) |
| O3-Zn1-O2 i | 92.02 (4) | O2 i-Zn-O1 | 92.60 (6) |
| O3 i-Zn1-O2i | 87.98 (4) | O2-Zn-O1 | 87.40 (6) |
| O3-Zn1-O1 | 89.98 (4) | C4-O2-Zn | 127.95 (13) |
| O3 i-Zn1-O1 | 90.02 (4) | O2B-Zn-O1 | 98.2 (3) |
| O2 i-Zn1-O1 | 89.59 (4) | O2B-Zn-O1 i | 81.8 (3) |
| O2 i-Zn1-O1 i | 90.41 (4) | O2 i-Zn-O2A | 88.41 (18) |
| C1-O3-Zn1 | 125.61 (8) | O2 i-Zn-O2A i | 91.59 (18) |
| O1-Zn1-O1 i | 180.00 (5) | O2-C4-C5 | 112.87 (17) |
| O4-C1-O3 | 125.65 (12) | O3-C4-O2 | 126.26 (19) |
| O4-C1-C2 | 121.81 (11) | O2A i-Zn-O2A | 180.00 (4) |
| O3-C1-C2 | 112.54 (11) | ||
| O5-C2-C1 | 110.41 (10) | ||
| Compound | IR (cm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| νOHCOOH | νOHwater | νCH, νCH(oxy) | νC=Ocoum | νC=OCOOH | νasymOCO | νsymOCO | Δν (OCO) | ν(M-Oc)/ν(M-OH2) | |
| C-3oxy-acetH (1) | 3428 m (3535) * | 3052 w, 2997 w (3084, 2968) c | 1713 vs (1711) | 1736 vs (1761) | - | - | - | ||
| [Zn(C-3oxy-acet)2.4H2O] (11) | 3517 m, 3417 br (3632, 3396) * | 3053 w, 2919 w, 2862 w (3087, 2941, 2901) | 1730 d,vs (1747) | 1610 s,sh (1653) | 1340 m (1304) | 270 (349) | (357,340/ 274, 236, 209) | ||
| C-4oxy-acetH (2) | 3426 m,br (3528) | 3080 w, 2925 w (3086, 2955) | 1720 vs (1729) | overlapped (1777) | - | - | - | - | |
| [Zn(C-4oxy-acet)2.4H2O] (12) | - | 3423 m, 3118s (3471, 3278, 3186, 3093) ** | 2941 v (3021, 2850) | 1675 vs (1687) | - | 1603 s (1615) | 1340 m (1308) | 263 (307) | (385/248) |
| [Cu(C-4oxy-acet)2.4H2O] (22) | - | 3425 m (3492, 3182) * | 3083 v, 2930 v (3086, 2924) | 1670 vs (1727) | 1601 s (1565) | 1306 m (1299) | 295 (266) | (386/274) | |
| 4CF3-C-7oxy-acetH (9) | 3439 m,br | 3076 w | 1730 vs | 1757 s,sh | |||||
| [Zn(4CF3-C-7oxy-acet)2.4H2O] (19) | 3440 m,br, 3241 w (3641, 3427, 3355, 3212) ** | 3091 w,2925 vw (3162, 3119, 3014) | 1727 vs (1705) | - | 1567 s (1583) | 1347 m (1340) | 220 (243) | (413/311) | |
| Ligands | Zinc(II) Complex | Copper(II) Complex | |||
|---|---|---|---|---|---|
| No. | λmax (ε) nm (M−1cm−1) | λmax (ε) nm (M−1cm−1) | λmax (ε) nm (M−1cm−1) | ||
| 1 | 310 (14220) ππ * 280 (18220) ππ * 255 (2100) ππ * - | Zn(C-3oxy-acet)2.4H2O (11) | 310 (24360) 295 (24400) 255 (2300) 245 (24400) | Cu(C-3oxy-acet)2.4H2O (21) | 765 (92) Cu(d)-Cu(dx2-y2) 310 (31960) 295 (31100) 255 240 235 |
| 2 | 320 ππ * 295 (10680) ππ * 280 (17080) ππ * 255 ππ * | Zn(C-4oxy-acet)2.4H2O (12) | 320 (9080) 305 (17040) 280 (23420) 255(42000) 240 (44000) | Cu(C-4oxy-acet)2.4H2O (22) | 785 (81) Cu(d)-Cu(dx2-y2) 320 (11540) 305 (21120) 280 (28900) 255 245 235 |
