Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production
Abstract
1. Introduction
2. Results and Discussion
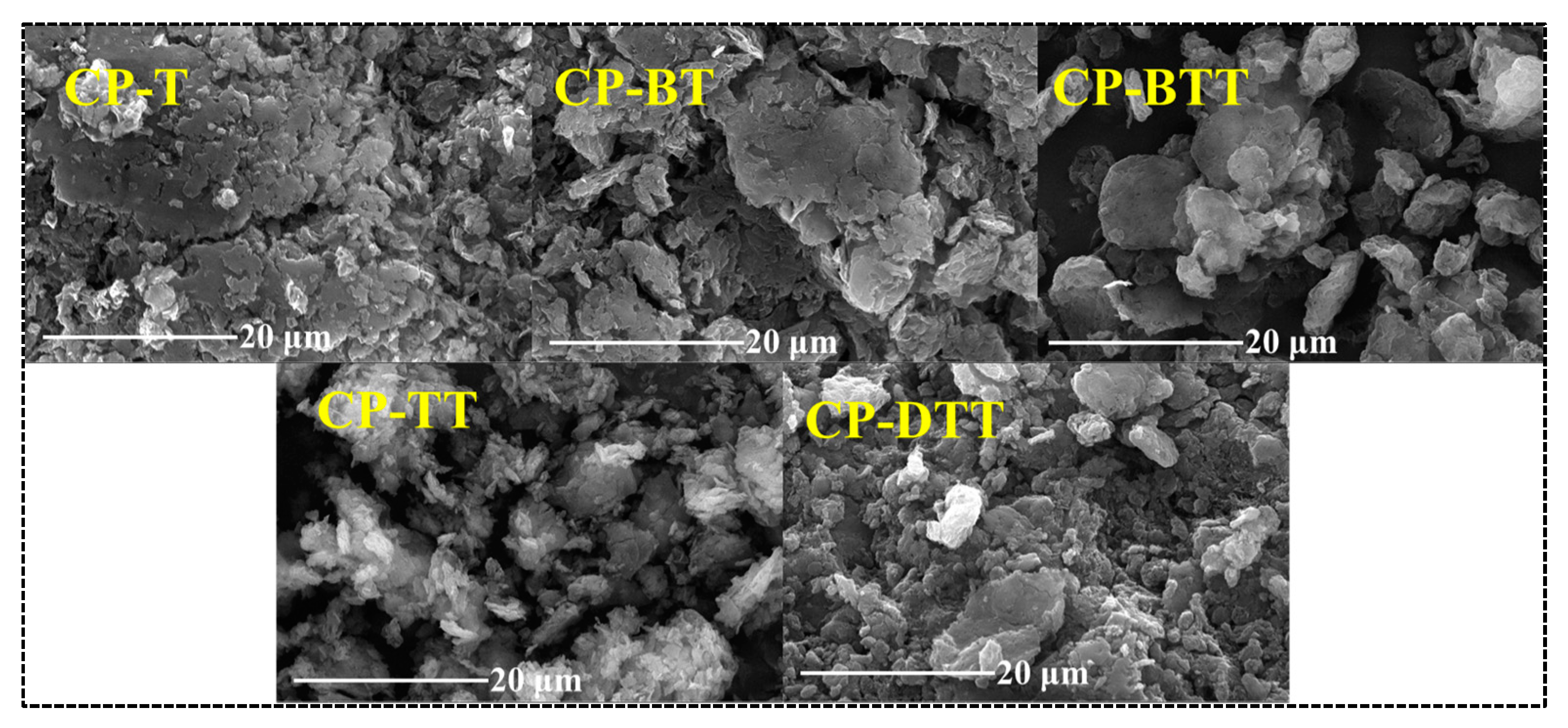
2.1. Synthesis and Characterization of the CPs
2.2. Opto-Electronic Properties of the CPs
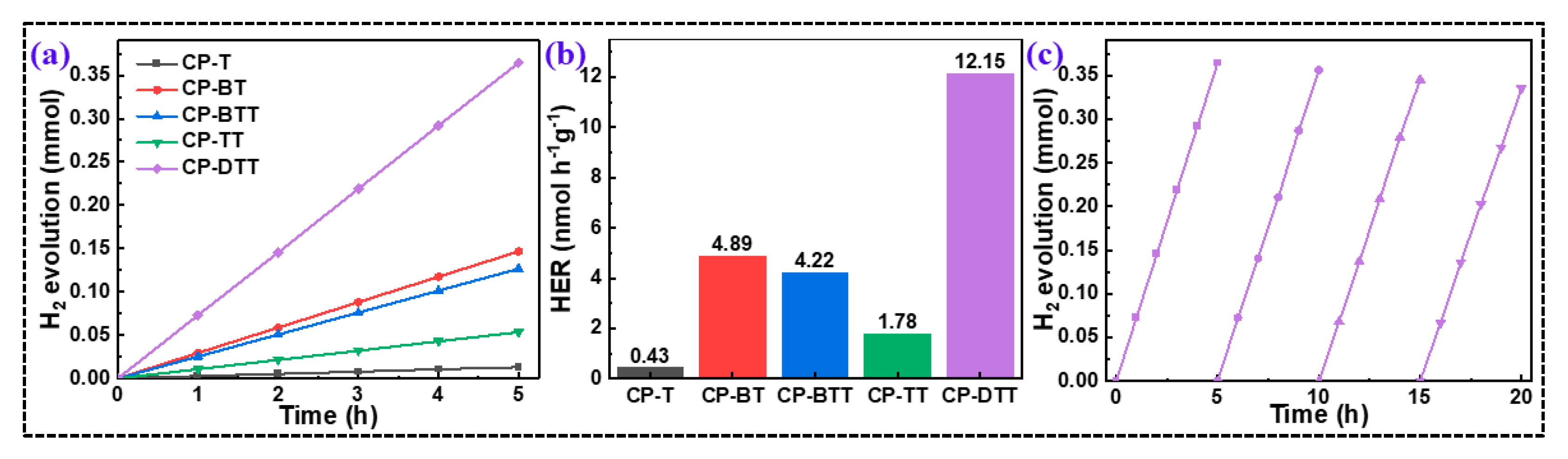
2.3. PHP of the CPs
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthesis of Dibromocyanostilbene (DBCS)
3.3. Synthesis of CPs
3.4. PHP Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yanagida, S.; Kabumoto, A.; Mizumoto, K.; Pac, C.; Yoshino, K. Poly (p-phenylene)-catalysed photoreduction of water to hydrogen. Chem. Commun. 1985, 8, 474–475. [Google Scholar] [CrossRef]
- Sprick, R.; Bonillo, B.; Clowes, R.; Guiglion, P.; Brownbill, N.; Slater, B.; Blanc, F.; Zwijnenburg, M.; Adams, D.; Cooper, A. Visible-light-driven hydrogen evolution using planarized conjugated polymer photocatalysts. Angew. Chem. Int. Ed. 2016, 55, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Sachs, M.; Sprick, R.; Pearce, D.; Hillman, S.; Monti, A.; Guilbert, A.; Brownbill, N.; Dimitrov, S.; Shi, X.; Blanc, F.; et al. Understanding structure-activity relationships in linear polymer photocatalysts for hydrogen evolution. Nat. Chem. 2018, 9, 4968. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Xing, Y.; Cheng, J.; Zhang, G.; Shen, Z.; Zhang, Y.; Liao, G.; Chen, L.; Liu, S. EDOT-based conjugated polymers accessed via C–H direct arylation for efficient photocatalytic hydrogen production. Chem. Sci. 2022, 13, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xiang, S.; Jin, S.; Luo, L.; Zhang, C.; Yan, C.; Jiang, J. Linear multiple-thiophene-containing conjugated polymer photocatalysts with narrow band gaps for achieving ultrahigh photocatalytic hydrogen evolution activity under visible light. J. Mater. Chem. A 2022, 10, 5255–5261. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Wang, F. Dibenzothiophene-S, S-dioxide-containing conjugated polymer with hydrogen evolution rate up to 147 mmol g−1 h−1. Appl. Catal. B Environ. 2022, 307, 121144. [Google Scholar] [CrossRef]
- Sprick, R.; Jiang, J.; Bonillo, B.; Ren, S.; Ratvijitvech, T.; Guiglion, P.; Zwijnenburg, M.; Adams, D.; Cooper, A. Tunable organic photocatalysts for visible-light-driven hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 3265–3270. [Google Scholar] [CrossRef]
- Gao, X.; Shu, C.; Zhang, C.; Ma, W.; Ren, S.; Wang, F.; Chen, Y.; Zeng, J.; Jiang, J. Substituent effect of conjugated microporous polymers on the photocatalytic hydrogen evolution activity. J. Mater. Chem. A 2020, 8, 2404–2411. [Google Scholar] [CrossRef]
