Abstract
The mechanisms of sulfoxidation and epoxidation mediated by previously synthesized and characterized iron(III)-iodosylbenzene adduct, FeIII(OIPh) were investigated using para-substituted thioanisole and styrene derivatives as model substrates. Based on detailed kinetic reaction experiments, including the linear free-energy relationships between the relative reaction rates (logkrel) and the σp (4R-PhSMe) with ρ = −0.65 (catalytic) and ρ = −1.13 (stoichiometric), we obtained strong evidence that the stoichiometric and catalytic oxidation of thioanisoles mediated by FeIII(OIPh) species involves direct oxygen transfer. The small negative slope −2.18 from log kobs versus Eox for 4R-PhSMe gives further clear evidence for the direct oxygen atom transfer mechanism. On the contrary, with the linear free-energy relationships between the relative reaction rates (logkrel) and total substituent effect (TE, 4R-PhCHCH2) parameters with slope = 0.33 (catalytic) and 2.02 (stoichiometric), the stoichiometric and catalytic epoxidation of styrenes takes place through a nonconcerted electron transfer (ET) mechanism, including the formation of the radicaloid benzylic radical intermediate in the rate-determining step. On the basis of mechanistic studies, we came to the conclusion that the title iron(III)-iodosylbenzene complex is able to oxygenate sulfides and alkenes before it is transformed into the oxo-iron form by cleavage of the O−I bond.
1. Introduction
Iodosylbenzene (PhIO) has attracted considerable attention for its various applications in organic synthesis and biomimetic studies. For example, PhIO has been widely used to produce high-valent metal oxo species, which have been identified as key intermediates in various oxidative reactions in biological systems [1,2,3,4,5,6,7,8,9,10,11,12]. Despite the widespread interest in oxoiron(IV)-mediated epoxidations and sulfoxidations [13,14], relatively little information is available on the mechanism of alkene and sulfide oxidation by synthetic non-heme iron-iodosylbenzene complexes. This is mainly a result of the low stability of the iron-iodosylbenzene intermediates, which prevented direct kinetic measurements of their reactions at ambient temperature. Recently, metal-iodosylbenzene adducts, [M-OIPh], have been successfully prepared, isolated and characterized by various spectroscopic methods, despite their transient and unstable formation. The structure and reactivity of the iodosylarene adduct of heme and non-heme Mn(III), Mn(IV) and Fe(III) complexes have been thoroughly investigated. Recent studies have shown that the metal-iodosylbenzene adduct itself can play an important role in transition metal-catalyzed oxidation reactions [15,16,17,18,19,20,21,22,23,24,25,26,27]. For some multiple oxidation mechanisms, some of showed better reactivity than the corresponding metal-oxo intermediates. The complex [FeII(PBI)3](CF3SO3)2 (1) (PBI = 2-(2-pyridyl)benzimidazole) proved suitable for the generation of various intermediates depending on the co-oxidant used (Figure 1). Its reaction with H2O2 and PhIO results in the formation of μ-1,2-peroxo-diiron(III) and iron(III)-iodosylbenzene complexes, respectively [28,29,30]. The spectral properties, reactivity and kinetics of FeIII(OIPh) bearing PBI ligands towards cycloketones in nucleophilic Baeyer-Villiger reactions [15], and towards benzaldehydes and triphenylmethane in electrophilic C-H oxygenation reactions, were investigated in detail [27].
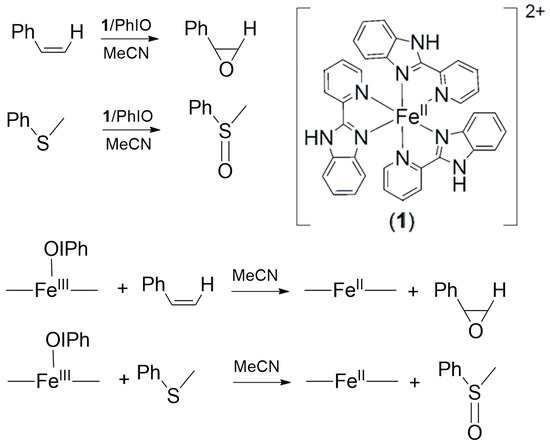
Figure 1.
Schematic structure of [FeII(PBI)3](CF3SO3)2 (1) used in the stoichiometric and catalytic oxidation of thioanisole and styrene.
Significant progress has been made in the last three decades in the understanding and application of nonheme iron complexes in oxidation catalysis. Several ligand systems have been demonstrated as able to form a reactive iron(III)-iodosylarene moiety. Although this part is less commonly examined than the related high-valent oxo congeners, there is no doubt that the iodosylarene entity is a crucial part of the oxidation catalysis in biomimetic model systems. Hence, understanding its properties and reactivity helps tremendously to understand processes in catalytic oxidations. In parallel, highly active and selective catalysts for epoxidation and sulfoxidation have been designed where this moiety is unequivocally involved. To obtain more insight into the mechanisms of the metastable iron(III)-iodosylbenzene mediated sulfoxidation and epoxidation reactions, catalytic and stoichiometric oxidation were carried out using [FeII(PBI)3](CF3SO3)2 (1) as catalyst and PhIO as oxidant for the catalytic, and the in situ formed FeIII(OIPh) species for the stoichiometric oxidation of thioanisole and styrene derivatives (Figure 1).
2. Results
2.1. Catalytic Sulfoxidation Reaction Mediated by In Situ Generated Iron(III)-Iodosylbenzene Adduct
The catalytic activity of the previously synthesized and characterized ferrous complex, [FeII(PBI)3](OTf)2 (1) was investigated in the oxidation of para-substituted thioanisole derivatives, utilizing PhIO as oxidant. The reactions were carried out under standard, optimal catalytic conditions (1:100:300 ratio for catalyst:oxidant:substrate) in acetonitrile at 293 K. A large excess of substrate was used to minimize over-oxidation of the product. It took around 1 h to have about 20–70% yields (based on the oxidant) depending on the substituent on the phenyl ring (Table 1). The iron(II)-catalyzed reactions of thioanisoles produced methyl phenyl sulfoxide derivatives as expected and as a major product in all cases, in addition to one minor product, namely methyl phenyl sulfone derivatives. The effect of electron-donating and electron-withdrawing substituents on the aromatic ring on the relative reactivity has also been studied and showed a significant impact on the catalytic sulfoxidation reaction (Table 1).

Table 1.
Catalytic oxidation of para-substituted thioanisole derivatives carried out by 1 with PhIO as co-oxidant in acetonitrile at 293 K 1.
Thioanisols with electron-donating groups, such as -OMe or -Me, on the phenyl ring gave better yields (66% for -OMe and 60% for -Me) than those with electron-withdrawing groups (21% for -NO2 and 37% for -Cl) (Figure 2a). The highest TON (turn over number) and TOF (turn over frequency) values can be observed for 4-OMe-PhSMe, at 66 and 80 h−1, respectively (Figure 2b).
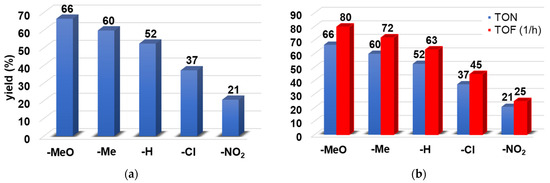
Figure 2.
