New Insight on In Vitro Biological Activities of Sulfated Polysaccharides from Ulvophyte Green Algae
Abstract
1. Introduction
2. Results
2.1. Characterization and Composition of SPs Samples
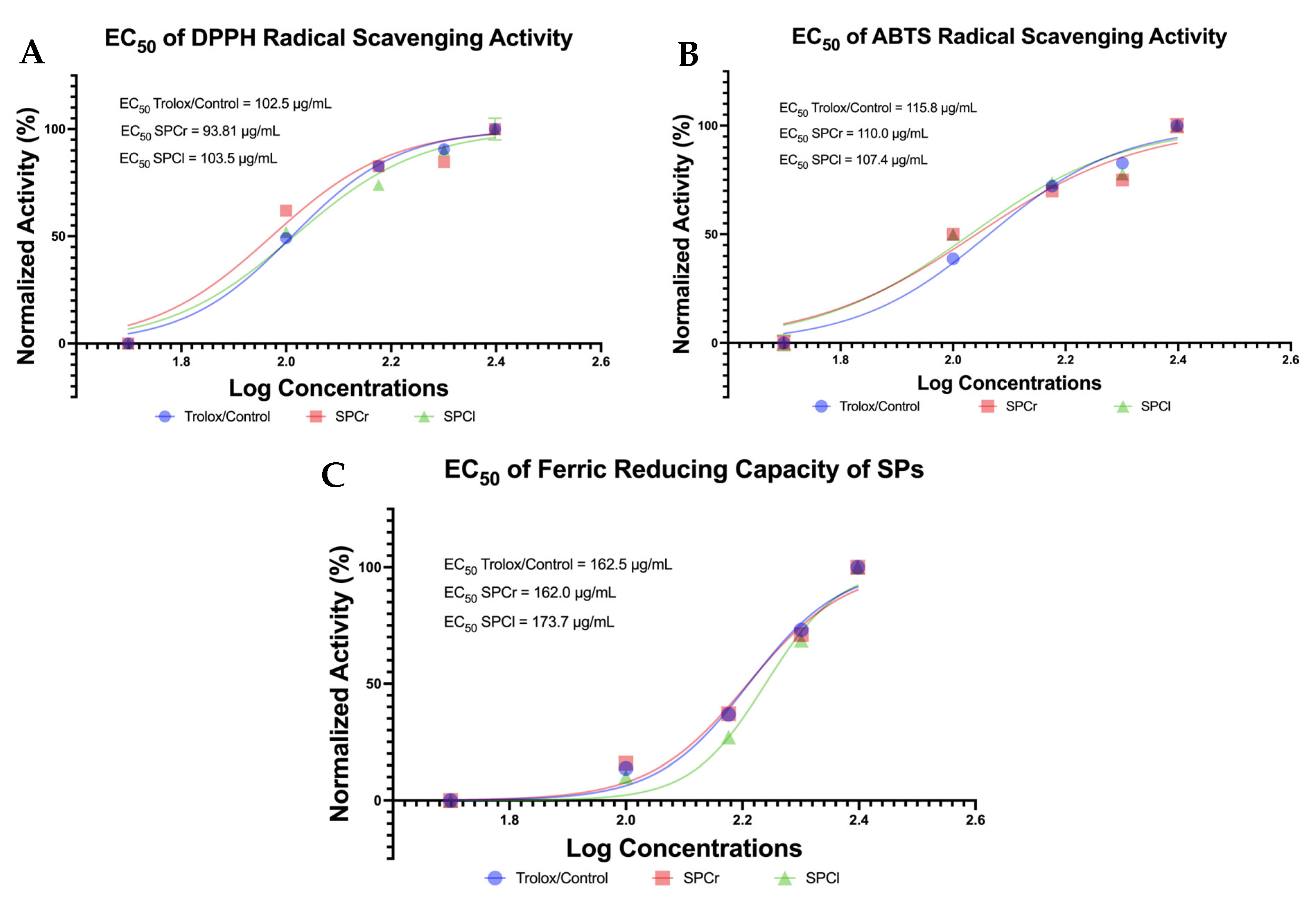
2.2. SPCr and SPCl Exhibit Promising Antioxidant Potential
2.3. Anti-Obesity Potential of Two Extracted SPs from Indonesian Green Algae
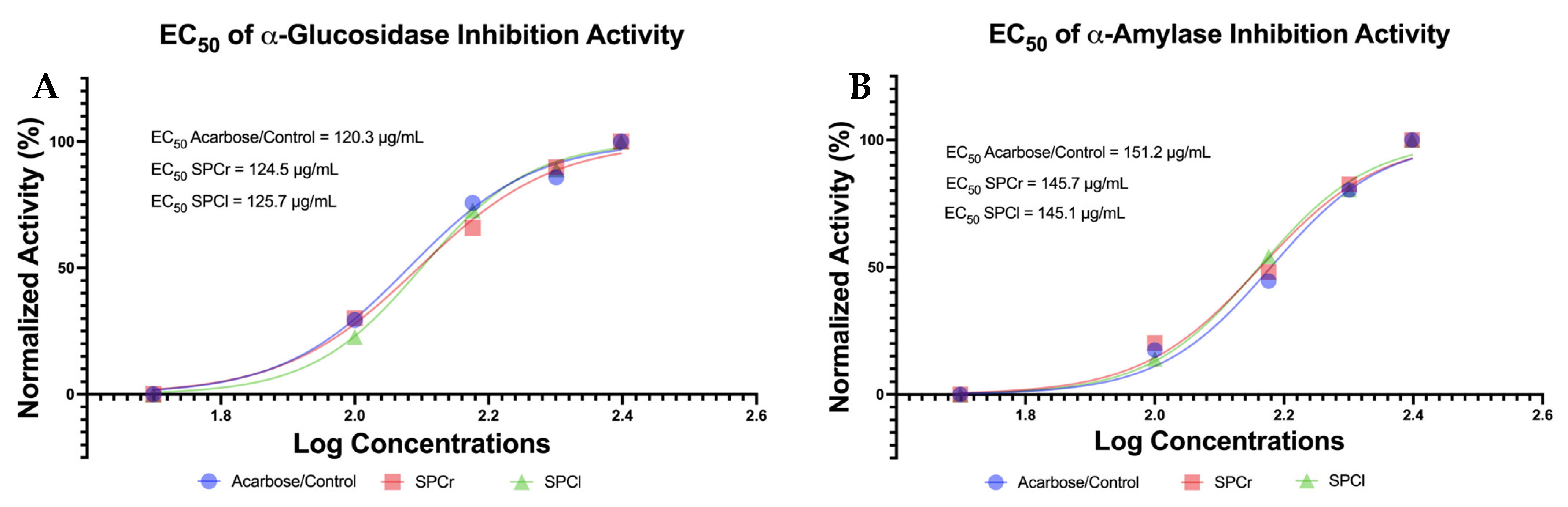
2.4. The Promising Antidiabetic of Two Isolated SPs from Indonesian Green Algae
2.5. Promising Anticancer Properties of Two Extracted SPs from Indonesian Green Algae
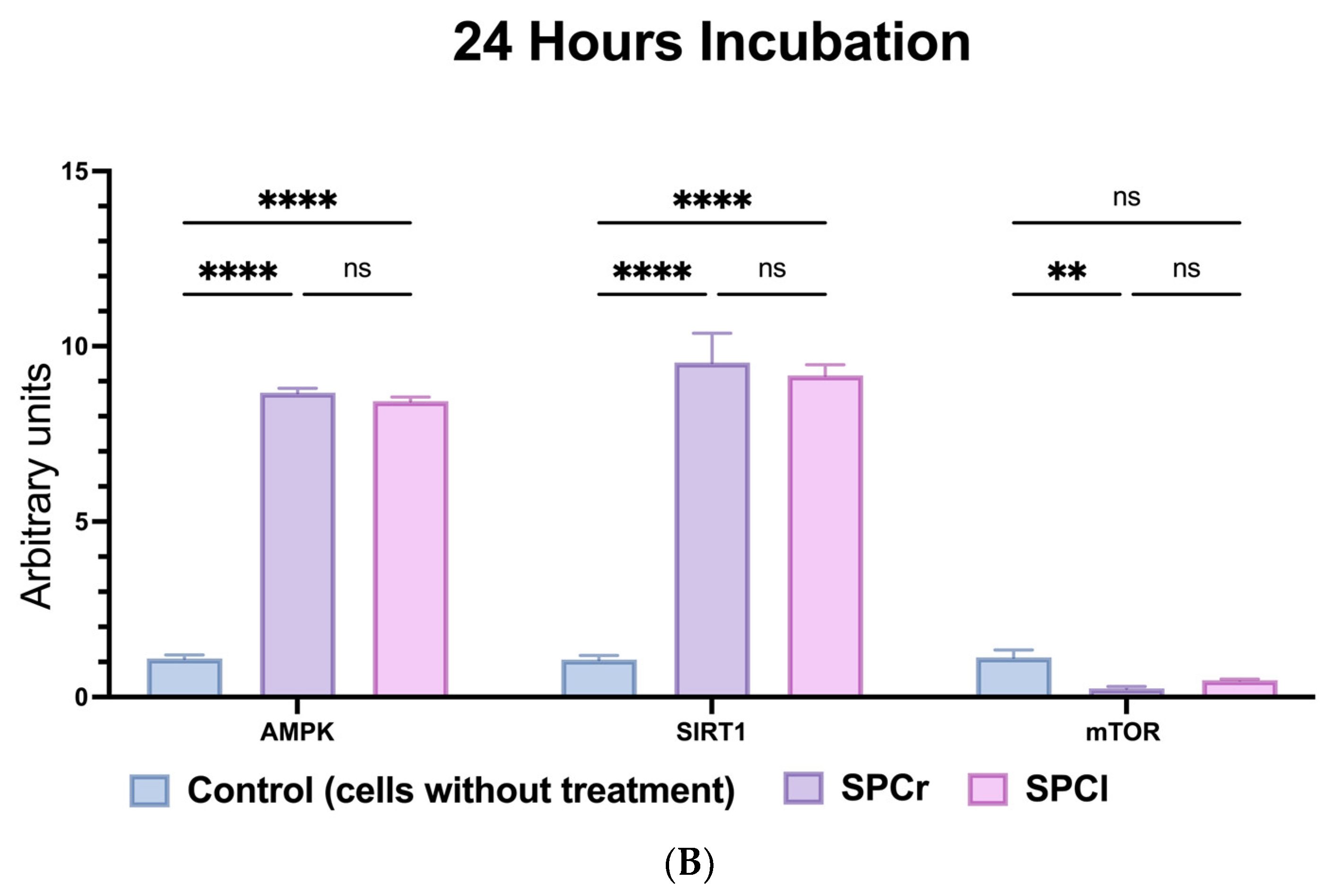
2.6. Anti-Ageing Capabilities of SPs via Modulation of mTOR-SIRT1-AMPK Pathways
3. Discussion
4. Materials and Methods
4.1. Preparation of the Indonesian Green Algae Samples
4.2. Isolation and Characterization of Sulfated Polysaccharides from Indonesian Caulerpa
4.3. Antioxidant Activity by DPPH and ABTS Radical Scavenging Activity, and FRAP (Ferric Reducing Antioxidant Power) Assay
4.4. In Vitro Anti-Obesity of SPs via Lipase Inhibition Assay (%)
4.5. In Vitro Antidiabetic Assay via α-Glucosidase and α-Amylase Inhibition Assay (%)
4.6. Anticancer Evaluation of SPs via Antiproliferative Activity
4.7. In Vitro Assays of Mammalian Target of Rapamycin (mTOR) Kinase, AMP-Activated Protein Kinase (AMPK), and Sirtuin 1 (SIRT1) Expressions
4.8. Management and Analysis of Data
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darmawan, M.; Fajarningsih, N.D.; Sihono; Irianto, H.E. Caulerpa: Ecology, Nutraceutical and Pharmaceutical Potential; Nathani, N.M., Mootapally, C., Gadhvi, I.R., Maitreya, B., Joshi, C.G., Eds.; Mar. Niche Appl. Pharm. Sci.; Springer: Singapore, 2020; pp. 299–318. [Google Scholar] [CrossRef]
- Magdugo, R.P.; Terme, N.; Lang, M.; Pliego-Cortés, H.; Marty, C.; Hurtado, A.Q.; Bedoux, G.; Bourgougnon, N. An analysis of the nutritional and health values of Caulerpa racemosa (Forsskål) and Ulva fasciata (Delile)—Two chlorophyta collected from the Philippines. Molecules 2020, 25, 2901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Che, X.; Huang, Z.; Chen, P.; Xia, G.; Zhao, M. Comparative Analysis of Nutrient Composition of Caulerpa lentillifera from Different Regions. J. Ocean Univ. China 2020, 19, 439–445. [Google Scholar] [CrossRef]
