Naringenin Induces HepG2 Cell Apoptosis via ROS-Mediated JAK-2/STAT-3 Signaling Pathways
Abstract
1. Introduction
2. Results and Discussion
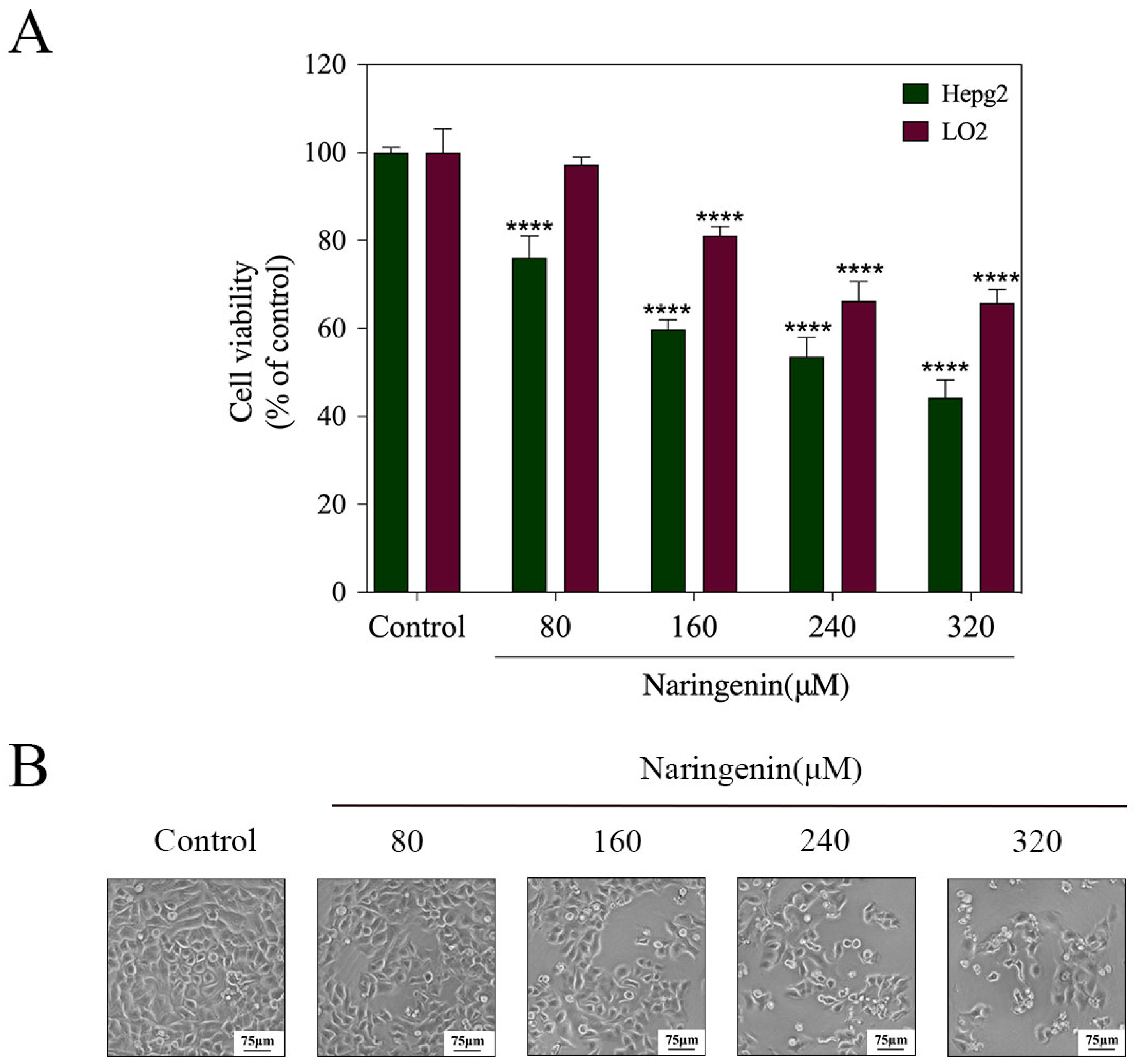
2.1. In Vitro Cytotoxicity of Naringenin
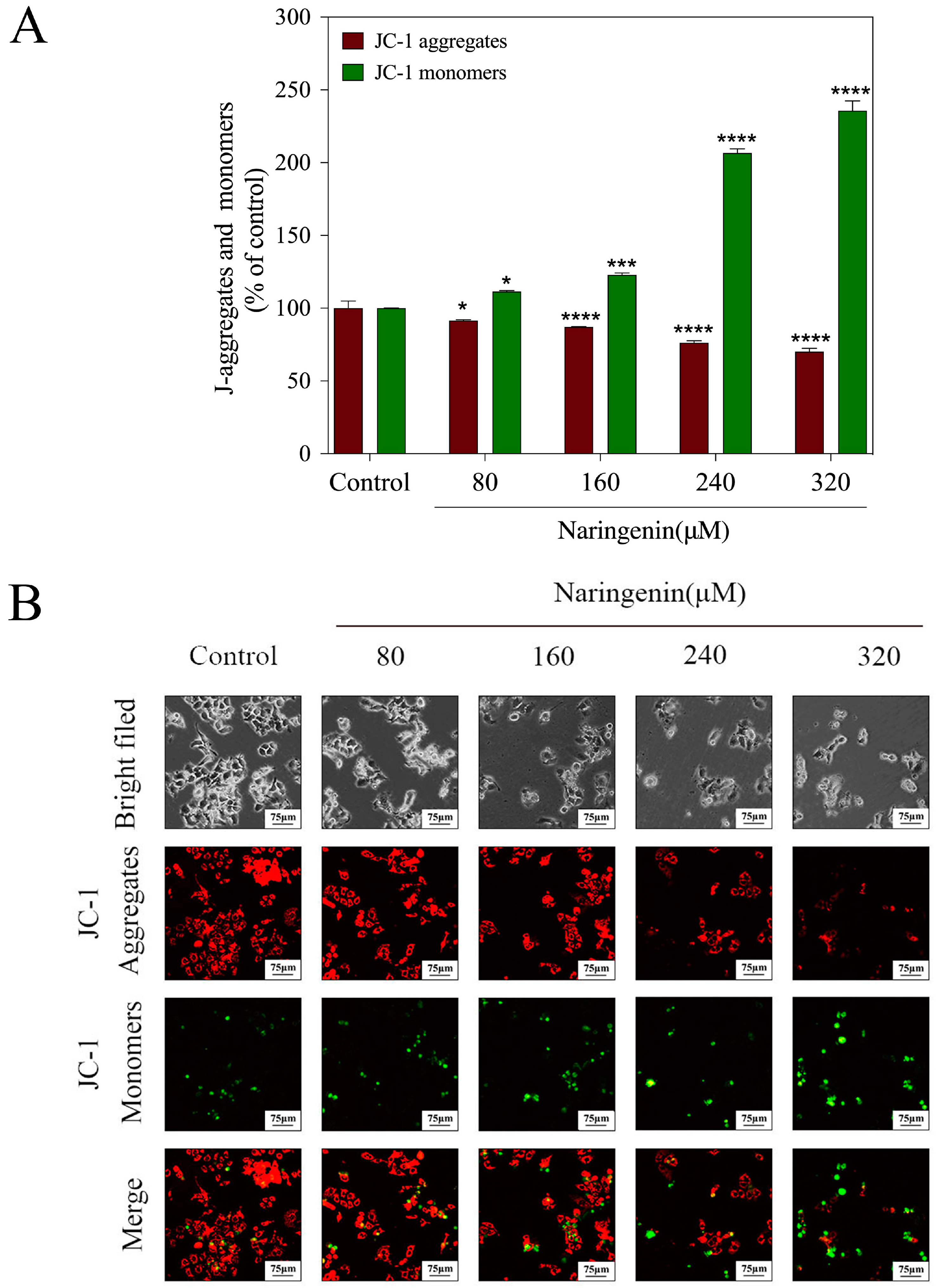
2.2. Depletion of Mitochondrial Membrane Potential (ΔΨm) Induced by Naringenin
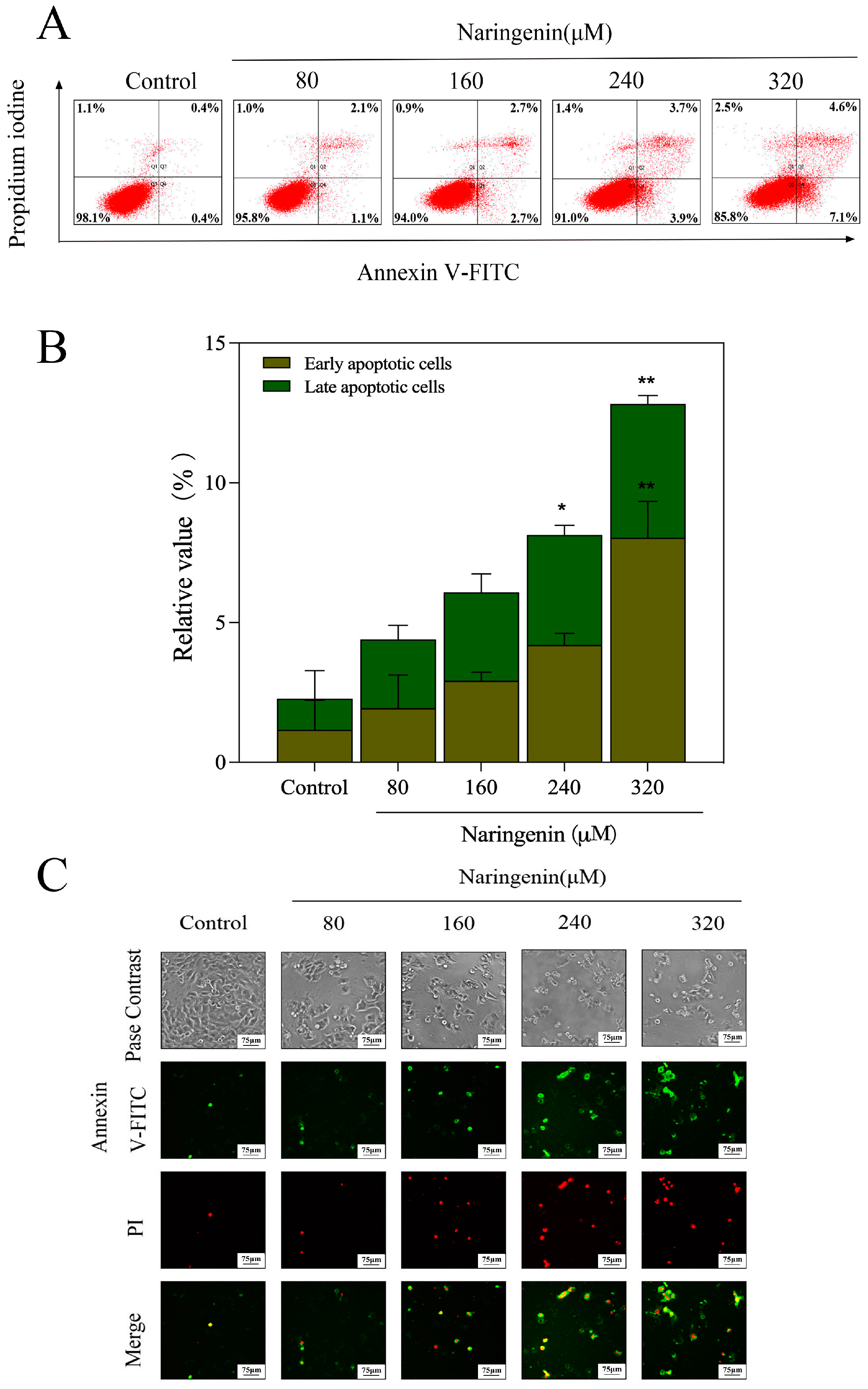
2.3. Translocation of Phosphatidylserine Induced by Naringenin
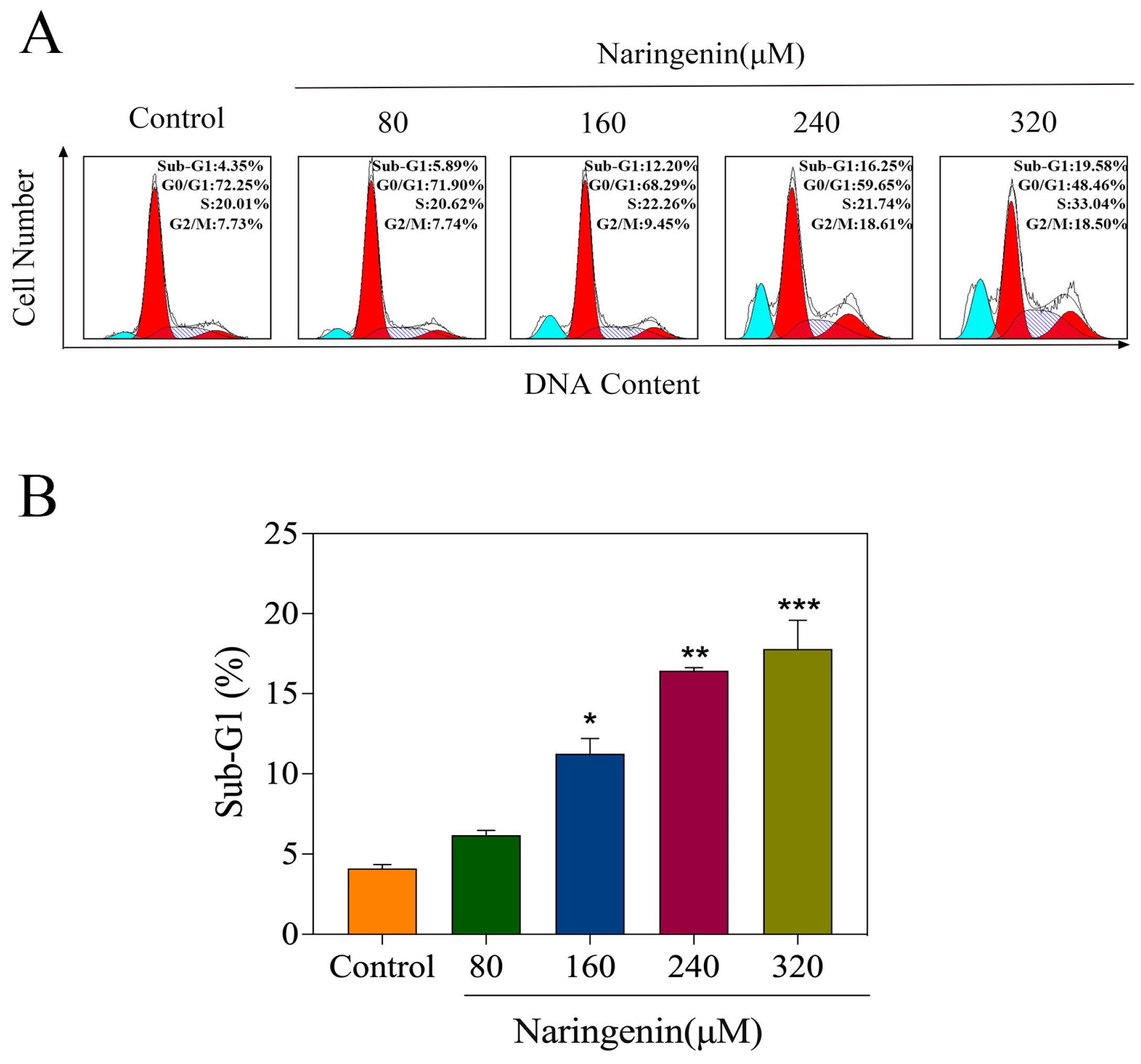
2.4. Naringenin-Induced HepG2 Cell Apoptosis
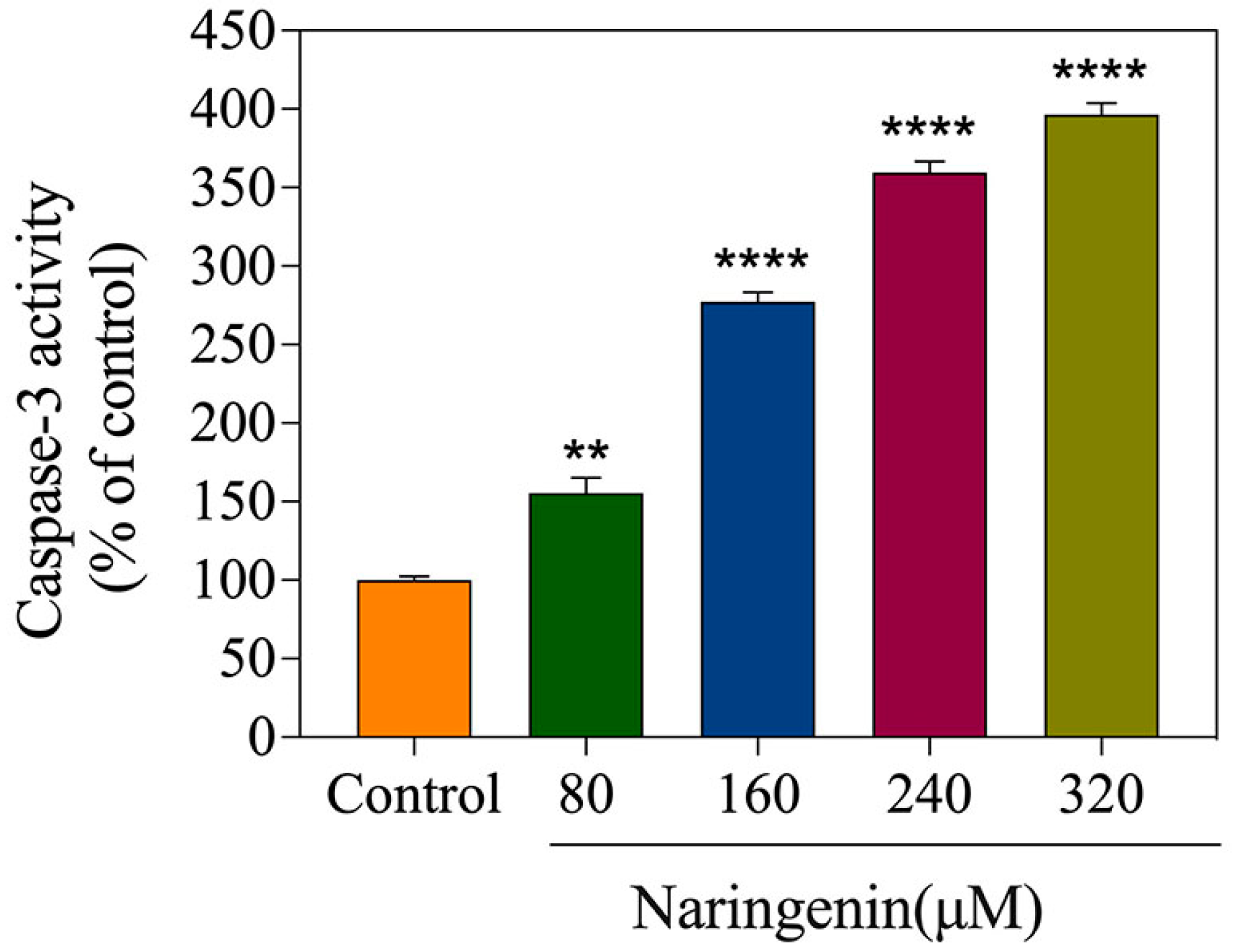
2.5. Detection of Caspase-3 Activity
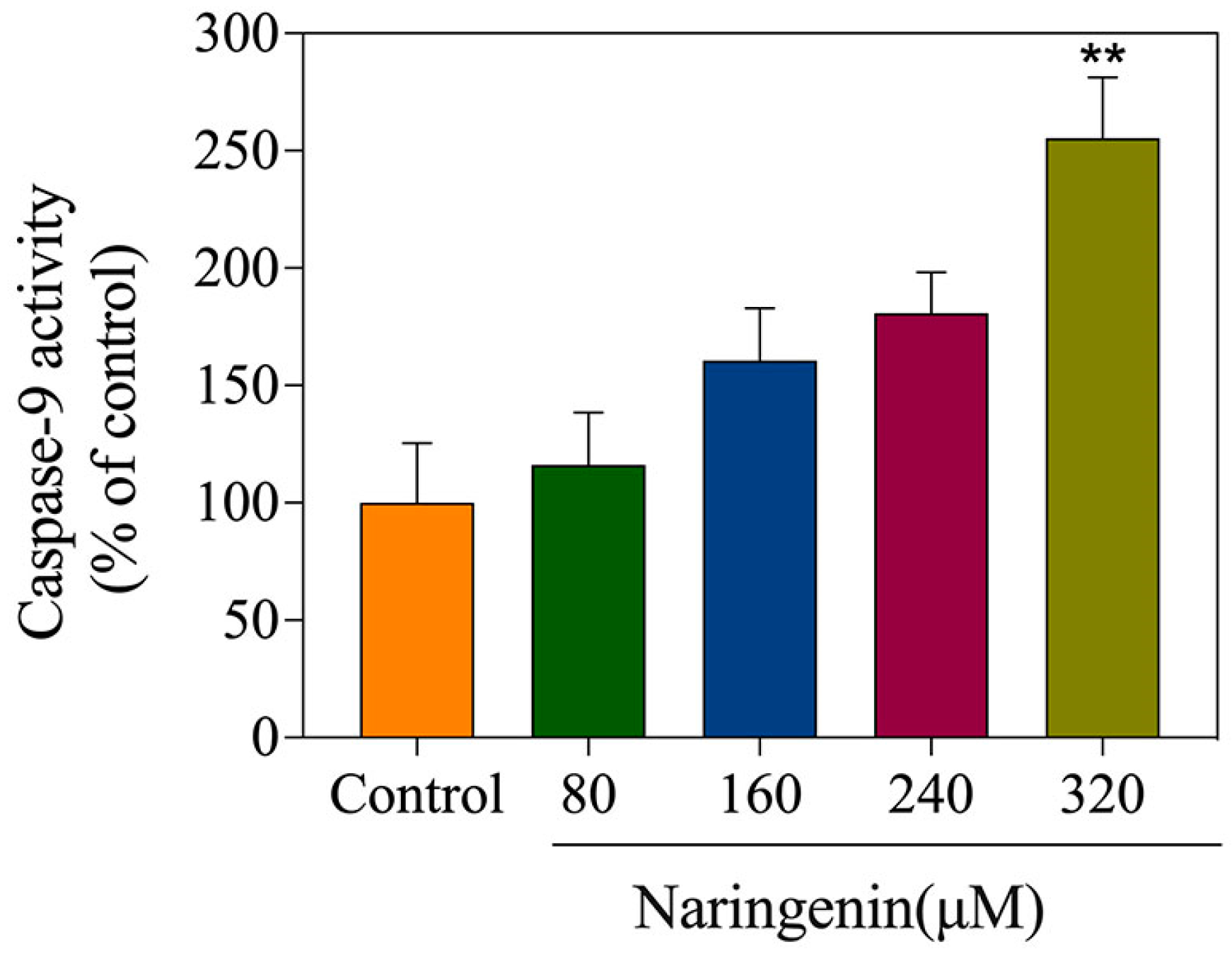
2.6. Detection of Caspase-9 Activity
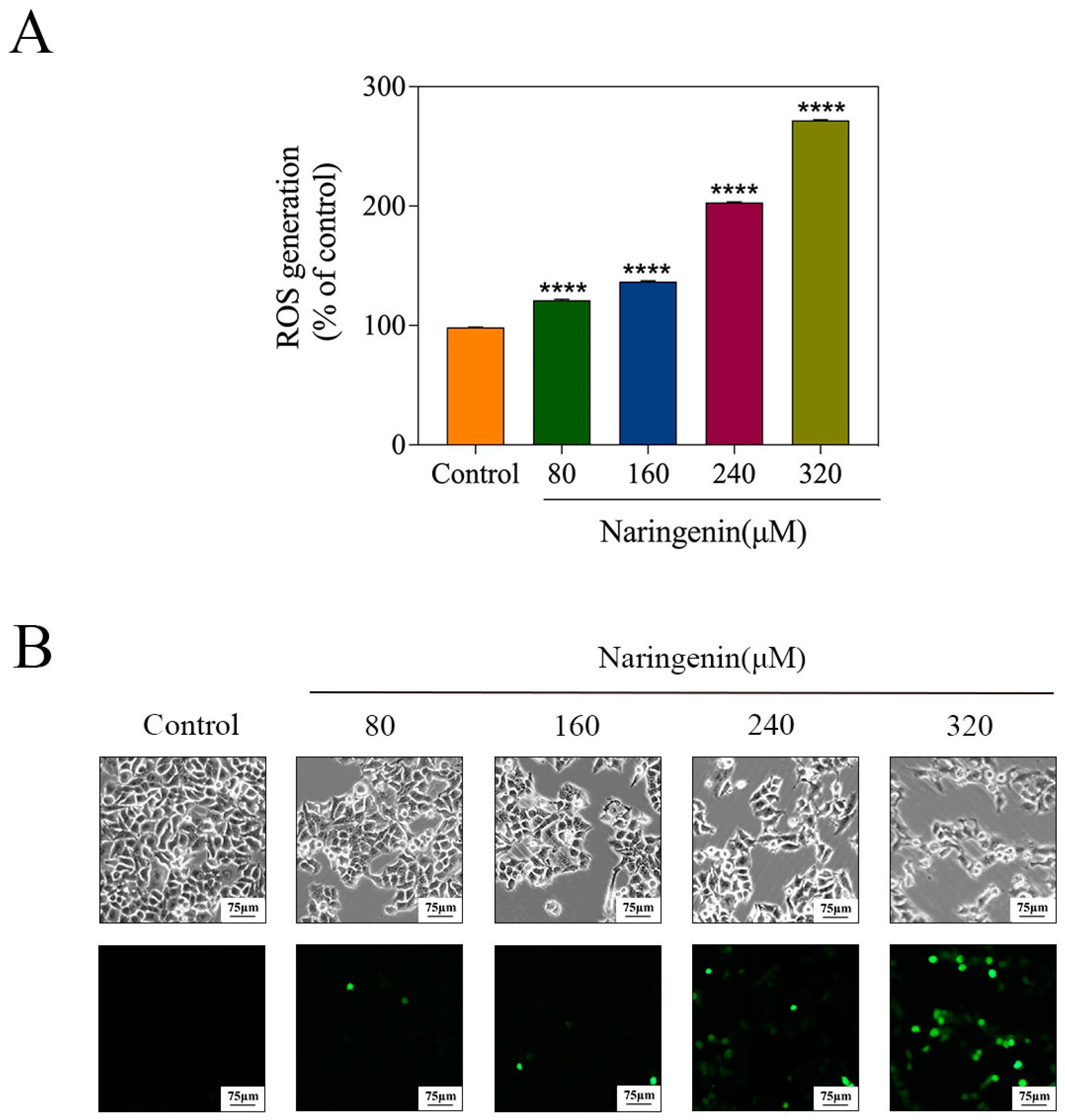
2.7. Induction of ROS Generation by Naringenin
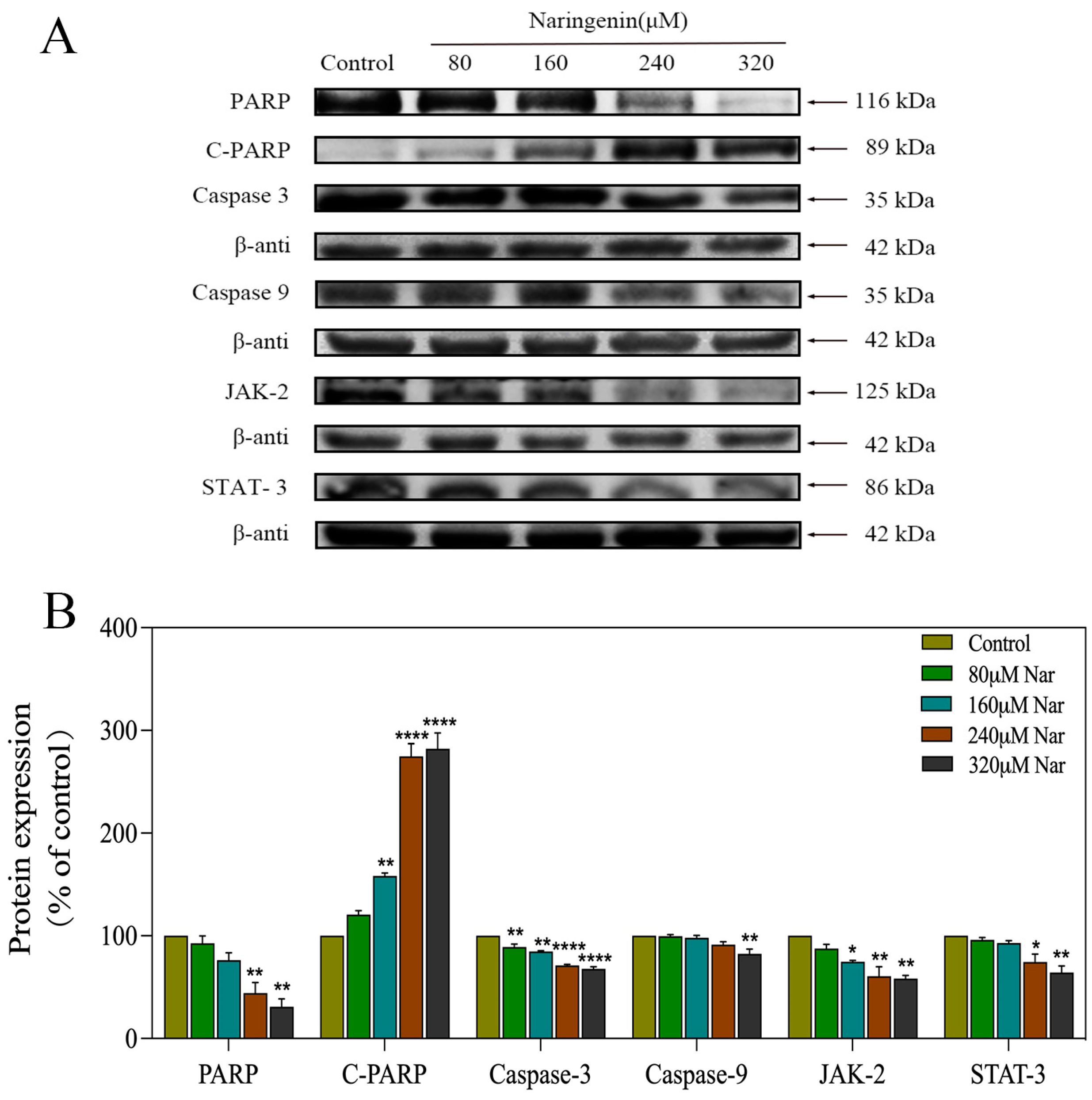
2.8. Activation of ROS-Mediated Signaling Pathways by Naringenin
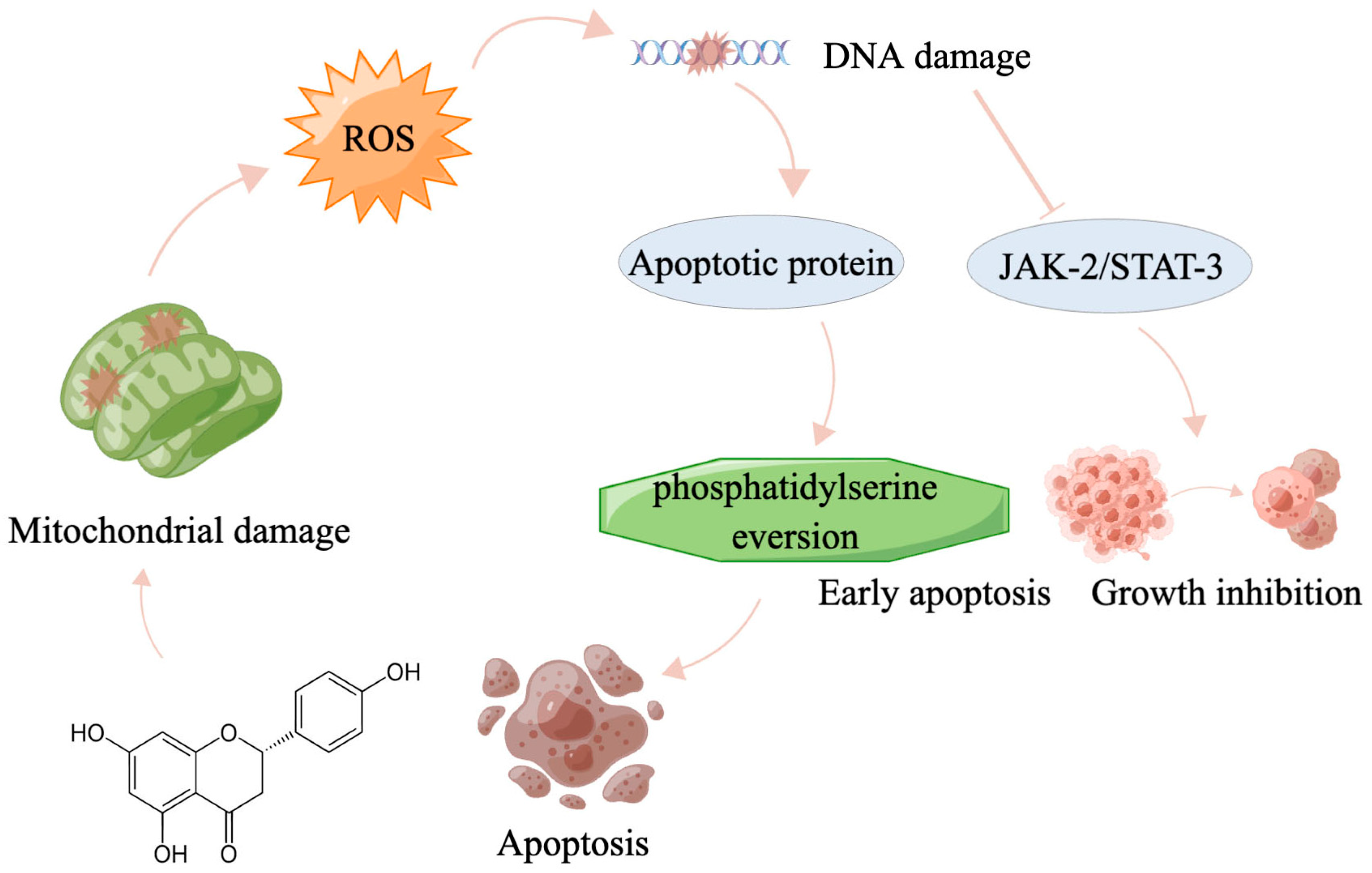
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of Naringenin
