Cellulose Modified with Polyethylenimine (PEI) Using Microwave Methodology for Adsorption of Chromium from Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Adsorbents
2.1.1. FTIR Analysis
2.1.2. SEM Analysis
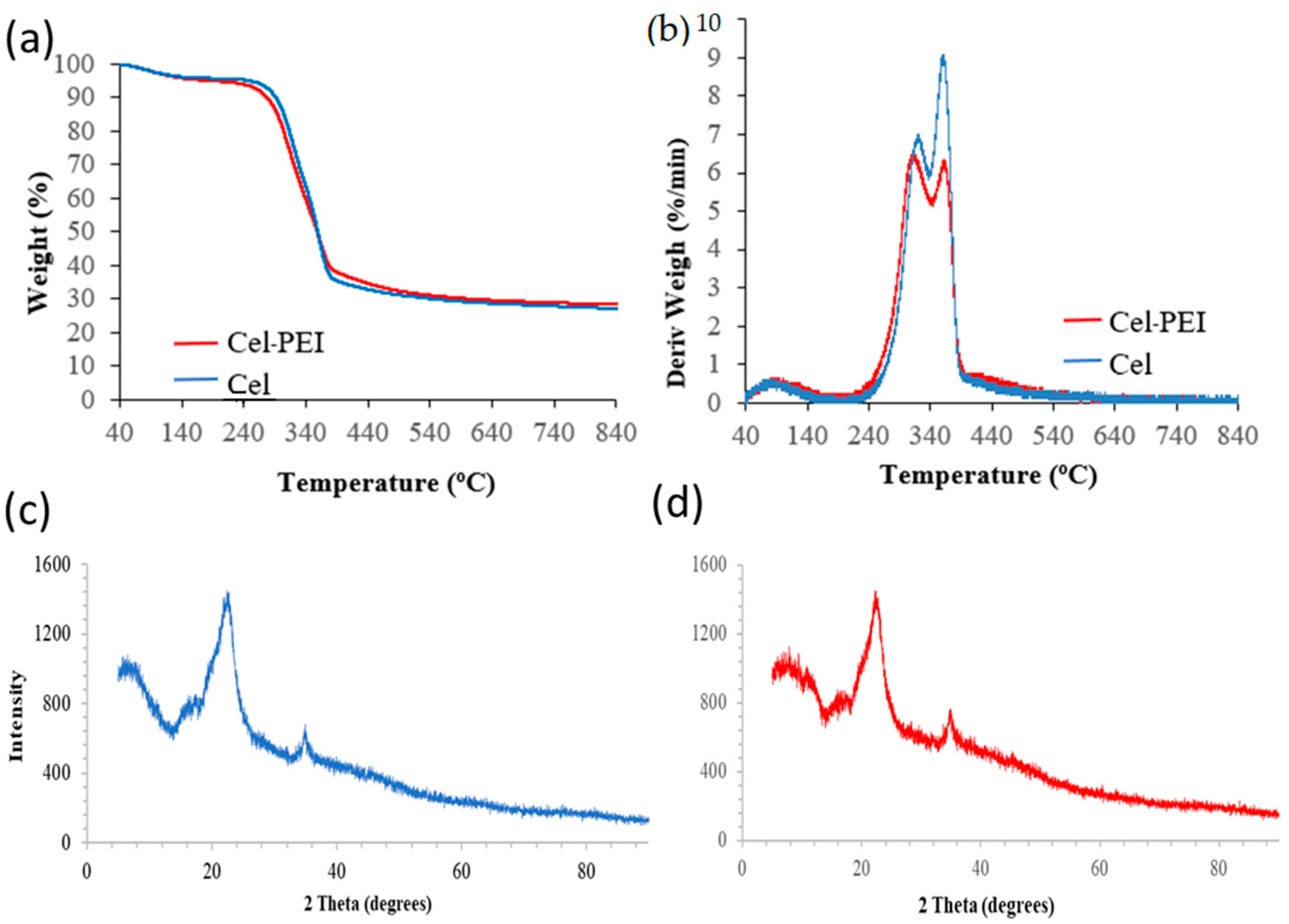
2.1.3. Thermogravimetric and XRD Analyses
2.2. Adsorption Studies
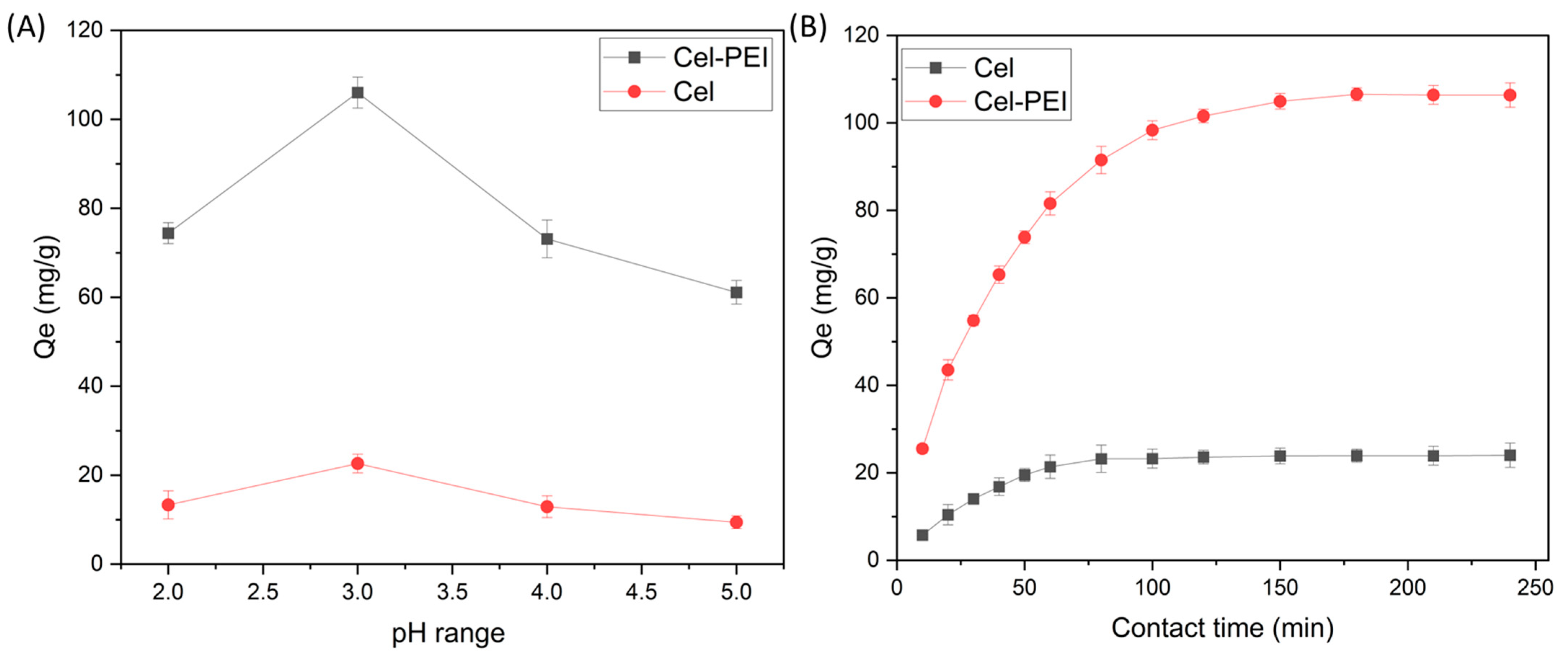
2.2.1. Effect of Solution pH and Time
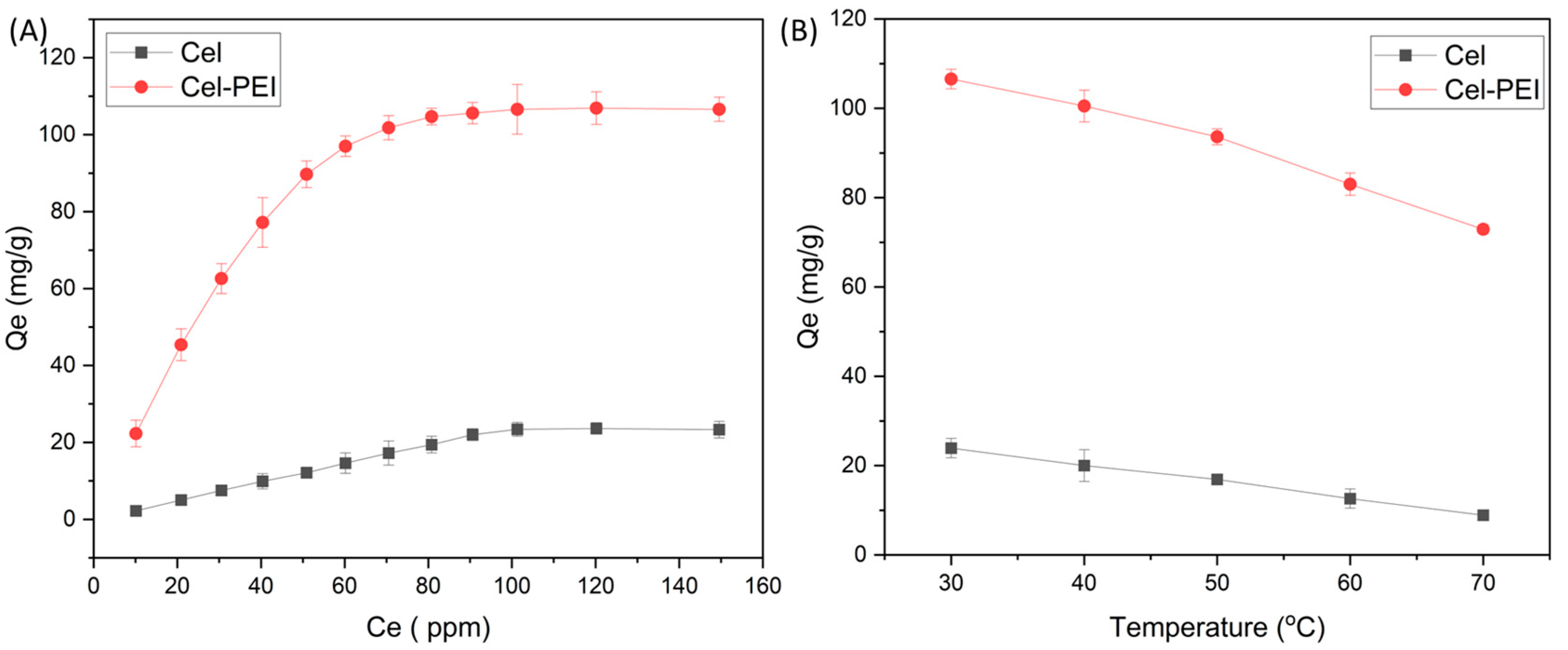
2.2.2. Effect of Initial Concentration of Cr and Adsorbent Dosage
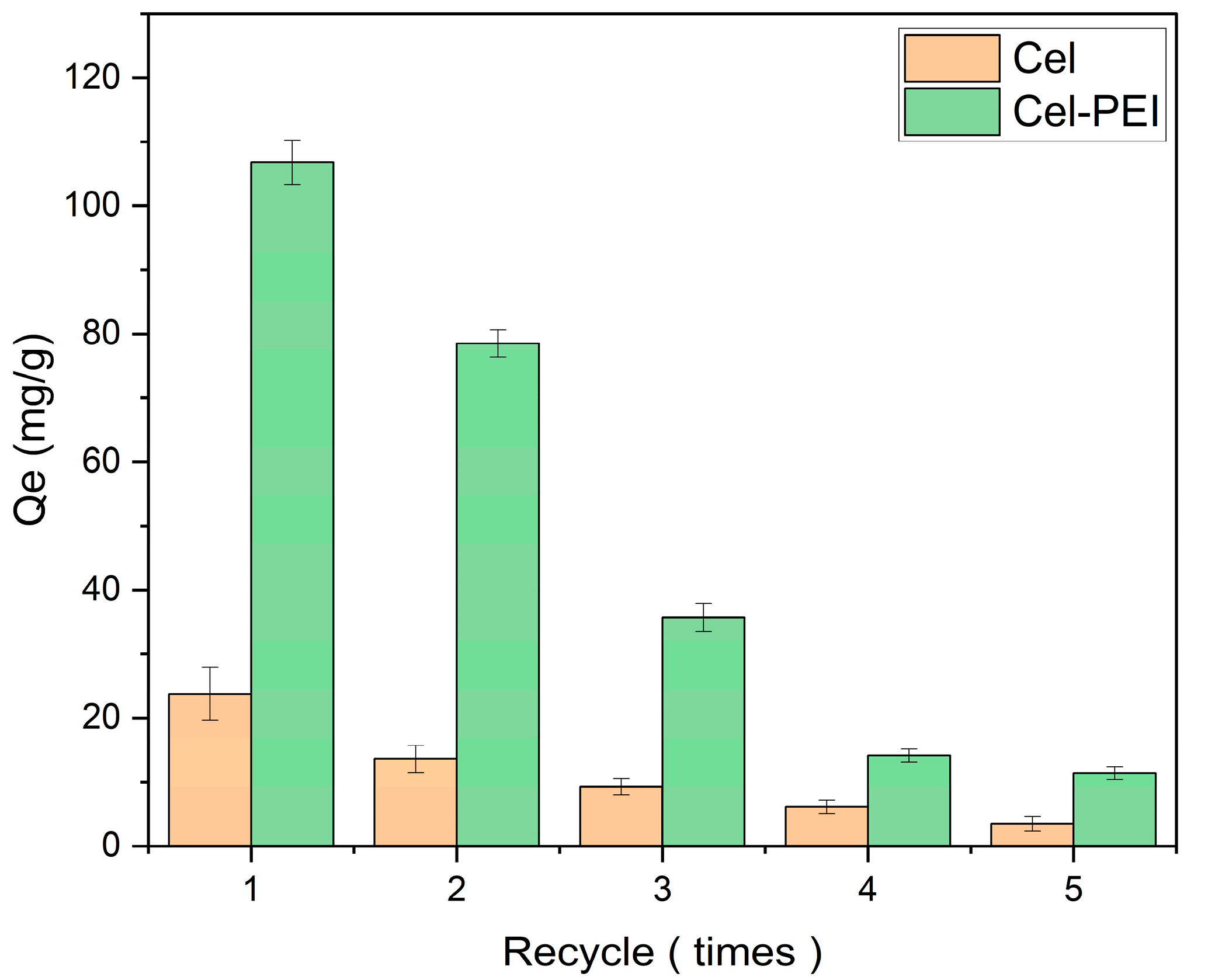
2.2.3. The Efficiency of Adsorbent Recovery
2.2.4. Absorption Isotherm Studies
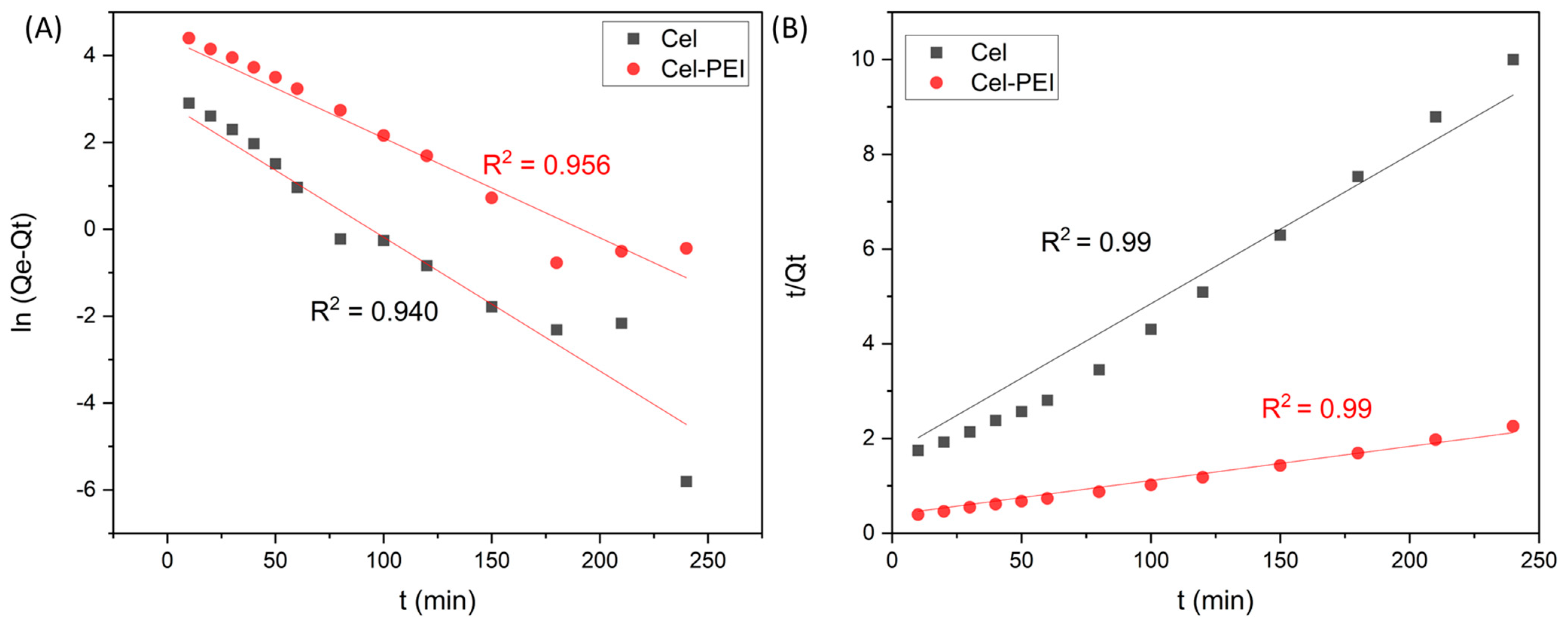
2.2.5. Kinetics of Chromium Adsorption
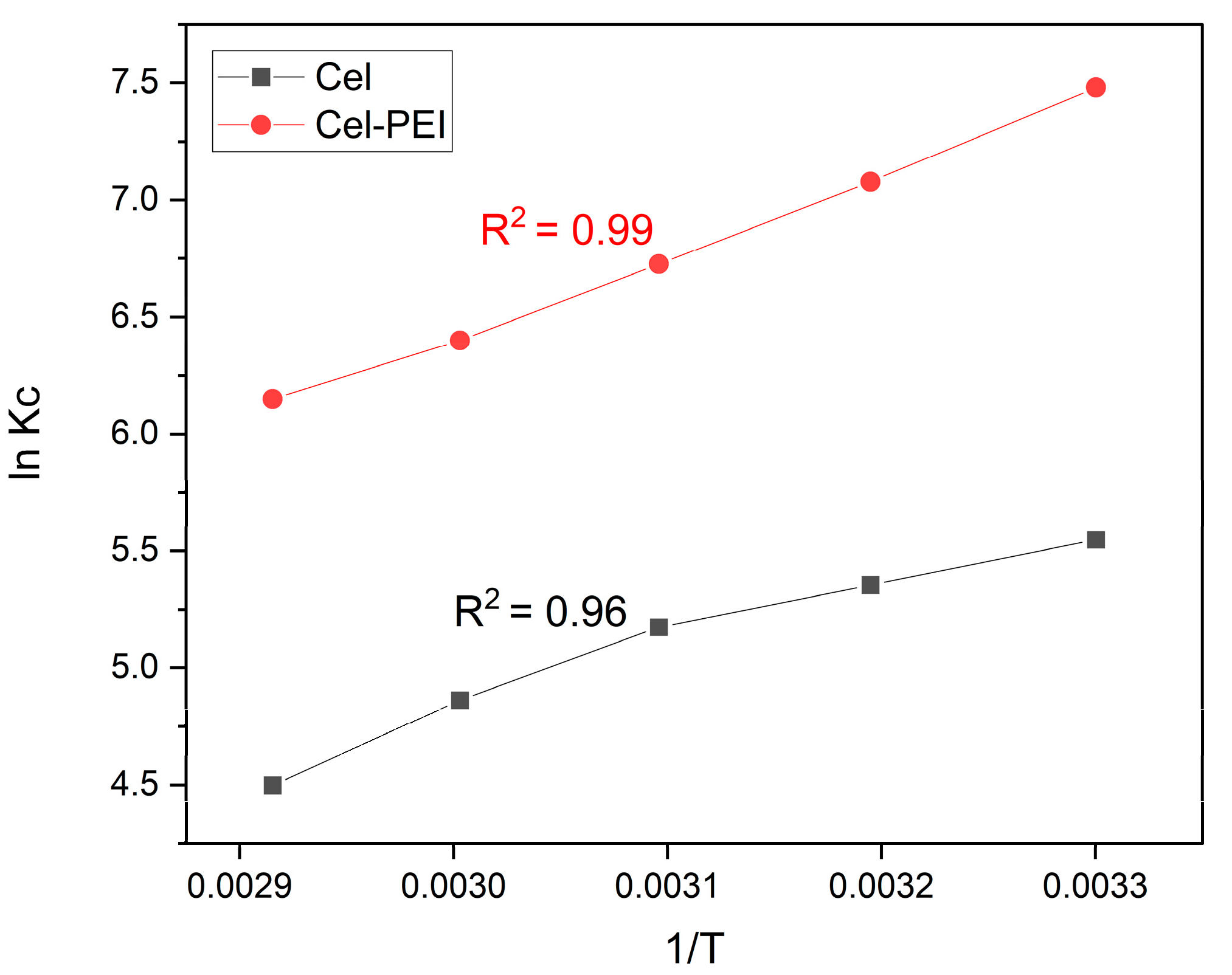
2.2.6. Thermodynamic Studies
3. Materials and Methods
3.1. Materials and Characterization
3.2. Preparation of Cellulose Fibers
Cellulose Fiber Improvement Using Microwave Method
3.3. Study of Factors Affecting the Batch Adsorption of Chromium by the Sorbent Material
3.3.1. pH Value
3.3.2. Adsorption Time
3.3.3. Initial Concentration Affecting Adsorption
3.3.4. Affecting Adsorption Temperature
3.3.5. Amount of Absorbent Material
3.4. Reuse of Absorbent Material
3.5. Analysis of Chromium Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, Q.; Liu, Q.; Wu, Y.; Qiao, C.; Yao, J. Toxic Cr removal from aqueous media using catechol-amine copolymer coating onto as-prepared cellulose. Carbohydr. Polym. 2019, 209, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; He, H.; Zhou, H.; Xue, F.; Zhu, H.; Zhou, S.; Wang, L.; Wang, S. Multiple active sites cellulose-based adsorbent for the removal of low-level Cu (II), Pb (II) and Cr (VI) via multiple cooperative mechanisms. Carbohydr. Polym. 2020, 233, 115860. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Jiang, S.; Huang, J.; Lv, Y.; Liu, Y.; Liu, M. Rapid removal of Cr (III) from high-salinity wastewater by cellulose-g-poly-(acrylamide-co-sulfonic acid) polymeric bio-adsorbent. Carbohydr. Polym. 2021, 270, 118356. [Google Scholar] [CrossRef] [PubMed]