- Nagappan, T.; Vairappan, C.S. Nutritional and bioactive properties of three edible species of green algae, genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2013, 26, 1019–1027. [Google Scholar] [CrossRef]
- Paul, N.A.; Neveux, N.; Magnusson, M.; de Nys, R. Comparative production and nutritional value of “sea grapes”—The tropical green seaweeds Caulerpa lentillifera and C. racemosa. J. Appl. Phycol. 2014, 26, 1833–1844. [Google Scholar] [CrossRef]
- de Gaillande, C.; Payri, C.; Remoissenet, G.; Zubia, M. Caulerpa consumption, nutritional value and farming in the Indo-Pacific region. J. Appl. Phycol. 2016, 29, 2249–2266. [Google Scholar] [CrossRef]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries—A review. J. Food Sci. Technol. 2021, 58, 2453–2466. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Z.; Song, S.; Zhu, B.; Zhao, L.; Jiang, J.; Liu, N.; Wang, J.; Chen, X. Anti-inflammatory activity and structural identification of a sulfated polysaccharide CLGP4 from Caulerpa lentillifera. Int. J. Biol. Macromol. 2020, 146, 931–938. [Google Scholar] [CrossRef]
- Filho, G.P.C.; Oliveira, R.D.P.; De Medeiros, S.R.B.; Rocha, H.A.O.; Moreira, S.M.G. Sulfated polysaccharides from green seaweed Caulerpa prolifera suppress fat accumulation. J. Appl. Phycol. 2020, 32, 4299–4307. [Google Scholar] [CrossRef]
- Pires, C.L.; Rodrigues, S.D.; Bristot, D.; Gaeta, H.H.; Toyama, D.D.O.; Farias, W.R.L.; Toyama, M.H. Sulfated polysaccharide extracted of the green algae Caulerpa racemosa increase the enzymatic activity and paw edema induced by sPLA2 from Crotalus durissus terrificus venom. Rev. Bras. Farm. 2013, 23, 635–643. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization of sulfated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef]
- Figueroa, F.A.; Abdala-Díaz, R.T.; Pérez, C.; Casas-Arrojo, V.; Nesic, A.; Tapia, C.; Durán, C.; Valdes, O.; Parra, C.; Bravo-Arrepol, G.; et al. Sulfated Polysaccharide Extracted from the Green Algae Codium bernabei: Physicochemical Characterization and Antioxidant, Anticoagulant and Antitumor Activity. Mar. Drugs 2022, 20, 458. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Hao, H.; Zhang, X.; Wong, I.N.; Chung, S.K.; Chen, Z.; Xu, B.; Huang, R. Immunomodulatory sulfated polysaccharides from Caulerpa racemosa var. peltata induces metabolic shifts in NF-κB signaling pathway in RAW 264.7 macrophages. Int. J. Biol. Macromol. 