Building Fucoidan/Agarose-Based Hydrogels as a Platform for the Development of Therapeutic Approaches against Diabetes
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical–Chemical Characterization of Fu, Aga, and AgaFu
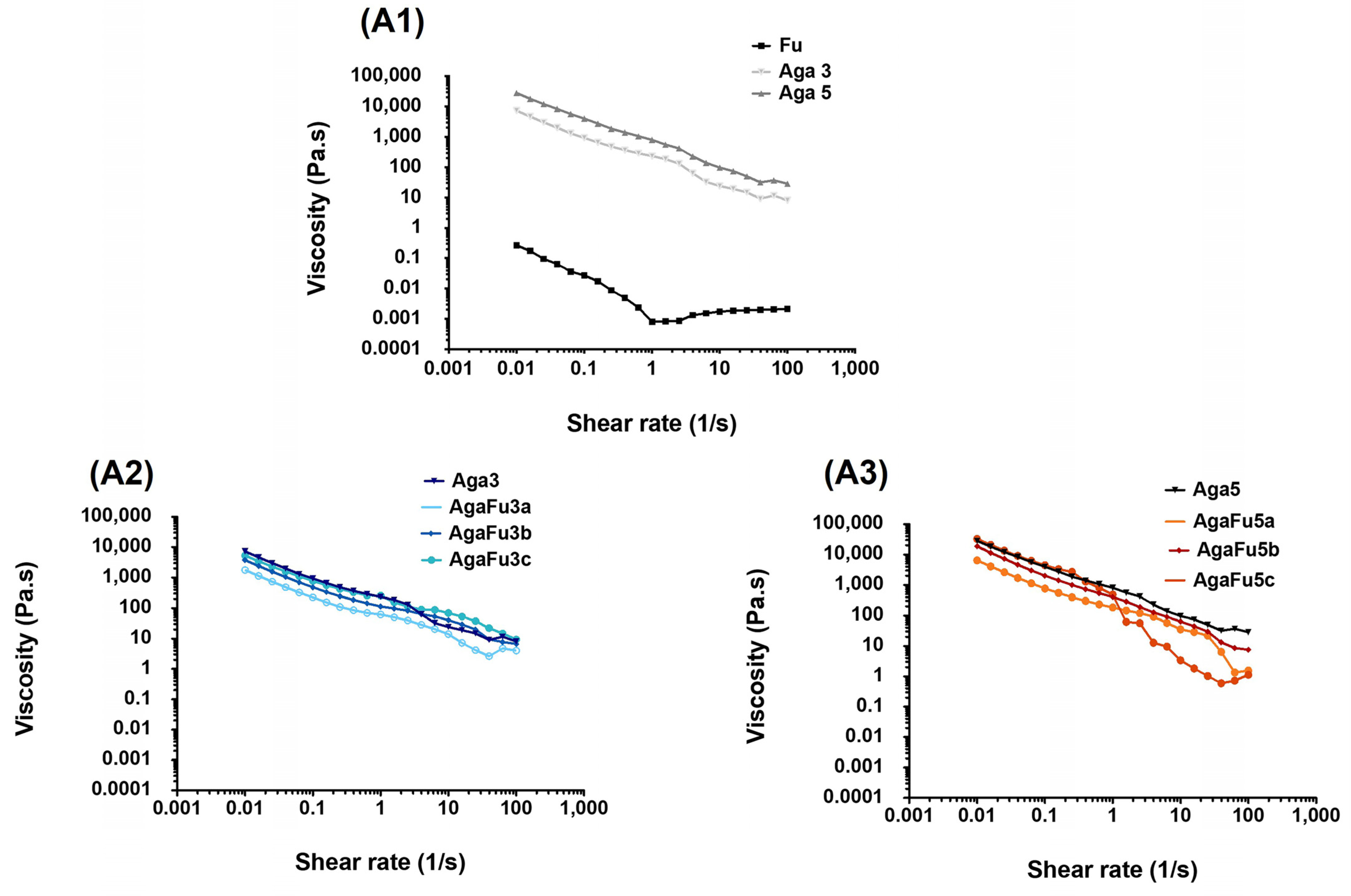
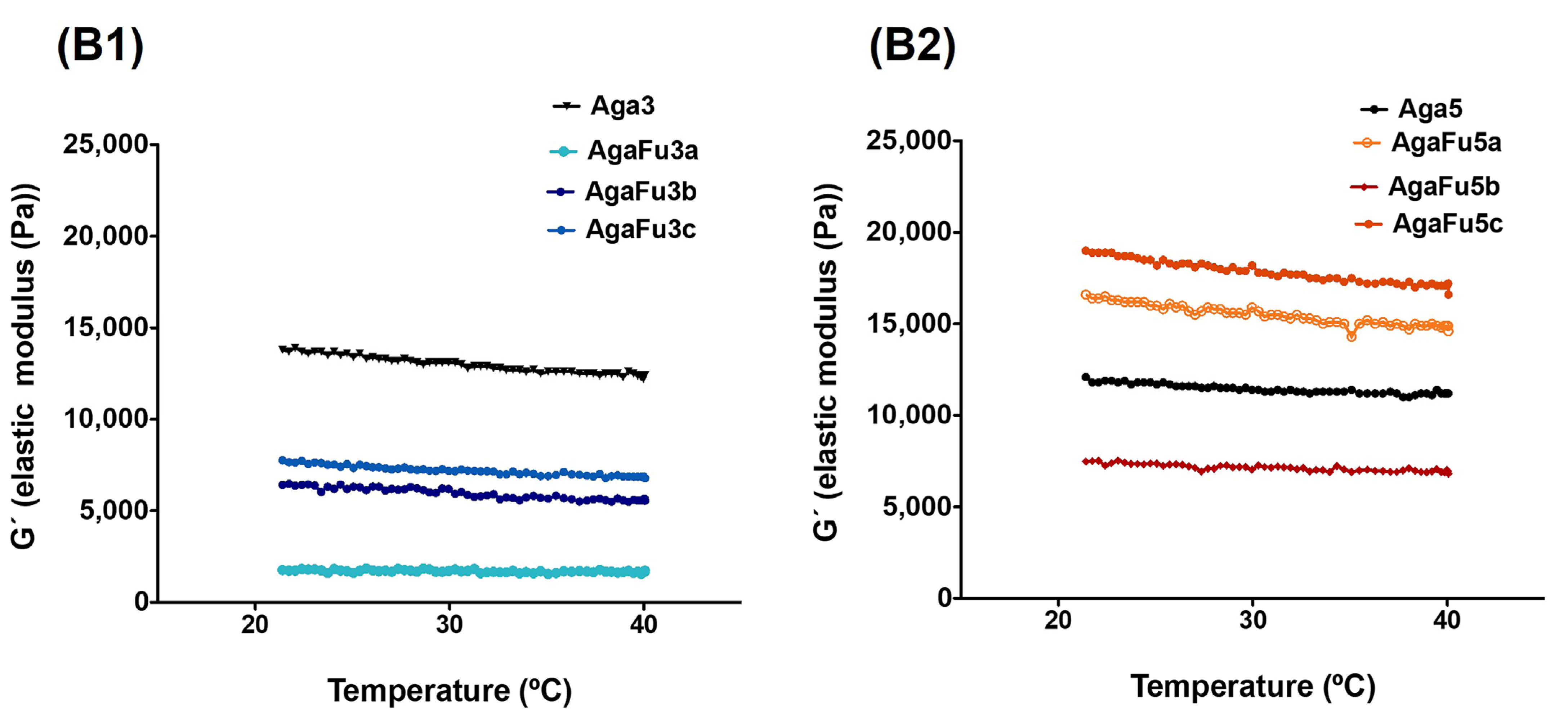
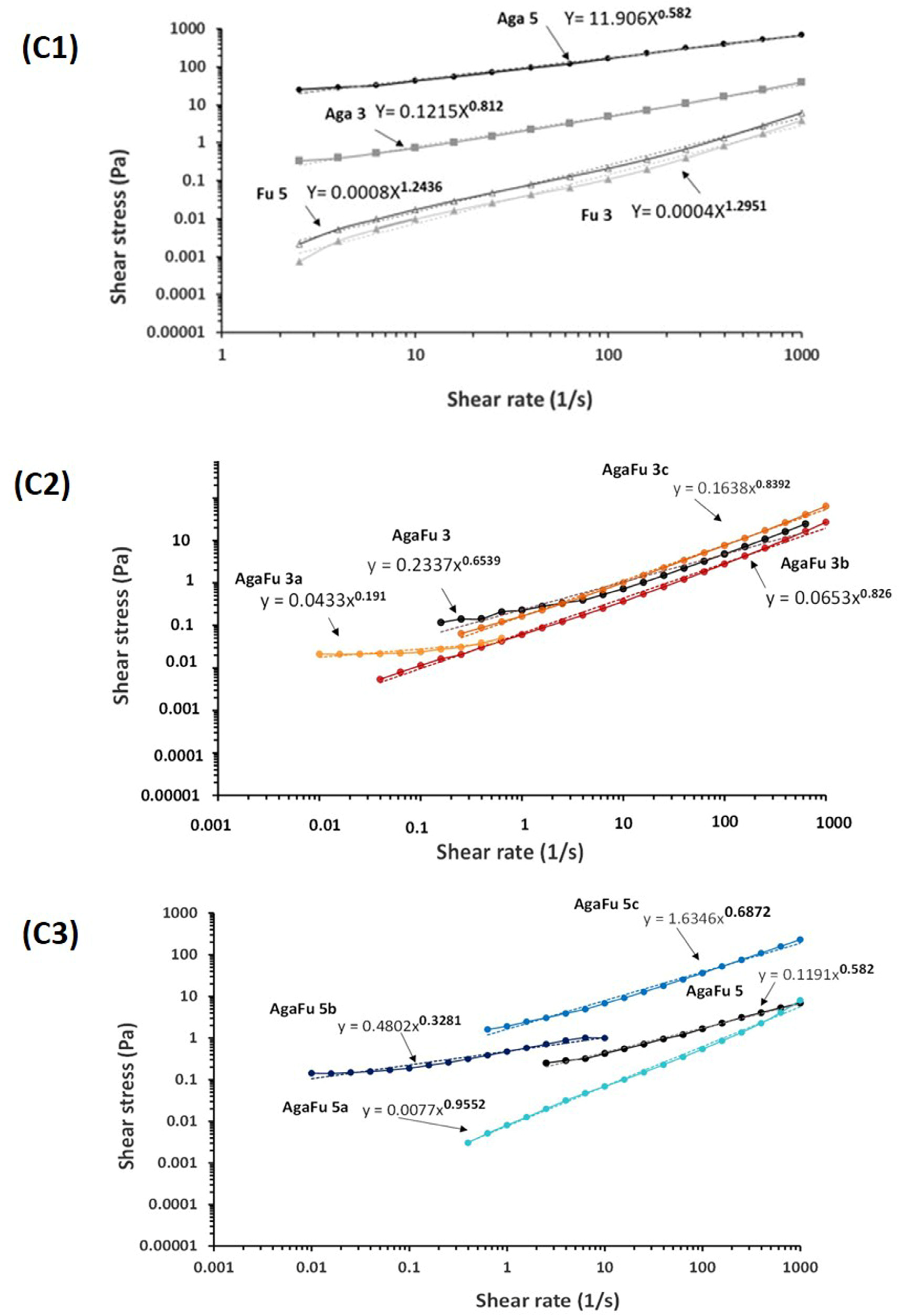
2.1.1. Rheology
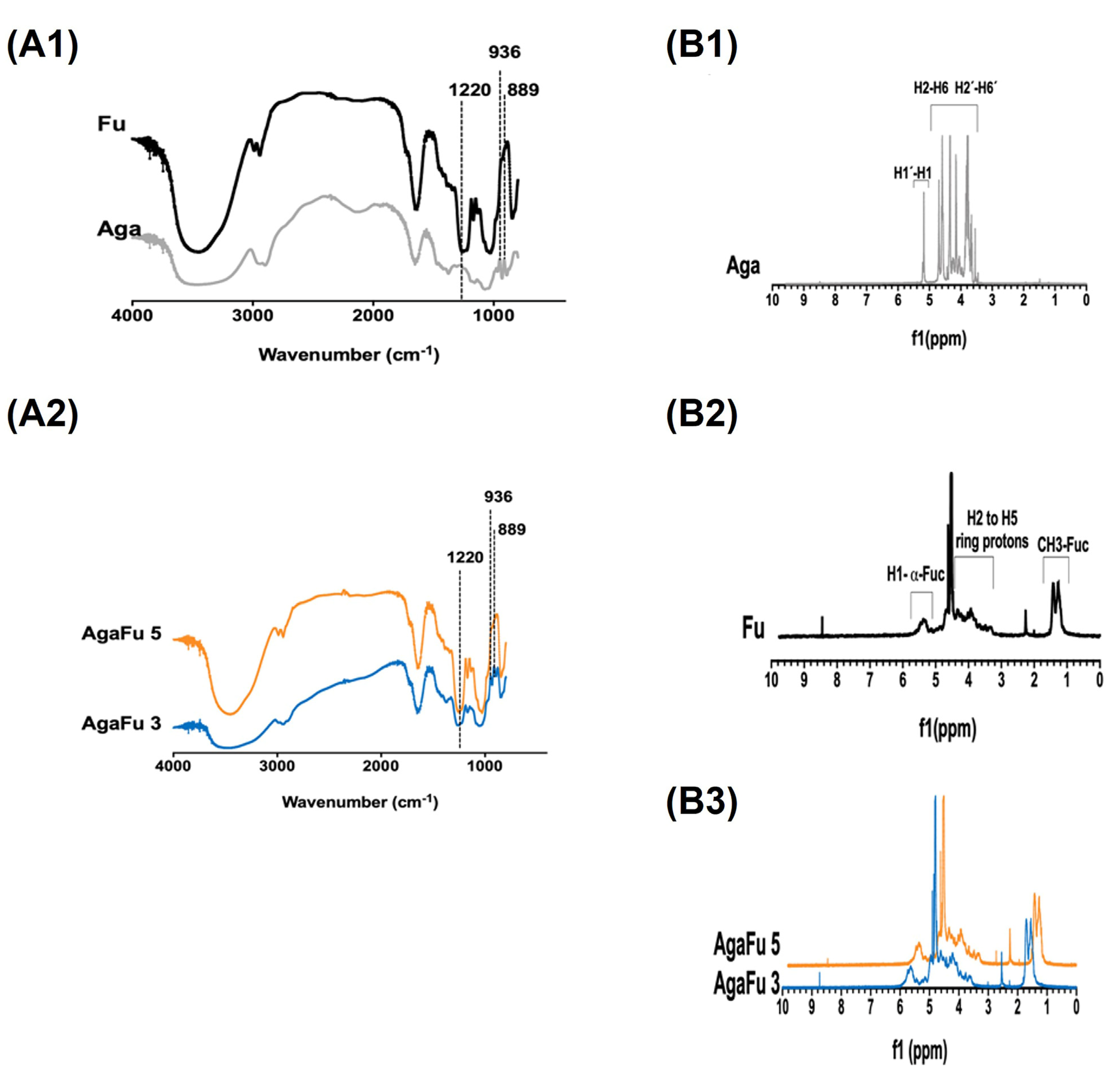
2.1.2. Chemical Characterization
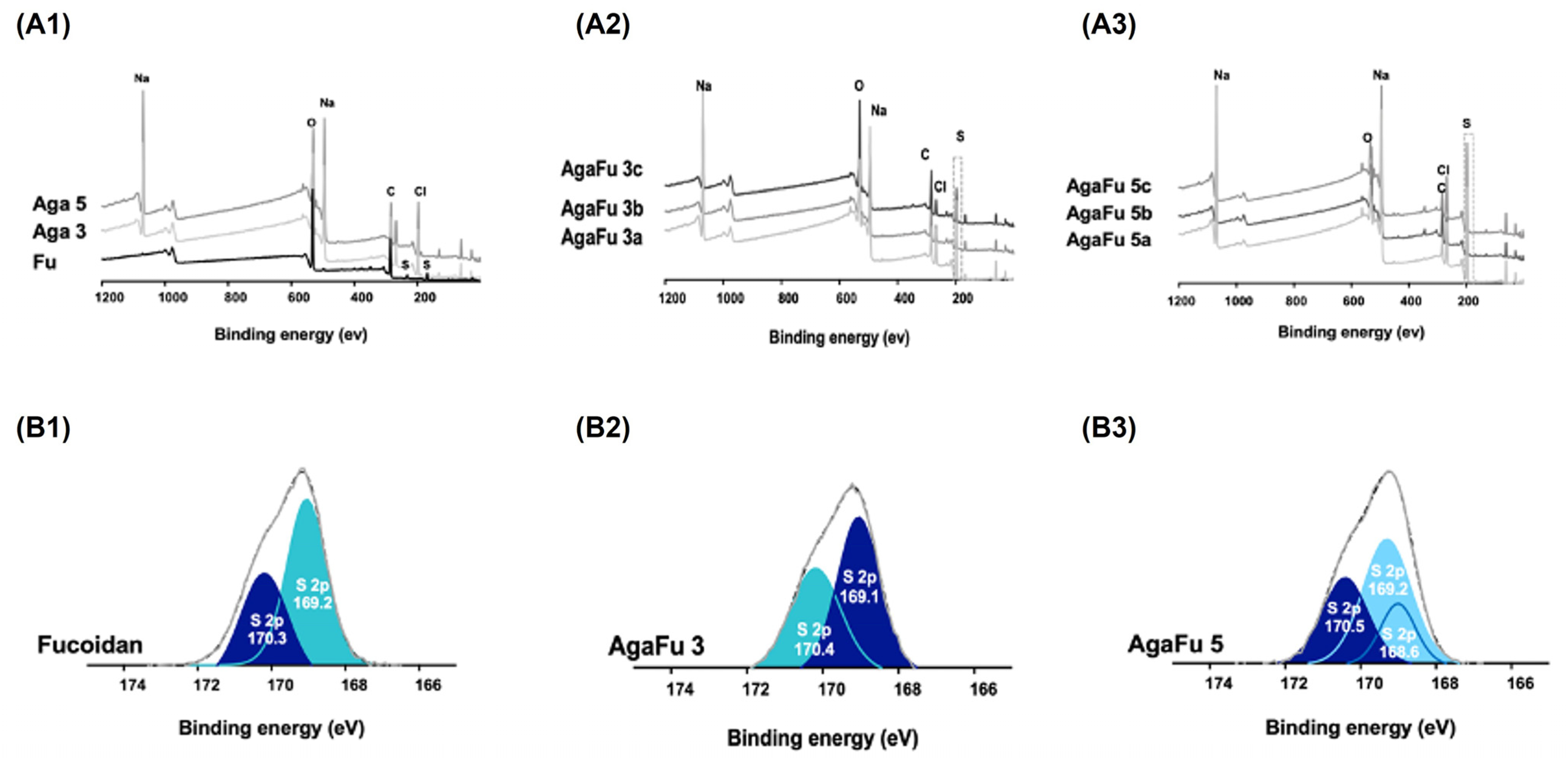
2.1.3. X-ray Photoelectron Spectroscopy (XPS)
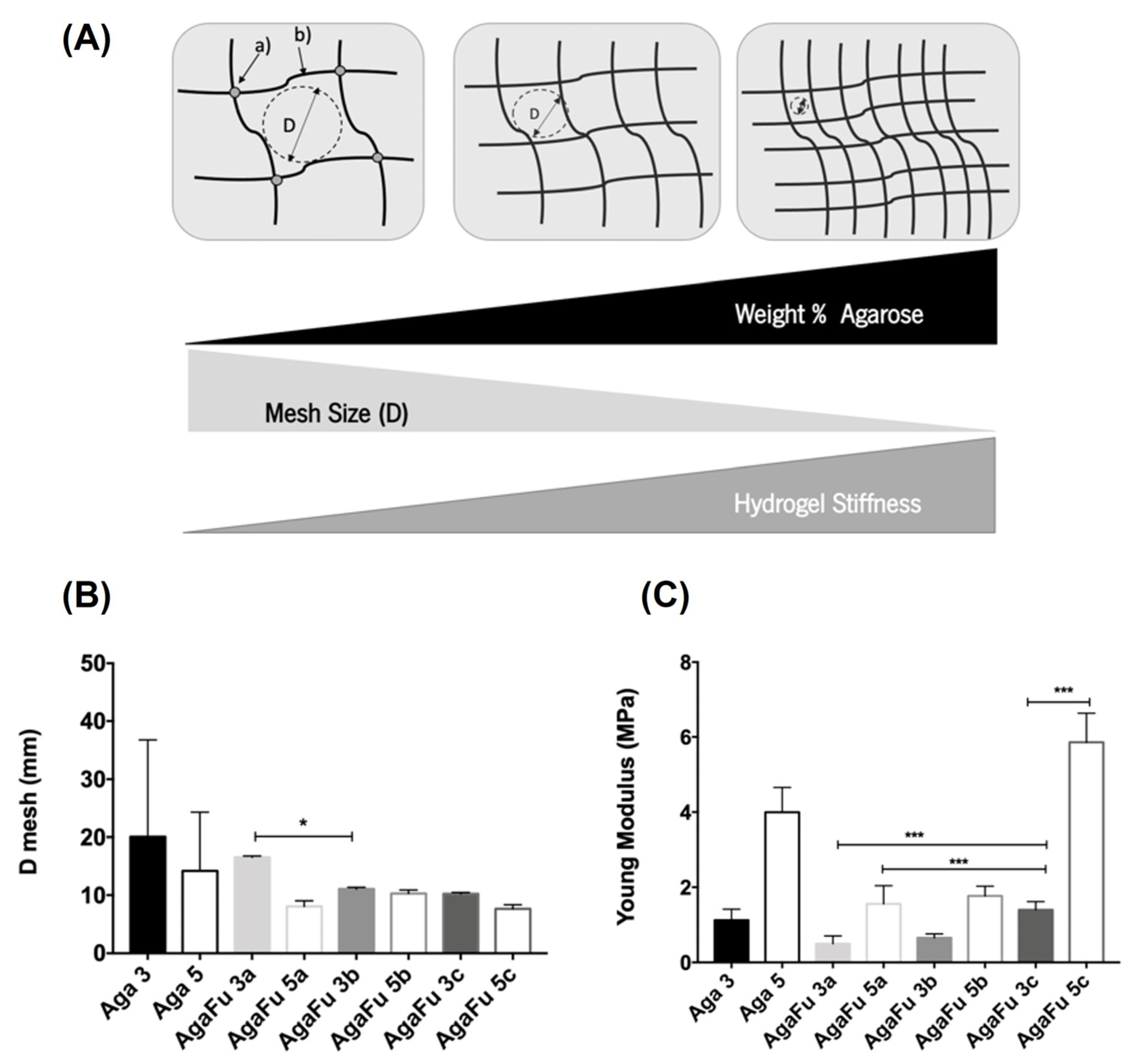
2.1.4. Mechanical Properties and Hydrogel Mesh Size
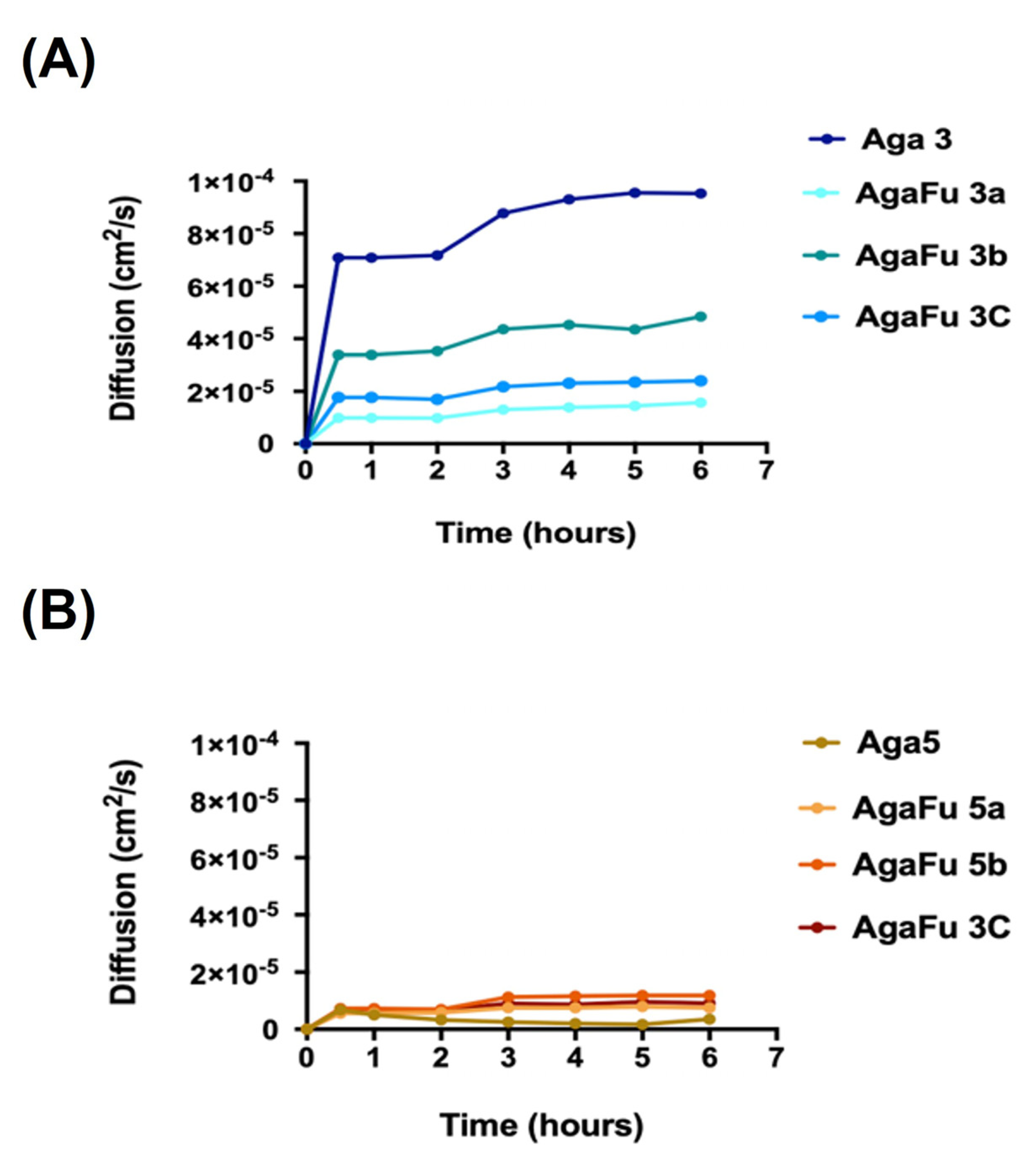
2.1.5. Diffusion Tests and Permeability to Glucose
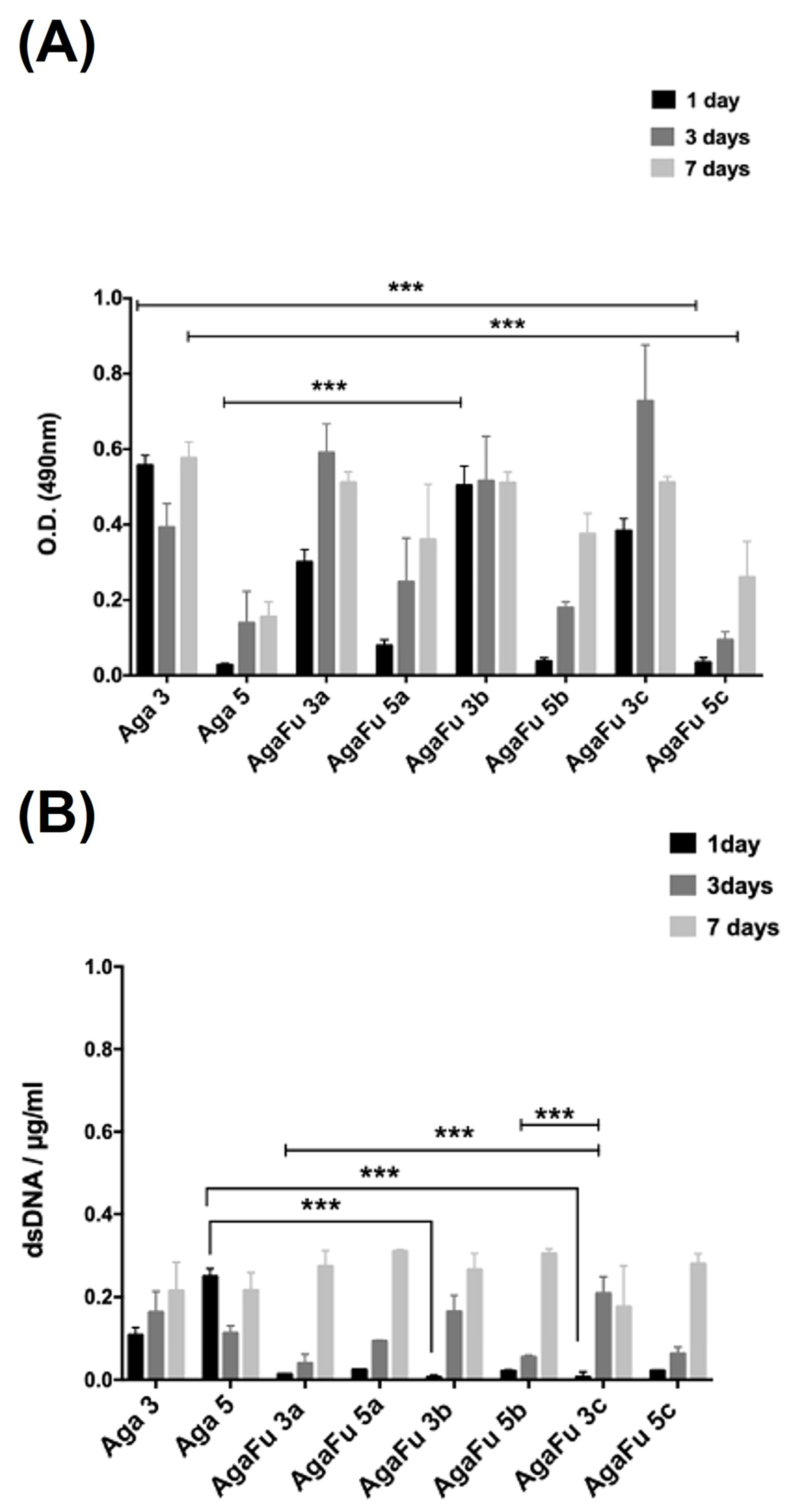
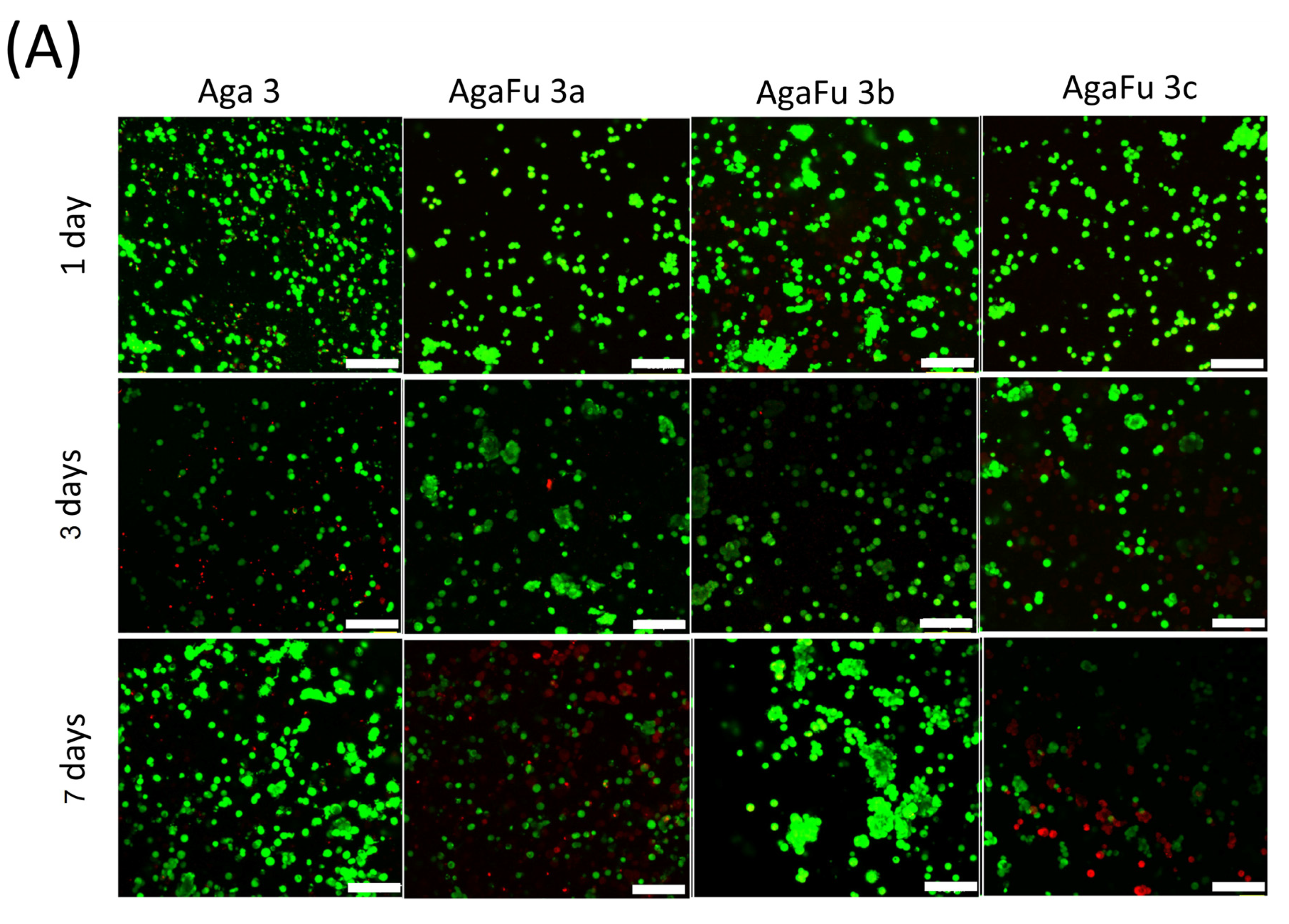
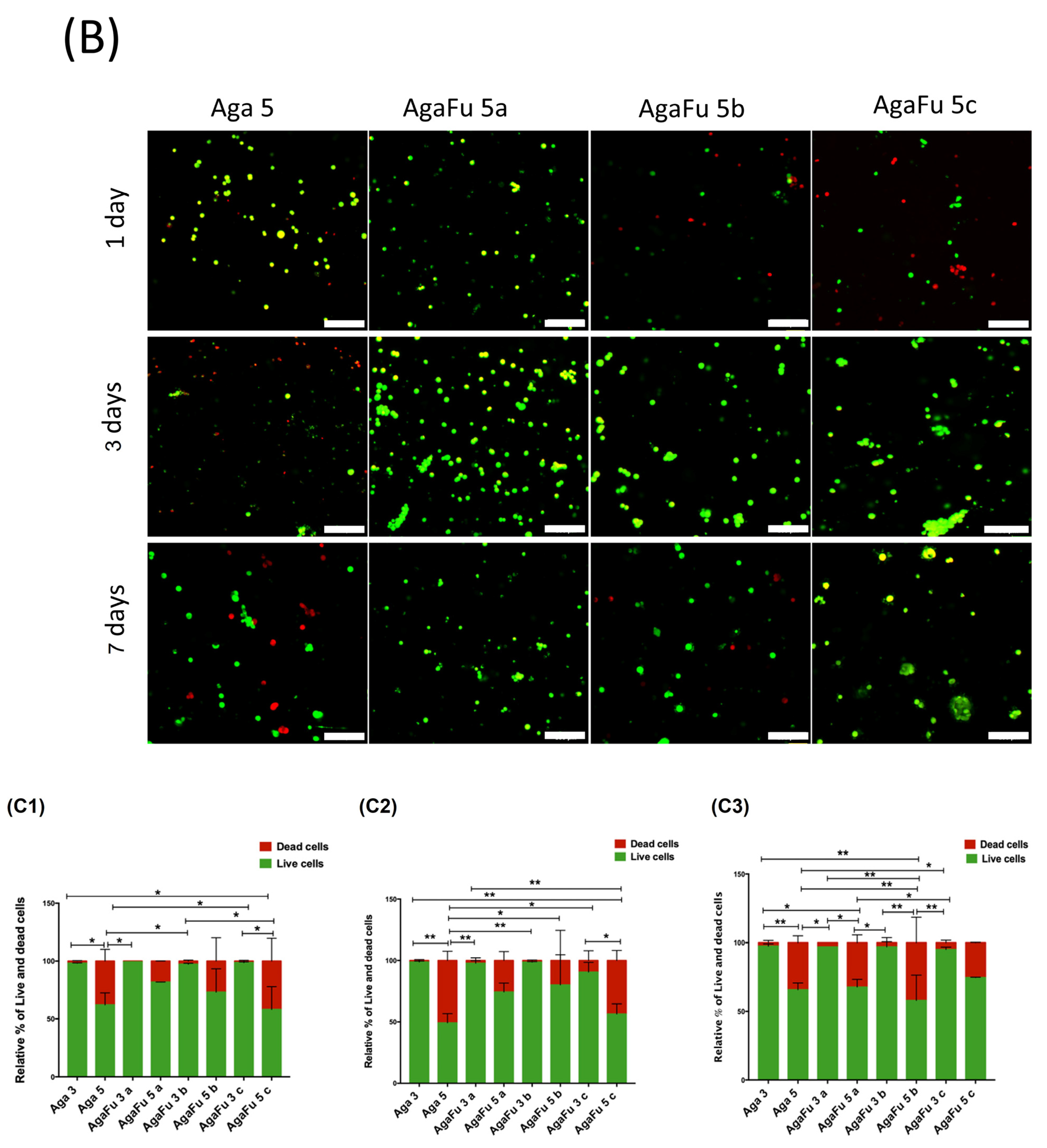
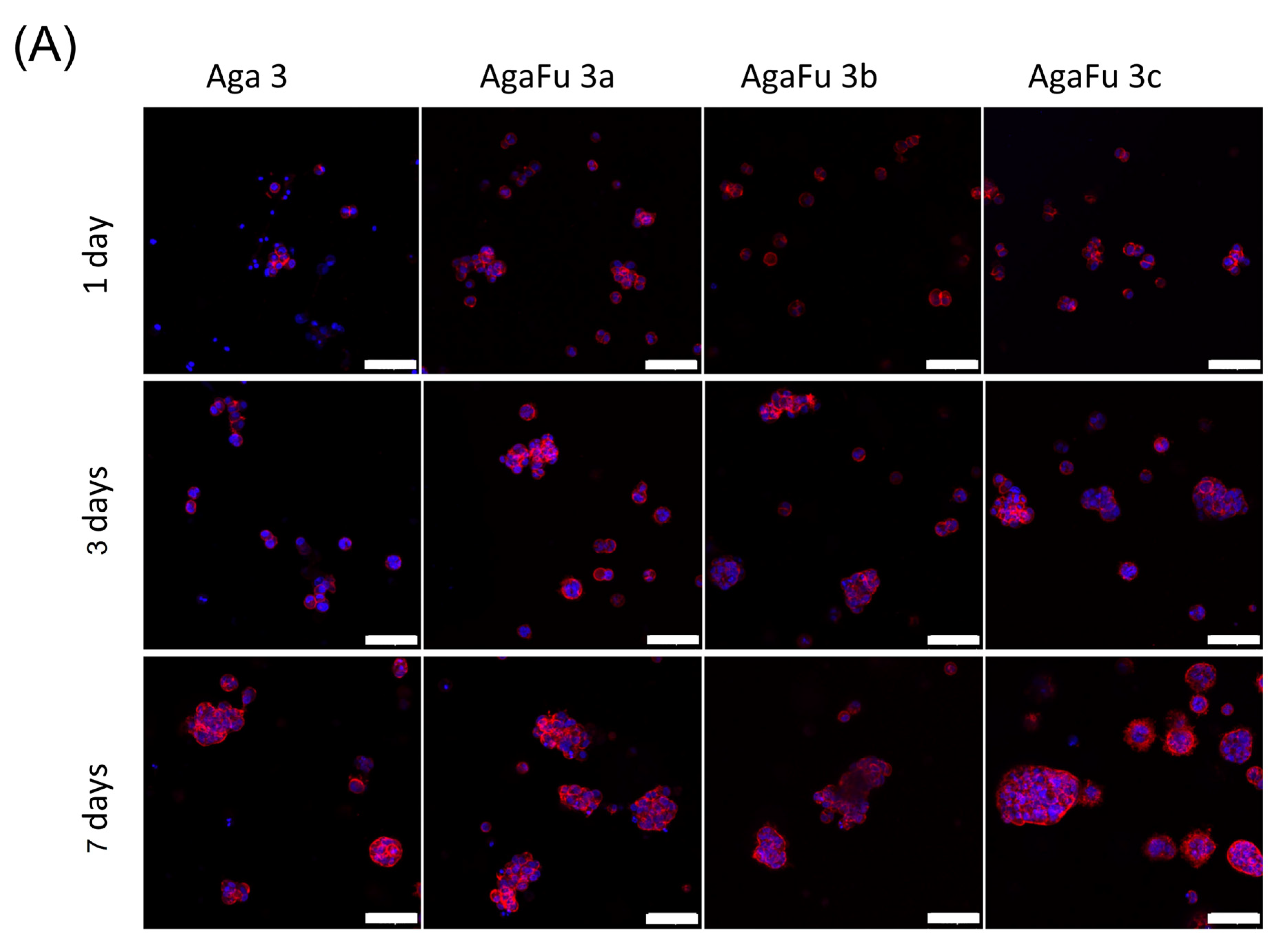
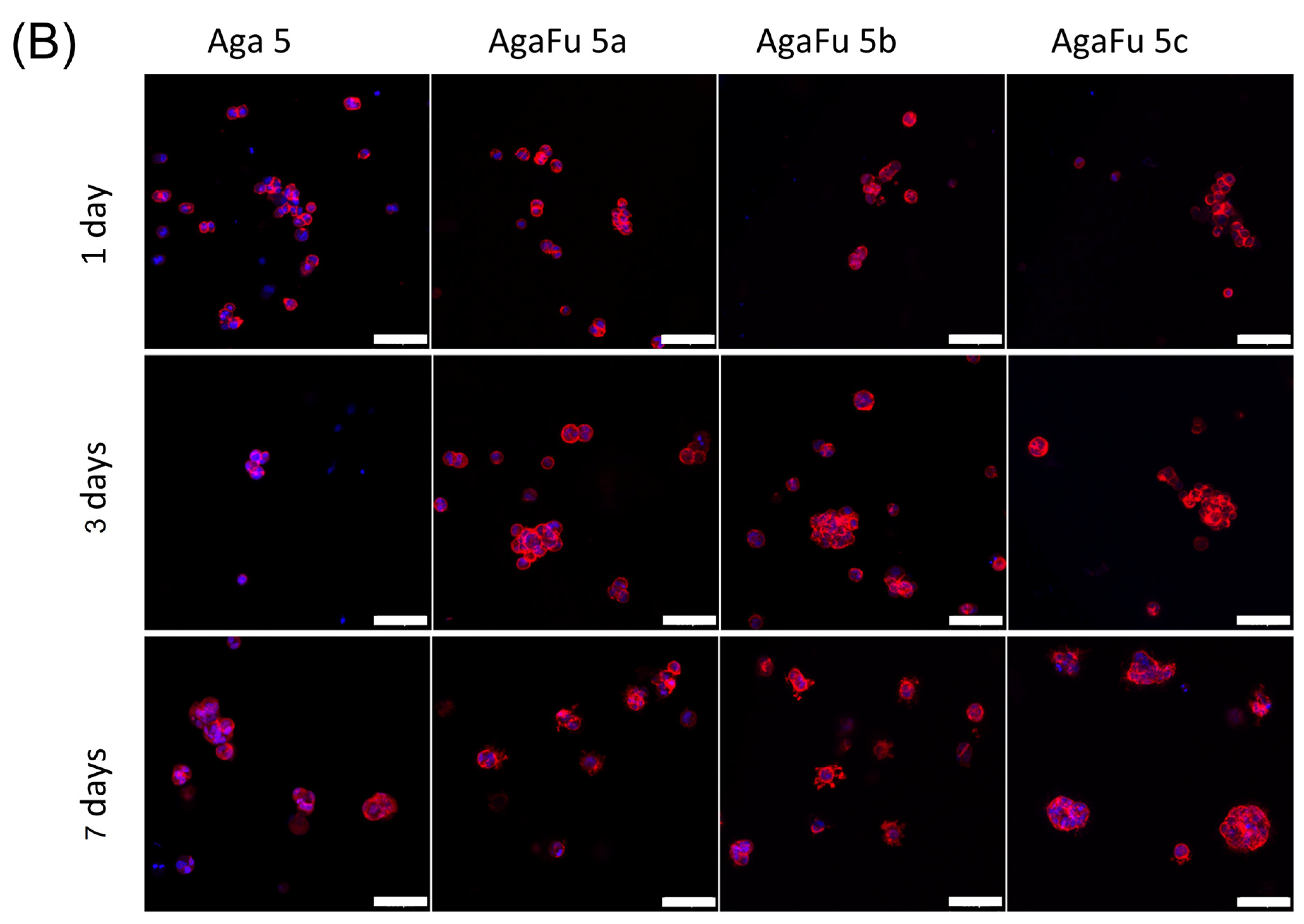
2.2. Biological Characterization
3. Materials and Methods
3.1. Materials
3.2. Purification of Fucoidan (PFu)
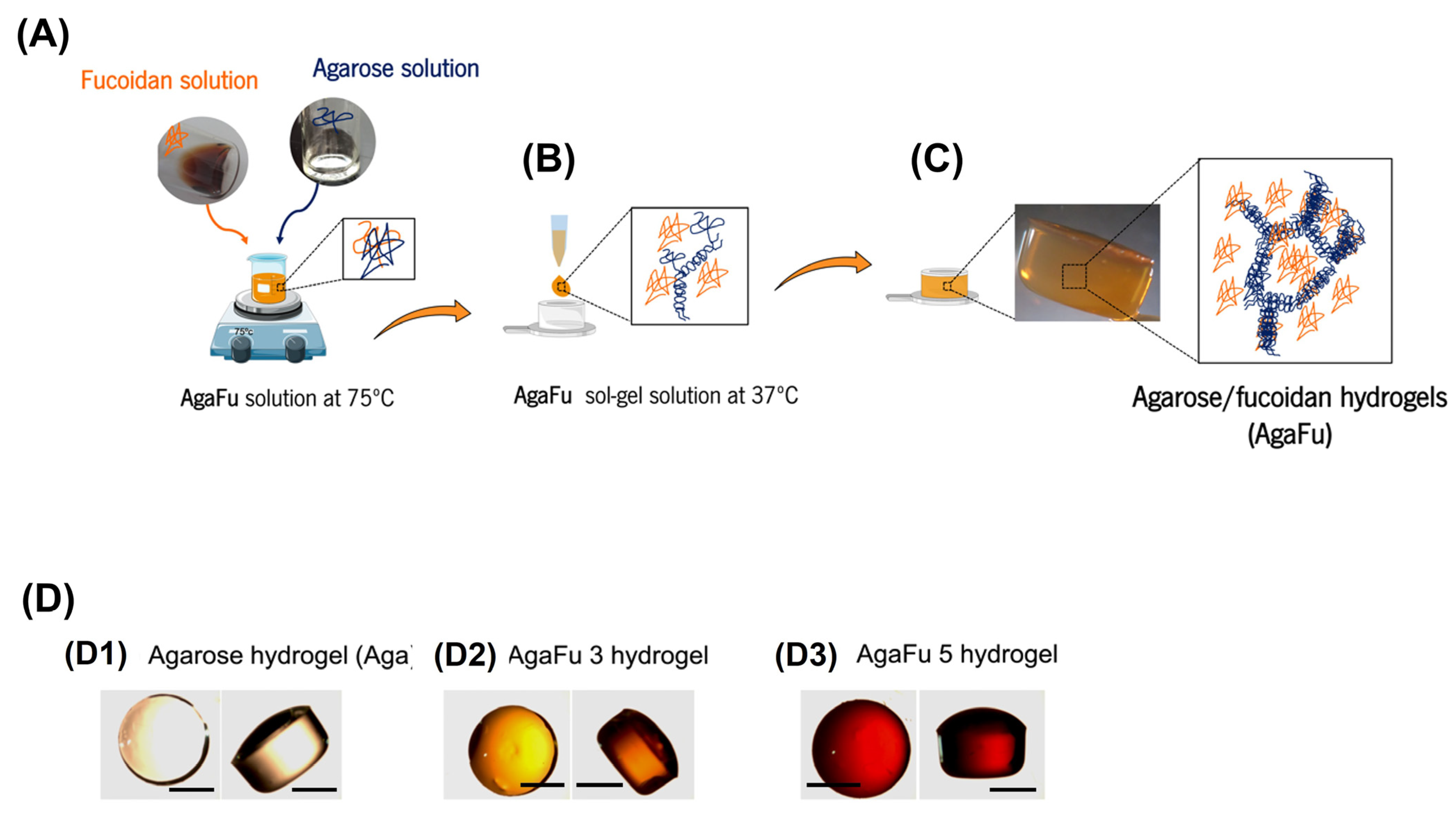
3.3. Preparation of Fucoidan-Based Hydrogels
3.4. Physical–Chemical Characterization of AgaFu Hydrogels
3.4.1. Rheology
3.4.2. Hydrogel Mesh Size
3.4.3. 1H NMR Spectroscopy
3.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.5. X-ray Photoelectron Spectroscopy (XPS)
3.4.6. Stability Test
3.4.7. Diffusion within Hydrogels
3.4.8. Permeability to Glucose
3.5. Biological Assessment of Developed Fucoidan-Based Hydrogel
3.5.1. Cell Culture
3.5.2. Cell Encapsulation
3.5.3. Metabolic Activity, Proliferation, and Morphology
3.5.4. Cell Morphology
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Fan, C.; Wang, D.-A. Macroporous Hydrogel Scaffolds for Three-Dimensional Cell Culture and Tissue Engineering. Tissue Eng. Part B Rev. 2017, 23, 451–461. [Google Scholar] [CrossRef]
- Jabbari, E. Bioconjugation of hydrogels for tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 655–660. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Wilson, A.N.; Guiseppi-Elie, A. Bioresponsive Hydrogels. Adv. Healthc. Mater. 2013, 2, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Reyes Martínez, J.E.; Ruiz-Pacheco, J.; Flores-Valdéz, M.; Elsawy, M.; Vallejo, A.A.; Castillo Díaz, L. Advanced hydrogels for treatment of diabetes. J. Tissue Eng. Regen. Med. 2019, 13, 1375–1393. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Sutapa Biswas, M., Ed.; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar]