- Tarabanko, N.; Baryshnikov, S.V.; Kazachenko, A.S.; Miroshnikova, A.; Skripnikov, A.M.; Lavrenov, A.V.; Taran, O.P.; Kuznetsov, B.N. Hydrothermal hydrolysis of microcrystalline cellulose from birch wood catalyzed by Al2O3-B2O3. Wood Sci. Technol. 2022, 56, 437–457. [Google Scholar] [CrossRef]
- Mokhena, T.; John, M. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2020, 227, 1149–1194. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, L. Covalently bonded ionic liquid onto cellulose for fast adsorption and efficient separation of Cr (VI): Batch, column and mechanism investigation. Carbohydr. Polym. 2018, 189, 190–197. [Google Scholar] [CrossRef]
- Singh, S.; Arputharaj, E.; Dahms, H.U.; Patel, A.K.; Huang, Y.L. Chitosan-based nanocomposites for removal of Cr (VI) and synthetic food colorants from wastewater. Bioresour. Technol. 2022, 351, 127018. [Google Scholar] [CrossRef]
- Qu, J.; Wang, S.; Jin, L.; Liu, Y.; Yin, R.; Jiang, Z.; Tao, Y.; Huang, J.; Zhang, Y. Magnetic porous biochar with high specific surface area derived from microwave-assisted hydrothermal and pyrolysis treatments of water hyacinth for Cr (VI) and tetracycline adsorption from water. Bioresour. Technol. 2021, 340, 125692. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Lala, M.A.; Thompson-Yusuff, K.A.; Babatunde, E.O. ZnCl2-modified eucalyptus bark biochar as adsorbent: Preparation, characterization and its application in adsorption of Cr (VI) from aqueous solutions. S. Afr. J. Chem. Eng. 2022, 42, 138–145. [Google Scholar] [CrossRef]