2021, 182, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.D.S.; Palhares, L.C.G.F.; Silva, C.H.F.; Sabry, D.A.; Chavante, S.F.; Rocha, H.A.O. In Vitro Antitumor Potential of Sulfated Polysaccharides from Seaweed Caulerpa cupressoides var. flabellata. Mar. Biotechnol. 2021, 23, 77–89. [Google Scholar] [CrossRef]
- Qin, L.; Yang, Y.; Hao, J.; He, X.; Liu, S.; Chu, X.; Mao, W. Antidiabetic-activity sulfated polysaccharide from Chaetomorpha linum: Characteristics of its structure and effects on oxidative stress and mitochondrial function. Int. J. Biol. Macromol. 2022, 207, 333–345. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Marudhupandi, T.; Rajasekar, P.; Prabhu, N.M. Sulfated polysaccharide from Sargassum tenerrimum attenuates oxidative stress induced reactive oxygen species production in in vitro and in zebrafish model. Carbohydr. Polym. 2019, 203, 441–449. [Google Scholar] [CrossRef]
- Aroyehun, A.Q.B.; Razak, S.A.; Palaniveloo, K.; Nagappan, T.; Rahmah, N.S.N.; Jin, G.W.; Chellappan, D.K.; Chellian, J.; Kunnath, A.P. Bioprospecting Cultivated Tropical Green Algae, Caulerpa racemosa (Forsskal) J. Agardh: A Perspective on Nutritional Properties, Antioxidative Capacity and Anti-Diabetic Potential. Foods 2020, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Bhushan, S.; Bast, F.; Singh, S. Marine macroalga Caulerpa: Role of its metabolites in modulating cancer signaling. Mol. Biol. Rep. 2019, 46, 3545–3555. [Google Scholar] [CrossRef]
- Zheng, L.-X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.-L. Preparation methods, biological activities, and potential applications of marine algae oligosaccharides: A review. Food Sci. Hum. Wellness 2023, 12, 359–370. [Google Scholar] [CrossRef]
- Nurkolis, F.; Taslim, N.A.; Qhabibi, F.R.; Kang, S.; Moon, M.; Choi, J.; Choi, M.; Park, M.N.; Mayulu, N.; Kim, B. Ulvophyte Green Algae Caulerpa lentillifera: Metabolites Profile and Antioxidant, Anticancer, Anti-Obesity, and In Vitro Cytotoxicity Properties. Molecules 2023, 28, 1365. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Je, J.G.; Lee, H.G.; Fernando, K.H.N.; Jeon, Y.J.; Ryu, B. Purification and structural characterization of sulfated polysaccharides derived from brown algae, sargassum binderi: Inhibitory mechanism of inos and cox-2 pathway interaction. Antioxidants 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Günal, S.; Hardman, R.; Kopriva, S.; Mueller, J.W. Sulfation pathways from red to green. J. Biol. Chem. 2019, 294, 12293–12312. [Google Scholar] [CrossRef] [PubMed]
- Zhong, O.; Hu, J.; Wang, J.; Tan, Y.; Hu, L.; Lei, X. Antioxidant for treatment of diabetic complications: A meta-analysis and systematic review. J. Biochem. Mol. Toxicol. 2022, 36, e23038. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Ji, J.; Petropavlovskaia, M.; Khatchadourian, A.; Patapas, J.; Makhlin, J.; Rosenberg, L.; Maysinger, D. Type 2 diabetes is associated with suppression of autophagy and lipid accumulation in β-cells. J. Cell. Mol. Med. 2019, 23, 2890–2900. [Google Scholar] [CrossRef]
- Kanbarkar, N.; Mishra, S.; Khanal, P. Beneficial effect of phospholipase A2 group IIA inhibitors from Acacia suma in obesity: An in silico and in vitro study. Adv. Tradit. Med. 2020, 20, 599–608. [Google Scholar] [CrossRef]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological management of obesity an endocrine society clinical practice guideline. Reprod. Endocrinol. 2015, 59, 342–362. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Altaber, S.; Bseiso, Y.; Oqal, M.; AbuDalo, R.; Alrosan, K.; Alrosan, A.Z.; Melhim, S.B.; et al. Isorhamnetin Reduces Glucose Level, Inflammation, and Oxidative Stress in High-Fat Diet/Streptozotocin Diabetic Mice Model. Molecules 2023, 28, 502. [Google Scholar] [CrossRef] [PubMed]
- Khodabandehloo, H.; Gorgani-Firuzjaee, S.; Panahi, G.; Meshkani, R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl. Res. 2016, 167, 228–256. [Google Scholar] [CrossRef]
- Kim, K.-S.; Yang, H.J.; Choi, E.-K.; Shin, M.H.; Kim, K.-H.; Um, J.Y.; Lee, B.-C.; Jang, H.-J. The effects of complex herbal medicine composed of Cornus fructus, Dioscoreae rhizoma, Aurantii fructus, and Mori folium in obese type-2 diabetes mice model. Orient. Pharm. Exp. Med. 2013, 13, 69–75. [Google Scholar] [CrossRef]
- Sekar, V.; Chakraborty, S.; Mani, S.; Sali, V.; Vasanthi, H. Mangiferin from Mangifera indica fruits reduces post-prandial glucose level by inhibiting α-glucosidase and α-amylase activity. S. Afr. J. Bot. 2019, 120, 129–134. [Google Scholar] [CrossRef]