- Salati, M.; Khazai, J.; Tahmuri, A.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.; Habibzadeh, S.; Seidi, F.; et al. Agarose-Based Biomaterials: Opportunities and Challenges in Cartilage Tissue Engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- Assanah, F.; Grassie, K.; Anderson, H.; Xin, X.; Rowe, D.; Khan, Y. Ultrasound-derived mechanical stimulation of cell-laden collagen hydrogels for bone repair. J. Biomed. Mater. Res. Part A 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, M.; Peng, Y.; He, S.; Zhu, X.; Hu, C.; Xia, G.; Zuo, T.; Zhang, X.; Yun, Y.; et al. A physicochemical double-cross-linked gelatin hydrogel with enhanced antibacterial and anti-inflammatory capabilities for improving wound healing. J. Nanobiotechnology 2022, 20, 426. [Google Scholar] [CrossRef] [PubMed]
- Craciun, A.M.; Morariu, S.; Marin, L. Self-Healing Chitosan Hydrogels: Preparation and Rheological Characterization. Polymers 2022, 14, 2570. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhao, S.; Zhang, X.; Wei, G.; Su, Z. Recent Advances in Peptide Engineering of PEG Hydrogels: Strategies, Functional Regulation, and Biomedical Applications. Macromol. Mater. Eng. 2022, 307, 2200385. [Google Scholar] [CrossRef]
- Olăreț, E.; Voicu, Ș.I.; Oprea, R.; Miculescu, F.; Butac, L.; Stancu, I.-C.; Serafim, A. Nanostructured Polyacrylamide Hydrogels with Improved Mechanical Properties and Antimicrobial Behavior. Polymers 2022, 14, 2320. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, D.; Hu, H.; Sun, S.; Li, Y.; Liu, X.; Li, G. Polydimethylsiloxane (PDMS)-containing hydrogels prepared by micellar copolymerization in aqueous media. Mater. Lett. 2020, 263, 127251. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Gradys, A.; Kolbuk, D.; Krzton-Maziopa, A.; Rogujski, P.; Stanaszek, L.; Lukomska, B.; Sajkiewicz, P. A methylcellulose/agarose hydrogel as an innovative scaffold for tissue engineering. RSC Adv. 2022, 12, 26882–26894. [Google Scholar] [CrossRef]
- Lee, J.E.; Heo, S.W.; Kim, C.H.; Park, S.J.; Park, S.H.; Kim, T.H. In-situ ionic crosslinking of 3D bioprinted cell-hydrogel constructs for mechanical reinforcement and improved cell growth. Biomater. Adv. 2023, 147, 213322. [Google Scholar] [CrossRef]
- Zhang, J.; Hurren, C.; Lu, Z.; Wang, D. pH-sensitive alginate hydrogel for synergistic anti-infection. Int. J. Biol. Macromol. 2022, 222, 1723–1733. [Google Scholar] [CrossRef]