- Feng, C.; Ren, P.; Huo, M.; Dai, Z.; Liang, D.; Jin, Y.; Ren, F. Facile synthesis of trimethylammonium grafted cellulose foams with high capacity for selective adsorption of anionic dyes from water. Carbohydr. Polym. 2020, 241, 116369. [Google Scholar] [CrossRef]
- Kim, U.J.; Kimura, S.; Wada, M. Highly enhanced adsorption of Congo red onto dialdehyde cellulose-crosslinked cellulose-chitosan foam. Carbohydr. Polym. 2019, 214, 294–302. [Google Scholar] [CrossRef]
- Neolaka, Y.A.; Lawa, Y.; Naat, J.N.; Riwu, A.A.; Iqbal, M.; Darmokoesoemo, H.; Kusuma, H.S. The adsorption of Cr (VI) from water samples using graphene oxide-magnetic (GO-Fe3O4) synthesized from natural cellulose-based graphite (kusambi wood or Schleichera oleosa): Study of kinetics, isotherms and thermodynamics. J. Mater. Res. Technol. 2020, 9, 6544–6556. [Google Scholar] [CrossRef]
- Rahaman, M.H.; Islam, M.A.; Islam, M.M.; Rahman, M.A.; Alam, S.N. Biodegradable composite adsorbent of modified cellulose and chitosan to remove heavy metal ions from aqueous solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Seid, S.M.; Gonfa, G. Adsorption of Cr (V) from aqueous solution using eggshell-based cobalt oxide-zinc oxide nano-composite. Environ. Chall. 2022, 8, 100574. [Google Scholar] [CrossRef]
- Zheng, Z.; Duan, X.; Tie, J. One-pot synthesis of a magnetic Zn/iron-based sludge/biochar composite for aqueous Cr (VI) adsorption. Environ. Technol. Innov. 2022, 28, 102661. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, G.; Liu, J.; Xiao, Y.; Wang, T.; Xue, Y. Magnetic biochar prepared by electromagnetic induction pyrolysis of cellulose: Biochar characterization, mechanism of magnetization and adsorption removal of chromium (VI) from aqueous solution. Bioresour. Technol. 2021, 337, 125429. [Google Scholar] [CrossRef]