- Shu, C.; Han, C.; Yang, X.; Zhang, C.; Chen, Y.; Ren, S.; Wang, F.; Huang, F.; Jiang, J. Boosting the photocatalytic hydrogen evolution activity for D-π-A conjugated microporous polymers by statistical copoly merization. Adv. Mater. 2021, 33, 2008498. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Xiang, S.; Ge, M.; Xie, P.; Zhang, C.; Jiang, J. An efficient electron donor for conjugated microporous polymer photocatalysts with high photocatalytic hydrogen evolution activity. Small 2022, 18, 2202072. [Google Scholar] [CrossRef]
- Saber, A.; Elewa, A.; Chou, H.; EL-Mahdy, A. Donor-acceptor carbazole-based conjugated microporous polymers as photocatalysts for visible-light-driven H2 and O2 evolution from water splitting. Appl. Catal. B Environ. 2022, 316, 121624. [Google Scholar] [CrossRef]
- Stegbauer, L.; Schwinghammer, K.; Lotsch, B. A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem. Sci. 2014, 5, 2789–2793. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Chong, S.; Little, M.; Wu, Y.; Zhu, W.; Clowes, R.; Yan, Y.; Zwijnenburg, M.; Sprick, R.; et al. Sulfone-containing covalent organic frameworks for photocatalytic hydrogen evolution from water. Nat. Chem. 2018, 10, 1180–1189. [Google Scholar] [CrossRef]
- Caballero, R.; Cohen, B.; Gutiérrez, M. Thiophene-based covalent organic frameworks: Synthesis, photophysics and light-driven applications. Molecules 2021, 26, 7666. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, T.; Ma, S.; Zhang, Z.; Wu, G.; Wang, J.; Li, Q.; Xia, H.; Yang, S.; Liu, X. Three-component donor-π-acceptor covalent-organic frameworks for boosting photocatalytic hydrogen evolution. J. Am. Chem. Soc. 2023, 145, 8364–8374. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Fang, W.; Li, L.; Wang, J.; Liang, S.; He, Y.; Liu, M.; Wu, L. Covalent triazine-based frameworks as visible light photocatalysts for the splitting of water. Macromol. Rapid Comm. 2015, 36, 1799–1805. [Google Scholar] [CrossRef]
- Xie, J.; Shevlin, S.; Ruan, Q.; Moniz, S.; Liu, Y.; Liu, X.; Li, Y.; Lau, C.; Guo, Z.; Tang, J. Efficient visible light-driven water oxidation and proton reduction by an ordered covalent triazine-based framework. Energy Environ. Sci. 2018, 11, 1617–1624. [Google Scholar] [CrossRef]
- Huang, W.; He, Q.; Hu, Y.; Li, Y. Molecular heterostructures of covalent triazine frameworks for enhanced photocatalytic hydrogen production. Angew. Chem. Int. Ed. 2019, 58, 8676–8680. [Google Scholar] [CrossRef]