Catalytic oxidation of para-substituted thioanisole derivatives carried out by 1 with PhIO as co-oxidant in acetonitrile at 293 K: (a) Reaction yields for para-substituted thioanisoles. (b) TON and TOF values for para-substituted thioanisoles. [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M, [PhIO]0 = 1 × 10−1 M, [4R-PhSMe]0 = 3 × 10−1 M.
The reactivities of para-substituted 4R-PhSMe relative to that of PhSMe were also investigated (Table 1 and Figure 3). It was found that thioanisoles with Hammett treatments of relative reactivities (krel = log(Xf/Xi)/log(Yf/Yi), where Xi and Xf are the initial and final concentration of 4R-PhSMe, and Yi and Yf are the initial and final concentration of PhSMe) of various substituents against σ gave a ρ value of −0.65, which suggests that the behavior of the oxidant generated from 1 and PhIO is mildly electrophilic (Figure 3a). When the logkrel values were plotted against the E0ox potentials of 4R-PhSMe, the plot gave a gradient of −1.23 (Figure 3b). The magnitude of these values may be consistent with a direct O-atom transfer from the substrate in the rate-determining step. Much higher values can be observed for an ET (electron transfer) mechanism [31].
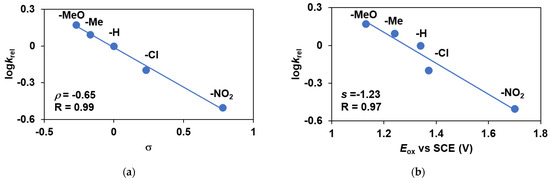
Figure 3.
Catalytic oxidation of para-substituted thioanisole derivatives carried out by 1 with PhIO as co-oxidant in acetonitrile at 293 K: (a) Hammett plot of logkrel against the σp of para-substituted thioanisoles. (b) Plot of logkrel against the E0ox of para-substituted thioanisoles. [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M, [PhIO]0 = 1 × 10−1 M, [4R-PhSMe]0 = 3 × 10−1 M.
As we noticed before, the reaction of 1 with PhIO results in the formation of reactive FeIII(OIPh) species, which suggests that it may have a key role in the catalytic cycles. To prove this assumption, we followed the formation and decomposition of FeIII(OIPh) intermediate (the rise and fall of their visible chromophores at λmax = 760 nm, as well as the appearance of the forming products (methyl phenyl sulfoxide and methyl phenyl sulfone) during the oxidation reaction of thioanisole, using parallel UV-Vis and GC measurements). The profile of the catalytic process shows the immediate formation and subsequent decomposition of the spectroscopically characterized FeIII(OIPh) species, as well as the formation of the oxidation products that appear parallel to it (Figure 4).
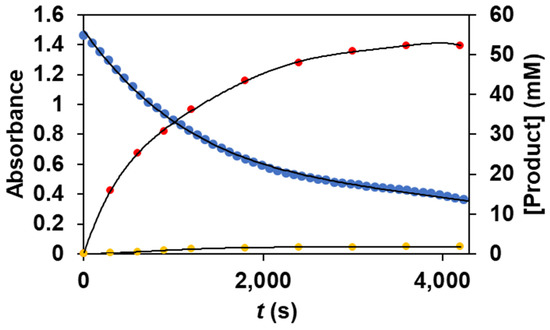
Figure 4.
Time course for the catalytic oxidation of thioanisole (•) carried out by 1 with PhIO as co-oxidant in acetonitrile at 293 K, monitored at 760 nm by UV-Vis and by GC, measuring the amounts of the forming phenyl sulfoxide (•) and methyl phenyl sulfone (•). [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M, [PhIO]0 = 1 × 10−1 M, [PhSMe]0 = 3 × 10−1 M.
2.2. Stoichiometric Sulfoxidation Reaction Mediated by In Situ Generated Iron(III)-Iodosylbenzene Adduct
To obtain direct evidence for the involvement of the iron(III)-iodosylbenzene adduct in the sulfoxidation reaction, the stoichiometric reaction of the in situ generated FeIII(OIPh) with various thioanisoles was investigated. The FeIII(OIPh) species was generated with 4 equivalent of PhIO, and the excess of oxidant was removed by filtration. It was found that this intermediate is able to selectively oxidize the thioanisole derivatives, resulting in an almost quantitative formation of the corresponding sulfoxides suggesting a direct O-atom transfer mechanism with two-electron process. The pseudo-first-order rate constants, kobs, in the reaction of FeIII(OIPh) with thioanisoles were determined from the absorbance change at 760 nm (Figure 5) by monitoring the decrease of the FeIII(OIPh) concentration. The yields of sulfoxides without a significant amount of sulfone formation (~1%) were about 80–90%, indicating that the UV-Vis spectral change corresponds to the OAT process. An isobestic point was observed at ~520 nm, indicating that there were no long-lived intermediates during the oxidation reaction (Figure 5a). The kinetic data show that the reaction is first-order in the oxidant, FeIII(OIPh) (Figure 5b and Figure 6b), and thioanisole (Figure 6a). The rates in the presence of excess of thioanisole (300–1500 equivalent) obeyed pseudo-first-order kinetics, and the pseudo-first-order rate constants (normalized with the rate of self-decay process without substrates) increased linearly with the thioanisole concentration (Figure 6a). The second-order constants, k2 were evaluated from the slope of the plots of kobs versus [thioanisole]0. From this linear plotting, the second-order rate constant (k2) was determined to be 1.69 × 10−3 M−1 s−1 with EA = 41.93 ± 4.45 kJ mol−1, ΔH≠ = 39.41 ± 6.53 kJ mol−1, ΔS≠ = −162.15 ± 21.8 J mol−1 K−1, ΔG≠ = 86.92 ± 12.91 kJ mol−1 at 293 K (Figure 7). The obtained value is 2 orders of magnitude smaller than those obtained for FeIV(O) species with pentadentate poly-pirydil ligands (k2 = 2.56 × 10−1 M−1 s−1 with ΔH≠ = 44 kJ mol−1, and ΔS≠ = −100 J mol−1 K−1 at 298 K) [12]. A krel(PhS(O)Me/PhSMe) value of 0.64 was also determined by comparing an individual reaction with methyl-phenyl-sulfoxide as substrate, with the PhSMe oxidation under identical conditions (Figure 6a). The kobs and k2 values are listed in Table 2.
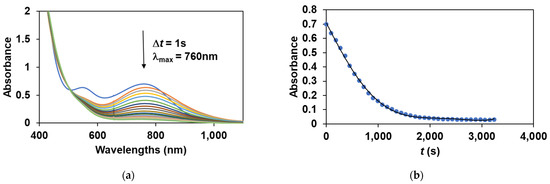
Figure 5.
Stoichiometric oxidation of thioanisole with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO. (a) UV-Vis spectral change of FeIII(OIPh) (0.4 mM) in the presence of PhSMe (60 mM). (b) Time course of the oxidation of PhSMe monitored at 760 nm (a).
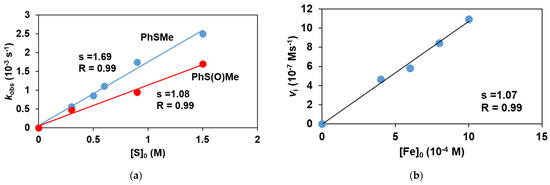
Figure 6.