4.3. Cell Culture and Viability Assay
4.4. Detection of Mitochondrial Membrane Potential (ΔΨm)
4.5. Annexin-V/PI Double-Staining Assay
4.6. Cell-Cycle Analysis
4.7. Caspase-3 and Caspase-9 Activity
4.8. Determination of Reactive Oxygen Species
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 26 April 2023).
- Global Health Estimates: Life Expectancy and Leading Causes of Death and Disability. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 26 April 2023).
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Khan, Z.; Alloghbi, A.; Said Ahmed, T.S.; Ashraf, M.; Hammouda, D.M. Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina 2019, 55, 526. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ma, X.; Duan, Z.; Chen, X. Diagnosis, Therapy, and Prognosis for Hepatocellular Carcinoma. Anal. Cell Pathol. 2020, 2020, 8157406. [Google Scholar] [CrossRef]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Chapter One—Hepatocellular Carcinoma (HCC): Epidemiology, Etiology and Molecular Classification. In Advances in Cancer Research; Sarkar, D., Fisher, P.B., Eds.; Mechanisms and Therapy of Liver Cancer; Academic Press: Cambridge, MA, USA, 2021; Volume 149, pp. 1–61. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Hao, X.; Sun, G.; Zhang, Y.; Kong, X.; Rong, D.; Song, J.; Tang, W.; Wang, X. Targeting Immune Cells in the Tumor Microenvironment of HCC: New Opportunities and Challenges. Front. Cell Dev. Biol. 2021, 9, 3149. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Pathak, M.P.; Saikia, R.; Gogoi, U.; Sahariah, J.J.; Zothantluanga, J.H.; Samanta, A.; Das, A. Cancer Chemotherapy via Natural Bioactive Compounds. Curr. Drug Discov. Technol. 2022, 19, e310322202888. [Google Scholar] [CrossRef]
- Deng, L.-J.; Qi, M.; Li, N.; Lei, Y.-H.; Zhang, D.-M.; Chen, J.-X. Natural Products and Their Derivatives: Promising Modulators of Tumor Immunotherapy. J. Leukoc. Biol. 2020, 108, 493–508. [Google Scholar] [CrossRef]