- Hu, X.; Zhan, Z.; Zhang, J.; Hussain, I.; Tan, B. Immobilized covalent triazine frameworks films as effective photocatalysts for hydrogen evolution reaction. Nat. Chem. 2021, 12, 6596. [Google Scholar] [CrossRef]
- Fang, X.; Shang, Q.; Wang, Y.; Jiao, L.; Yao, T.; Li, Y.; Zhang, Q.; Luo, Y.; Jiang, H. Single Pt atoms confined into a metal-organic framework for efficient photocatalysis. Adv. Mater. 2018, 30, 1705112. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Sun, Y.; Chen, Q.; He, L.; Zhang, K.; Zhang, J.; Liu, B.; Chen, L. Design of metal-organic framework-based photocatalysts for hydrogen generation. Coord. Chem. Rev. 2020, 413, 213266. [Google Scholar] [CrossRef]
- Hu, N.; Cai, Y.; Li, L.; Wang, X.; Gao, J. Amino-functionalized titanium-based metal-organic framework for photocatalytic hydrogen production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef] [PubMed]
- Navalón, S.; Dhakshinamoorthy, A.; Álvaro, M.; Ferrer, B.; García, H. Metal-organic frameworks as photocatalysts for solar-driven overall water splitting. Chem. Rev. 2023, 123, 445–490. [Google Scholar] [CrossRef]
- Liang, Z.; Xue, Y.; Wang, X.; Zhang, X.; Tian, J. Structure engineering of 1T/2H multiphase MoS2 via oxygen incorporation over 2D layered porous g-C3N4 for remarkably enhanced photocatalytic hydrogen evolution. MT Nano 2022, 18, 100204. [Google Scholar] [CrossRef]
- Fang, B.; Xing, Z.; Sun, D.; Li, Z.; Zhou, W. Hollow semiconductor photocatalysts for solar energy conversion. APM 2022, 1, 100021. [Google Scholar] [CrossRef]
- Sun, B.; Lu, S.; Qian, Y.; Zhang, X.; Tian, J. Recent progress in research and design concepts for the characterization, testing, and photocatalysts for nitrogen reduction reaction. Carbon Energy 2023, 5, e305. [Google Scholar] [CrossRef]
- Wang, J.; Ouyang, G.; Wang, D.; Li, J.; Yao, J.; Li, W.; Li, H. Enhanced photocatalytic performance of donor-acceptor-type polymers based on a thiophene-contained polycyclic aromatic unit. Macromolecules 2021, 54, 2661–2666. [Google Scholar] [CrossRef]
- Leriche, P.; Piron, F.; Ripaud, E.; Frère, P.; Allain, M.; Roncali, J. Star-shaped triazine-thiophene conjugated systems. Tetrahedron Lett. 2009, 50, 5673–5676. [Google Scholar] [CrossRef]
- Cheng, J.; Tan, Z.; Xing, Y.; Shen, Z.; Zhang, Y.; Liu, L.; Yang, K.; Chen, L.; Liu, S. Exfoliated conjugated porous polymer nanosheets for highly efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2021, 9, 5787–5795. [Google Scholar] [CrossRef]