- Georgouvelas, D.; Abdelhamid, H.N.; Li, J.; Edlund, U.; Mathew, A.P. All-cellulose functional membranes for water treatment: Adsorption of metal ions and catalytic decolorization of dyes. Carbohydr. Polym. 2021, 264, 118044. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, K.; Sun, W.; Fu, Z.; Ahmad, H.; Fan, M.; Gao, H. Revealing the adsorption energy and interface characteristic of cellulose-graphene oxide composites by first-principles calculations. Compos. Sci. Technol. 2022, 218, 109209. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Preparation and applications of chitosan and cellulose composite materials. J. Environ. Manag. 2022, 301, 113850. [Google Scholar] [CrossRef]
- Jiang, R.; Zhu, H.Y.; Fu, Y.Q.; Zong, E.M.; Jiang, S.T.; Li, J.B.; Zhu, J.Q.; Zhu, Y.Y. Magnetic NiFe2O4/MWCNTs functionalized cellulose bioadsorbent with enhanced adsorption property and rapid separation. Carbohydr. Polym. 2021, 252, 117158. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, Y.E.; Lee, G.H.; Kim, M.J.; Eom, Y.; Chae, H.G. The effect of cellulose nanocrystals (CNCs) on the microstructure of amorphous polyetherimide (PEI)-based nanocomposite fibers and its correlation with the mechanical properties. Compos. Sci. Technol. 2020, 200, 108452. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, S.B.; Yun, H.J.; Won, S.W. Polyethylenimine-crosslinked chitin biosorbent for efficient recovery of Pd (II) from acidic solution: Characterization and adsorption mechanism. Carbohydr. Polym. Technol. Appl. 2021, 2, 100091. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, S.; Xu, Y.; Wei, N.; Wan, B.; Qian, L.; Liu, Y. A polyethylenimine/salicylaldehyde modified cellulose Schiff base for selective and sensitive Fe3+ detection. Carbohydr. Polym. 2020, 228, 115379. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Guo, Q.; Han, H.; Zhang, S.; Han, R. Waste peanut shell modified with polyethyleneimine for enhancement of hexavalent chromium removal from solution in batch and column modes. Bioresour. Technol. Rep. 2020, 12, 100576. [Google Scholar] [CrossRef]
