A Reliable Production System of Large Quantities of [13N]Ammonia for Multiple Human Injections
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
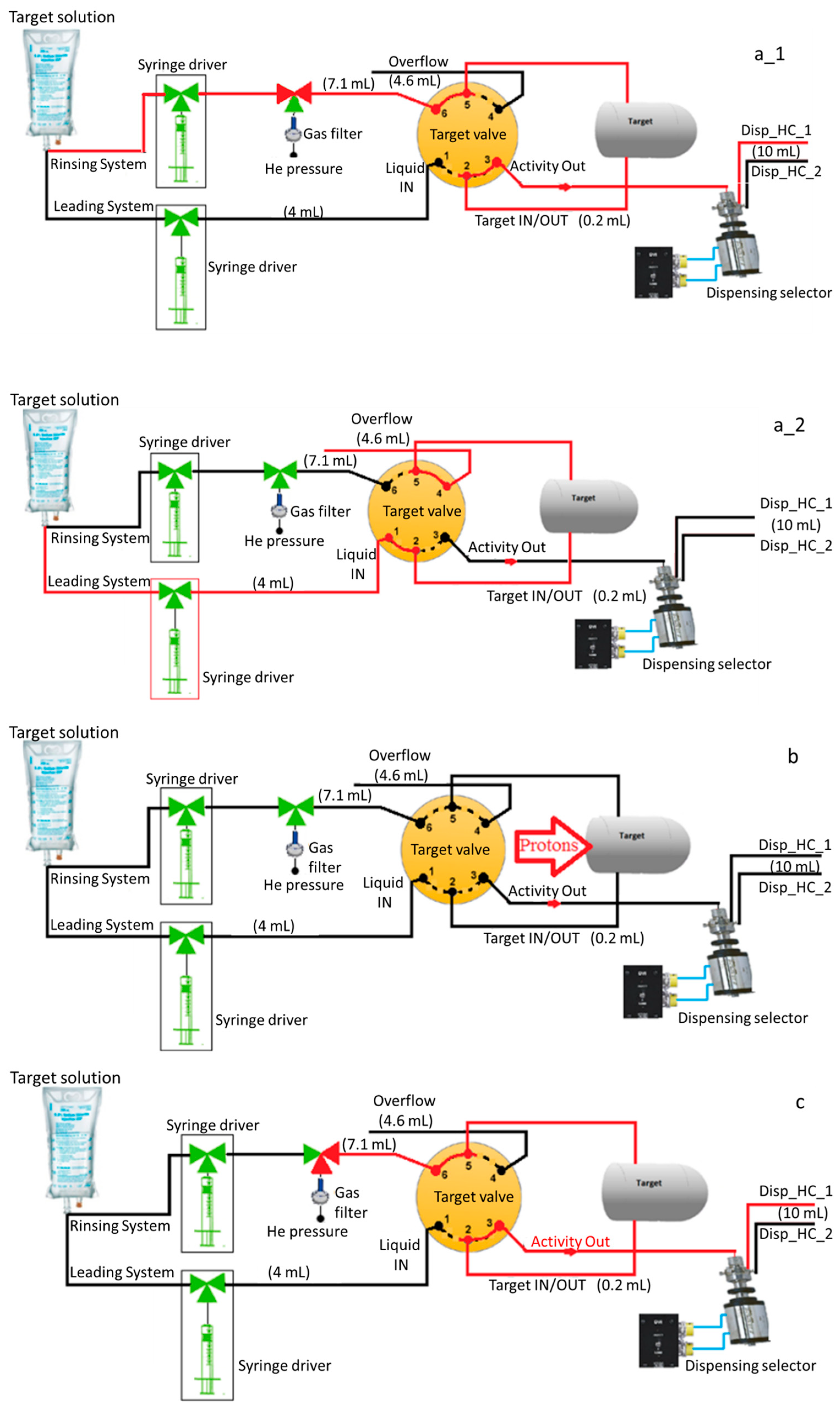
4.2. System Description
- Materials:
- System operation:
4.3. Production of [13N]Ammonia Injection
- [13N]Ammonia is produced with a Cyclone® 18/9 cyclotron (IBA, Louvain-La-Neuve, Belgium) via the nuclear reaction 16O(p,α)13N with 18-MeV protons degraded to 16 MeV using an aluminum degrader (300–400 µm) installed in the target collimator to prevent the production of oxygen-15 via the 16O(p,pn)15O nuclear reaction. The 2XL target is filled with 3.5 mL of target solution (10 mM ethanol in WFI) (Figure 2(a2)) and bombarded at a current of 55 µA for 20–40 min (Figure 2b). The target body is made of niobium, and the entrance windows are made of a 12.5 µm thick titanium foil (ø 23.5 mm) and a 35 µm thick Havar foil (ø 43 mm).
