1. Introduction
With the obvious exception of carbon, it is boron that amongst all the elements boasts the greatest number and diversity of hydride compounds [
1]. These species produce polyhedral cluster assemblies with an ostensible 12-vertex icosahedral size limit. This size constraint, however, can be surpassed by the intimate fusion of two or more clusters to form ‘macropolyhedral’ species, in which constituent subclusters conjoin via shared polyhedral edges or faces [
2]. We are interested in how the chemical modification of such macropolyhedral boron hydride clusters produces changes in their physical properties, and in particular, with regard to the interaction of these molecules with laser light. At very high excitation energies, our investigations hold relevance to the generation of boron–proton plasmas, and, at lower excitation energies, emission from electronically excited states that form the basis for an important class of light sources. Here, there is a renewed interest in the binary boron hydride cluster compound
anti-B
18H
22 1 due to the discovery of its utility as a new class of laser material [
3]. It is also of particular interest as it fluoresces in the elusive blue region of the spectrum, with a quantum yield approaching unity [
4]; it is highly photostable [
3], and it is readily soluble in organic polymer matrices [
3,
5]. These are all factors of general practical benefit regarding the fabrication of optical devices. The photophysics of
1 may be modified by replacing some of its cluster terminal hydrogen atoms with substituents or ligands such as –SH [
6], bromine [
7,
8], iodine [
9], alkyls [
10], and pyridine [
11,
12] to give molecules capable of, for example, environment-sensitive thermochromic luminescence [
11], single-molecule multiple emissions [
7], and the photosensitisation of oxygen [
9]. Recently, metal-catalysed and metal-free nucleophilic substitution of the iodine group in 7-I-B
18H
21 [
9,
13] has been demonstrated [
14], leading to a series of luminescent B-N, B-O, and B-S substituted octadecaborane derivatives [
14]. In addition, a monochlorinated derivative of
1, the blue fluorescent 7-Cl-B
18H
21, has been reported, featuring a quantum yield of luminescence of 0.8 [
15]. Collectively, these entries to the literature are the beginnings of a systematic survey of the functionalisation of
1, from which there are starting to emerge several broader patterns linking the photophysical behaviour of these molecules to their chemical structure. Accompanying these trends are tentative explanations based on, for example, the principles of ‘Total Cluster Volume’ [
10], cluster electron density mapping [
10], and the cluster-position of substitution [
14]. Here, we report an extension to this growing portfolio of compounds by the synthesis of several chlorinated derivatives of
1, the highest yielding examples of which are blue-fluorescent isomers 3,3′-Cl
2-B
18H
20 (
2) and 3,4′-Cl
2-B
18H
20 (
3). Compounds
2 and
3 are the first examples of halogenation of
1 at the B3 or B3′ positions, and thus afford further opportunity to deepen the understanding on what are the chemical factors influencing luminescence from
anti-B
18H
22 and its derivatives.
2. Results and Discussion
Synthesis and structural characterization. Addition of aluminium chloride to room-temperature tetrachloromethane solutions of
anti-B
18H
22 1 results primarily in the formation of a mixture of two of its dichlorinated derivatives, isomers 3,3′-Cl
2-B
18H
20 (
2) and 3,4′-Cl
2-B
18H
20 (
3) (
Figure 1), which, after work-up and purification via sublimation, were obtained in an non-optimised yield of 76%. The mass spectrum of the crude product mixture (
Figure S1) indicates the additional presence of lesser amounts of mono- and trichlorinated derivatives of
1. The generally smaller quantities of these species precluded their full examination, but we were able to collect good NMR, UV-vis, and crystallographic data on some of these minor products that we present later in this manuscript. Temperature and duration of reaction seem to bear an influence on both the rate at which the chlorinated derivatives are formed as well as the ratio in which they are produced. Thus, typically, a room temperature reaction requires a period of 4–5 days for the complete consumption of
1. The same synthesis carried out at 55 °C gives a full conversion to products in only 90 min, whereas at an ice-bath temperature of 2 °C a period of 30 days is required. The product mix ratios (
Table 1) were quantitatively determined by analytical HPLC and an analysis of the
11B NMR spectra of the reaction mixtures and, in particular, a comparison of the integrations of the singlets at δ(
11B) +23.1 ppm for the substituted B3 and/or B3′ position(s), and at −24.3 ppm for the substituted B4 and/or B4′ position(s) (
Figure S2). These ratios show that at higher temperatures, substitution at the B4 and/or B4′ position(s) becomes more probable, and the formation of compound
3 and trichlorinated derivatives
9 and
10 are therefore more likely. Lower temperatures or shorter reaction times boost the amount of monochlorinated derivatives
7 and
8 in the product mix (primarily
7), and longer reaction times, over a certain temperature, support trichlorination and the formation of
9 and
10 (primarily
9).
HPLC, fractional sublimation, and crystallization were used to separate the various products of chlorination of 1. Our discussion of these new species begins with the two main products, dichlorinated 2 and 3.