Stoichiometric oxidation of thioanisole and methyl phenyl sulfoxide with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO. (a) Dependence of kobs on the initial thioanisole and methyl phenyl sulfoxide concentrations, [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M at 293 K in acetonitrile. (b) Dependence of Vi on the initial complex (1) contratio, [PhSMe]0 = 6 × 10−1 M at 293 K.
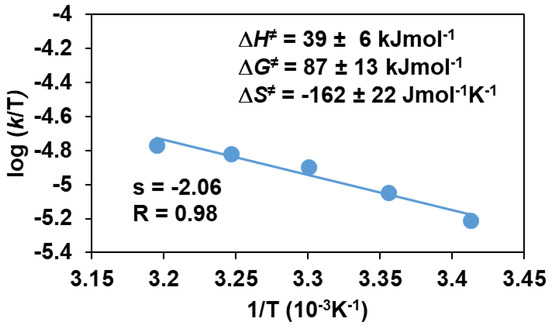
Figure 7.
Eyring plot of logk/T versus 1/T for the stoichiometric oxidation of thioanisole with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO in acetonitrile.

Table 2.
Kinetic data for the stoichiometric oxidation of para-substituted thioanisole and methyl phenyl sulfoxide derivatives with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO in acetonitrile 1.
In general, two alternative mechanisms can be proposed for the sulfoxidation of sulfides with oxo-metal complexes, namely direct oxygen atom transfer (OAT) to afford the sulfoxide (pathway a in Scheme 1), and the formation of sulfenium radical via electron transfer (ET) mechanism (pathway b in Scheme 1) [12].
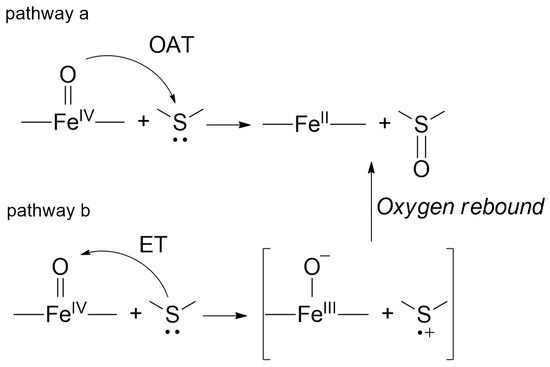
Scheme 1.
Alternative mechanisms for the oxo-iron-mediated sulfoxidation reactions.
To confirm the mechanism of the reaction as direct OAT rather than electron transfer, we investigated the effect of the substituents in the para position of PhSMe, and the observed kinetic data were treated in terms of the Hammett equation. Electron-attracting substituents in the sulfides reduce the rate of oxidation. The ρ value is −1.13, suggesting that the metal-based oxidant is electrophilic (Figure 8a). By plotting logkobs against the one-electron oxidation potentials (Eox) of the thioanisols, a linear line with a slope of −2.18 was obtained, which is consistent with a direct OAT mechanism (Figure 8b). These values are of the same order of magnitude, although slightly smaller than the values obtained for FeIV(O) species with pentadentate poly-pirydil ligands (ρ value is −1.78 and −1.51, respectively), indicating the less electrophilic nature of the FeIII(OIPh) species compared to the oxo-iron(IV) intermediates with similar ligand environment [12,32]. Gradients of much larger magnitude (−8.5 and −10.5) were observed when reactions proceeded through an ET pathway [33].
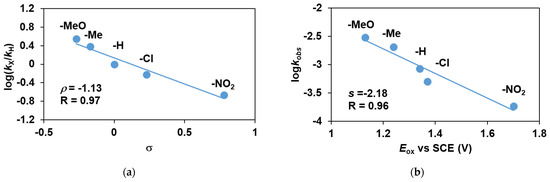
Figure 8.
Stoichiometric oxidation of para-substituted thioanisole derivatives with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO in acetonitrile at 293 K: (a) Hammett plot of logkrel against the σp of para-substituted thioanisoles. (b) Plot of logkobs against the E0ox of para-substituted thioanisoles. [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M, [4R-PhSMe]0 = 5 × 10−1 M.
Based on these results, we ruled out the participation of the oxo-iron(IV) species, which can be formed by homolytic O-I bond cleavage of iron(III)-iodosyl-benzene adducts, as a reactive intermediate in our stoichiometric and catalytic sulfoxidation reactions. However, we cannot rule out the possibility that the oxo-iron(V) species is formed via heterolytic cleavage of the O-I bond of the iron(III)-iodosyl-benzene adducts in a pre-equilibrium process, and this intermediate participates as a reactive element in the oxidation process. In iron porphyrin systems, an equilibrium exists between an iron(IV) oxo porphyrin π-cation radical complex and an iron(III) iodosylarene porphyrin complex in the presence of iodoarene and it is the iron(IV) center which is the reactive species in olefin epoxidation reactions [19]. If there is an equilibrium between an iron(III)–iodosylarene adduct and an iron(V) oxo species, which is formed by OI bond cleavage of the iron(III)–iodosylarene adduct, the presence of an excess amount of iodoarene should decrease the reaction rate. Compound FeIII(OIPh) was employed in the thioanisole oxidation reactions in the presence of PhI (50 equivalent). It was found that the reaction rates were significantly higher than those obtained in the reactions carried out in the absence of PhI (Figure 9). These results demonstrate unambiguously that the iron(III) iodosylarene adduct (Scheme 2), not the iron(V)oxo complex, is the reactive species for the sulfoxidation reactions. When the stoichiometric reaction is carried out with an excess of PhI, a nearly three-fold reaction rate (kobs = 2.459 × 10−3 s−1 (with 50 mmol PhI) compared to kobs = 0.97 × 10−3 s−1 (without PhI)) is observed, suggesting that the FeIII(IOPh)-mediated reaction is more favored compared to the oxo-iron(V) species (Figure 9a). The saturation curve clearly shows that the equilibrium is shifted towards the FeIlI(OIPh) species (Figure 9b).
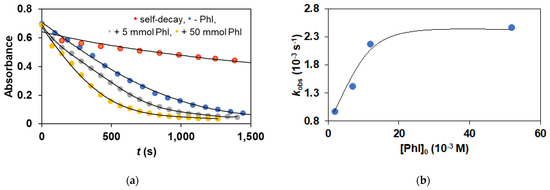
Figure 9.
Stoichiometric oxidation of thioanisole with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO. (a) Time course of the oxidation of PhSMe monitored at 760 nm in the absence and in the presence of PhI. (b) Dependence of kobs on the initial PhI concentrations, [[FeII(PBI)3](OTf)2]0 = 0.6 × 10−3 M at 293 K in acetonitrile. [PhSMe]0 = 6 × 10−1 M at 293 K.
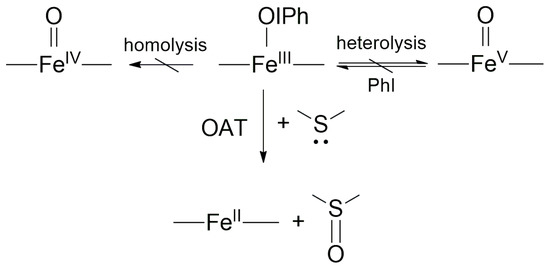
Scheme 2.
Suggested mechanism for the FeIII(OIPh)-mediated sulfoxidation reaction.
The obtained results are consistent with the results obtained for our catalytic system, based on which the substituent effect tests indicate a direct O-atom transfer from the FeIII(OIPh) intermediate to the substrate in the rate-determining step of both systems (Scheme 2). The mechanism of direct oxygen atom transfer mediated by FeIII(IOPh) and its differences compared to the ET mechanism were also supported by detailed DFT calculations [21].
