Thermogravimetric, Morphological and Infrared Analysis of Blends Involving Thermoplastic Starch and Poly(ethylene-co-methacrylic acid) and Its Ionomer Form
Abstract
1. Introduction
- The capacity of strong interactions coming from inclusion complex formation and ionic plasticization in the case of EMAA-54Na used;
- The optimization of the viscosity ratio, thanks to glycerol plasticization of starch;
- Working without a supplementary compatibilizer, which allows us to avoid controlling its dilution and its saturation at blend interface.
2. Experimental Procedures
2.1. Materials
2.2. TPS and Blends Preparation
2.2.1. TPS Preparation
2.2.2. Blends Preparations
2.3. Characterizations
2.3.1. Rheological Behavior of EMMA, Ionomer and TPS
2.3.2. Scanning Electron Microscopy (MEB)
2.3.3. Thermogravimetric Analysis
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
3.1. Viscosity Ratio Evaluation
3.2. Thermal Properties
3.2.1. Blend Components
3.2.2. TPS/EMAA and TPS/EMAA-54Na Blends
3.3. Morphology of Blends
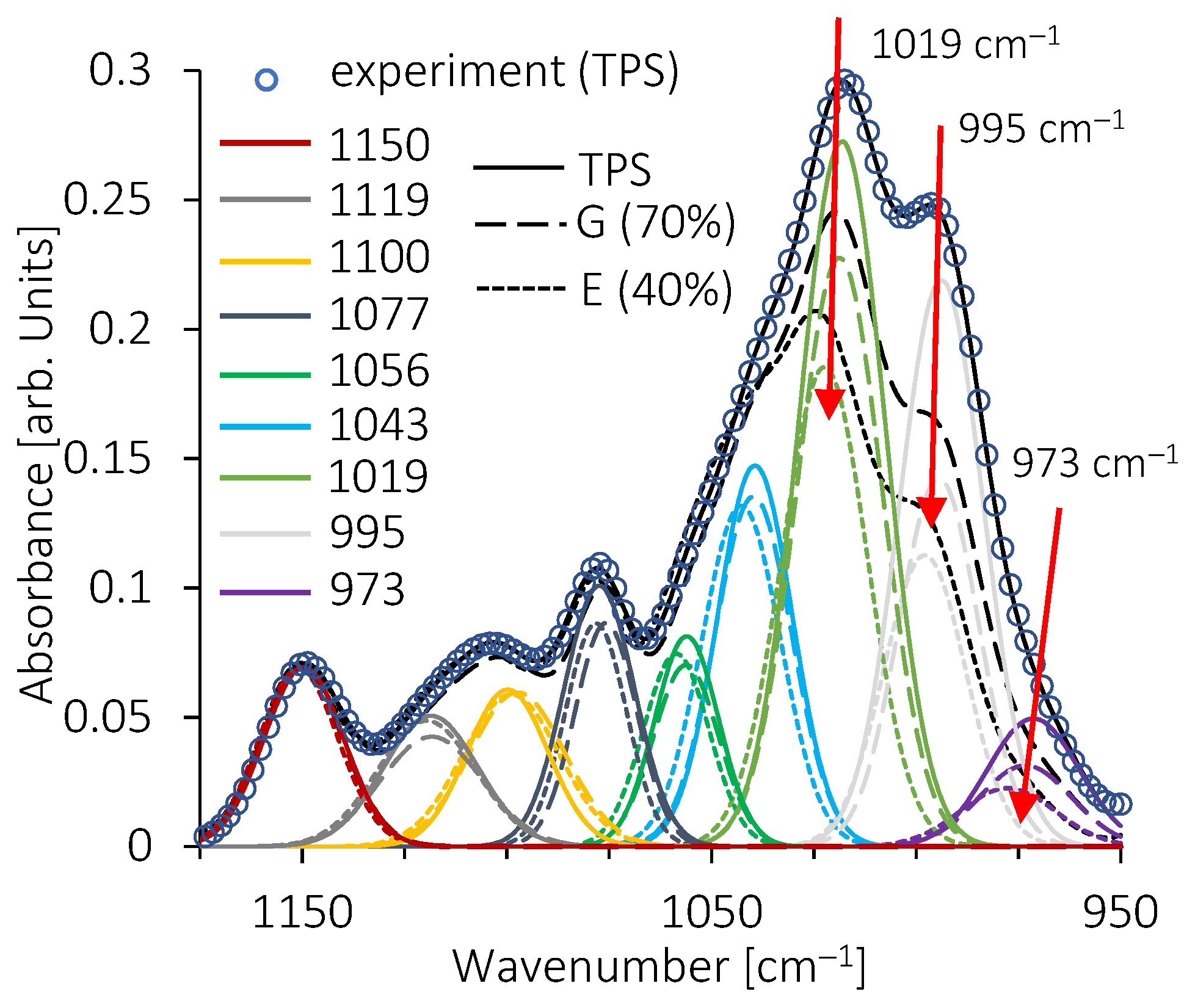
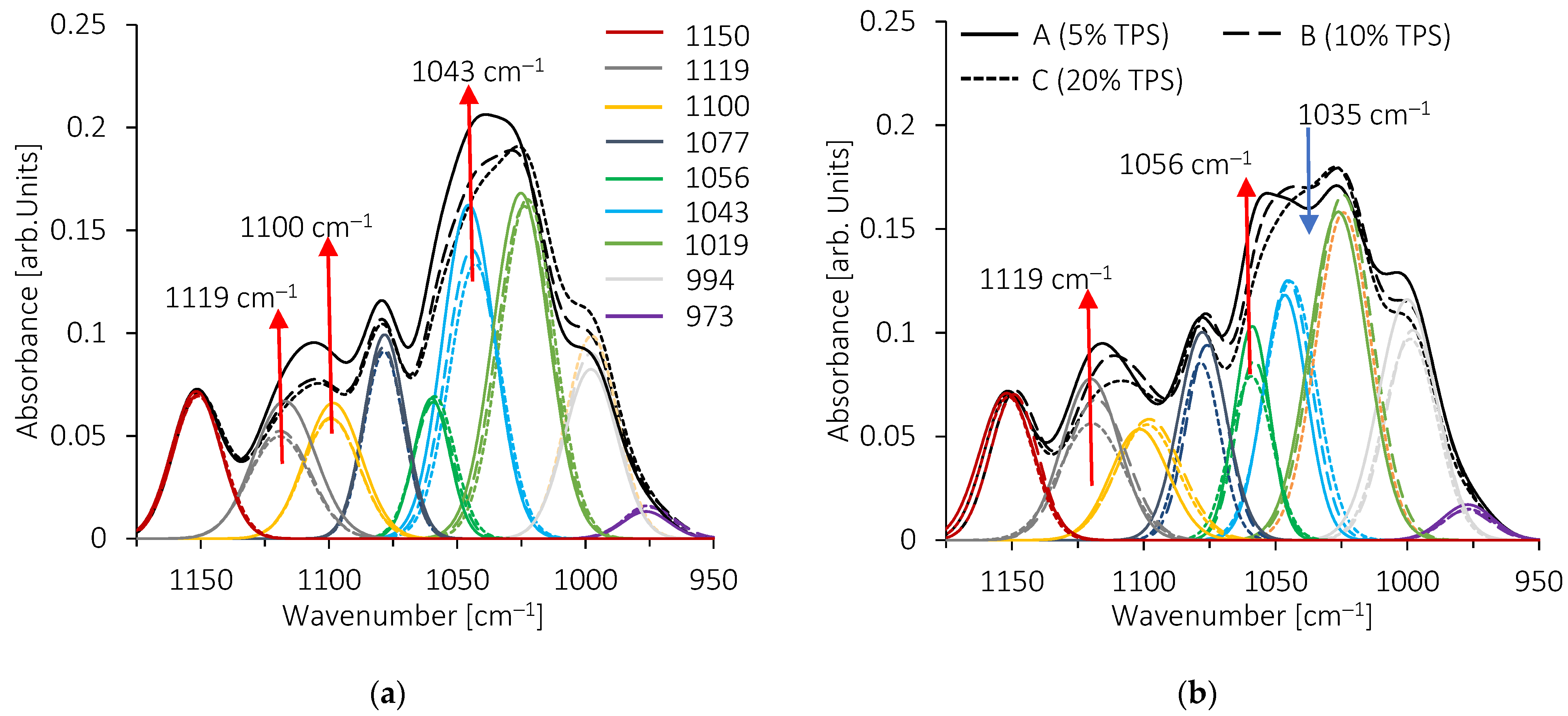
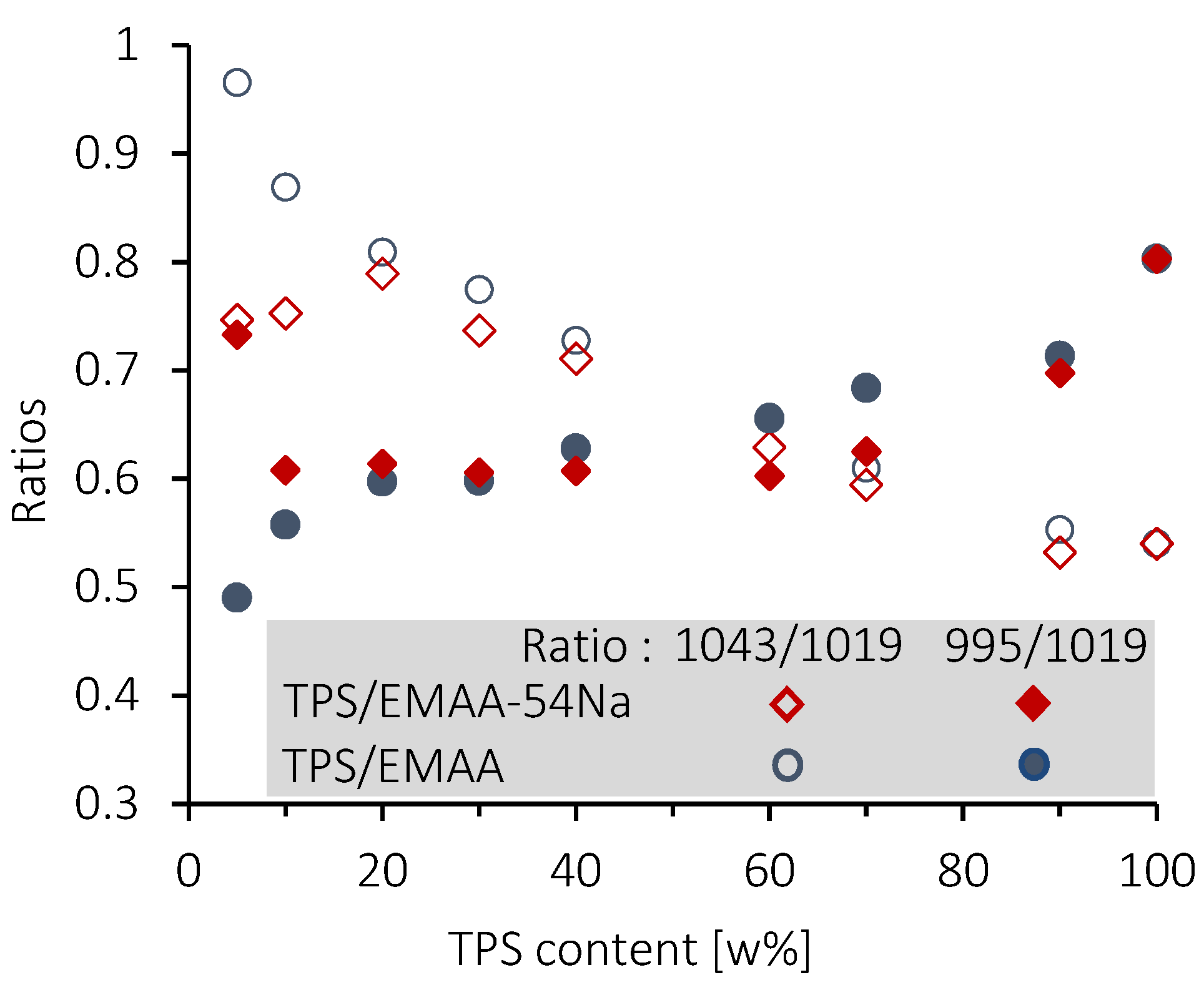
3.4. Structural Features of Blends by FTIR Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat Starch Production, Structure, Functionality and Applications—A Review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Ogunsona, E.; Ojogbo, E.; Mekonnen, T. Advanced Material Applications of Starch and Its Derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar] [CrossRef]
- Xie, F.; Luckman, P.; Milne, J.; McDonald, L.; Young, C.; Tu, C.Y.; Pasquale, T.D.; Faveere, R.; Halley, P.J. Thermoplastic Starch: Current Development and Future Trends. J. Renew. Mater. 2014, 2, 95–106. [Google Scholar] [CrossRef]
- Cai, C.; Wei, C. In Situ Observation of Crystallinity Disruption Patterns during Starch Gelatinization. Carbohydr. Polym. 2013, 92, 469–478. [Google Scholar] [CrossRef]
- Kuang, Q.; Xu, J.; Liang, Y.; Xie, F.; Tian, F.; Zhou, S.; Liu, X. Lamellar Structure Change of Waxy Corn Starch during Gelatinization by Time-Resolved Synchrotron SAXS. Food Hydrocoll. 2017, 62, 43–48. [Google Scholar] [CrossRef]