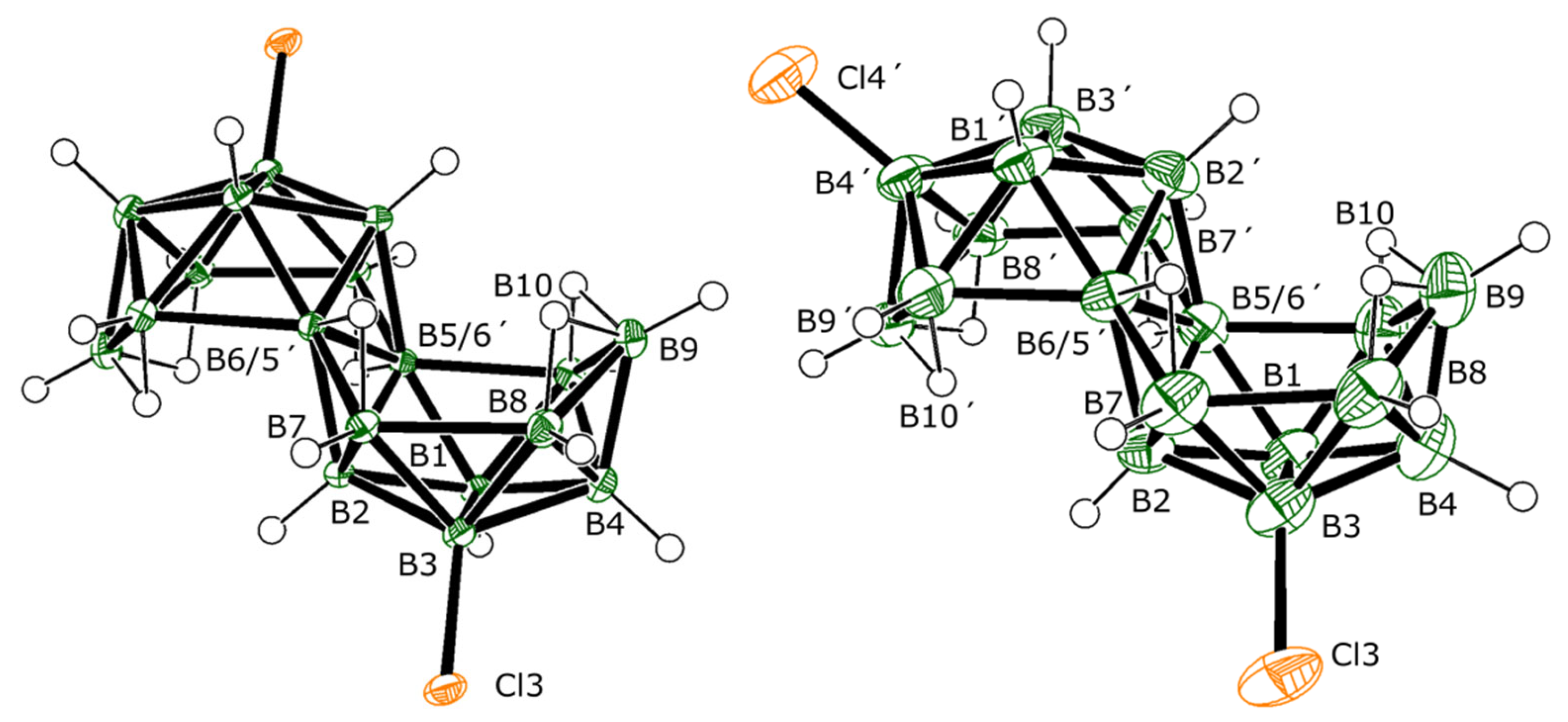
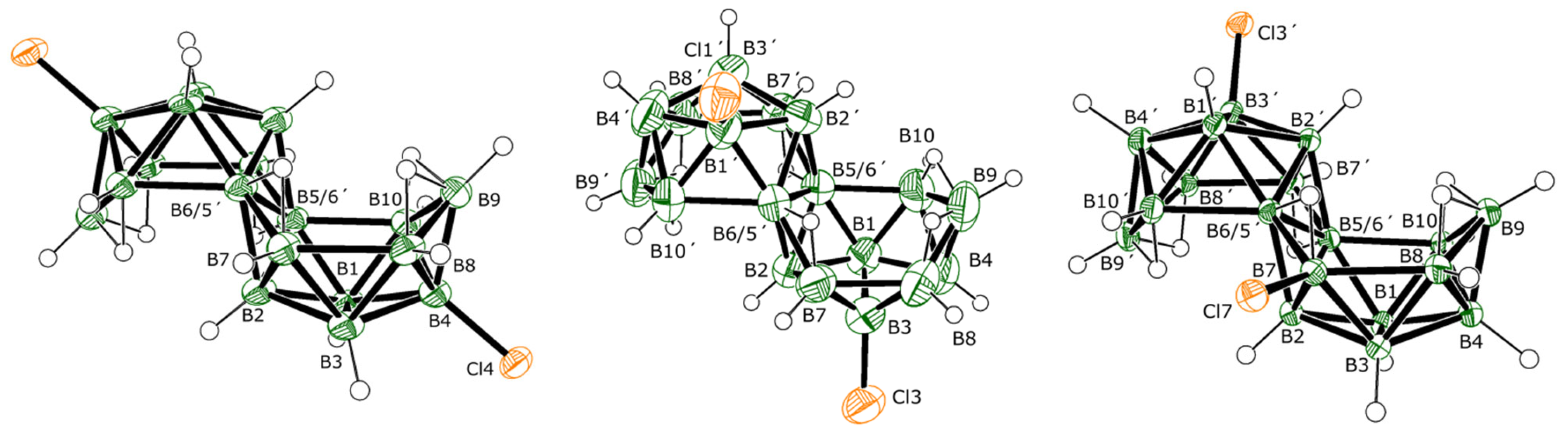
The molecular structures of
2 and
3, as determined by single-crystal X-ray diffraction studies (SCXRD), are shown in
Figure 1. Both isomers preserve the classical octadecaborane polyhedral architecture of
1, with no perturbation of significance in any of the B-B connectivities. Equally, there is very little difference between B-Cl bond lengths for the different substituent positions that all measure approximately 1.8 Å, which is a similar value to B-Cl bonds in other known macropolyhedral borane systems [