- Lim, B.; Baeg, K.J.; Jeong, H.G.; Jo, J.; Kim, H.; Park, J.W.; Noh, Y.Y.; Vak, D.; Park, J.H.; Park, J.W.; et al. A new poly(thienylenevinylene) derivative with high mobility and oxidative stability for organic thin-film transistors and solar cells. Adv. Mater. 2009, 21, 2808–2814. [Google Scholar] [CrossRef]
- Ye, D.; Zhang, Y.; Tan, Z.; Xing, Y.; Chen, Z.; Qiu, J.; Liu, S. Tunable cyano substituents in D-A conjugated polymers accessed via direct arylation for photocatalytic hydrogen production. Chem. Commun. 2022, 58, 12680–12683. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Liu, L.; Zhang, Y.; Qiu, J.; Tan, Z.; Xing, Y.; Liu, S. Tunable donor-acceptor linear conjugated polymers involving cyanostyrylthiophene linkages for visible-light-driven hydrogen production. Molecules 2023, 28, 2203. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Zhang, J.; Housekeeper, J.B.; Marder, S.R.; Luscombe, C.K. C–H arylation reaction: Atom efficient and greener syntheses of π-conjugated small molecules and macromolecules for organic electronic materials. Macromolecules 2013, 46, 8059–8078. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Wang, L.; Yu, G. Synthetic strategies, molecular engineering and applications of semiconducting polymers based on diarylethylene units in electronic devices. J. Mater. Chem. C 2022, 10, 18091–18119. [Google Scholar] [CrossRef]
- Huang, X.; Chen, N.; Ye, D.; Zhong, A.; Liu, H.; Li, Z.; Liu, S. Structurally complementary star-shaped unfused ring electron acceptors with simultaneously enhanced device parameters for ternary organic solar cells. Sol. RRL 2023, 7, 2300143. [Google Scholar] [CrossRef]
- Kim, G.; Choi, M.; Song, M.; Jin, S.; Liaw, D.; Wu, H.; Huang, Y.; Ha, C. Synthesis and characterization of new donor-acceptor type copolymers based on fluorene derivatives for photovoltaic solar cells. J. Nanosci. Nanotechnol. 2012, 12, 5735–5741. [Google Scholar] [CrossRef]
- Yan, H.J.; Huang, Y. Polymer composites of carbon nitride and poly(3-hexylthiophene) to achieve enhanced hydrogen production from water under visible light. Chem. Commun. 2011, 47, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dong, C.L.; Zhao, D.; Huang, Y.C.; Wang, X.X.; Samad, L.; Dang, L.N.; Shearer, M.; Shen, S.H.; Guo, L.J. Molecular design of polymer heterojunctions for efficient solar–hydrogen conversion. Adv. Mater. 2017, 29, 1606198. [Google Scholar] [CrossRef]
- Hang, X.H.; Wang, X.P.; Xiao, J.; Wang, S.Y.; Huang, D.K.; Ding, X.; Xiang, Y.G.; Chen, H. Synthesis of 1,4-diethynylbenzene-based conjugated polymer photocatalysts and their enhanced visible/near-infrared-light-driven hydrogen production activity. J. Catal. 2017, 350, 64–71. [Google Scholar]