- Sinawang, G.; Osaki, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Biofunctional hydrogels based on host–guest interactions. Polym. J. 2020, 52, 839–859. [Google Scholar] [CrossRef]
- Pamplona, R.; González-Lana, S.; Romero, P.; Ochoa, I.; Martín-Rapún, R.; Sánchez-Somolinos, C. Tuning of Mechanical Properties in Photopolymerizable Gelatin-Based Hydrogels for In Vitro Cell Culture Systems. ACS Appl. Polym. Mater. 2023, 5, 1487–1498. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Y. Application of “Click” Chemistry in Biomedical Hydrogels. ACS Omega 2022, 7, 36918–36928. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.G.; Nita, L.E.; Simionescu, N.; Ghilan, A.; Chiriac, A.P.; Mititelu-Tartau, L. Enzymatically-Crosslinked Gelatin Hydrogels with Nanostructured Architecture and Self-Healing Performance for Potential Use as Wound Dressings. Polymers 2023, 15, 780. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.; Vangala, A.; Buonocore, F.; Yarandi, N.; Calabrese, G. Chitosan Hydrogels Cross-Linked with Trimesic Acid for the Delivery of 5-Fluorouracil in Cancer Therapy. Pharmaceutics 2023, 15, 1084. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Rumon, M.M.H.; Akib, A.A.; Sultana, F.; Moniruzzaman, M.; Niloy, M.S.; Shakil, M.S.; Roy, C.K. Self-Healing Hydrogels: Development, Biomedical Applications, and Challenges. Polymers 2022, 14, 4539. [Google Scholar] [CrossRef] [PubMed]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef]
- Reys, L.L.; Silva, S.S.; Soares da Costa, D.; Oliveira, N.M.; Mano, J.F.; Reis, R.L.; Silva, T.H. Fucoidan Hydrogels Photo-Cross-Linked with Visible Radiation As Matrices for Cell Culture. ACS Biomater. Sci. Eng. 2016, 2, 1151–1161. [Google Scholar] [CrossRef]
- Oliveira, C.; Ferreira, A.S.; Novoa-Carballal, R.; Nunes, C.; Pashkuleva, I.; Neves, N.M.; Coimbra, M.A.; Reis, R.L.; Martins, A.; Silva, T.H. The Key Role of Sulfation and Branching on Fucoidan Antitumor Activity. Macromol. Biosci. 2017, 17, 1600340. [Google Scholar] [CrossRef]
- Isnansetyo, A.; Lutfia, F.; Nursid, M.; Trijoko; Susidarti, R. Cytotoxicity of Fucoidan from Three Tropical Brown Algae Against Breast and Colon Cancer Cell Lines. Pharmacogn. J. 2017, 9, 14–20. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In Vitro Anti-Inflammatory Activities of Fucoidans from Five Species of Brown Seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef]