2.3. Catalytic Epoxidation Reaction Mediated by In Situ Generated Iron(III)-Iodosylbenzene Adduct
Similarly to the catalytic sulfoxidation reaction, the catalytic activity of the [FeII(PBI)3](OTf)2 (1) was also investigated in the oxidation of para-substituted styrene derivatives, utilizing PhIO as oxidant. The reactions were carried out under standard, optimal catalytic conditions (1:100:300 ratio for catalyst:oxidant:substrate) in acetonitrile at 293 K. The large excess of substrate was used to minimize over-oxidation of the product. It took around 1 h to have about 14–29% yields (based on the oxidant) depending on the substituent on the phenyl ring (Table 3). The iron(II)-catalyzed reactions of styrenes produced epoxide derivatives as expected, and as a major product in all cases, in addition to one minor product, namely benzaldehyde derivatives (less than 1%). The effect of electron-donating and electron-withdrawing substituents on the aromatic ring on the relative reactivity has also been studied and showed a significant impact on the catalytic epoxidation reaction (Table 1 and Figure 10). Styrenes with both electron-donating (-OMe or -Me) and electron-withdrawing (-Cl or -CN) groups on the phenyl ring gave better yields (29% for -OMe, 19% for -Me, and 16% for -Cl and 27% for -CN) (Figure 10a). The highest TON (TOF) values can be observed for 4-OMe-PhCHCH2; 59 (59 h−1) (Figure 10b).

Table 3.
Catalytic oxidation of para-substituted styrene derivatives carried out by 1 with PhIO as co-oxidant in acetonitrile at 293 K 1.
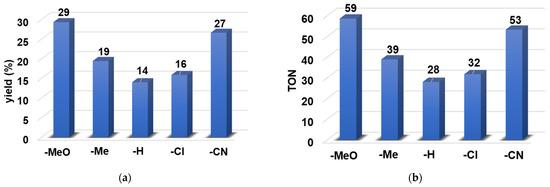
Figure 10.
Catalytic oxidation of para-substituted styrene derivatives carried out by 1 with PhIO as co-oxidant in acetonitrile at 293 K: (a) Reaction yields for para-substituted styrenes. (b) TON and TOF values for para-substituted styrenes. [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M, [PhIO]0 = 1 × 10−1 M, [4R-PhCHCH2]0 = 3 × 10−1 M.
The reactivities of para-substituted 4R-PhCHCH2 relative to that of PhCHCH2 were also investigated (Figure 10 and Figure 11). It was found that styrenes displayed Hammett treatments of relative reactivities (krel = log(Xf/Xi)/log(Yf/Yi), where Xi and Xf are the initial and final concentration of 4R-PhCHCH2, and Yi and Yf are the initial and final concentration of PhCHCH2) of various substituents against σ gave concave Hammett curve, which means that both electron-donating and electron-withdrawing groups on the para-position of the aromatic ring increase the rate of the epoxidation reaction (Figure 11a). A linear free-energy relationship between the relative rate (logkrel) for the para-substituted styrene oxidations and the total substituent effect (TE) parameters has been established: ρTE• = +0.33 (Figure 11b) [33]. Based on the obtained results, it can be assumed that the oxidation of aromatic alkenes catalyzed by 1 with PhIO as oxidant takes place through the formation of a rate-limiting radical intermediate. The profile of the catalytic process shows the immediate formation and subsequent decomposition of the spectroscopically characterized FeIII(OIPh) species, as well as the formation of the oxidation products that appear parallel to it (Figure 12).
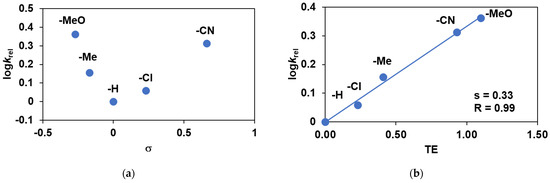
Figure 11.
Catalytic oxidation of para-substituted styrene derivatives carried out by 1 with PhIO as co-oxidant in acetonitrile at 293 K: (a) Hammett plot of logkrel against the σp of para-substituted styrenes. (b) Plot of logkrel against the TE of para-substituted styrenes. [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M, [PhIO]0 = 1 × 10−1 M, [4R-PhCHCH2]0 = 3 × 10−1 M.
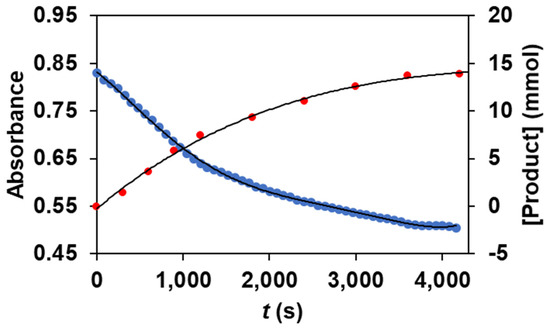
Figure 12.
Time course for the catalytic oxidation of styrene (•) carried out by 1 with PhIO as co-oxidant in acetonitrile at 293 K, monitored at 760 nm by UV-Vis and by GC measuring the amounts of the forming styrene oxide (•). [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M, [PhIO]0 = 1 × 10−1 M, [PhCHCH2]0 = 3 × 10−1 M.
2.4. Stoichiometric Epoxidation Reaction Mediated by In Situ Generated Iron(III)-Iodosylbenzene Adduct
The epoxidation of styrene derivatives with 1 and PhIO as an oxidant was carried out at room temperature under catalytic reaction conditions, and the corresponding epoxides were obtained as the main products in these reactions. Since the product distributions were identical to those observed in the stoichiometric reactions with in situ generated iron(III)-iodosylbenzene adduct, this lead us to suggest that the active oxidizing species responsible for the olefin epoxidation in these catalytic reactions is the iron(III)-iodosylbenzene adduct (Figure 12). In order to clarify the elementary step of the catalytic reaction, we performed detailed reaction kinetic measurements. The reactivity of the in situ generated iron(III)-iodosylbenzene was investigated in the epoxidation reaction of styrene derivatives. The FeIII(OIPh) intermediate was generated by the reaction of 1 with 1.2 equivalent of PhIO, and the rate of its decay at 760 nm was measured as a function of the concentration of added styrene derivatives (Figure 13). An isobestic point was observed at ~520 nm, indicating that there were no long-lived intermediates during the oxidation reaction (Figure 13a). The rates in the presence of a large excess of styrene (300–1500 equiv.) obeyed pseudo-first order kinetics −d[FeIII(OIPh)]/dt = kobs[FeIII(OIPh)], where kobs = ksd + k2[S] and ksd << k2[PhCHCH2]), which is evident from the linear log(absorbance) versus time plots (Figure 13b), and the pseudo-first order rate constants (kobs = k2[PhCHCH2)] increased proportionally with the substrate concentration (Figure 14a). At constant styrene concentration the linear plot of the reaction rate values (Vi = kobs[FeIII(OIPh)]) against the initial concentration of FeIII(OIPh) states that the reaction is first-order with respect to the FeIII(OIPh) concentration (Figure 14b).