- Kong, S.H.; Chin, C.Y.J.; Yek, P.N.Y.; Wong, C.C.; Cheong, K.Y.; Liew, R.K.; Lam, S.S. Removal of heavy metals using activated carbon from microwave steam activation of palm kernel shell. Environ. Adv. 2022, 9, 100272. [Google Scholar] [CrossRef]
- Bazzi, L.; Ayouch, I.; Tachallait, H.; Hankari, S.E. Ultrasound and microwave assisted-synthesis of ZIF-8 from zinc oxide for the adsorption of phosphate. Results Eng. 2022, 13, 100378. [Google Scholar] [CrossRef]
- Krishnan, R.; Shibu, S.N.; Poelman, D.; Badyal, A.K.; Kunti, A.; Swart, H.C.; Menon, S.G. Recent advances in microwave synthesis for photoluminescence and photocatalysis. Mater. Today Commun. 2022, 32, 103890. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Ramontja, J. Microwave-assisted green synthesis of xanthan gum grafted diethylamino ethyl methacrylate: An efficient adsorption of hexavalent chromium. Carbohydr. Polym. 2019, 222, 114989. [Google Scholar] [CrossRef]
- Ajravat, K.; Rajput, S.; Brar, L.K. Microwave assisted hydrothermal synthesis of N doped graphene for supercapacitor applications. Diam. Relat. Mater 2022, 129, 109373. [Google Scholar] [CrossRef]
- Qu, J.; Bi, F.; Li, S.; Feng, Z.; Li, Y.; Zhang, G.; Wang, L.; Wang, Y.; Zhang, Y. Microwave-assisted synthesis of polyethylenimine-grafted nanocellulose with ultra-high adsorption capacity for lead and phosphate scavenging from water. Bioresour. Technol. 2022, 362, 127819. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhang, L.; Yang, Y.; Liu, X. Diethylenetriaminepentaacetic acid–thiourea-modified magnetic chitosan for adsorption of hexavalent chromium from aqueous solutions. Carbohydr. Polym. 2021, 274, 118555. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Mäkilä, E.; Willför, S.; Xu, C. Ultralight and porous cellulose nanofibers/polyethyleneimine composite aerogels with exceptional performance for selective anionic dye adsorption. Ind. Crops Prod. 2022, 177, 114513. [Google Scholar] [CrossRef]