- Gopal, S.S.; Lakshmi, M.J.; Sharavana, G.; Sathaiah, G.; Sreerama, Y.N.; Baskaran, V. Lactucaxanthin-a potential anti-diabetic carotenoid from lettuce (Lactuca sativa) inhibits α-amylase and α-glucosidase activity in vitro and in diabetic rats. Food Funct. 2017, 8, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Li, B.; Wang, X.; Xu, X.; Liu, X.; Li, W.; Chang, X.; Li, H.; Qi, H. The antioxidant and antihyperlipidemic activities of phosphorylated polysaccharide from Ulva pertusa. Int. J. Biol. Macromol. 2020, 145, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chrienova, Z.; Nepovimova, E.; Kuca, K. The role of mTOR in age-related diseases. J. Enzym. Inhib. Med. Chem. 2021, 36, 1679–1693. [Google Scholar] [CrossRef]
- Giovannini, L.; Bianchi, S. Role of nutraceutical SIRT1 modulators in AMPK and mTOR pathway: Evidence of a synergistic effect. Nutrition 2017, 34, 82–96. [Google Scholar] [CrossRef]
- Filho, G.P.C.; de Sousa, A.F.G.; Câmara, R.B.G.; Rocha, H.A.O.; de Medeiros, S.B.; Moreira, S.M.G. Genotoxicity and osteogenic potential of sulfated polysaccharides from Caulerpa prolifera seaweed. Int. J. Biol. Macromol. 2018, 114, 565–571. [Google Scholar] [CrossRef]
- Sanniyasi, E.; Venkatasubramanian, G.; Anbalagan, M.M.; Raj, P.P.; Gopal, R.K. In vitro anti-HIV-1 activity of the bioactive compound extracted and purified from two different marine macroalgae (seaweeds) (Dictyota bartayesiana J.V.Lamouroux and Turbinaria decurrens Bory). Sci. Rep. 2019, 9, 12185. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Nurkolis, F.; Ben Gunawan, W.; Yusuf, V.M.; Yusuf, M.; Kusuma, R.J.; Sabrina, N.; Muharram, F.R.; Taslim, N.A.; Mayulu, N.; et al. Modulation of gut microbiota and markers of metabolic syndrome in mice on cholesterol and fat enriched diet by butterfly pea flower kombucha. Curr. Res. Food Sci. 2022, 5, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.S.; Kim, Y.-J.; Na, H.J.; Jung, H.R.; Song, C.K.; Kang, S.Y.; Kim, J.Y. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions. Food Sci. Biotechnol. 2019, 28, 201–207. [Google Scholar] [CrossRef]