- Das, R.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Hossain, M.d.J.; Alqahtani, A.M.; Alghazwani, Y.; Dhama, K.; Simal-Gandara, J. Medicinal Plants Used against Hepatic Disorders in Bangladesh: A Comprehensive Review. J. Ethnopharmacol. 2022, 282, 114588. [Google Scholar] [CrossRef]
- Faramarzi, F.; Alimohammadi, M.; Rahimi, A.; Alizadeh-Navaei, R.; Shakib, R.J.; Rafiei, A. Naringenin Induces Intrinsic and Extrinsic Apoptotic Signaling Pathways in Cancer Cells: A Systematic Review and Meta-Analysis of in Vitro and in Vivo Data. Nutr. Res. 2022, 105, 33–52. [Google Scholar] [CrossRef]
- Bhia, M.; Motallebi, M.; Abadi, B.; Zarepour, A.; Pereira-Silva, M.; Saremnejad, F.; Santos, A.C.; Zarrabi, A.; Melero, A.; Jafari, S.M.; et al. Naringenin Nano-Delivery Systems and Their Therapeutic Applications. Pharmaceutics 2021, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Tutunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a Flavanone with Antiviral and Anti-Inflammatory Effects: A Promising Treatment Strategy against COVID-19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef]
- Rivoira, M.A.; Rodriguez, V.; Talamoni, G.; Talamoni, N.T. de New Perspectives in the Pharmacological Potential of Naringin in Medicine. Curr. Med. Chem. 2021, 28, 1987–2007. [Google Scholar] [CrossRef] [PubMed]
- Motallebi, M.; Bhia, M.; Rajani, H.F.; Bhia, I.; Tabarraei, H.; Mohammadkhani, N.; Pereira-Silva, M.; Kasaii, M.S.; Nouri-Majd, S.; Mueller, A.-L.; et al. Naringenin: A Potential Flavonoid Phytochemical for Cancer Therapy. Life Sci. 2022, 305, 120752. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic Potential of Flavonoids in Cancer: ROS-Mediated Mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Elefantova, K.; Lakatos, B.; Kubickova, J.; Sulova, Z.; Breier, A. Detection of the Mitochondrial Membrane Potential by the Cationic Dye JC-1 in L1210 Cells with Massive Overexpression of the Plasma Membrane ABCB1 Drug Transporter. Int. J. Mol. Sci. 2018, 19, 1985. [Google Scholar] [CrossRef]