- Huang, H.-K.; Sheu, H.-S.; Chuang, W.-T.; Jeng, U.-S.; Su, A.-C.; Wu, W.-R.; Liao, K.-F.; Chen, C.-Y.; Chang, S.-Y.; Lai, H.-M. Correlated Changes in Structure and Viscosity during Gelatinization and Gelation of Tapioca Starch Granules. Int. Union Crystallogr. 2014, 1, 418–428. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Molecular Disassembly of Starch Granules during Gelatinization and Its Effect on Starch Digestibility: A Review. Food Funct. 2013, 4, 1564–1580. [Google Scholar] [CrossRef]
- Tajuddin, S.; Xie, F.; Nicholson, T.M.; Liu, P.; Halley, P.J. Rheological Properties of Thermoplastic Starch Studied by Multipass Rheometer. Carbohydr. Polym. 2011, 83, 914–919. [Google Scholar] [CrossRef]
- Yu, L.; Christie, G. Microstructure and Mechanical Properties of Orientated Thermoplastic Starches. J. Mater. Sci. 2005, 40, 111–116. [Google Scholar] [CrossRef]
- Chang, Q.; Zheng, B.; Zhang, Y.; Zeng, H. A Comprehensive Review of the Factors Influencing the Formation of Retrograded Starch. Int. J. Biol. Macromol. 2021, 186, 163–173. [Google Scholar] [CrossRef]
- Matignon, A.; Tecante, A. Starch Retrogradation: From Starch Components to Cereal Products. Food Hydrocoll. 2017, 68, 43–52. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Ottenhof, M.-A.; Hill, S.E.; Farhat, I.A. Comparative Study of the Retrogradation of Intermediate Water Content Waxy Maize, Wheat, and Potato Starches. J. Agric. Food Chem. 2005, 53, 631–638. [Google Scholar] [CrossRef]
- Paluch, M.; Ostrowska, J.; Tynski, P.; Sadurski, W.; Konkol, M. Structural and Thermal Properties of Starch Plasticized with Glycerol/Urea Mixture. J. Polym. Environ. 2022, 30, 728–740. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Okawa, Y.; Ninomiya, K.; Kumagai, H.; Kumagai, H. Evaluation and Suppression of Retrogradation of Gelatinized Rice Starch. J. Nutr. Sci. Vitaminol. 2019, 65, S134–S138. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, J.; Gladden, I.; Contreras, A.; Kong, L. Complexation Ability and Physicochemical Properties of Starch Inclusion Complexes with C18 Fatty Acids. Food Hydrocoll. 2022, 123, 107175. [Google Scholar] [CrossRef]
- Putseys, J.A.; Lamberts, L.; Delcour, J.A. Amylose-Inclusion Complexes: Formation, Identity and Physico-Chemical Properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, F.; Lin, Y.; Zhou, M.; Zhu, P.; Wu, D. Synergistic Effects of Sodium Adipate/Triethylene Glycol on the Plasticization and Retrogradation of Corn Starch. Carbohydr. Polym. 2020, 496, 108112. [Google Scholar] [CrossRef]