- Anisha, G.S.; Padmakumari, S.; Patel, A.K.; Pandey, A.; Singhania, R.R. Fucoidan from Marine Macroalgae: Biological Actions and Applications in Regenerative Medicine, Drug Delivery Systems and Food Industry. Bioengineering 2022, 9, 472. [Google Scholar] [CrossRef]
- Fang, C.Y.; Chen, S.H.; Huang, C.C.; Liao, Y.-W.; Chao, S.-C.; Yu, C.-C. Fucoidan-Mediated Inhibition of Fibrotic Properties in Oral Submucous Fibrosis via the MEG3/miR-181a/Egr1 Axis. Pharmaceuticals 2022, 15, 833. [Google Scholar] [CrossRef] [PubMed]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial Properties of Fucoidans from the Brown Algae Fucus vesiculosus L. of the Barents Sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef]
- Cabral, E.M.; Mondala, J.R.M.; Oliveira, M.; Przyborska, J.; Fitzpatrick, S.; Rai, D.K.; Sivagnanam, S.P.; Garcia-Vaquero, M.; O’Shea, D.; Devereux, M.; et al. Influence of molecular weight fractionation on the antimicrobial and anticancer properties of a fucoidan rich-extract from the macroalgae Fucus vesiculosus. Int. J. Biol. Macromol. 2021, 186, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Xu, Z.; Xue, Y.; Zhang, D.; Zhang, Y.; Li, W.; Li, X. Fucoidan protects the pancreas and improves glucose metabolism through inhibiting inflammation and endoplasmic reticulum stress in T2DM rats. Food Funct. 2022, 13, 2693–2709. [Google Scholar] [CrossRef] [PubMed]
- Fitton, H.J.; Stringer, D.S.; Park, A.Y.; Karpiniec, S.N. Therapies from Fucoidan: New Developments. Mar. Drugs 2019, 17, 571. [Google Scholar] [CrossRef]
- Fu, X.T.; Kim, S.M. Agarase: Review of major sources, categories, purification method, enzyme characteristics and applications. Mar. Drugs 2010, 8, 200–218. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Roberts, J.J.; Martens, P.J. 9—Engineering biosynthetic cell encapsulation systems. In Biosynthetic Polymers for Medical Applications; Poole-Warren, L., Martens, P., Green, R., Eds.; Woodhead Publishing: Swaston, Cambridge, UK, 2016; pp. 205–239. [Google Scholar]
- Normand, V.; Lootens, D.L.; Amici, E.; Plucknett, K.P.; Aymard, P. New Insight into Agarose Gel Mechanical Properties. Biomacromolecules 2000, 1, 730–738. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- García, F.; Solís, J.; Calderón, J.; Luque, E.; Neyra, L.; Manrique, H.; Cancino, R.; Castillo, O.; Cornejo, S.; Rodríguez, E.; et al. Prevalencia de diabetes mellitus y factores de riesgo relacionados en una población urbana. Rev. Soc. Peru. Med. Interna 2007, 20, 90–94. [Google Scholar]