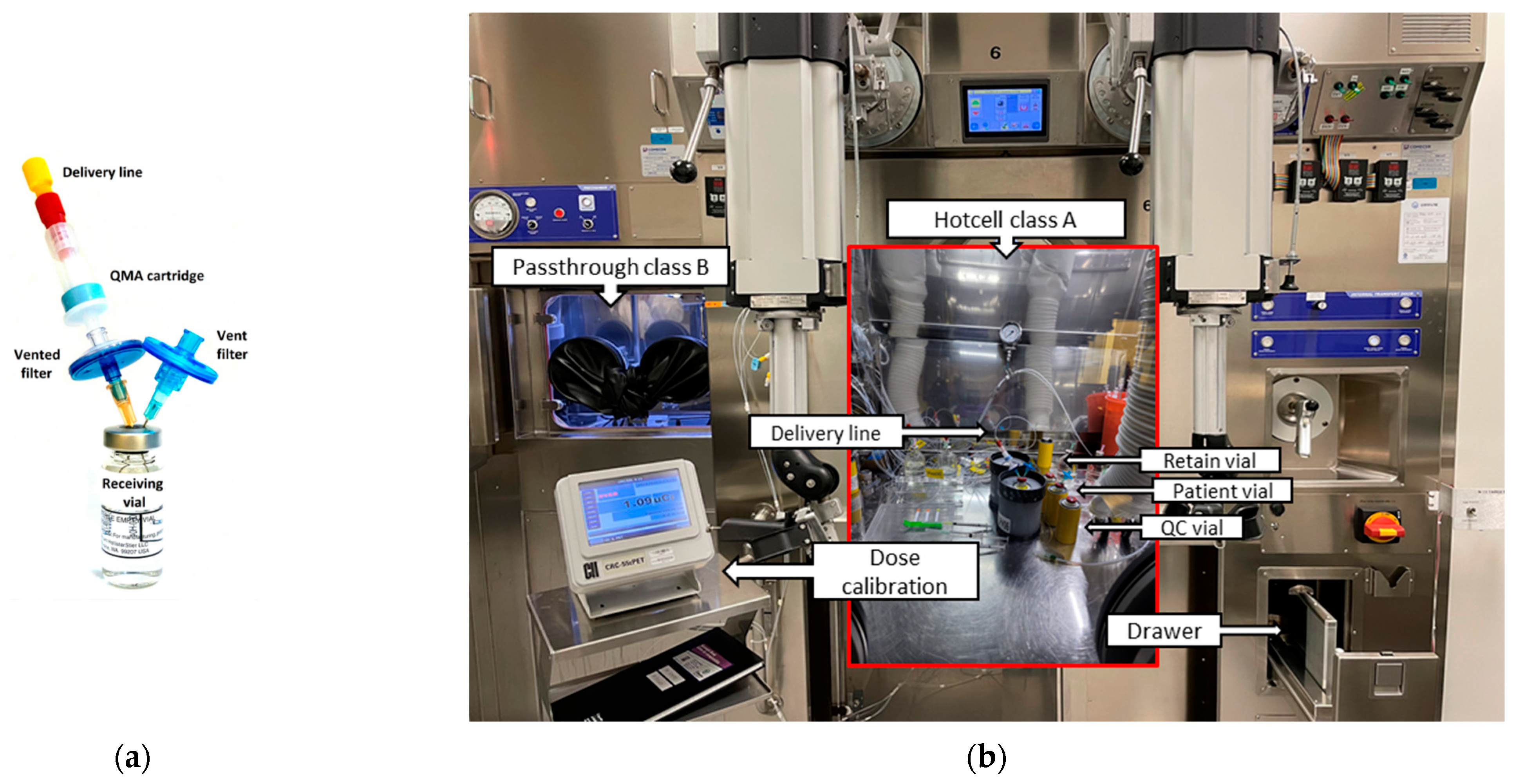
- The set-up of the Class A dispensing hot cell consists of two vial assemblies containing either four (first batch of the day) or three (second batch) 10 mL vials (Hollister Stier, Montréal, QC, Canada), enough syringes and needles of the appropriate size for the manipulation to be performed, along with a 3 mL syringe containing 2.5 mL of 3% saline. Using the manipulating gloves, before the transfer of the dose, a hydrophilic 0.22 μm vented filter (Millex GV, Millipore, Burlington, MA, USA) and a venting 0.22 μm filter (PharmAssure, Pall, Port Washington, NY, USA) are inserted into the patient (receiving) 10 mL vial. The vented filter is then connected to the rinsed and dried (see above) QMA + delivery line, as shown in Figure 3a. The remaining three vials are used for QC, retain and sterility/endotoxin samples, respectively (Figure 3b). Since the sterility sample of the second batch is pooled with the first one, the vial assembly for the second batch contains only three 10 mL vials. An acrylic plate with three pH strips (0 to 6) and a vented 100 mL vial full of target solution to perform the filter integrity test complete the setup for the [13N]ammonia production.
- At the EOB, the irradiated water/ethanol/[13N]NH3 solution is flushed out of the target (Figure 2c) with 0.25–0.5 MPa (2.5–5 bar) flow of He through an in-line QMA cartridge to trap any anionic impurities such as [18F]fluoride and [13N]NOx−, into a dispensing hot cell previously set up for the aseptic preparation (see above). Right after, an additional target rinse with 2.5 mL of target solution is performed to recover the remaining activity.
- Approximately 0.1 mL of [13N]NH3 is withdrawn to start the QC, then 2.5 mL of 3% saline is added to the bulk vial to produce isotonic [13N]ammonia final product. From this, it is understood that the QC steps start before finishing the sampling and reformulation of the final product (both stages overlapping). Sterility/endotoxin and retain samples (0.3 mL sterility/endotoxin + 0.1 mL retain) are taken at this step of the process (Figure 3b) while visual inspection, pH and filter integrity tests are performed subsequently. Sterility/endotoxin samples of all batches of the day are pooled, while retained samples from each batch are kept separately in case a sterility and/or endotoxin re-test is required afterwards.