16,
17]. Isomers
2 and
3 provide identical mass spectra with
m/
z 284.32, due to their [B
18H
19Cl
2]
− ions (
Figure S5), but may be distinguished by their different absorption spectra and retention times when passed through an HPLC system prior to injection into the mass spectrometric device (
Figure S6). The relative positions of substitution in isomers
2 and
3 result in the preservation of
Ci symmetry in
2 and asymmetry in
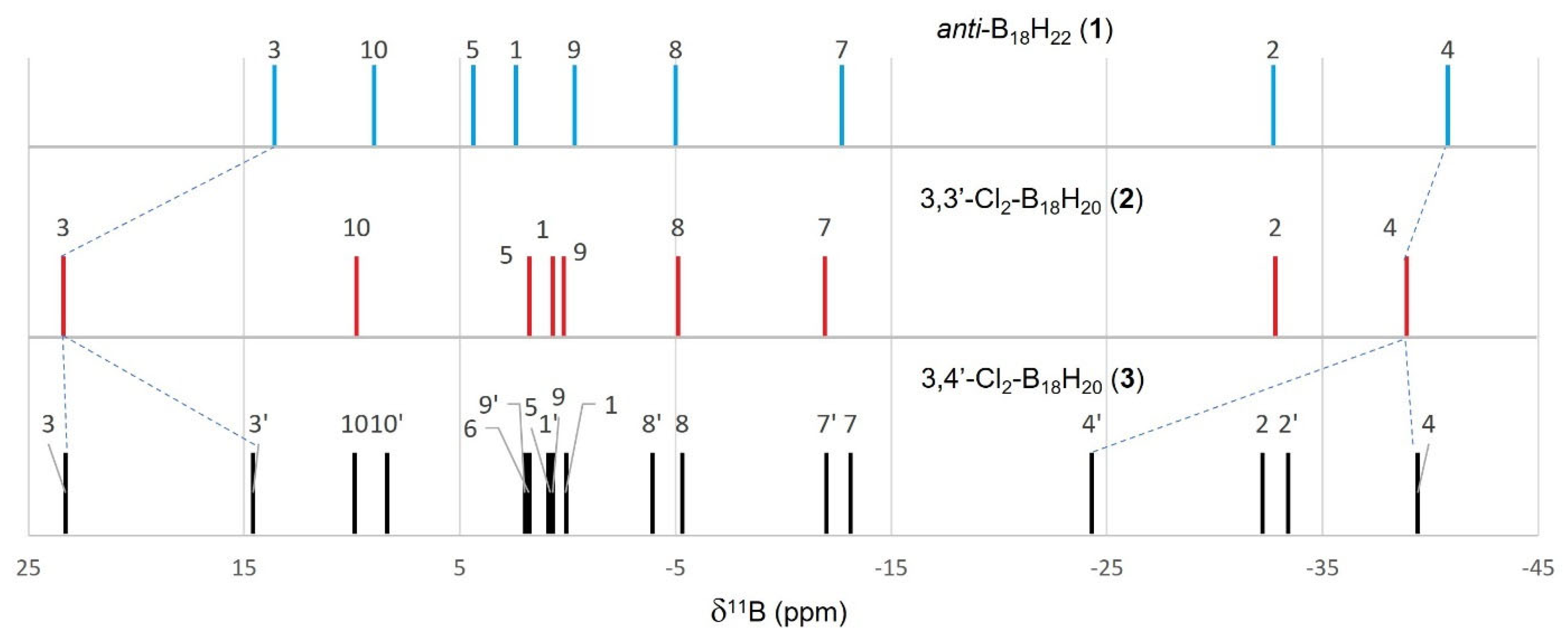
3. These differences are clearly seen in the NMR data for the compounds (see
Figure S7 vs.