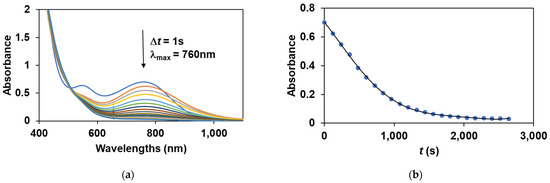
Figure 13.
Stoichiometric oxidation of styrene with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO. (a) UV-Vis spectral change of FeIII(OIPh) (0.4 mM) in the presence of PhCHCH2 (60 mM). (b) Time course of the oxidation of PhCHCH2 monitored at 760 nm (a).
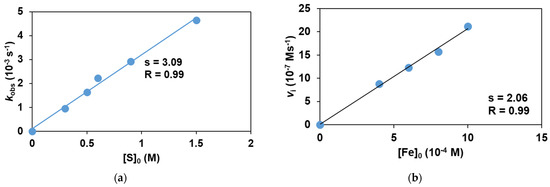
Figure 14.
Stoichiometric oxidation of styrene with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO. (a) Dependence of kobs on the initial concentrations, [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M at 293 K in acetonitrile. (b) Dependence of Vi on the initial complex (1) contration, [PhCHCH2]0 = 6 × 10−1 M at 293 K.
The above results establish a rate law of −d[FeIII(OIPh)]/dt = k2[FeIII(OIPh)][PhCHCH2] for the styrene derivatives. From these experiments, the second order rate constant (k2) was determined to be 3.09 × 10−3 M−1 s−1 with ΔH≠ = 32 ± 4 kJ mol−1, ΔS≠ = −182 ± 14 J mol−1 K−1, ΔG≠ = 85 ± 8 kJ mol−1 at 293 K (Table 4 and Figure 15). This value is an order of magnitude larger than that was found for FeIV(O) species with pentadentate poly-pirydil ligands (k2 = 2.94 × 10−4 M−1 s−1 with ΔG≠ = 93 ± 8 kJ mol−1 at 298 K) [34]. Comparing the obtained values with the values calculated for sulfoxidation, it can be concluded that the epoxidation is slightly more favorable, approximately twice as fast, if the reactions are carried out under the same conditions. The lower ΔG≠ value obtained for epoxidation is also consistent with the above result. The relatively large and negative entropies are typical of associative processes.

Table 4.
Kinetic data for the stoichiometric oxidation of para-substituted styrenes with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO in acetonitrile 1.
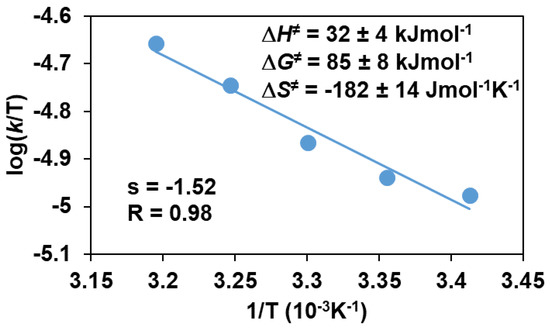
Figure 15.
Eyring plot of logk/T versus 1/T for the stoichiometric oxidation of styrene with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO in acetonitrile.
Competitive reactions were also performed with para-substituted styrene derivatives to evaluate the effect of electronic factors on the reaction. Since both electron-donating (-OMe, -Me) and electron-withdrawing (-Cl, -CN) substituents can accelerate the reaction, the correlation of relative reactivity (log krel) on the substituent constants (σ+) of para-substituted styrenes is not linear, resulting in a concave Hammett curve (16a) [13]. This result is in contrast to the styrene oxidations by oxo-iron(IV) systems, wherein typically linear Hammett correlations of logkrel versus σ+ with ρ+ of ~−2.0 were obtained [14,34]. A linear free-energy relationship was established with ρTE• = +2.02 value between the relative rates (logkrel) of para-substituted styrene oxidations and the total substituent effect (TE, stabilities of the benzylic radicals including spin delocalization and polar effects (Figure 16b) [33]).
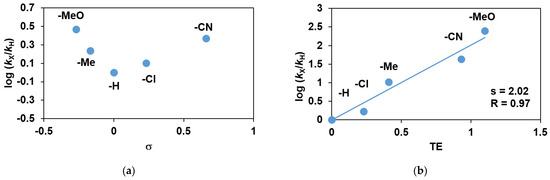
Figure 16.
Stoichiometric oxidation of para-substituted styrene derivatives with FeIII(OIPh) generated in situ by the reaction of 1 with 4 equivalents of PhIO in acetonitrile at 293 K: (a) Hammett plot of logkrel against the σp of para-substituted styrenes. (b) Plot of logkrel against the TE of para-substituted styrenes. [[FeII(PBI)3](OTf)2]0 = 1 × 10−3 M, [4R-PhCHCH2]0 = 3 × 10−1 M.
In general, three alternative mechanisms, including three possible intermediates, can be proposed for the epoxidation of alkenes with oxo-metal complexes, namely one concerted via [2 + 1] oxene insertion (pathway a in Scheme 3), and two nonconcerted via an alkene-derived benzylic radical (pathway b in Scheme 3) or a carbocation intermediate (pathway c in Scheme 3).
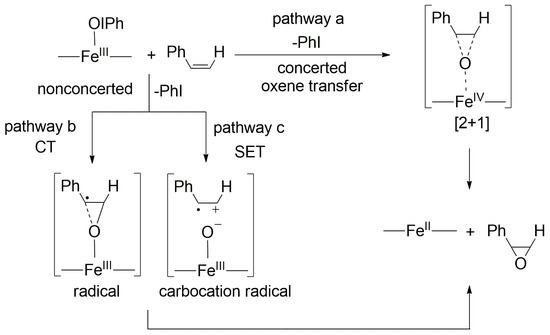
Scheme 3.
Alternative mechanisms for the FeIII(OIPh)-mediated epoxidation reactions.
When the stoichiometric reaction is carried out with an excess of PhI, no inhibition was observed, suggesting that the FeIII(IOPh)-mediated reaction is more favored compared to the oxo-iron(V) species. The sensitivity of the k2 values to the para-substituent effect and the concave Hammett curve support the radical mechanism, including the rate-limiting formation of benzyl radical species. If the carbocation ion is the assumed intermediate, a more negative ρ+ value is expected in the electrophilic process (~−3.5). The benzylic radical formed then undergoes ring closure to form an epoxide. Since a similar trend and behavior was observed during the catalytic and stoichiometric reactions, the reaction mediated by FeIII(IOPh) intermediate can be considered as an elementary step in the catalytic process.
3. Experimental
3.1. Materials and Methods
All chemicals including PBI ligands and substrates were obtained from Aldrich Chemical Co. and used without further purification unless otherwise indicated. Solvents were dried according to published procedures and distilled, stored under argon [35]. [Fe(PBI)3](CF3SO3)2 (1) was synthesized according to literature methods [28]. Iodosylbenzene (PhIO) was prepared by literature methods [36]. UV-visible spectra were recorded on an Agilent 8453 diode-array spectrophotometer using quartz cells. IR spectra were recorded using a Thermo Nicolet Avatar 330 FT-IR instrument (Thermo Nicolet Corporation, Madison, WI, USA). Samples were prepared in the form of KBr pellets. GC analyses were performed on an Agilent 6850 (Budapest, Hungary) gas chromatograph equipped with a flame ionization detector and a 30 m HP-5MS column. GC-MS analyses were carried out on a Shimadzu QP2010SE (Budapest, Hungary) equipped with a secondary electron multiplier detector with conversion dynode and a 30 m HP5MS column.