- The final [13N]ammonia product is removed from the dispensing hot cell using a dedicated drawer (Figure 3b) and transferred to the Nuclear Medicine department in less than a minute by a built-in pneumatic system while the QC assays are performed in order to save time.
4.4. Quality Control Testing of [13N]Ammonia Injection
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bateman, T.M.; Heller, G.V.; Beanlands, R.; Calnon, D.A.; Case, J.; deKemp, R.; DePuey, E.G.; Di Carli, M.; Guler, E.C.; Murthy, V.L.; et al. Practical guide for interpreting and reporting cardiac PET measurements of myocardial blood flow: An information statement from the american society of nuclear cardiology, and the society of nuclear medicine and molecular imaging. J. Nucl. Med. 2021, 62, 1599–1615. [Google Scholar] [CrossRef] [PubMed]
- Dorbala, S.; Di Carli, M.F.; Delbeke, D.; Abbara, S.; DePuey, E.G.; Dilsizian, V.; Forrester, J.; Janowitz, W.; Kaufmann, P.A.; Mahmarian, J.; et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J. Nucl. Med. 2013, 54, 1485. [Google Scholar] [CrossRef] [PubMed]
- Rosenspire, K.C.; Schwaiger, M.; Mangner, T.J.; Hutchins, G.D.; Sutorik, A.; Kuhl, D.E. Metabolic fate of [13N]Ammonia in human and canine blood. J. Nucl. Med. 1990, 31, 163–167. [Google Scholar] [PubMed]
- Herzog, B.A.; Husmann, L.; Valenta, I.; Gaemperli, O.; Siegrist, P.T.; Tay, F.M.; Burkhard, N.; Wyss, C.A.; Kaufmann, P.A. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography: Added value of coronary flow reserve. J. Am. Coll. Cardiol. 2009, 54, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Fiechter, M.; Ghadri, J.R.; Gebhard, C.; Fuchs, T.A.; Pazhenkottil, A.P.; Nkoulou, R.N.; Herzog, B.A.; Wyss, C.A.; Gaemperli, O.; Kaufmann, P.A. Diagnostic value of 13N-ammonia myocardial perfusion PET: Added value of myocardial flow reserve. J. Nucl. Med. 2012, 53, 1230. [Google Scholar] [CrossRef] [PubMed]