- Juikar, S.K.; Warkar, S.G. Biopolymers for Packaging Applications: An Overview. Packag. Technol. Sci. 2023, 36, 229–251. [Google Scholar] [CrossRef]
- Gunawardene, O.H.P.; Gunathilake, C.; Amraweera, S.M.; Fernando, N.M.L.; Wanninayaka, D.B.; Manamperi, A.; Kulatunga, A.K.; Rajapaksha, S.M.; Dassanayake, R.S.; Fernando, C.A.N.; et al. Compatibilization of Starch/Synthetic Biodegradable Polymer Blends for Packaging Applications: A Review. J. Compos. Sci. 2021, 5, 300. [Google Scholar] [CrossRef]
- Charfeddine, I.; Majeste, J.C.; Carrot, C.; Lhost, O. A Model for the Prediction of the Morphology of Immiscible Blends of Polymers. Polymer 2020, 193, 122334. [Google Scholar] [CrossRef]
- Mazerolles, T.; Heuzey, M.C.; Soliman, M.; Martens, H.; Kleppinger, R.; Huneault, M.A. Development of Co-Continuous Morphology in Blends of Thermoplastic Starch and Low-Density Polyethylene. Carbohydr. Polym. 2019, 206, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, F.J.; Ramsay, B.A.; Favis, B.D. High Performance LDPE/Thermoplastic Starch Blends: A Sustainable Alternative to Pure Polyethylene. Polymer 2003, 44, 1517–1526. [Google Scholar] [CrossRef]
- Huitric, J.; Moan, M.; Carreau, P.J.; Dufaure, N. Effect of Reactive Compatibilization on Droplet Coalescence in Shear Flow. J. Non-Newton. Fluid Mech. 2007, 145, 139–149. [Google Scholar] [CrossRef]
- Wang, X.; Huang, L.; Zhang, C.; Deng, Y.; Xie, P.; Liu, L.; Cheng, J. Research Advances in Chemical Modifications of Starch for Hydrophobicity and Its Applications: A Review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef]
- Sabetzadeh, M.; Bagheri, R.; Masoomi, M. Study on Ternary Low Density Polyethylene/Linear Low Density Polyethylene/Thermoplastic Starch Blend Films. Carbohydr. Polym. 2015, 119, 126–133. [Google Scholar] [CrossRef]
- Sailaja, R.R.N.; Chanda, M. Use of Maleic Anhydride-Grafted Polyethylene as Compatibilizer for HDPE-Tapioca Starch Blends: Effects on Mechanical Properties. J. Appl. Polym. Sci. 2001, 80, 863–872. [Google Scholar] [CrossRef]
- Taghizadeh, A.; Sarazin, P.; Favis, B.D. High Molecular Weight Plasticizers in Thermoplastinc Starch/Polyethylene Blends. J. Mater. Sci. 2013, 48, 1799–1811. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Preparation and Properties of Extruded Thermoplastic Starch/Polymer Blends. J. Appl. Polym. Sci. 2012, 126, E96–E108. [Google Scholar] [CrossRef]