- Wang, C.; Okubayashi, S. Polyethyleneimine-crosslinked cellulose aerogel for combustion CO2 capture. Carbohydr. Polym. 2019, 225, 115248. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.J.; Yu, H.; Park, M.; Jeong, H.S. Recovery of platinum from waste effluent using polyethyleneimine-modified nanocelluloses: Effects of the cellulose source and type. Carbohydr. Polym. 2019, 210, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Buljan, I.; Kosanovic, C.; Subotic, B.; Novak Tusăr, N.; Ristic, A.; Gabrovsek, R.; Kaucic, V.; Misĭć Radić, T. Kinetic analysis of isothermal crystallization of potassium aluminosilicate ceramics (leucite and kalsilite) from amorphous potassium aluminosilicate precursors. Cryst. Growth Des. 2010, 10, 838–844. [Google Scholar] [CrossRef]
- Anah, L.; Astrini, N. Influence of pH on Cr (VI) ions removal from aqueous solutions using carboxymethyl cellulose-based hydrogel as adsorbent. IOP Conf. Ser. Earth Environ. Sci. 2017, 60, 012010. [Google Scholar] [CrossRef]
- Barad, J.M.; Kohli, H.P.; Chakraborty, M. Adsorption of hexavalent chromium from aqueous stream by maghemite nanoparticles synthesized by the microemulsion method. Energy Nexus 2022, 5, 100035. [Google Scholar] [CrossRef]
- Menkiti, M.C.; Aniagor, C.O.; Onuzulike, C.M.; Ejimofor, M.I.; Okonkwo, S.S. Chromium adsorption from petroleum refinery wastewater using biocomposites. Surf. Interfaces 2022, 8, 100064. [Google Scholar] [CrossRef]
- Khan, A.; Wang, X.; Gul, K.; Khuda, F.; Aly, Z.; Elseman, A.M. Microwave-assisted spent black tea leaves as cost-effective and powerful green adsorbent for the efficient removal of Eriochrome black T from aqueous solutions. Egypt. J. Basic Appl. Sci. 2018, 5, 171–182. [Google Scholar] [CrossRef]