- Muzik, O.; Beanlands, R.S.B.; Hutchins, G.D.; Mangner, T.J.; Nguyen, N.; Schwaiger, M. Validation of nitrogen-13-ammonia tracer kinetic model for quantification of myocardial blood flow using PET. J. Nucl. Med. 1993, 34, 83–91. [Google Scholar] [PubMed]
- Pieper, J.; Patel, V.N.; Escolero, S.; Nelson, J.R.; Poitrasson-Rivière, A.; Shreves, C.K.; Freiburger, N.; Hubers, D.; Rothley, J.; Corbett, J.R.; et al. Initial clinical experience of N13-ammonia myocardial perfusion PET/CT using a compact superconducting production system. J. Nucl. Cardiol. 2021, 28, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, K.; Vandamme, M.; Bormans, G.; Cleeren, F. Design and challenges of radiopharmaceuticals. Semin. Nucl. Med. 2019, 49, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Wieland, B.; Bida, G.; Padgett, H.; Hendry, G.; Zippi, E.; Kabalka, G.; Morelle, J.-L.; Verbruggen, R.; Ghyoot, M. In-target production of [13N]ammonia via proton irradiation of dilute aqueous ethanol and acetic acid mixtures. Int. J. Rad. Appl. Instrum. A 1991, 42, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, H.; Jacob, M.; Anand, S.P.; Bandopadhyaya, G.P. Production of nitrogen-13-labeled ammonia by using 11MeV medical cyclotron: Our experience. Hell. J. Nucl. Med. 2009, 12, 248–250. [Google Scholar] [PubMed]
- Frank, C.; Winter, G.; Rensei, F.; Samper, V.; Brooks, A.F.; Hockley, B.G.; Henderson, B.D.; Rensch, C.; Scott, P.J.H. Development and implementation of ISAR, a new synthesis platform for radiopharmaceutical production. EJNMMI Radiopharm. Chem. 2019, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Yokell, D.L.; Rice, P.A.; Neelamegam, R.; El Fakhri, G. Development, validation and regulatory acceptance of improved purification and simplified quality control of [13N]Ammonia. EJNMMI Radiopharm. Chem. 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Vaalburg, W.; Kamphuis, J.A.A.; Beerling-van der Molen, H.D.; Reiffers, S.; Rijskamp, A.; Woldring, M.G. An improved method for the cyclotron production of 13N-labelled ammonia. Int. J. Appl. Radiat. Isot. 1975, 26, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.J.H. Synthesis of [13N]ammonia ([13N]NH3). In Radiochemical Syntheses; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 313–320. ISBN 9781118140345. [Google Scholar]