- Cercle, C.; Favis, B.D. Generalizing Interfacial Modification in Polymer Blends. Polymer 2012, 53, 4338–4343. [Google Scholar] [CrossRef]
- Cercle, C.; Sarazin, P.; Favis, B.D. High Performance Polyethylene/Thermoplastic Starch Blends through Controlled Emulsification Phenomena. Carbohydr. Polym. 2013, 92, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Taguet, A.; Huneault, M.A.; Favis, B.D. Interface/Morphology Relationships in Polymer Blends with Thermoplastic Starch. Polymer 2009, 50, 5733–5743. [Google Scholar] [CrossRef]
- Taguet, A.; Bureau, M.N.; Huneault, M.A.; Favis, B.D. Toughening Mechanisms in Interfacially Modified HDPE/Thermoplastic Starch Blends. Carbohydr. Polym. 2014, 114, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.; Ghasemi, I.; Oromiehie, A. Effect of Phase Inversion on the Physical and Mechanical Properties of Low Density Polyethylene/Thermoplastic Starch. Polym. Test. 2013, 32, 482–491. [Google Scholar] [CrossRef]
- Mortazavi, S.; Ghasemi, I.; Oromiehie, A. Morphological and Rheological Properties of (Low-Density Polyethylene/Thermoplastic Starch Blend: Investigation of the Role of High Elastic Network. J. Vinyl Addit. Technol. 2014, 20, 250–259. [Google Scholar] [CrossRef]
- Rahmat, A.R.; Rahman, W.A.W.A.; Sin, L.T.; Yussuf, A.A. Approaches to Improve Compatibility of Starch Filled Polymer System: A Review. Mater. Sci. Eng. C 2009, 29, 2370–2377. [Google Scholar] [CrossRef]
- Yu, F.; Prashantha, K.; Soulestin, J.; Lacrampe, M.-F.; Krawczak, P. Plasticized-Starch/Poly(Ethylene Oxide) Blends Prepared by Extrusion. Carbohydr. Polym. 2013, 91, 253–261. [Google Scholar] [CrossRef]
- Orts, W.J.; Nobes, G.A.R.; Glenn, G.M.; Gray, G.M.; Imam, S.; Chiou, B.-S. Blends of Starch with Ethylene Vinyl Alcohol Copolymers: Effect of Water, Glycerol, and Amino Acids as Plasticizers. Polym. Adv. Technol. 2007, 18, 629–635. [Google Scholar] [CrossRef]
- Otey, F.H.; Westhoff, R.P.; Doane, W.M. Starch-Based Blown Films. 2. Ind. Eng. Chem. Prod. Res. Dev. 1987, 26, 1659–1663. [Google Scholar] [CrossRef]
- Fanta, G.F.; Shogren, R.L.; Salch, J.H. Steam Jet Cooking of High-Amylose Starch-Fatty Acid Mixtures. An Investigation of Complex Formation. Carbohydr. Polym. 1999, 38, 1–6. [Google Scholar] [CrossRef]
- Fanta, G.F.; Swanson, C.L.; Doane, W.M. Complexing between Starch and Poly(Ethylene-Co-Acrylic Acide)—A Comparison of Starch Varieties and Complexing Conditions. Carbohydr. Polym. 1992, 17, 51–58. [Google Scholar] [CrossRef]