- Payel, S.; Hashem, M.A.; Hasan, M.A. Recycling biochar derived from tannery liming sludge for chromium adsorption in static and dynamic conditions. Environ. Technol. Innov. 2021, 24, 102010. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Filipkowska, U.; Modrzejewska, Z. Adsorption of the dye Acid Blue 158 premetalized with chromium on chitin/chitosan. Carbohydr. Polym. 2022, 298, 120122. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, I.; Stankovic, D.; Jovic, M.; Markovic, M.; Krstic, J.; Manojlovic, D.; Roglic, G. Microwave-hydrothermal synthesis of TiO2 and zirconium doped TiO2 adsorbents for removal of As (III) and As (V). J. Saudi Chem. Soc. 2015, 19, 429–435. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abd Malek, N.N.; Khadiran, T.; ALOthman, Z.A.; Yaseen, Z.M. Mesoporous high-surface-area activated carbon from biomass waste via microwave-assisted-H3PO4 activation for methylene blue dye adsorption: An optimized process. Diam. Relat. Mater. 2022, 128, 109288. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Xiong, L.; Huang, C.; Zhang, H.; Luo, M.; Tian, L.; Chen, X. Equilibrium, kinetic and thermodynamic studies of acid soluble lignin adsorption from rice straw hydrolysate by a self-synthesized macro/mesoporous resin. RSC Adv. 2017, 7, 23896–23906. [Google Scholar] [CrossRef]
- Nguyen, L.H.T.; Nguyen, H.T.T.; Le, B.Q.G.; Dang, M.H.D.; Nguyen, T.T.T.; Mai, N.X.D.; Doan, T.L.H. Microwave-assisted solvothermal synthesis of defective zirconium-organic framework as a recyclable nano-adsorbent with superior adsorption capacity for efficient removal of toxic organic dyes. Colloids Interface Sci. Commun. 2022, 46, 100511. [Google Scholar] [CrossRef]



| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | b (mL/mg) | R2 | Kf (L/g) | n (L/mg) | R2 | |