- Wang, X.P.; Chen, B.; Dong, W.B.; Zhang, X.H.; Li, Z.B.; Xiang, Y.G.; Chen, H. Hydrophilicity-controlled conjugated microporous polymers for enhanced visible-light-driven photocatalytic H2 evolution. Macromol. Rapid Commun. 2018, 40, 1800494. [Google Scholar] [CrossRef]
- Dai, C.; Xu, S.; Liu, W.; Gong, X.; Panahandeh-Fard, M.; Liu, Z.; Zhang, D.; Xue, C.; Loh, K.P.; Liu, B. Dibenzothiophene-S,S-dioxide-based conjugated polymers: Highly efficient photocatalyts for hydrogen production from water under visible light. Small 2018, 14, e1801839. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.A.; Zhang, G.G.; Chen, X.; Zhang, Y.F.; Zhang, K.A.I.; Wang, X.C. Reducing the exciton binding energy of donor–acceptor-based conjugated polymers to promote charge-induced reactions. Angew. Chem. Int. Ed. 2019, 58, 10236–10240. [Google Scholar] [CrossRef]
- Wang, Z.J.; Mao, N.; Zhao, Y.B.; Yang, T.J.; Wang, F.; Jiang, J.X. Building an electron push–pull system of linear conjugated polymers for improving photocatalytic hydrogen evolution efciency. Polym. Bull. 2019, 76, 3195–3206. [Google Scholar] [CrossRef]
- Bai, Y.; Woods, D.C.J.; Wilbraham, L.; Aitchison, C.M.; Zwijnenburg, M.A.; Sprick, R.S.; Cooper, A.I. Hydrogen evolution from water using heteroatom substituted fluorene conjugated co-polymers. J. Mater. Chem. A 2020, 8, 8700–8705. [Google Scholar] [CrossRef]
- Chen, R.K.; Hu, P.W.; Xian, Y.X.; Hu, X.H.; Zhang, G.B. Incorporation of sequence aza-substitution and thiophene bridge in linear conjugated polymers toward highly efficient photo-catalytic hydrogen evolution. Macromol. Rapid Commun. 2022, 43, 2100872. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xiao, Z.H.; Hu, J.H.; Gao, X.F.; Asim, M.; Pan, L.; Shi, C.X.; Zhang, X.W.; Zou, J.J. Rational design of alkynyl-based linear donor−π−acceptor conjugated polymers with accelerated exciton dissociation for photocatalysis. Macromolecules 2022, 55, 5412–5421. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09 (Revision D.01) I; Gaussian: Wallingford, CT, USA, 2013. [Google Scholar]
- Jacquemin, D.; Perpe‘te, E.; Ciofini, I.; Adamo, C.; Valero, R.; Zhao, Y.; Truhlar, D. On the performances of the m06 family of density functionals for electronic excitation energies. J. Chem. Theory Comput. 2010, 6, 2071–2085. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Huang, W.; Shen, Z.; Cheng, J.; Liu, L.; Yang, K.; Wen, H.; Liu, S. C−H activation derived CPPs for photocatalytic hydrogen production excellently accelerated by a DMF cosolvent. J. Mater. Chem. A 2019, 7, 24222–24229. [Google Scholar] [CrossRef]