Figure S8 for direct comparison, and
Figure 2 for wider comparison with all chlorinated derivatives of
1 described in this manuscript).
Table 2 shows a listing of the measured and calculated NMR data for compound
2 that are entirely consistent with its
Ci symmetric molecular structure shown in
Figure 1. The excellent correlation between the measured data and those calculated for the molecular configuration shown in
Figure 1 provide further weight to this structure being representative of the bulk sample of
2. The degree of perturbation from the δ(
11B) chemical shifts for parent compound
1 is small for
2, with only the chlorine-substituted B(3,3′) chemical shift changing significantly (
Figure 3). The chlorine atoms deshield the B(3,3′) nuclei, shifting the resonance downfield by about 10 ppm.
Table 3 lists the measured and calculated NMR data for compound
3. The asymmetry of
3 is immediately apparent in its
11B NMR spectrum, which displays 18 peaks. These peaks may be conveniently split into two sets of nine resonances for each of the molecule’s two subclusters: {3-Cl-B
9} and {4′-Cl-B
9}. The nine peaks for the {3-Cl-B
9}-subcluster essentially overlap with those for the symmetrical compound
2. The {4′-Cl-B
9}-subcluster, on the contrary, displays change; the B3′-H peak shifts upfield by about 9 ppm to a position similar to that in parent compound