| 3 | 310 (7540) ππ * 280 (15080) ππ * | Zn(6Cl-C-4oxy-acet)2.4H2O (13) | 325 (11140) 315 (14540 280 (20320) | Cu(6Cl-C-4oxy-acet)2.4H2O (23) | 785 (75) Cu(d)-Cu(dx2-y2) 325 (13200) 310 (17880) 280 (25340) |
| 4 | 275 (20900) ππ * | [Zn(4Me-C-6oxy-acet)2.4H2O] (14) | 340 (7140) 275 (21900) | [Cu(4Me-C-6oxy-acet)2.4H2O] (24) | 750 (126) Cu(d)-Cu(dx2-y2) 340 (11400) |
| 5 | 320 (15400) ππ * | [Zn(C-7oxy-acet)2.4H2O] (15) | 335 (23200) 325 (29580) | [Cu(C-7oxy-acet)2.4H2O] (25) | 760 (85) Cu(d)-Cu(dx2-y2) 335 (25980) 325 (31120) |
| 6 | 320 (17560) ππ * 285 (13760) ππ * | [Zn(4Me-C-7oxy-acet)2.4H2O] (16) | 335 (22560) 320 (31900) | [Cu(4Me-C-7oxy-acet)2.4H2O] (26) | 755 (124) Cu(d)-Cu(dx2-y2) 335 (28620) 320 (38660) |
| 7 | 315 (15540) ππ * | [Zn(3,4,8triMe-C-7oxy-acet)2.4H2O] (17) | 335 (24260) 320 (34340) | [Cu(3,4,8-triMe-C-7oxy-acet)2.4H2O] (27) | 750 (87) Cu(d)-Cu(dx2-y2) 320 (25460) |
| 8 | 325 (17780) ππ * | [Zn(3Cl-4Me-C-7oxy-acet)2.4H2O] (18) | 335 (29100) 325 (33680) | [Cu(3Cl-4Me-C-7oxy-acet)2.4H2O] (28) | 760 (104) Cu(d)-Cu(dx2-y2) 335 (33780) 325 (35260) |
| 9 | 340 (14200) ππ * 325 ππ * 300 ππ * 245 ππ * | [Zn(4CF3-C-7oxy-acet)2.4H2O] (19) | 340 (17760) | [Cu(4CF3-C-7oxy-acet)2.4H2O] (29) | 765 (98) Cu(d)-Cu(dx2-y2) 340 (30320) 325 (28320) 255 245 |
| 10 | 335 (1200) ππ * 320 (17220) ππ * 290 (14780) ππ * 280 ππ * 240 ππ * | [Zn(8acetyl-C-7oxy-acet)2.2H2O] (20) | 335 (24680) 325 (32440) 290 (23000) | [Cu(8acetyl-C-7oxy-acet)2.4H2O] (30) | 765 (98) Cu(d)-Cu(dx2-y2) 335 (30100) 325 (37120) 255 245 |
| 1H NMR Signals in ppm, Peak Multiplicity, Coupling Constant in Hz | ||||||||
|---|---|---|---|---|---|---|---|---|
| Complex | H3 | H4 | H5 | H6 | H7 | H8 | H11 | OH |
| C-3oxy-acetH (1) | acet. gr. | 7.41 s | 7.60 d J = 7.7 | 7.32 td J = 7.6, 1.1 | 7.46 ddd J=8.7, 7.3, 1.6 | 7.38 d J = 8.1 | 4.88 s | 13.26 |
| [Zn(C-3oxy-acet)2.4H2O] (11) | acet. gr. | 7.10 (7.35) s | 7.53 (7.77) dd J = 7.7, 1.4 | 7.23 (7.61) m | 7.38 (7.87) m | 7.23 (7.59) m | 4.56 (4.47) s | |
| C-4oxy-acetH (2) | 5.90 (5.69) a s | acet. gr. | 7.84 (8.20) dd J= 7.9, 1.4 | 7.40 (7.67) m | 7.65 (7.96) m | 7.40 (7.59) m | 5.00 (5.13) s | 13.43 (7.24) b |
| [Zn(C-4oxy-acet)2.4H2O] (12) | 5.69 (5.72) s | acet. gr. | 7.86 (8.38) dd J = 7.9, 1.4 | 7.38 (7.75) m | 7.66 (8.00) m | 7.38 (7.56) m | 4.77 (4.61) s | |
| 4CF3-C-7oxy-acetH (9) | 6.87 s | CF3 * | 7.63 dd J = 8.8, 1.4 | 7.07 dd J = 9.0, 2.5 | acet. gr. | 7.14 d J = 2.5 | 4.88 s | 13.21 |
| [Zn(4CF3-C-7oxy-acet)2.4H2O] (19) | 6.80 s | CF3 * | 7.55 dd J = 8.9, 1.9 | 7.00 dd J = 8.9, 2.5 | acet. gr. | 6.96 d J = 2.4 | 4.62 s | |
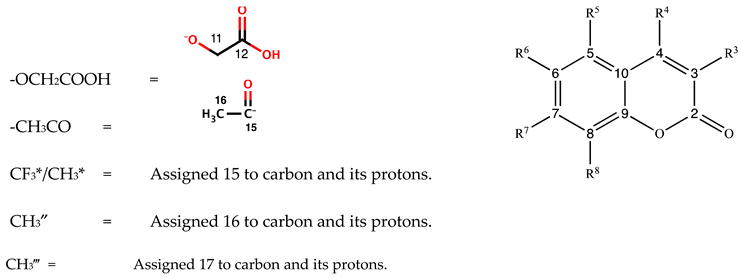
| 13C NMR Signals in ppm, Peak Multiplicity, Coupling Constant in Hz | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C15 |