3.2. Reactivity Studies and Product Analysis
All stoichiometric reactions were run in a 1.0 cm UV cuvette and followed by monitoring UV-vis spectral changes in the reaction solutions, rate constants were determined under pseudo-first-order conditions (e.g., [substrate]/[FeIII(OIPh)] > 10) by fitting the absorbance changes at 760 nm in CH3CN at 293–313 K, respectively, and the resulting solutions were used directly in reactions with substrates, such as thioanisoles, and styrenes, for oxygenation reactions. Reactions were run at least in triplicate, and the data reported are the average of the reactions.
Stoichiometric reactions: In a typical reaction [FeII(PBI)3](OTf)2 (1 × 10−3 M) was dissolved in acetonitrile (2 mL) and iodosylbenzene (4 × 10−3 M) was added to the solution. The mixture was stirred for 4 min and then the excess of iodosylbenzene was removed by filtration. Substrate (0.3–1.5 M) was added to the solution and the reaction was monitored with a UV-Vis spectrophotometer at 760 nm. The product was identified by GC-MS and the yields were determined by GC using bromobenzene as internal standard.
Catalytic reactions: [FeII(PBI)3](OTf)2 (1 × 10−3 M) was dissolved in acetonitrile (3 mL), and iodosylbenzene (1 × 10−1 M) and substrate (3–5 × 10−1 M) was added to the solution. The reaction was monitored by UV-Vis spectroscopy at 293 K in a cuvette. These reactions were also followed by GC: [FeII(PBI)3](OTf)2 (1 × 10−3 M) was dissolved in acetonitrile (10 mL), and PhIO (1 × 10−1 M) and substrate (3–5 × 10−1 M) were added to the solution. Bromobenzene (1 × 10−1 M) was added to the solution as an internal standard. The mixture was stirred for 1 h and, during this time, the product yields were determined every 10 min by GC.
Products of substituted thioanisole oxidation:
Thioanisole/methyl phenyl sulfoxide: (m/z) relative intensity 140 (M+, 77.02); 124.95 (100); 97 (86.14); 94 (22.79); 91 (10.05); 77 (70.10); 74 (10.88); 65 (22.7); 53 (15.7); 51 (75.36); 50 (30.96);45 (16.48); 39 (16.69)/methyl phenyl sulfone: (m/z) relative intensity 155.95 (M+, 12.62); 141 (15.54); 94 (29.93); 77 (100); 51 (50.10); 50 (18.33); 39.05 (8.89).
4-metoxythioanisole/p-methoxyphenyl methyl sulfoxide: (m/z) relative intensity 170 (M+, 48.93); 154.95 (100); 139 (34.61); 103.95 (25.71); 90 (37.52); 89 (15.75); 77 (36.95); 67.05 (24.86); 65 (38.52); 63 (19.43); 51 (10.86); 45 (16.54); 39.05 (28.97)/p-methoxyphenyl methyl sulfone: (m/z) relative intensity 186.2 (M+, 18.53);171 (36.36); 155 (18.52); 139 (15.61); 106.9 (28.90); 92 (14.54); 91.05 (58.18); 89.1 (17.26); 79 (18.92); 77 (100); 65.05 (40.92); 63 (18.93); 51.1 (16.33); 39 (37.54).
Methyl p-tolyl sulfide/methyl p-tolyl sulfoxide: (m/z) relative intensity 153.95 (M+, 50.80); 139 (100); 111 (17.52); 108 (12.39); 90 (36.92); 89 (13.38); 79 (10.70); 78 (14.96); 77 (43.63); 67.05 (22.84); 65 (35.81); 63 (21.10); 51 (11.45); 45 (14.57); 39.05 (25.46)/methyl p-tolyl sulfone: (m/z) relative intensity 170.05 (M+, 15.08); 154.9 (16.37); 139.1 (15.61); 107.1 (23.60); 92.2 (11.44); 91.05 (100); 89.1 (12.26); 79 (14.09); 77 (23.1); 65.05 (45.96); 63 (20.24); 51.1 (13.66); 39.05 (34.54).
4-chlorothioanisole/p-chlorophenyl methyl sulfoxide: (m/z) relative intensity 175.9 (M+, 17.26); 17.9 (49.04); 160.9 (37.02); 158.9 (100); 132.95 (21.51); 130.95 (58.38); 128 (18.89); 126.95 (12.67); 110.95 (32.58); 108 (13.97); 98.95 (15.1); 77 (5.29); 76 (11.74); 75 (65.71); 74 (25.12); 73 (11.39); 63 (18.47); 51.05 (15.50); 50.05 (36.25); 45 (18.56)/p-chlorophenyl methyl sulfone: (m/z) relative intensity 190 (M+, 11.60); 175 (19.60); 129 (11.20); 128 (13.20); 127 (31.60); 113 (32.4); 112 (8.4); 111 (100); 99 (15.6); 77 (8.16); 76 (11.2); 75 (59.2); 74 (23.2); 73 (9.2); 51 (11.2); 50 (31.2).
4-nitrothioanisole/p-nitrophenyl methyl sulfoxide: (m/z) relative intensity 185.9 (M+, 9.76); 184.9 (100); 169.95 (51.51); 139.95 (33.51); 139 (10.81); 124 (26.41); 123 (7.85); 112 (34.61); 111 (16.21); 110 (11.17); 108 (12.88); 96 (28.54); 95 (17.13); 92 (20.43); 82.05 (19.09); 77 (20.14); 76 (47.21); 75 (33.45); 74 (31.63); 70 (30.94); 68.95 (21.28); 65.05 (13.62); 64 (22.02); 63 (44.94); 62.05 (10.33); 58 (12.09); 51 (20.36); 50 (78.27); 45 (32.47); 39 (16.16)/p-nitrophenyl methyl sulfone: (m/z) relative intensity 201.9 (M+, 12.8); 201 (100); 186 (31.21); 156 (21.56); 155 (12.31); 139.9 (17.41); 139 (11.35); 128 (43.81); 137 (14.71); 125.95 (13.27); 124 (14.93); 112 (25.84); 111 (21.13); 108 (26.03); 97.95 (18.52); 93 (18.54); 77 (17.14); 76 (38.21); 75 (28.57); 74 (35.39); 70 (26.37); 69 (23.87); 65 (17.58); 63.95 (24.72); 63 (40.85); 62 (11.73); 58 (16.82); 51 (19.43); 50 (77.3); 45 (31.96); 39 (14.54).
Products of substituted styrene oxidation:
Styrene/styrene oxide: (m/z) relative intensity 120.05 (M+, 24.05); 119 (45.13); 92 (43.84); 91.05 (100); 90.05 (61.15); 89 (70.51); 77.1 (19.47); 73.8 (8.24); 65 (25.7); 64 (10.72); 63 (33.95); 62.05 (9.73); 51.05 (32.36);50.05 (27.81); 41 (10.75); 39 (30.49); 38 (14.5).
4-vinylanisole/2-(4-methoxyphenyl)oxirane: (m/z) relative intensity 150.1 (M+, 33.25); 135 (74.19); 119.05 (57.95); 92 (37.14); 90.95 (100); 90 (58.91); 89.05 (66.43); 77 (27.93); 74 (10.86); 65 (31.8); 64 (13.64); 63 (36.64); 62 (10.65); 51.05 (28.54); 50.05 (24.87); 41 (15.85); 39 (35.85); 38 (12.64).