1, and there is a roughly 16 ppm downfield shift of the B4′-Cl peak (see dotted lines in
Figure 3).
Overall, the
11B NMR properties of compounds
2 and
3 suggest them to be the same two dichlorinated
anti-B
18H
20Cl
2 species mentioned, but structurally undefined and uncharacterised, in a previous report describing the chlorination of
1 with SO
2Cl
2 [
15].
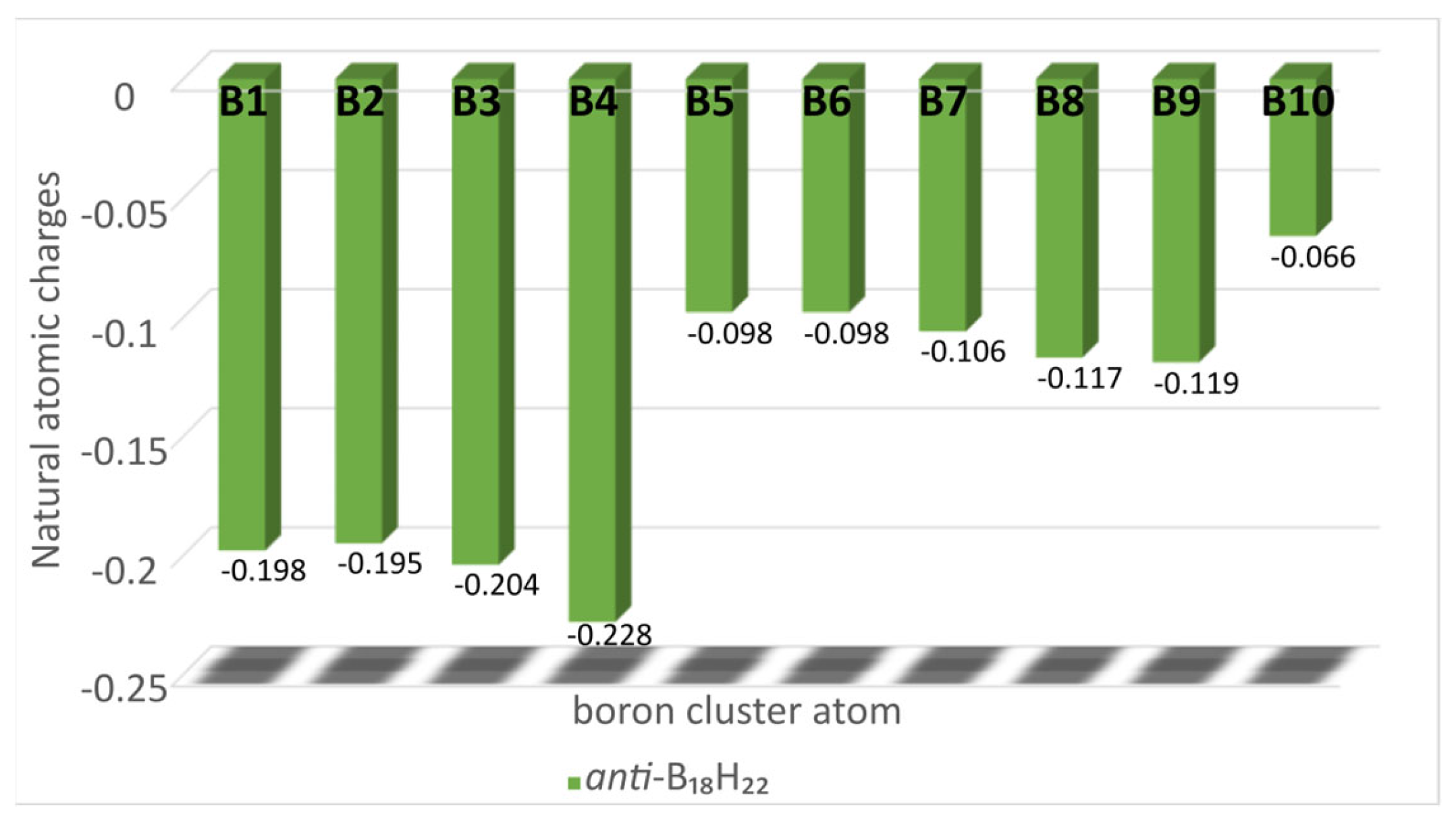
The charge distribution in
anti-B
18H
22 is such that the basal boron atoms B(1), B(2), B(3) and, in particular, B(4) (see
Figure 4, and
Figure 1 for numbering) possess the greatest share of the available electron density. This distinction is apparent in the products of electrophilic substitution of
1 recorded in the literature so far. Thus, it is the case for recently published brominated and iodinated derivatives of
1 that, under similar reaction conditions to those used here, bromination results mainly in the monohalogenated product 4-Br-B
18H
21 (although a partially characterised dibrominated derivate 4,4′-Br
2-B
18H
20 was also observed in mass spectrometric analysis and NMR) [
7], and iodination to high yields of the dihalogenated species 4,4′-I
2-B
18H
20. (n.b. the reaction of the dianionic form of
1 with iodine in alcohol solutions gives the monosubstituted 7-I-B
18H
21) [
9]. It is therefore apparent from this work that the chlorination of