- Ito, S.; Sakane, H.; Deji, S.; Saze, T.; Nishizawa, K. Radioactive byproducts in [18O]H2O used to produce 18F for [18F]FDG synthesis. Appl. Radiat. Isot. 2006, 64, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Marengo, M.; Lodi, F.; Magi, S.; Cicoria, G.; Pancaldi, D.; Boschi, S. Assessment of radionuclidic impurities in 2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG) routine production. Appl. Radiat. Isot. 2008, 66, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Degering, D.; Zessin, J.; Füchtner, F.; Konheiser, J. Radionuclide impurities in [18F]F− and [18F]FDG for positron emission tomography. Appl. Radiat. Isot. 2013, 81, 268–271. [Google Scholar] [CrossRef]
- Kamar, F.; Kovacs, M.S.; Hicks, J.W. Low cost and open source purification apparatus for GMP [13N]ammonia production. Appl. Radiat. Isot. 2022, 185, 110214. [Google Scholar] [CrossRef] [PubMed]
- Bars, E.; Sajjad, M. Automated system for the production of multiple doses of nitrogen-13 labeled ammonia. J. Nucl. Med. 2012, 53, 1459. [Google Scholar]
- Akhilesh, S.; Shanker, N.; Subhash, K.; Sanjay, G.; Dixit, M. Fully automated synthesis of nitrogen-13-NH3 by SHIs HM-18 cyclotron and dedicated module for routine clinical studies: Our institutional experiences. Indian J. Nucl. Med. 2022, 37, 50–53. [Google Scholar] [CrossRef]
- Bormans, G.; Langendries, W.; Verbruggen, A.; Mortelmans, L. On-line anion exchange purification of [13N]NH3 produced by 10 MeV proton irradiation of dilute aqueous ethanol. Appl. Radiat. Isot. 1995, 46, 83–86. [Google Scholar] [CrossRef]
| Specification | Acceptance Criteria | Day 1 * | Day 2 * | Day 3 * |
|---|---|---|---|---|
| Activity EOB GBq/mCi | - | 43.03/1163 44.55/1204 | 40.33/1090 42.18/1140 | 42.74/1155 43.07/1164 |
| Activity EOM (GBq/mCi) | - | 28.19/762 28.82/779 | 29.23/790 29.97/810 | 29.49/797 29.49/797 |
| Elapsed time EOB to EOM | - | 5 min 5 min, 15 s | 4 min, 40 s 4 min, 42 s | 4 min, 55 s 5 min, 15 s |