4-methylstyrene/2-(4-methylphenyl)oxirane: (m/z) relative intensity 134.1 (M+, 28.53); 119 (100); 91.95 (47.32); 91 (63.29); 90.05 (57.05); 89.05 (64.64); 77 (27.92); 74.05 (10.74); 64.95 (23.95); 64 (14.92); 63.05 (36.17); 62 (15.96); 51.05 (28.95);50.05 (35.17); 41.05 (13.47); 39.05 (34.75); 38 (17.94).
4-chlorostyrene/2-(4-chlorophenyl)oxirane: (m/z) relative intensity 154.1 (M+, 36.86); 119.05 (37.75); 92 (47.98); 91 (100); 90.05 (56.91); 89 (69.43); 77.1 (23.97); 74 (11.52); 65.05 (28.93); 64.05 (15.72); 63 (38.91); 62 (13.84); 51 (37.47);50.05 (36.81); 41.05 (14.74); 39 (31.65); 38.05 (17.43).
4-cianostyrene/2-(4-cianophenyl)oxirane: (m/z) relative intensity 145.1 (M+, 41.96); 119.05 (100); 91.95 (42.57); 91 (48.24); 90.05 (47.93); 89 (53.96); 77 (29.34); 74 (16.83); 65 (33.83); 64 (12.54); 63 (35.94); 62 (16.83); 51.05 (42.65); 50 (39.75); 41 (18.63); 39 (37.75); 38 (22.83).
4. Conclusions
In summary, we reported the catalytic oxidation of thioanisole and styrene derivatives using a non-heme complex, FeII(PBI)3 as catalyst and PhIO as oxidant. The catalytic activity was moderate, but proved to be selective for both substrates, producing sulfoxides and epoxides, respectively, as the main products. Based on Hammett correlation for para-substituted thioanisoles, electrophilic iron-based oxidation can be assumed via a direct O-atom transfer mechanism. In contrast, in the case of para-substituted styrenes, a nonconcerted electron transfer mechanism can be proposed through the formation of the radicaloid benzylic radical intermediate in the rate-determining step. The results of the stoichiometric sulfoxidation and epoxidation reactions of the in situ generated FeIII(OIPh) intermediate supported our proposed hypotheses for the investigated catalytic reactions. These results provide further example of metal–oxidant adducts which can transfer their oxygen atom to organic substrates prior to the conversion into metal oxo species. Efforts to develop asymmetric catalyzed oxidation of various alkenes and sulfides of higher technological interest by introducing the chiral moiety to ligands or oxidants as well as their detailed mechanistic aspects are under progress. Future studies will also be focused on elucidating the intriguing reactivity differences between high- and low-spin Fe(III)-iodosylarene complexes. This type of reactivity difference has been demonstrated recently in the reactions of high- and low-spin iron(III)-hydroperoxo and high-valent oxoiron complexes.
Author Contributions
Resources, D.L.-B. and P.T.; writing—original draft preparation and supervision, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Financial support of the Hungarian National Research, Development and Innovation Fund, OTKA K142212 (J.K.), TKP2021-NKTA-21 (J.K.) and ÚNKP-22-3 (P.T.) New National Excellence Program of the Ministry for Culture and Innovation are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available.
References
- Lakk-Bogáth, D.; Kripli, B.; Meena, B.I.; Speier, G.; Kaizer, J. Catalytic and stoichiometric oxidation of N,N-dimethylanilines mediated by nonheme oxoiron(IV) complex with tetrapyridyl ligand. Polyhedron 2019, 169, 169–175. [Google Scholar] [CrossRef]
- Nehru, K.; Seo, M.S.; Kim, J.; Nam, W. Oxidative N-Dealkylation Reactions by Oxoiron(IV) Complexes of Nonheme and Heme Ligands. Inorg. Chem. 2007, 46, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Nam, W. Dioxygen Activation by Metalloenzymes and Models. Acc. Chem. Res. 2007, 40, 465. [Google Scholar] [CrossRef]
- Nam, W. High-valent Iron(IV)-Oxo Complexes of Heme and Non-Heme Ligands in Oxygenation Reactions. Acc. Chem. Res. 2007, 40, 522–531. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.R.; Que, L., Jr. High-valent nonheme iron-oxo complexes: Synthesis, structure, and spectroscopy. Coord. Chem. Rev. 2013, 257, 414–428. [Google Scholar] [CrossRef]
- Kaizer, J.; Klinker, E.J.; Oh, N.Y.; Rohde, J.U.; Song, W.J.; Stubna, A.; Kim, J.; Munck, E.; Nam, W.; Que, L., Jr. Nonheme FeIVO Complexes That Can Oxidize the C-H Bonds of Cyclohexane at Room Temperature. J. Am. Chem. Soc. 2004, 126, 472–473. [Google Scholar] [CrossRef]
- Klinker, E.J.; Kaizer, J.; Brennessel, W.W.; Woodrum, N.L.; Cramer, C.J.; Que, L., Jr. Structures of Nonheme Oxoiron(IV) Complexes from X-ray Crystallography, NMR Spectroscopy, and DFT Calculations. Angew. Chem. Int. Ed. 2005, 44, 3690–3694. [Google Scholar] [CrossRef]
- Nam, W.; Lee, Y.-M.; Fukuzumi, S. Tuning Reactivity and Mechanism in Oxidation Reactions by Mononuclear Nonheme Iron(IV)-Oxo Complexes. Acc. Chem. Res. 2014, 47, 1146–1154. [Google Scholar] [CrossRef]
- Turcas, R.; Lakk-Bogáth, D.; Speier, G.; Kaizer, J. Steric control and the mechanism of benzaldehyde oxidation by polypyridyl oxoiron(iv) complexes: Aromatic versus benzylic hydroxylation of aromatic aldehydes. Dalton. Trans. 2018, 47, 3248–3252. [Google Scholar] [CrossRef]
- Turcas, R.; Kripli, B.; Attia, A.A.A.; Lakk-Bogáth, D.; Speier, G.; Giorgi, M.; Silaghi-Dumitrescu, R.; Kaizer, J. Catalytic and stoichiometric flavanone oxidation mediated by nonheme oxoiron(IV) complexes as flavone synthase mimics: Kinetic, mechanistic and computational studies. Dalton. Trans. 2018, 47, 14416–14420. [Google Scholar] [CrossRef]
- Oloo, W.N.; Que, L., Jr. Bioinspired Nonheme Iron Catalysts for C–H and C═C Bond Oxidation: Insights into the Nature of the Metal-Based Oxidants. Acc. Chem. Res. 2015, 48, 2612–2621. [Google Scholar] [CrossRef]
- Lakk-Bogáth, D.; Csonka, R.; Speier, G.; Reglier, M.; Simaan, A.J.; Naubron, J.V.; Giorgi, M.; Lazar, K.; Kaizer, J. Oxoiron(IV) Complex Derived from Chiral Pentadentate Ligand asN4Py. Inorg. Chem. 2016, 55, 10090–10093. [Google Scholar] [CrossRef]