1 is far more versatile, and the substitution of its octadecaborane cluster is more multi-directed than is the case for bromination or iodination, in particular with regard to the activation of the B3 cluster position towards substitution. The relatively lower energy of the 3,3′-Cl
2-B
18H
20 isomer
2, calculated at 2.3 kcal/mol, would, all other things being equal, lead to a Boltzmann distribution of 98% for
2 and 2% for isomer
3. However, the considerably more negative charge associated with the B(4 and 4′) cluster atoms (see
Figure 4) presumably competes with the differences in zero-point total energies, especially at higher temperatures, leading to the observed ratios of
2:
3 formed during synthesis at 55 °C.
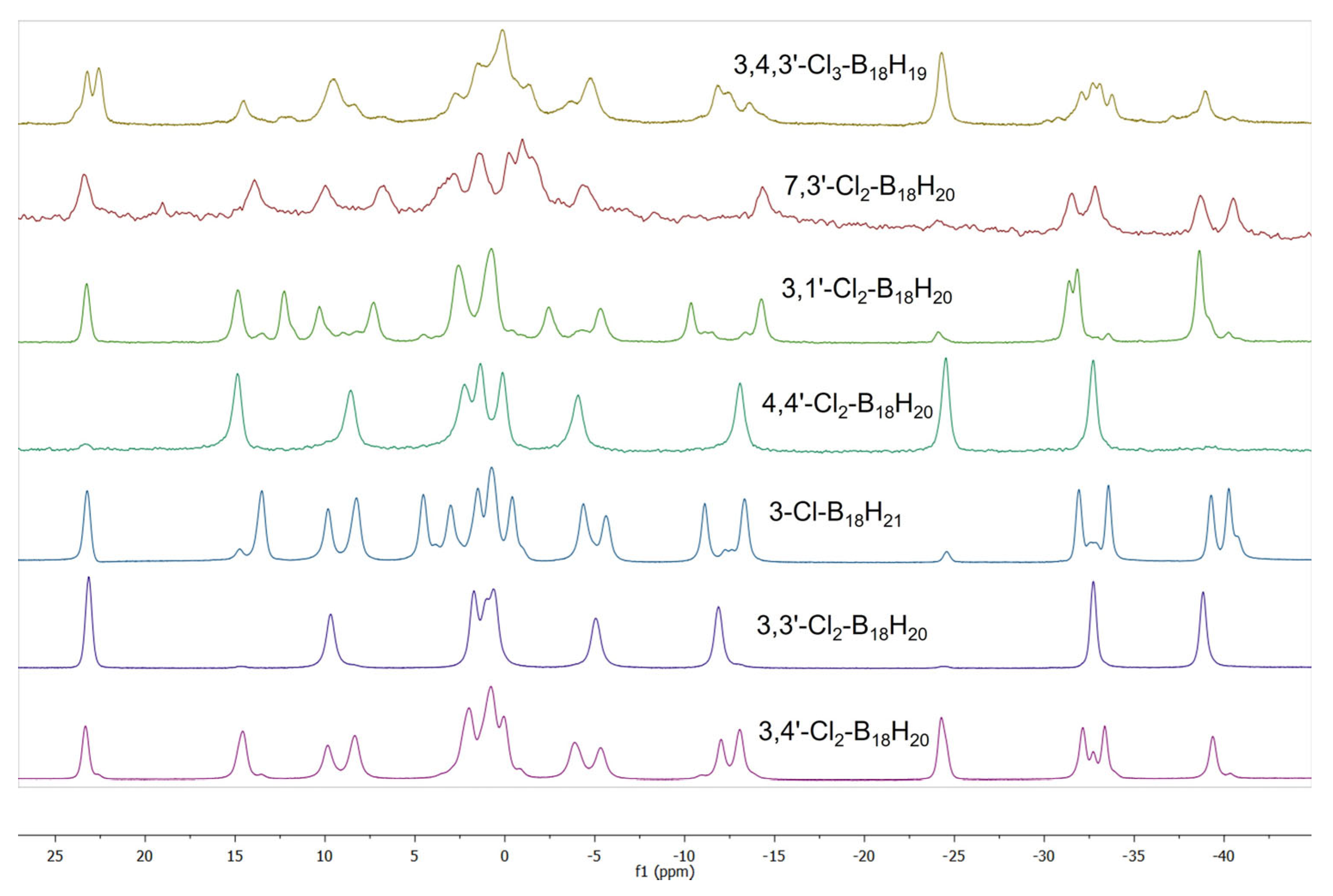
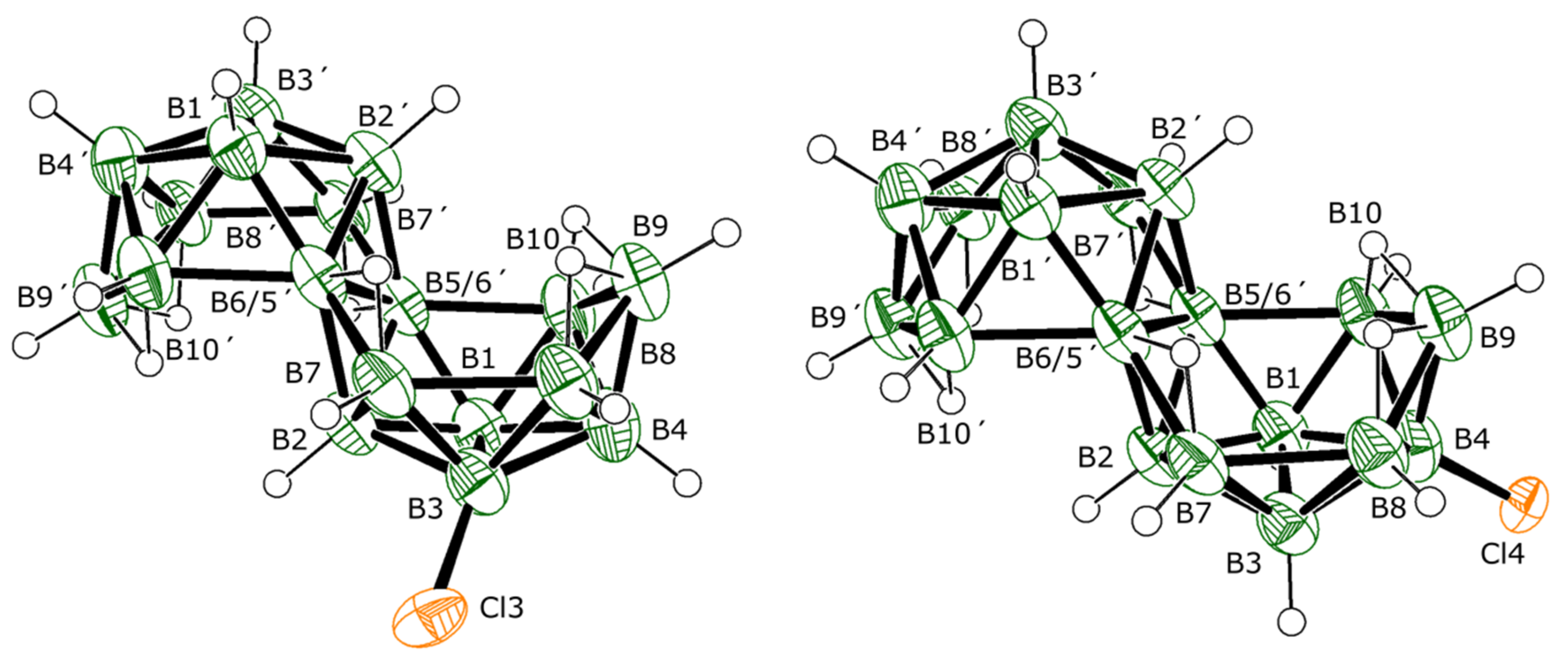
Above and beyond the two main products from this reaction, compounds
2 and
3, an effort was made to isolate and characterise the lower-yielding side products. Laborious work involving HPLC separation and identification using mass spectrometry and UV absorption spectroscopy resulted in the imperfect isolation of an additional six chlorinated derivatives of
1. Of these six compounds, three are further isomers of dichlorinated derivatives of
1, specifically 4,4′-Cl
2-B
18H
20 (
4), 3,1′-Cl
2-B
18H
20 (
5), and 7,3′-Cl
2-B
18H
20 (
6). The molecular structures of each of these isomers were established by SCXRD analyses, the results of which are shown in
Figure 5.