| Yield (d.c. to EOB) | - | 0.93 0.93 | 1.00 0.99 | 0.97 0.99 |
| Appearance | Clear colourless solution with no particulate matter | Pass | Pass | Pass |
| pH | 3.5–8.5 | 4.0 4.0 | 4.7 4.4 | 4.5 4.0 |
| Filter integrity | ≥manufacturer specification of 0.34 MPa (50 psi) | Pass | Pass | Pass |
| Radionuclide identity | Half-life: t1/2 = 10 ± 1 min | 9.03 min 9.84 min | 9.86 min 9.91 min | 10.39 min 9.95 min |
| Photopeak: 466 keV < E < 556 keV | Pass | Pass | Pass | |
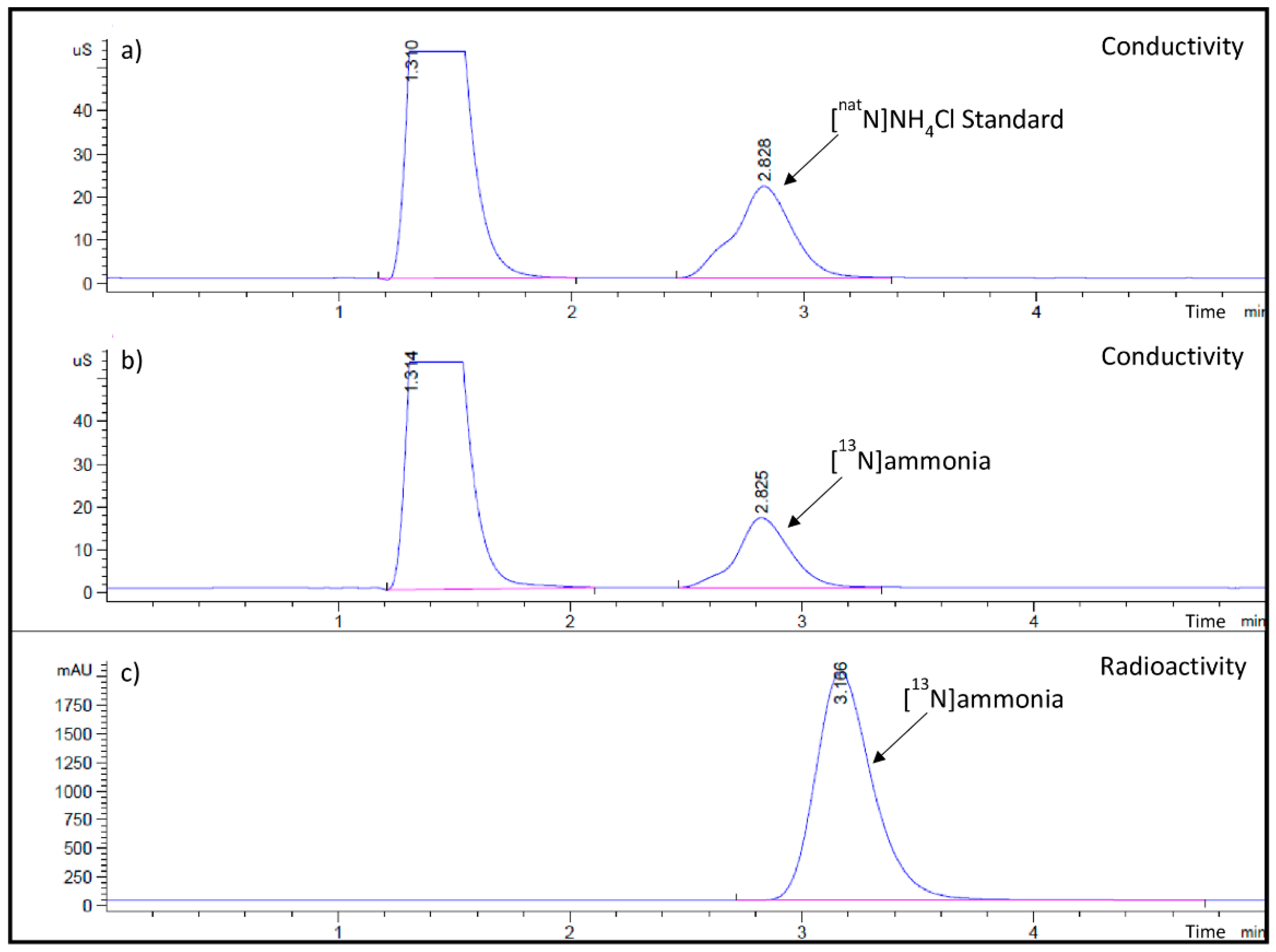
| Radiochemical identity and Purity | HPLC tR 3.0 to 3.8 min | 3.17 min 3.27 min | 3.33 min 3.25 min | 3.26 min 3.19 min |
| Purity ≥ 95% | 99.99% 99.98% | 99.99% 99.99% | 99.98% 99.99% | |
| Radionuclidic purity | Purity ≥ 99.5% | 99.98% 99.92% | 99.99% 99.99% | 99.74% 99.98% |
| Bacterial endotoxins # | ≤175 EU/vial | Pass | Pass | Pass |
| Sterility # | No growth after 14 days | Pass | Pass | Pass |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso Martinez, L.M.; Naim, N.; Saiz, A.H.; Simard, J.-M.; Boudjemeline, M.; Juneau, D.; DaSilva, J.N. A Reliable Production System of Large Quantities of [13N]Ammonia for Multiple Human Injections. Molecules 2023, 28, 4517. https://doi.org/10.3390/molecules28114517
Alonso Martinez LM, Naim N, Saiz AH, Simard J-M, Boudjemeline M, Juneau D, DaSilva JN. A Reliable Production System of Large Quantities of [13N]Ammonia for Multiple Human Injections. Molecules. 2023; 28(11):4517. https://doi.org/10.3390/molecules28114517
Chicago/Turabian StyleAlonso Martinez, Luis Michel, Nabil Naim, Alejandro Hernandez Saiz, José-Mathieu Simard, Mehdi Boudjemeline, Daniel Juneau, and Jean N. DaSilva. 2023. "A Reliable Production System of Large Quantities of [13N]Ammonia for Multiple Human Injections" Molecules 28, no. 11: 4517. https://doi.org/10.3390/molecules28114517
APA StyleAlonso Martinez, L. M., Naim, N., Saiz, A. H., Simard, J.-M., Boudjemeline, M., Juneau, D., & DaSilva, J. N. (2023). A Reliable Production System of Large Quantities of [13N]Ammonia for Multiple Human Injections. Molecules, 28(11), 4517. https://doi.org/10.3390/molecules28114517