- Ren, Z.; Li, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Weng, W.; Yang, H.; Li, B. Characteristics and Application of Fish Oil-in-Water Pickering Emulsions Structured with Tea Water-Insoluble Proteins/κ-Carrageenan Complexes. Food Hydrocoll. 2021, 114, 106562. [Google Scholar] [CrossRef]
- Reich, N.C.; Liu, L. Tracking STAT Nuclear Traffic. Nat. Rev. Immunol. 2006, 6, 602–612. [Google Scholar] [CrossRef]
- Plaza, A.; Merino, B.; Del Olmo, N.; Ruiz-Gayo, M. The Cholecystokinin Receptor Agonist, CCK-8, Induces Adiponectin Production in Rat White Adipose Tissue. Br. J. Pharmacol. 2019, 176, 2678–2690. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chipot, C.; Scheuring, S. Annexin-V Stabilizes Membrane Defects by Inducing Lipid Phase Transition. Nat. Commun. 2020, 11, 230. [Google Scholar] [CrossRef]
- Hu, K.; Relton, E.; Locker, N.; Phan, N.T.N.; Ewing, A.G. Electrochemical Measurements Reveal Reactive Oxygen Species in Stress Granules**. Angew. Chem. Int. Ed. 2021, 60, 15302–15306. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, M.; Migliaccio, E.; Orsini, F.; Paolucci, D.; Moroni, M.; Contursi, C.; Pelliccia, G.; Luzi, L.; Minucci, S.; Marcaccio, M.; et al. Electron Transfer between Cytochrome c and P66Shc Generates Reactive Oxygen Species That Trigger Mitochondrial Apoptosis. Cell 2005, 122, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Zhang, J.; Ren, Y.; Feng, H.; Chen, W.N. HBx Genotype D Represses GSTP1 Expression and Increases the Oxidative Level and Apoptosis in HepG2 Cells. Mol. Oncol. 2009, 3, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Oxidative Stress and Aging. Adv. Exp. Med. Biol. 2003, 543, 191–200. [Google Scholar] [CrossRef]
- Yang, D.; Gao, S.; Yang, H. Effects of Sucrose Addition on the Rheology and Structure of Iota-Carrageenan. Food Hydrocoll. 2020, 99, 105317. [Google Scholar] [CrossRef]