- Shogren, R.L.; Fanta, G.F.; Felker, F.C. X-ray Diffraction Study of Crystal Transformations in Spherulitic Amylose/Lipid Complexes from Jet-Cooked Starch. Carbohydr. Polym. 2006, 64, 444–451. [Google Scholar] [CrossRef]
- Shogren, R.L.; Greene, R.V.; Wu, Y.V. Complexes of Starch Polysaccharides and Poly(Ethylene Co-Acrylic Acid): Structure and Stability in Solution. J. Appl. Polym. Sci. 1991, 42, 1701–1709. [Google Scholar] [CrossRef]
- Fanta, G.F.; Dintzis, F.R.; Bagley, E.B.; Christianson, D.D. The Influence of PH on the Viscous Behavior of Starch-Poly(Ethylene-Co-Acrylic Acid) Complexes. Carbohydr. Polym. 1992, 19, 253–259. [Google Scholar] [CrossRef]
- Fredrickson, G.H.; Xie, S.; Edmund, J.; Le, M.L.; Sun, D.; Grzetic, D.J.; Vigil, D.L.; Delaney, K.T.; Chabinyc, M.L.; Segalman, R.A. Ionic Compatibilization of Polymers. ACS Polym. AU 2022, 2, 299–312. [Google Scholar] [CrossRef]
- Silva, P.A.P.; da Silva, A.B.; Santos, J.P.F.; Orefice, R.L. Self_healing Polymer Blend Based on PETG and EMAA. J. Appl. Polym. Sci. 2021, 138, 50148. [Google Scholar] [CrossRef]
- Zhang, L.; Brostowitz, N.R.; Cavicchi, K.A.; Weiss, R.A. Perspective: Ionomer Research and Applications. Macromol. React. Eng. 2014, 8, 81–99. [Google Scholar] [CrossRef]
- Vogler, E.A.; Bussian, R.W. Short-Term Cell-Attachment Rates: A Surface-Sensitive Test of Cell-Substrate Compatibility. J. Biomed. Mater. Res. A 1987, 21, 1197–1211. [Google Scholar] [CrossRef]
- Brouillet-Fourmann, S.; Carrot, C.; Mignard, N.; Prochazka, F. On the Use of an Internal Mixer for the Rheological Characterization of Maize Starch. Appl. Rheol. 2002, 12, 192–199. [Google Scholar] [CrossRef]
- Bousmina, M.; Ait-Kadi, A.; Faisant, J.B. Determination of Shear Rate and Viscosity from Batch Mixer Data. J. Rheol. 1999, 43, 415–433. [Google Scholar] [CrossRef]
- Vega, D.; Villar, M.A.; Failla, M.D.; Valles, E.M. Thermogravimetric Analysis of Starch-Based Biodegradable Blends. Polym. Bull. 1996, 37, 229–235. [Google Scholar] [CrossRef]
- Wu, S. Formation of Dispersed Phase in Incompatible Polymer Blends: Interfacial and Rheological Effects. Polym. Eng. Sci. 1987, 27, 335–343. [Google Scholar] [CrossRef]
- Cai, J.; Wang, Y.; Zhou, L.; Huang, Q. Thermogravimetric Analysis and Kinetics of Coal/Plastic Blends during Co-Pyrolysis in Nitrogen Atmosphere. Fuel Process. Technol. 2008, 1, 21–27. [Google Scholar] [CrossRef]