- Marchioli, G.; van Gurp, L.; van Krieken, P.P.; Stamatialis, D.; Engelse, M.; van Blitterswijk, C.A.; Karperien, M.B.J.; de Koning, E.; Alblas, J.; Moroni, L.; et al. Fabrication of three-dimensional bioplotted hydrogel scaffolds for islets of Langerhans transplantation. Biofabrication 2015, 7, 25009. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Alexander, J.F.; Thekkedath, U.; Ferrari, M.; Grattoni, A. Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev. 2019, 139, 92–115. [Google Scholar] [CrossRef]
- Paez-Mayorga, J.; Capuani, S.; Hernandez, N.; Farina, M.; Chua, C.Y.X.; Blanchard, R.; Sizovs, A.; Liu, H.-C.; Fraga, D.W.; Niles, J.A.; et al. Neovascularized implantable cell homing encapsulation platform with tunable local immunosuppressant delivery for allogeneic cell transplantation. Biomaterials 2020, 257, 120232. [Google Scholar] [CrossRef] [PubMed]
- Pierre, S.; Frederic, C.; Francois, R.; Faisal, A.-S.; Rana Ben, A.; Nadia, B.; Luc, P.; Liliane, L.; Murielle, M.; Lydia, R.; et al. Purification of a low molecular weight fucoidan for SPECT molecular imaging of myocardial infarction. Mar. Drugs 2014, 12, 4851–4867. [Google Scholar] [CrossRef]
- Lee, J.; Jin, G.; Yeo, M.; Jang, C.; Lee, H.; Kim, G. Fabrication of electrospun biocomposites comprising polycaprolactone/fucoidan for tissue regeneration. Carbohydr. Polym. 2012, 90, 181–188. [Google Scholar] [CrossRef]
- Fernández, E.; López, D.; Mijangos, C.; Duskova-Smrckova, M.; Ilavsky, M.; Dusek, K. Rheological and thermal properties of agarose aqueous solutions and hydrogels. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 322–328. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Wellard, M.; Woodruff, M.; Klein, T. Tailoring Hydrogel Viscoelasticity with Physical and Chemical Crosslinking. Polymers 2015, 7, 2650–2669. [Google Scholar] [CrossRef]
- Silva, S.S.; Rodrigues, L.C.; Reis, R.L. An alternative approach to prepare alginate/acemannan 3D architectures. SN Appl. Sci. 2019, 1, 684. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. Physicochemical Properties and the Gelation Process of Supramolecular Hydrogels: A Review. Gels 2017, 3, 1. [Google Scholar] [CrossRef]
- Schurz, J. Rheology of polymer solutions of the network type. Prog. Polym. Sci. 1991, 16, 1–53. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Silva, S.S.; Reis, R.L. Acemannan-based films: An improved approach envisioning biomedical applications. Mater. Res. Express 2019, 6, 095406. [Google Scholar] [CrossRef]