These molecular structures were supported by mass spectrometry and, in the cases of
4 and
5, a comparison of measured NMR data with calculated NMR chemical shifts for specific optimised molecular structures (
Table 4 and
Table 5). Unfortunately, only
11B NMR data were collected for compound
6, which was isolated as a single crystal in one of many fractional sublimation procedures used to purify other dichlorinated species. These measured data nevertheless match well with those calculated for the
11B chemical shifts (
Table 6) of a molecular geometry for
6 shown in
Figure 5.
Two mono-chlorinated derivatives of
1, 3-Cl-B
18H
21 (
7) and 4-Cl-B
18H
21 (
8), were also observed. Although the isolation of these monochlorinated derivatives from other, multichlorinated, compounds was facile, the complete separation of
7 from
8 proved too difficult. However, SCXRD studies on the solid solutions of these compounds resulted in the molecular structures shown in
Figure 6. Again, the octadecaborane cluster structure of
1 is unperturbed; however, the B-Cl distances appear to be about 0.1 Å shorter than those lengths for the dichlorinated species described here, although this may be primarily due to the disorder present in the solid solution.
Analytical chromatography of the multi-chlorinated mixture led to the separation of compounds
7 and
8 from the other multi-chlorinated species. Initial separations generally gave a mixture of
7 and
8 with a mass spectrum of
m/z 250.32, due to their [B
18H
20Cl]
− ions (
Figure S16). NMR of these mixtures indicated the presence of about 85% of 3-Cl-B
18H
21 (
7) to 15% of 4-Cl-B
18H
21 (
8), based on an integration of the B3-Cl singlet peak (located at δ(
11B) +23.2 ppm) for the former and the B4-Cl singlet peak (located at −24.5 ppm) for the latter compound. Crystals (of the three samples measured by SCXRD) of this sample that were obtained via sublimation show, however, a reversal of this ratio (approximately 75% of
8 to 25% of
7, with small variations in different crystals), suggesting that
8 is the more volatile of the two isomers. Indeed, this physical difference enabled the effective separation of
8 from
7 via careful sublimation and the isolation of the 3-Cl derivative in good purity for NMR and UV-vis studies. There is a very good correlation between measured NMR data for
7 and chemical shifts calculated for the monochlorinated 3-Cl-B
18H
21 structure (
Table 7).
Finally, an attempt was made to determine the location of the chlorine substituents on the very small amount of isolated trichlorinated species using a comparison of the collected experimental
11B NMR spectroscopic data with the computed chemical shifts for a series of seven trichlorinated isomers with the lowest zero-point corrected total energies, as shown in
Table 8.
Figure S18 shows the measured
11B NMR spectrum of the HPLC-separated fraction with a single mass spectrometric peak at
m/
z 319.22 (
Figure S19), which computes precisely to the expected mass of [Cl
3-B
18H
18]
−. This spectrum appears to comprise two molecular species present in an approximate 2:1 ratio.
Figures S20–S33 and
Tables S20–S33 are the calculated spectra for all of the seven lowest energy trichlorinated isomers listed in
Table 8. A comparison of these measured and calculated data suggest that the main component is very likely 3,4,3′-Cl
3-B
18H
19 (
9) and the minor component most probably 3,4,4′-Cl
3-B
18H
19 (
10). However, in absence of any reliable crystallographic data, these are tentative assignments. The measured and calculated NMR data for 3,4,3′-Cl
3-B
18H
19, compound
9, are shown in
Table 9.
A detailed comparison of the molecular geometry of these various chlorinated clusters, together with the parent
anti-B
18H
22 (
1) [
18], shows that the B–B (and B–Cl) bond lengths are rather insensitive to the substituent positions. Because structural disorder has an averaging effect on parts of the molecules in which disorder is not resolved and twinning adversely affects precision, such an analysis can be carried out reliably only on ordered structures, which here means one form of compound
2 (not the forms in the Supporting Information), and compounds
3,
4, and
6; two of these (
2 and
4) have more than one crystallographically independent molecule, all of which can be included in the comparison. A full list of bond lengths and their differences from the corresponding bonds in compound
1 are given in
Table S38. These differences between the substituted and parent clusters range from −0.045 Å to +0.034 Å, while the total range of all B–B bond lengths is 0.294 Å (from 1.706 to 2.002 Å, with individual crystallographic standard uncertainties between about 0.002 and 0.02 Å); they follow no clear pattern relative to the positions of substitution. We therefore conclude that the cluster bonding is essentially unaffected by chlorination.
Photophysical properties of some of the chlorinated derivatives of 1. As mentioned in the Introduction, compound
1 [
3,
4,
5] and its derivatives [
6,
7,
8,
9,
10,
11,
12,
14,
15,
16] display various modes of useful luminescence. As such, we were keen to understand the photophysics of the new chlorinated derivatives of
1 reported here.