- Pineda-Gomez, P.; Angel-Gil, N.C.; Valencia-Munoz, C.; Rosales-Rivera, A.; Rodriguez-Garcia, M.E. Thermal Degradation of Starch Sources: Green Banana, Potato, Cassava, and Corn—Kinetic Study by Non-Isothermal Procedures. Starch/Stärke 2014, 66, 691–699. [Google Scholar] [CrossRef]
- Aggarwal, P.; Dollimore, D. A Thermal Analysis Investigation of Partially Hydrolyzed Starch. Thermochim. Acta 1998, 319, 17–25. [Google Scholar] [CrossRef]
- Ma, X.-F.; Yu, J.-G.; Wan, J.J. Urea and Ethanolamine as a Mixed Plasticizer for Thermoplastic Starch. Carbohydr. Polym. 2006, 64, 267–273. [Google Scholar] [CrossRef]
- McNeill, I.C.; Alston, A. Thermal Degradation Behaviour of Acrylic Salt Polymers and Ionomers. Angew. Makromol. Chem. 1998, 261–262, 157–172. [Google Scholar] [CrossRef]
- Rufino, E.S.; Monteiro, E.E.C. Characterisation of Lithium and Sodium Salts of Poly(Methacrylic Acid) by FTIR and Thermal Analysis. Polymer 2000, 41, 4213–4222. [Google Scholar] [CrossRef]
- Lizymol, P.P.; Thomas, S. Thermal Behaviour of Polymer Blends: A Comparison of the Thermal Properties of Miscible and Immiscible Systems. Polym. Degrad. Stab. 1993, 41, 59–64. [Google Scholar] [CrossRef]
- Luo, X.; Li, J.; Lin, X. Effect of Gelatinization and Additives on Morphology and Thermal Behavior of Corn Starch/PVA Blend Films. Carbohydr. Polym. 2012, 90, 1595–1600. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahman, W.A.W.A.; Rahmat, A.R.; Mokhtar, M. Determination of Thermal Stability and Activation Energy of Polyvinyl Alcohol-Cassava Starch Blends. Carbohydr. Polym. 2011, 83, 303–305. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M.; Botta, L.; Mistretta, M.C.; Ceraulo, M.; Scaffaro, R. Degradation of Polymer Blends: A Brief Review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Tajvidi, M.; Takemura, A. Effect of Fiber Content and Type, Compatibilizer, and Heating Rate on Thermogravimetric Properties of Natural Fiber High Density Polyethylene Composites. Polym. Compos. 2009, 30, 1226–1233. [Google Scholar] [CrossRef]
- Li, Z.; Wei, C. Morphology, Structure, Properties and Applications of Starch Ghost: A Review. Int. J. Biol. Macromol. 2020, 163, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Impact of Ghosts on the Mechanical, Optical, and Barrier Properties of Corn Starch Films. Starch/Stärke 2017, 69, 1600308. [Google Scholar] [CrossRef]