- Agnello, S.; Gasperini, L.; Mano, J.F.; Pitarresi, G.; Palumbo, F.S.; Reis, R.L.; Giammona, G. Synthesis, mechanical and thermal rheological properties of new gellan gum derivatives. Int. J. Biol. Macromol. 2017, 98, 646–653. [Google Scholar] [CrossRef]
- Trivedi, T.; Kumar, A. Efficient Extraction of Agarose from Red Algae Using Ionic Liquids. Green Sustain. Chem. 2014, 4, 190–201. [Google Scholar] [CrossRef]
- Kandemir, N.; Xia, Y.; Duan, P.; Yang, W.; Chen, J. Rheological Characterization of Agarose and Poloxamer 407 (P407) Based Hydrogels. MRS Adv. 2018, 3, 1719–1724. [Google Scholar] [CrossRef]
- Guvendiren, M.; Lu, H.D.; Burdick, J.A. Shear-thinning hydrogels for biomedical applications. Soft Matter 2012, 8, 260–272. [Google Scholar] [CrossRef]
- Duarte, M.E.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr Res. 2001, 333, 281–293. [Google Scholar] [CrossRef]
- Indovina, P.L.; Tettamanti, E.; Micciancio-Giammarinaro, M.S.; Palma, M.U. Thermal hysteresis and reversibility of gel–sol transition in agarose–water systems. J. Chem. Phys. 1979, 70, 2841–2847. [Google Scholar] [CrossRef]
- Sinurat, E.; Peranginangin, R.; Saepudin, E. Purification and Characterization of Fucoidan from the Brown Seaweed Sargassum binderi Sonder. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2016, 10, 547–558. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yang, Y.T. Effect of basic fibroblast growth factor released from chitosan-fucoidan nanoparticles on neurite extension. J. Tissue Eng. Regen. Med. 2016, 10, 418–427. [Google Scholar] [CrossRef]
- Alwarsamy, M.; Gooneratne, R.; Ravichandran, R. Effect of fucoidan from Turbinaria conoides on human lung adenocarcinoma epithelial (A549) cells. Carbohydr Polym. 2016, 152, 207–213. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P.; et al. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Pernodet, N.; Maaloum, M.; Tinland, B. Pore size of agarose gels by atomic force microscopy. Electrophoresis 1997, 18, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Lahaye, M.; Rochas, C. Chemical structure and physico-chemical properties of agar. Hydrobiologia 1991, 221, 137–148. [Google Scholar] [CrossRef]
- You, K.; Kim, D.; Kwon, B.-J.; Vagaska, B.; Kang, J.; Seo, H.; Lee, M.; Park, C. Methods for Evaluation of the Chemotactic Migration of the Cells in 3D Biomimetic Matrix. Biomater. Res. 2012, 16, 129–134. [Google Scholar]
- Silva, J.M.; Duarte, A.R.C.; Caridade, S.G.; Picart, C.; Reis, R.L.; Mano, J.F. Tailored Freestanding Multilayered Membranes Based on Chitosan and Alginate. Biomacromolecules 2014, 15, 3817–3826. [Google Scholar] [CrossRef]