| C-3oxy-acetH (1) | 156.4 (156.4) | 142.2 (141.1) | 115.1 (114.2) | 127.1 (124.8) | 124.8 (123.0) | 128.8 (126.7) | 115.7 (114.2) | 149.2 (148.8) | 119.5 (119.6) | 65.1 (65.9) | 169.0 (166.9) | - |
| [Zn(C-3oxy-acet)2.4H2O] (11) | 156.6 (156.9) | 142.8 (140.6) | 114.4 (110.2) | 126.9 (123.3) | 124.6 (122.5) | 128.3 (125.3) | 115.5 (114.5) | 148.9 (148.0) | 119.7 (120.4) | 66.9 ↑ (67.6) | N/A (172.7) ↑ | - |
| C-4oxy-acetH (2) | 161.5 (160.5) | 91.3 (88.7) | 164.3 (163.4) | 122.9 (121.8) | 124.3 (121.9) | 132.9 (130.6) | 116.5 (114.8) | 152.8 (153.0) | 115.0 (114.6) | 65.4 (66.0) | 168.5 (166.4) | - |
| [Zn(C-4oxy-acet)2.4H2O] (12) | 161.7 (161.1) | 90.9 (87.4) | 164.8 (164.2) | 123.1 (121.2) | 124.2 (121.4) | 132.7 (130.6) | 116.4 (114.6) | 152.8 (152.9) | 115.4 (114.1) | 67.1 ↑ (68.2) | 170.8 ↑ (174.5) | - |
| 4CF3-C-7oxy-acetH (9) | 161.3 | 113.8 | 139.5 | 125.9 | 113.5 | 158.7 | 102.5 | 155.7 | 107.0 | 65.0 | 168.0 | 121.7 |
| [Zn(4CF3-C-7oxy-acet)2.4H2O] (19) | 162.3 | 113.0 q J3F = 5.5 | 139.5 q J2F =31.9 | 125.6 | 113.7 | 158.7 | 102.4 | 155.6 | 106.2 | 66.6 ↑ | 171.9 ↑ | 121.6 q J1F = 275.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujahid, M.; Trendafilova, N.; Rosair, G.; Kavanagh, K.; Walsh, M.; Creaven, B.S.; Georgieva, I. Structural and Spectroscopic Study of New Copper(II) and Zinc(II) Complexes of Coumarin Oxyacetate Ligands and Determination of Their Antimicrobial Activity. Molecules 2023, 28, 4560. https://doi.org/10.3390/molecules28114560
Mujahid M, Trendafilova N, Rosair G, Kavanagh K, Walsh M, Creaven BS, Georgieva I. Structural and Spectroscopic Study of New Copper(II) and Zinc(II) Complexes of Coumarin Oxyacetate Ligands and Determination of Their Antimicrobial Activity. Molecules. 2023; 28(11):4560. https://doi.org/10.3390/molecules28114560
Chicago/Turabian StyleMujahid, Muhammad, Natasha Trendafilova, Georgina Rosair, Kevin Kavanagh, Maureen Walsh, Bernadette S. Creaven, and Ivelina Georgieva. 2023. "Structural and Spectroscopic Study of New Copper(II) and Zinc(II) Complexes of Coumarin Oxyacetate Ligands and Determination of Their Antimicrobial Activity" Molecules 28, no. 11: 4560. https://doi.org/10.3390/molecules28114560
APA StyleMujahid, M., Trendafilova, N., Rosair, G., Kavanagh, K., Walsh, M., Creaven, B. S., & Georgieva, I. (2023). Structural and Spectroscopic Study of New Copper(II) and Zinc(II) Complexes of Coumarin Oxyacetate Ligands and Determination of Their Antimicrobial Activity. Molecules, 28(11), 4560. https://doi.org/10.3390/molecules28114560