- Nagayama, K.; Morris, B.A.; Matsuba, G.; Nakata, K. Influence of Co-Neutralization by Sodium and Zinc on the Ethylene Ionomer Structure and Properties. J. Appl. Polym. Sci. 2022, 139, 52126. [Google Scholar] [CrossRef]
- Walters, R.M.; Sohn, K.E.; Winey, K.I.; Composto, R.J. Local Acid Environment in Poly(Ethylene-Ran-Methacrylic Acid) Ionomers. J. Polym. Sci. Part B Polym. Phys. 2002, 41, 2833–2841. [Google Scholar] [CrossRef]
- Coleman, M.M.; Lee, J.Y.; Painter, P.C. Acid Salts and the Structure of Ionomers. Macromolecules 1990, 23, 2339–2345. [Google Scholar] [CrossRef]
- Brozoski, B.A.; Painter, P.C.; Coleman, M.M. Concerning the Origin of Broad Bands Observed in the FT-IR Spectra of Ionomers. Cluster Formation or Water Absorption? Macromolecules 1984, 17, 1591–1594. [Google Scholar] [CrossRef]
- Chen, X.; Sha, J.; Chen, T.; Zhao, H.; Ji, H.; Xie, L.; Ma, Y. Effect of Ionomer Interfacial Compatibilization on Highly Filled HDPE/AL2O3/Ionomer Composites: Morphology and Rheological Behavior. Compos. Sci. Technol. 2019, 170, 7–14. [Google Scholar] [CrossRef]
- Kutsumizu, S.; Nakamura, M.; Yano, S. Pressure-Induced Coordination—Structural Change around Zinc(II) in Zinc(II)—Neutralized Ethylene-Methacrylic Acid Ionomers. 1. Infrared Spectroscopic Studies. Macromolecules 2001, 34, 3033–3040. [Google Scholar] [CrossRef]
- Kutsumizu, S.; Nagao, N.; Tadano, K.; Tachino, H.; Hirasawa, E.; Yano, S. Effects of Water Sorption on the Structure and Properties of Ethylene Ionomers. Macromolecules 1992, 25, 6829–6835. [Google Scholar] [CrossRef]
- Ishioka, T.; Shimizu, M.; Watanabe, I.; Kawauchi, S.; Harada, M. Infrared and XAFS Study on the Internal Structural Change of Ion Aggregate in a Zinc Salt of Poly(Ethylene-Co-Methacrylic Acid) Ionmer on Water Absorption. Macromolecules 2000, 33, 2722–2727. [Google Scholar] [CrossRef]
- Tachino, H.; Hara, H.; Hirasawa, E.; Kutsumizu, S.; Yano, S. Structure and Properties of Ethylene Ionomers Neutralized with Binary Metal Cations. Macromolecules 1994, 27, 372–378. [Google Scholar] [CrossRef]
- Kutsumizu, S.; Hara, H.; Tachino, H.; Shimabayashi, K.; Yano, S. Infrared Spectroscopic Study of the Binary Blends of Sodium and Zinc Salt Ionomers Produced from Poly(Ethylene-Co-Methacrylic Acid). Macromolecules 1999, 32, 6340–6347. [Google Scholar] [CrossRef]