- Matsuyama, H.; Tamura, T.; Kitamura, Y. Permeability of ionic solutes in a polyamphoteric membrane. Sep. Purif. Technol. 1999, 16, 181–187. [Google Scholar] [CrossRef]
- Singh, N.K.; Lee, D.S. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Control. Release 2014, 193, 214–227. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [CrossRef]
- Strand, B.L.; Coron, A.E.; Skjak-Braek, G. Current and Future Perspectives on Alginate Encapsulated Pancreatic Islet. Stem Cells Transl. Med. 2017, 6, 1053–1058. [Google Scholar] [CrossRef]
- McCluskey, J.T.; Hamid, M.; Guo-Parke, H.; McClenaghan, N.H.; Gomis, R.; Flatt, P.R. Development and functional characterization of insulin-releasing human pancreatic beta cell lines produced by electrofusion. J. Biol. Chem. 2011, 286, 21982–21992. [Google Scholar] [CrossRef]
- Haggag, Y.A.; Abd Elrahman, A.A.; Ulber, R.; Zayed, A. Fucoidan in Pharmaceutical Formulations: A Comprehensive Review for Smart Drug Delivery Systems. Mar. Drugs 2023, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Guo-Parke, H.; McCluskey, J.T.; Kelly, C.; Hamid, M.; McClenaghan, N.H.; Flatt, P.R. Configuration of electrofusion-derived human insulin-secreting cell line as pseudoislets enhances functionality and therapeutic utility. J. Endocrinol. 2012, 214, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Radhouani, H.; Bicho, D.; Gonçalves, C.; Maia, F.R.; Reis, R.L.; Oliveira, J.M. Kefiran cryogels as potential scaffolds for drug delivery and tissue engineering applications. Mater. Today Commun. 2019, 20, 100554. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.M.; Feijen, J.; De Smedt, S.C.; Meyvis, T.K.L.; Demeester, J.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J. Characterization of the Network Structure of Carbodiimide Cross-Linked Gelatin Gels. Macromolecules 1999, 32, 3325–3333. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Castle, J.E. Practical surface analysis by Auger and X-ray photoelectron spectroscopy. Surf. Interface Anal. 1984, 6, 302. [Google Scholar] [CrossRef]
- Jan, H. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. American Society for Microbiology: Washington DC, USA, 2016. [Google Scholar]
| Formulation | Polymer Solution Concentration (%) | Hydrogel | |
|---|---|---|---|
| Agarose (Aga) | Fucoidan (Fu) | Aga:Fu Ratio | |
| Aga 3 | 3 | - | - |
| Aga 5 | 5 | - | - |