| Cel | 81.3008 | 0.0038 | 0.4868 | 2.8471 | 0.9557 | 0.9671 |
| Cel-PEI | 108.6957 | 0.7480 | 0.9997 | 49.6352 | 0.2561 | 0.8168 |
| Absorbent | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| k1 (min−1) | Qe, cal (mg/g) | R2 | k2 (g/mg min) | Qe, cal (mg/g) | R2 | |
| Cel | 0.0322 | 16.28 | 0.9402 | 0.0019 | 33.67 | 0.9909 |
| Cel-PEI | 0.0244 | 74.03 | 0.9596 | 0.0003 | 138.88 | 0.9958 |
| ln Kc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 60 °C | 30 °C | 40 °C | 50 °C | 60 °C | (KJ mol−1) | (KJ mol−1) | |
| Cel | −13.97 | −13.93 | −13.89 | −13.45 | 5.54 | 5.35 | 5.17 | 4.86 | −22.26 | −0.02 |
| Cel-PEI | −19.46 | −18.41 | −18.06 | −17.71 | 7.48 | 7.07 | 6.72 | 6.39 | −36.42 | −0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattarith, K.; Nugroho, D.; Nanan, S.; Benchawattananon, R. Cellulose Modified with Polyethylenimine (PEI) Using Microwave Methodology for Adsorption of Chromium from Aqueous Solutions. Molecules 2023, 28, 4514. https://doi.org/10.3390/molecules28114514
Pattarith K, Nugroho D, Nanan S, Benchawattananon R. Cellulose Modified with Polyethylenimine (PEI) Using Microwave Methodology for Adsorption of Chromium from Aqueous Solutions. Molecules. 2023; 28(11):4514. https://doi.org/10.3390/molecules28114514
Chicago/Turabian StylePattarith, Kongsak, David Nugroho, Suwat Nanan, and Rachadaporn Benchawattananon. 2023. "Cellulose Modified with Polyethylenimine (PEI) Using Microwave Methodology for Adsorption of Chromium from Aqueous Solutions" Molecules 28, no. 11: 4514. https://doi.org/10.3390/molecules28114514
APA StylePattarith, K., Nugroho, D., Nanan, S., & Benchawattananon, R. (2023). Cellulose Modified with Polyethylenimine (PEI) Using Microwave Methodology for Adsorption of Chromium from Aqueous Solutions. Molecules, 28(11), 4514. https://doi.org/10.3390/molecules28114514