- Goodfellow, B.J.; Wilson, R.H. A Fourrier Transform IR Study of the Gelation of Amylose and Amylopectin. Biopolymers 1990, 30, 1183–1189. [Google Scholar] [CrossRef]
- van Soest, J.J.G.; Tournois, H.; de Wit, D.; Vliegenthart, J.F.G. Short-Range Structure in (Partially) Crystalline Potato Starch Determined with Attenuated Total Reflectance Fourrier-Transform IR Spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of External Region of the Starch Granule as Determined by Infrared Spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Capron, I.; Robert, P.; Colonna, P.; Brogly, M.; Planchot, V. Starch in Rubbery and Glassy States by FTIR Spectroscopy. Carbohydr. Polym. 2007, 68, 249–259. [Google Scholar] [CrossRef]
- Yang, S.; Dhital, S.; Shan, C.-S.; Zhang, M.-N.; Chen, Z.-G. Ordered Structural Changes of Retrograded Starch Gel over Long-Term Storage in Wet Starch Noodles. Carbohydr. Polym. 2021, 270, 118367. [Google Scholar] [CrossRef] [PubMed]
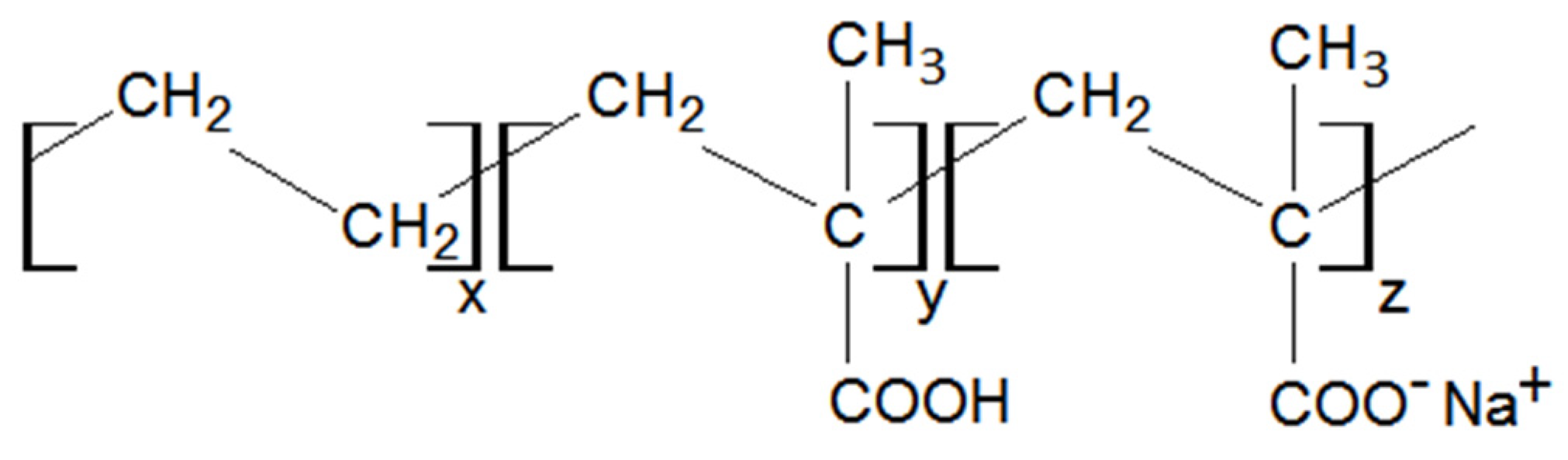



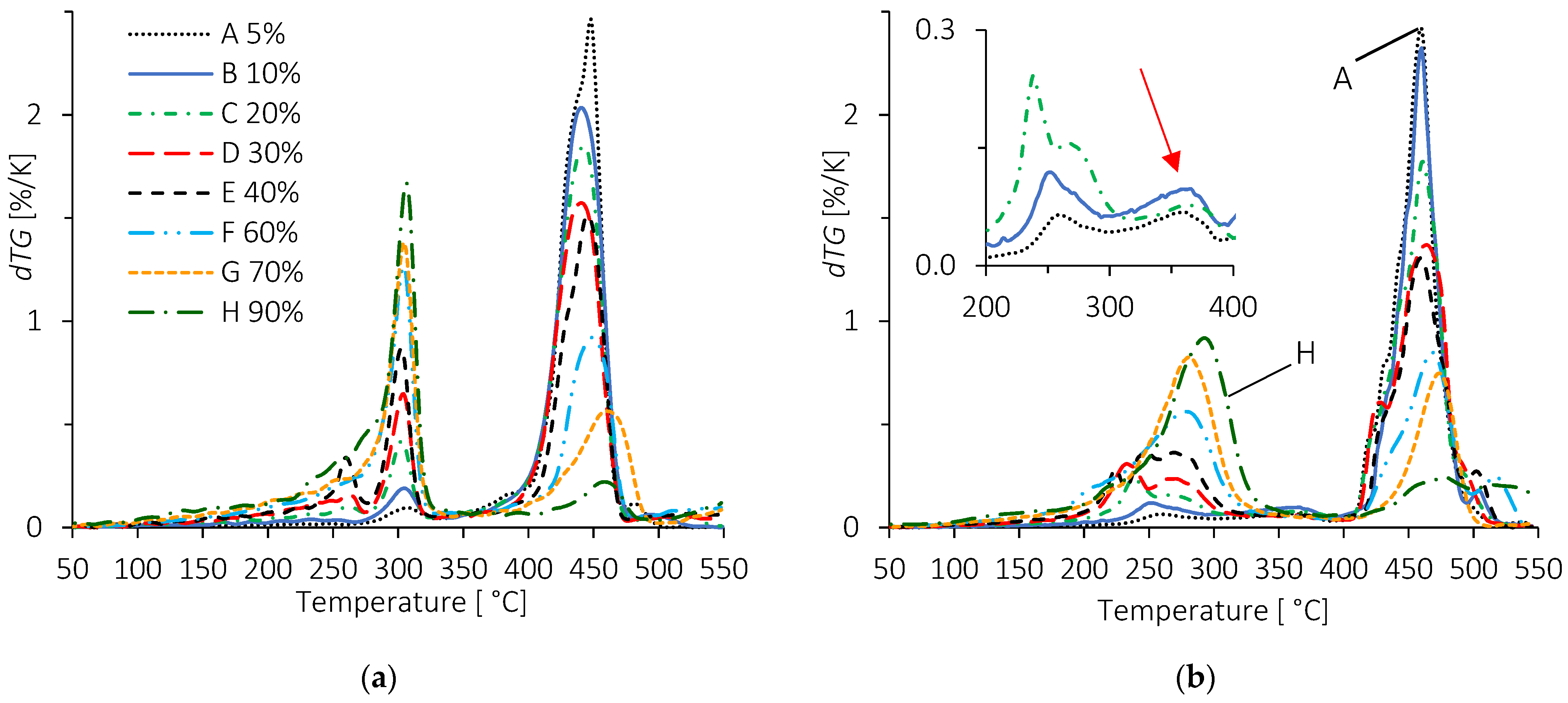


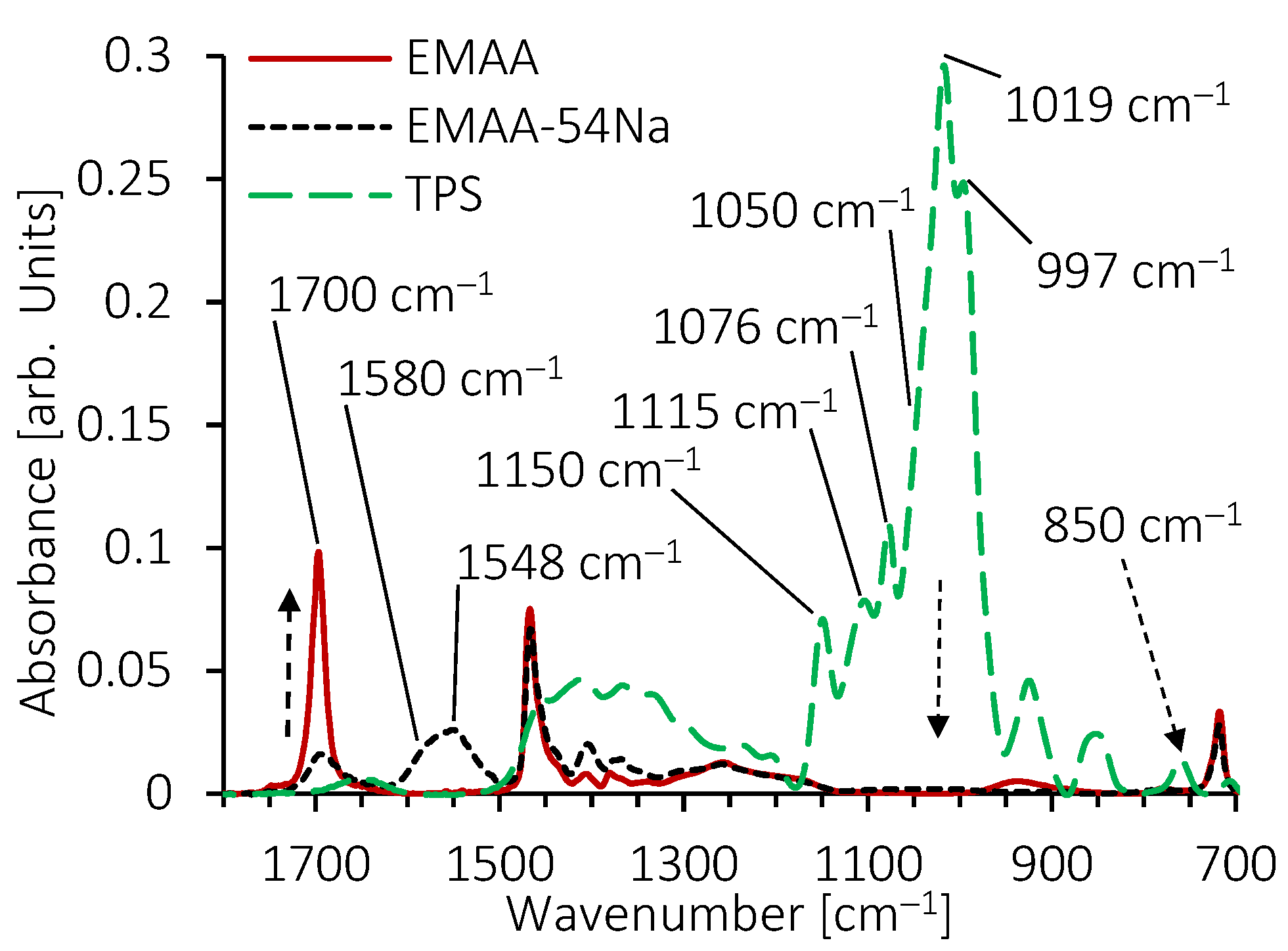
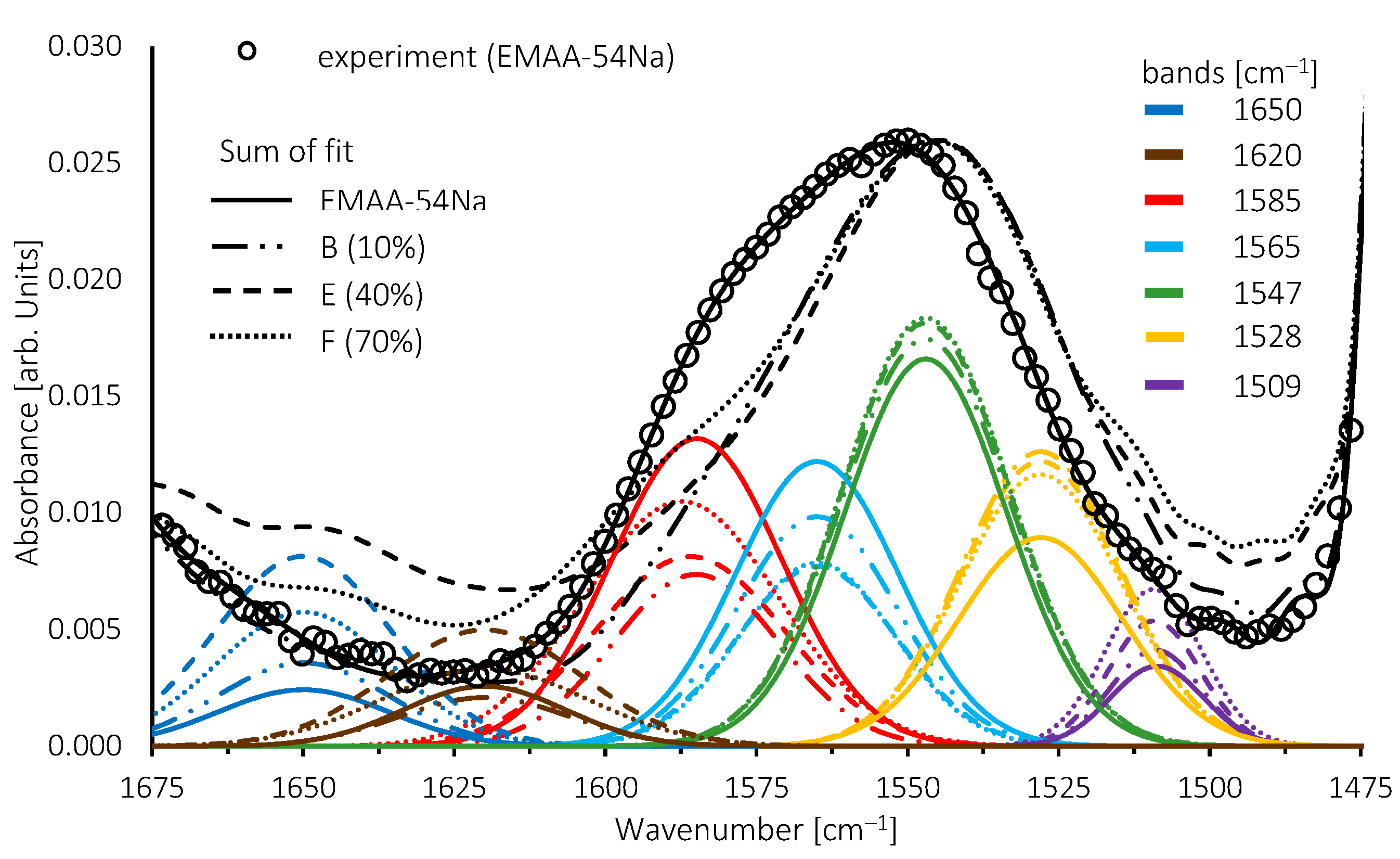
| Dispersed Phase | Matrix Phase | ||
|---|---|---|---|
| TPS | EMAA | EMAA-54Na | |
| TPS | 0.64 | 0.35 | |
| EMAA | 1.5 | ||
| EMAA-54Na | 2.82 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dony, P.; Berzin, F. Thermogravimetric, Morphological and Infrared Analysis of Blends Involving Thermoplastic Starch and Poly(ethylene-co-methacrylic acid) and Its Ionomer Form. Molecules 2023, 28, 4519. https://doi.org/10.3390/molecules28114519
Dony P, Berzin F. Thermogravimetric, Morphological and Infrared Analysis of Blends Involving Thermoplastic Starch and Poly(ethylene-co-methacrylic acid) and Its Ionomer Form. Molecules. 2023; 28(11):4519. https://doi.org/10.3390/molecules28114519
Chicago/Turabian StyleDony, Philippe, and Françoise Berzin. 2023. "Thermogravimetric, Morphological and Infrared Analysis of Blends Involving Thermoplastic Starch and Poly(ethylene-co-methacrylic acid) and Its Ionomer Form" Molecules 28, no. 11: 4519. https://doi.org/10.3390/molecules28114519
APA StyleDony, P., & Berzin, F. (2023). Thermogravimetric, Morphological and Infrared Analysis of Blends Involving Thermoplastic Starch and Poly(ethylene-co-methacrylic acid) and Its Ionomer Form. Molecules, 28(11), 4519. https://doi.org/10.3390/molecules28114519