- Suksaeree, J.; Wunnakup, T.; Monton, C. Synergistic antioxidant activity of plant compositions contained in Chatuphalathika herbal recipe: Terminalia chebula Retz. var. chebula, Terminalia arjuna Wight and Arn., Terminalia bellirica (Gaertn.) Roxb., and Phyllanthus emblica L. Adv. Tradit. Med. 2022, 22, 547–556. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Firani, N.K.; Prijadi, B.; Irnandi, D.F.; Riawan, W.; Yusuf, M.; Amar, N.; Chandra, L.A.; Yusuf, V.M.; Subali, A.D.; et al. Kombucha drink enriched with sea grapes (Caulerpa racemosa) as potential functional beverage to contrast obesity: An in vivo and in vitro approach. Clin. Nutr. ESPEN 2022, 49, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Permatasari, H.K.; Nurkolis, F.; Hardinsyah, H.; Taslim, N.A.; Sabrina, N.; Ibrahim, F.M.; Visnu, J.; Kumalawati, D.A.; Febriana, S.A.; Sudargo, T.; et al. Metabolomic Assay, Computational Screening, and Pharmacological Evaluation of Caulerpa racemosa as an Anti-obesity With Anti-aging by Altering Lipid Profile and Peroxisome Proliferator-Activated Receptor-γ Coactivator 1-α Levels. Front. Nutr. 2022, 9, 939073. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, G.; Liang, Y.; Song, T.; Zhu, Y.; Ni, H.; Yamaguchi, K.; Oda, T. Inhibitory effects of a sulfated polysaccharide isolated from edible red alga Bangia fusco-purpurea on α-amylase and α-glucosidase. Biosci. Biotechnol. Biochem. 2019, 83, 2065–2074. [Google Scholar] [CrossRef]
- Dissanayake, I.H.; Bandaranayake, U.; Keerthirathna, L.R.; Manawadu, C.; Silva, R.M.; Mohamed, B.; Ali, R.; Peiris, D.C. Integration of in vitro and in-silico analysis of Caulerpa racemosa against antioxidant, antidiabetic, and anticancer activities. Sci. Rep. 2022, 12, 20848. [Google Scholar] [CrossRef]
- Salawu, K.M.; Ajaiyeoba, E.O.; Ogbole, O.O.; Adeniji, J.A.; Faleye, T.C.; Agunu, A. Antioxidant, Brine Shrimp Lethality, and Antiproliferative Properties of Gel and Leaf Extracts of Aloe schweinfurthii and Aloe vera. J. Herbs Spices Med. Plants 2017, 23, 263–271. [Google Scholar] [CrossRef]


| Sample | Yield (%) | Protein (%) | Sulfate-to-Total-Sugar Ratio | * Monosaccharides | Observed Methylated Sugars (Peak Area; MW) |
|---|---|---|---|---|---|
| SPCr | 28.55 ± 1.00 a | nd | 0.55 ± 0.01 a | Arabinose, mannose, rhamnose, and xylose | 2,3-di-O-methyl-1,4,5-tri-O-acetyl arabinitol (20%; 306.31), 2,3,4,6-tetra-O-methyl-D-mannopyranose (24%; 236.26), and type B ulvanobiuronic acid 3-sulfate (36%) |