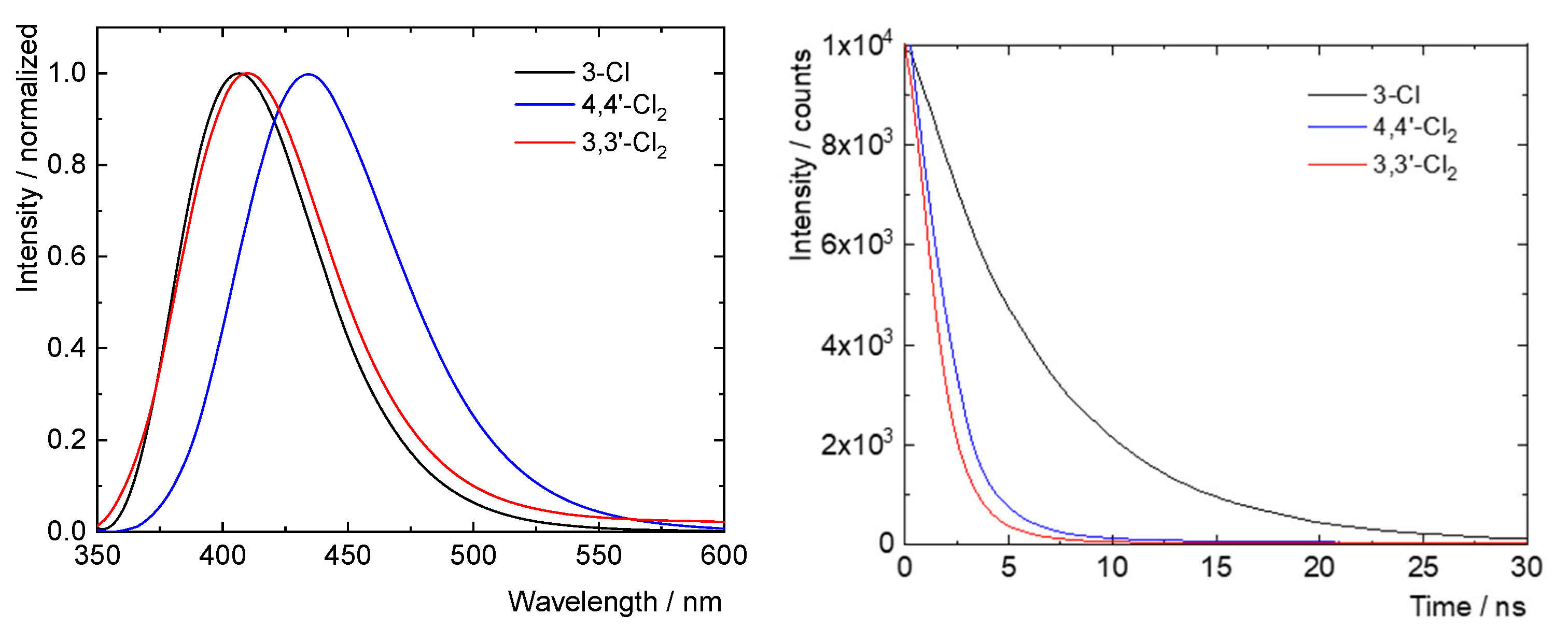
Table 10 and
Figure 7 show the main photophysical data recorded for new compounds
2,
4, and
7. Absorption and excitation spectra for these species may be found in the SI (
Figures S34–S36). Overall, there is little difference in the absorption and emission maxima for these derivatives, which all absorb in the UV-A region (absorption maxima in the 324–344 nm range) and emit in the blue region (emission maxima in the 406–435 nm range). Thus, chlorination seems to have only a small influence over the relative energies of the contributing HOMO and LUMO systems. However, from these data a trend is apparent that increasing the number of chlorine substituents both shortens the lifetimes of the fluorescent excited states (
τL) and decreases the quantum yield of fluorescence (
ΦL). So, for example, mono-chlorinated derivative
7 has about half of the fluorescent lifetime of parent compound
1 and approximately half of its
ΦL, and dichlorinated
2 has just over a tenth of the fluorescent lifetime of
1 and roughly a twelfth of its
ΦL.
A recent article by Spokoyny et al. [
15] proposes that the location of substituents on the octadecaborane cluster plays a crucial role in the efficiency of emission from the molecule (its quantum yield of luminescence). In their paper [
15], a comparison was made between 7-I-B
18H
21 and 4-I-B
18H
21 isomers, with the former displaying a two-fold greater quantum yield of phosphorescence. The mono-chlorinated species 7-Cl-B
18H
21 (
11) is also reported, the photophysical properties of which are also shown here in
Table 10. Comparison of the data for
7 and
10 reveals that chlorination of the basal B3 position in
7 leads to a roughly 25% shorter fluorescent lifetime and 25% lower quantum yield than in
11, where chlorination is at the open-face ‘gunwale’ B7 position. A further interesting comparison is between the photophysics of compounds
2 and
4: although both species have a similarly short excited-state lifetime, compound
4 has more than twice the quantum yield of fluorescence than
2. This suggests that chlorination (and by tentative extrapolation, substitution) of
1 at the B4/4′ positions reduces the efficiency of fluorescence less than when at the B3/3′ cluster sites.