| Fu 3 | - | 3 | - |
| Fu 5 | - | 5 | - |
| AgaFu 3a | 1.2 | 3 | 4:10 |
| AgaFu 3b | 1.5 | 5:10 | |
| AgaFu 3c | 2.1 | 7:10 | |
| AgaFu 5a | 2 | 5 | 4:10 |
| AgaFu 5b | 2.5 | 5:10 | |
| AgaFu 5c | 3.5 | 7:10 | |
| Composition % | O | C | S | O/C | S/C |
|---|---|---|---|---|---|
| Fu | 35.29 ± 0.20 | 61.52 ± 0.21 | 3.19 ± 0.05 | 0.57 | 0.052 |
| Aga 3 | 42.95 ± 0.57 | 56.88 ± 0.06 | 0.16 ± 0.05 | 0.75 | 0.002 |
| Aga 5 | 47.14 ± 1.04 | 52.49 ± 1.05 | 0.38 ± 0.09 | 0.90 | 0.007 |
| AgaFu 3a | 40.66 ± 0.49 | 55.06 ± 0.54 | 4.28 ± 0.12 | 0.74 | 0.081 |
| AgaFu 3b | 42.77 ± 0.49 | 53.43 ± 0.52 | 3.79 ± 0.08 | 0.80 | 0.070 |
| AgaFu 3c | 45.70 ± 0.59 | 51.39 ± 0.62 | 2.91 ± 0.09 | 0.89 | 0.057 |
| AgaFu 5a | 46.06 ± 0.84 | 52.26 ± 0.86 | 1.68 ± 0.13 | 0.88 | 0.032 |
| AgaFu 5b | 31.93 ± 0.65 | 66.96 ± 0.67 | 1.09 ± 0.08 | 0.48 | 0.016 |
| AgaFu 5c | 54.22 ± 0.84 | 45.13 ± 0.85 | 0.65 ± 0.10 | 1.20 | 0.014 |
| Samples | P (10−6 cm s−1) | D (10−5 cm2 s−1) | Kd |
|---|---|---|---|
| Aga 3 | 8.03 | 8.35 | 0.02 |
| AgaFu 3a | 24.36 | 1.24 | 0.45 |
| AgaFu 3b | 9.91 | 4.05 | 0.05 |
| AgaFu 3c | 9.94 | 2.07 | 0.11 |
| Aga 5 | 2.38 | 0.35 | 0.38 |
| AgaFu 5a | 9.90 | 0.68 | 0.32 |
| AgaFu 5b | 3.99 | 1.14 | 0.10 |
| AgaFu 5c | 10.61 | 0.82 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reys, L.L.; Silva, S.S.; Soares da Costa, D.; Rodrigues, L.C.; Reis, R.L.; Silva, T.H. Building Fucoidan/Agarose-Based Hydrogels as a Platform for the Development of Therapeutic Approaches against Diabetes. Molecules 2023, 28, 4523. https://doi.org/10.3390/molecules28114523
Reys LL, Silva SS, Soares da Costa D, Rodrigues LC, Reis RL, Silva TH. Building Fucoidan/Agarose-Based Hydrogels as a Platform for the Development of Therapeutic Approaches against Diabetes. Molecules. 2023; 28(11):4523. https://doi.org/10.3390/molecules28114523
Chicago/Turabian StyleReys, Lara L., Simone S. Silva, Diana Soares da Costa, Luísa C. Rodrigues, Rui L. Reis, and Tiago H. Silva. 2023. "Building Fucoidan/Agarose-Based Hydrogels as a Platform for the Development of Therapeutic Approaches against Diabetes" Molecules 28, no. 11: 4523. https://doi.org/10.3390/molecules28114523
APA StyleReys, L. L., Silva, S. S., Soares da Costa, D., Rodrigues, L. C., Reis, R. L., & Silva, T. H. (2023). Building Fucoidan/Agarose-Based Hydrogels as a Platform for the Development of Therapeutic Approaches against Diabetes. Molecules, 28(11), 4523. https://doi.org/10.3390/molecules28114523