| SPCl | 27.60 ± 2.55 a | nd | 0.40 ± 0.05 a | Xylose, galactose, and rhamnose | 2,3,4-tri-O-methyl-1,5-di-O-acetyl xylitol (17%; 278.30), 2,3,4,6-tetra-O-methyl-D-galactopyranose (20%; 236.26), and type A ulvanobiuronic acid 3-sulfate (28%) |
| SPs Sample | Colorectal | Hepatoma | Breast Cancer Cell Lines | Leukemia | Control Cell Lines | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCT-8 | Hep G2 | KAIMR C1 | MDA-MB-231 | MCF-7 | KG-1a | K-562 | HL-60 | Human Epithelial | PBMC | |
| SPCr | 1007.50 | 175.10 | 320.10 | 550.60 | 201.50 | 403.90 | 1200.50 | 1615.50 | 1260.80 | 5005.45 |
| SPCl | 482.10 | 1690.00 | 401.40 | 310.90 | 330.53 | 950.35 | 1001.70 | 306.90 | 3065.10 | 6095.00 |
| M/ Control | 0.201 | 0.150 | 0.955 | 0.890 | 1.850 | 0.185 | 0.458 | 1.056 | 0.122 | 0.189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurkolis, F.; Kurniawan, R.; Kurniatanty, I.; Park, M.N.; Moon, M.; Fatimah, S.; Gunawan, W.B.; Surya, R.; Taslim, N.A.; Song, H.; et al. New Insight on In Vitro Biological Activities of Sulfated Polysaccharides from Ulvophyte Green Algae. Molecules 2023, 28, 4531. https://doi.org/10.3390/molecules28114531
Nurkolis F, Kurniawan R, Kurniatanty I, Park MN, Moon M, Fatimah S, Gunawan WB, Surya R, Taslim NA, Song H, et al. New Insight on In Vitro Biological Activities of Sulfated Polysaccharides from Ulvophyte Green Algae. Molecules. 2023; 28(11):4531. https://doi.org/10.3390/molecules28114531
Chicago/Turabian StyleNurkolis, Fahrul, Rudy Kurniawan, Isma Kurniatanty, Moon Nyeo Park, Myunghan Moon, Siti Fatimah, William Ben Gunawan, Reggie Surya, Nurpudji Astuti Taslim, Hangyul Song, and et al. 2023. "New Insight on In Vitro Biological Activities of Sulfated Polysaccharides from Ulvophyte Green Algae" Molecules 28, no. 11: 4531. https://doi.org/10.3390/molecules28114531
APA StyleNurkolis, F., Kurniawan, R., Kurniatanty, I., Park, M. N., Moon, M., Fatimah, S., Gunawan, W. B., Surya, R., Taslim, N. A., Song, H., & Kim, B. (2023). New Insight on In Vitro Biological Activities of Sulfated Polysaccharides from Ulvophyte Green Algae. Molecules, 28(11), 4531. https://doi.org/10.3390/molecules28114531