- Liu, H.; Tao, Y.; Wang, L.; Ye, D.; Huang, X.; Chen, N.; Li, C.; Liu, S. C−H direct arylation: A robust tool to tailor the π-conjugation lengths of non-fullerene acceptors. ChemSusChem 2022, 15, e202200034. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, L.; Zhang, K.; Liu, S. Carbazole and diketopyrrolopyrrole-based D-A π-conjugated oligomers accessed via direct C–H arylation for opto-electronic property and performance study. Molecules 2022, 27, 9031. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.F.; Wang, L.H.; Chen, Y.; Ye, D.N.; Chen, L.; Wen, H.R.; Liu, S.Y. One-Pot Synthesis of 3- to 15-Mer π-Conjugated Discrete Oligomers with Widely Tunable Optical Properties. Chin. J. Chem. 2021, 39, 577–584. [Google Scholar] [CrossRef]




| Photocatalyst | Synthesis Methods | Co-Catalyst | SED | λ (nm) a | HER (mmol h−1 g−1) | Ref. |
|---|---|---|---|---|---|---|
| CP-DTT | DArP | -- | AA | >420 | 12.15 | This work |
| P10 | Suzuki | -- | TEA | >420 | 3.26 | [3] |
| CP4 | DArP | -- | AA/SA | >420 | 0.17 | [29] |
| CP3 | DArP | -- | AA | >420 | 7.60 | [32] |
| P3HT | Ni-catalyzed | Pt | Na2S/ Na2SO3 | >400 | 0.005 | [37] |
| PFBT/CN | Suzuki | Pt | TEOA | >420 | 0.72 | [38] |
| P7-E | Sonogashira | -- | TEOA | >420 | 6.02 | [39] |
| L-PDBT-O | Sonogashira | -- | TEOA | >420 | 4.43 | [40] |
| Flu-SO | Suzuki | -- | TEA | >420 | 5.04 | [41] |
| FSO-FS | Suzuki | -- | TEOA | >420 | 3.40 | [42] |
| PyPm | Suzuki | Pt | TEOA | >300 | 0.37 | [43] |
| p-FuS | Suzuki | -- | TEA | >420 | 5.88 | [44] |
| FSO-TPdT | Suzuki | Pt | TEOA | >420 | 7.39 | [45] |
| PEB−DBT−0.1PY | Sonogashira | -- | TEOA | >420 | 0.54 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, D.; Liu, L.; Peng, Q.; Qiu, J.; Gong, H.; Zhong, A.; Liu, S. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules 2023, 28, 4507. https://doi.org/10.3390/molecules28114507
Ye D, Liu L, Peng Q, Qiu J, Gong H, Zhong A, Liu S. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules. 2023; 28(11):4507. https://doi.org/10.3390/molecules28114507
Chicago/Turabian StyleYe, Dongnai, Lei Liu, Qimin Peng, Jiabin Qiu, Hao Gong, Aiguo Zhong, and Shiyong Liu. 2023. "Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production" Molecules 28, no. 11: 4507. https://doi.org/10.3390/molecules28114507
APA StyleYe, D., Liu, L., Peng, Q., Qiu, J., Gong, H., Zhong, A., & Liu, S. (2023). Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules, 28(11), 4507. https://doi.org/10.3390/molecules28114507