- Fung, W.-H.; Yu, W.-Y.; Che, C.-M. Mechanistic Investigation of the Oxidation of Aromatic Alkenes by Monooxoruthenium(IV). Asymmetric Alkene Epoxidation by Chiral Monooxoruthenium(IV) Complexes. J. Org. Chem. 1998, 63, 7715. [Google Scholar] [CrossRef]
- Groves, J.T.; Watanabe, Y. The mechanism of olefin epoxidation by oxo-iron porphyrins. Direct observation of an intermediate. J. Am. Chem. Soc. 1986, 108, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Lakk-Bogáth, D.; Szávuly, M.; Török, P.; Kaizer, J. Catalytic and Stoichiometric Baeyer-Villiger Oxidation Mediated by Nonheme Peroxi-Diiron(III), Acylperoxo, and Iodosylbenzene Iron(III) Intermediates. Molecules 2022, 27, 2814. [Google Scholar] [CrossRef]
- Wang, B.; Lee, Y.M.; Seo, M.S.; Nam, W. Mononuclear Nonheme Iron(III)-Iodosylarene and High-Valent Iron-Oxo Complexes in Olefin Epoxidation Reactions. Angew. Chem. Int. Ed. 2015, 127, 11906–11910. [Google Scholar] [CrossRef]
- Wang, C.; Kurahashi, T.; Inomata, K.; Hada, M.; Fujii, H. Oxygen-Atom Transfer from Iodosylarene Adducts of a Manganese(IV) Salen Complex: Effect of Arenes and Anions on I(III) of the Coordinated Iodosylarene. Inorg. Chem. 2013, 52, 9557–9566. [Google Scholar] [CrossRef]
- Lennartson, A.; McKenzie, C.J. An Iron(III) Iodosylbenzene Complex: A Masked Non-Heme FeVO. Angew. Chem. 2012, 124, 6871–6874. [Google Scholar] [CrossRef]
- Hong, S.; Wang, B.; Seo, M.S.; Lee, Y.-M.; Kim, M.J.; Kim, H.R.; Ogura, T.; Garcia-Serres, R.; Clemancey, M.; Latour, J.-M.; et al. Highly Reactive Nonheme Iron(III) Iodosylarene Complexes in Alkane Hydroxylation and Sulfoxidation Reactions. Angew. Chem. Int. Ed. 2014, 53, 6388–6392. [Google Scholar] [CrossRef]
- Guo, M.; Dong, H.; Li, J.; Cheng, B.; Huang, Y.-Q.; Feng, Y.-Q.; Lei, A. Spectroscopic Observation of Iodosylarene Metalloporphyrin Adducts and Manganese(V)-Oxo Porphyrin Species in a Cytochrome P450 Analogue. Nat. Commun. 2012, 3, 1190. [Google Scholar] [CrossRef]
- Guo, M.; Lee, Y.-M.; Fukuzumi, S.; Nam, W. Biomimetic metal-oxidant adducts as active oxidants in oxidation reactions. Coord. Chem. Rev. 2021, 435, 213807. [Google Scholar] [CrossRef]
- Nam, W.; Choi, S.K.; Lim, M.H.; Rohde, J.-U.; Kim, I.; Kim, J.; Kim, C.; Que, L., Jr. Reversible formation of iodosylbenzene-iron porphyrin intermediates in the reaction of oxoiron(IV) porphyrin pi-cation radicals and iodobenzene. Angew. Chem. Int. Ed. 2003, 42, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.A.; Kelty, M.L.; Filatov, A.S.; Anderson, J.S. Isolable iodosylarene and iodoxyarene adducts of Co and their O-atom transfer and C–H activation reactivity. Chem. Sci. 2018, 9, 4493–4499. [Google Scholar] [CrossRef]
- Wang, C.; Kurahashi, T.; Fujii, H. Structure and Reactivity of an Iodosylarene Adduct of a Manganese(IV)-Salen Complex. Angew. Chem. 2012, 124, 7929–7931. [Google Scholar] [CrossRef]
- Guo, M.; Lee, Y.-M.; Seo, M.S.; Kwon, Y.-J.; Li, X.-X.; Ohta, T.; Kim, W.-S.; Sarangi, R.; Fukuzumi, S.; Nam, W. Mn(III)-Iodosylarene Porphyrins as an Active Oxidant in Oxidation Reactions: Synthesis, Characterization, and Reactivity Studies. Inorg. Chem. 2018, 57, 10232–10240. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Ohta, T.; Cho, J.J. Structure and Reactivity of a Mononuclear Nonheme Manganese(III)–Iodosylarene Complex. J. Am. Chem. Soc. 2018, 140, 16037–16041. [Google Scholar] [CrossRef]
- Török, P.; Lakk-Bogáth, D.; Kaizer, J. Stoichiometric Alkane and Aldehyde Hydrtoxylation Reactions Mediated by In Situ Generated Iron(III)-Iodosylbenzene Adduct. Molecules 2023, 28, 1855. [Google Scholar] [CrossRef]
- Pap, J.S.; Draksharapu, A.; Giorgi, M.; Browne, W.R.; Kaizer, J.; Speier, G. Stabilisation of μ-peroxido-bridged Fe(III) intermediates with non-symmetric bidentate N-donor ligands. Chem. Commun. 2014, 50, 1326–1329. [Google Scholar] [CrossRef]
- Kripli, B.; Csendes, V.F.; Török, P.; Speier, G.; Kaizer, J. Stoichiometric Aldehyde Deformylation Mediated by nucleophilic Peroxo-diiron(III) Complex as a Functional Model of Aldehyde Deformylating Oxygenase. Chem.-Eur. J. 2019, 25, 14290–14294. [Google Scholar] [CrossRef]
- Szávuly, M.I.; Surducan, M.; Nagy, E.; Surányi, M.; Speier, G.; Silaghi-Dumitrescu, R.; Kaizer, J. Functional models on nonheme diiron enzymes: Kinetic and computational evidence for the formation of oxoiron(IV) species from peroxo-diiron(III) complexes, and their reactivity towards phenols and H2O2. Dalton Trans. 2016, 45, 14709–14718. [Google Scholar] [CrossRef]
- Goto, J.; Matsui, T.; Ozaki, S.-I.; Watanabe, Y.; Fukuzumi, S. Mechanisms of Sulfoxidation Catalyzed by High-Valent Intermediates of Heme Enzymes: Electron-Transfer vs Oxygen-Transfer Mechanism. J. Am. Chem. Soc. 1999, 121, 9497–9502. [Google Scholar] [CrossRef]
- Lakk-Bogáth, D.; Speier, G.; Kaizer, J. Comparison of the stability and reactivity of achiral versus chiral nonheme oxoiron(IV9 complexes supported by pentadentate N5 ligands in oxygen.atom and hydrogen-atom transfer reactions. Inorg. Chem. Commun. 2019, 107, 107446. [Google Scholar] [CrossRef]
- Wu, Y.-D.; Wong, C.-L.; Chan, K.W.K.; Ji, G.-Z.; Jiang, X.-K. Substituent Effects on the C−H Bond Dissociation Energy of Toluene. A Density Functional Study. J. Org. Chem. 1996, 61, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Meena, B.I.; Lakk-Bogáth, D.; Kripli, B.; Speier, G.; Kaizer, J. Kinetics and mechanism of epoxidation of olefins by chiral tetrapyridyl oxoiron(IV) complex. Polyhedron 2018, 151, 141–145. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Pergamon Press: Oxford, UK, 2009. [Google Scholar]
- Saltzman, H.; Sharefkin, J.G. Organic Syntheses; Wiley: New York, NY, USA, 1973; Collective Volume V, pp. 658–659. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).