- Checker, R.; Pal, D.; Patwardhan, R.S.; Basu, B.; Sharma, D.; Sandur, S.K. Modulation of Caspase-3 Activity Using a Redox Active Vitamin K3 Analogue, Plumbagin, as a Novel Strategy for Radioprotection. Free. Radic. Biol. Med. 2019, 143, 560–572. [Google Scholar] [CrossRef]
- Han, J.; Goldstein, L.A.; Hou, W.; Watkins, S.C.; Rabinowich, H. Involvement of CASP9 (Caspase 9) in IGF2R/CI-MPR Endosomal Transport. Autophagy 2021, 17, 1393–1409. [Google Scholar] [CrossRef]
- Wu, X.; Tao, P.; Zhou, Q.; Li, J.; Yu, Z.; Wang, X.; Li, J.; Li, C.; Yan, M.; Zhu, Z.; et al. IL-6 Secreted by Cancer-Associated Fibroblasts Promotes Epithelial-Mesenchymal Transition and Metastasis of Gastric Cancer via JAK2/STAT3 Signaling Pathway. Oncotarget 2017, 8, 20741–20750. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, Z.; Wei, Y.; Shu, L.; Peng, Y. Hepatic Inflammation-Fibrosis-Cancer Axis in the Rat Hepatocellular Carcinoma Induced by Diethylnitrosamine. J. Cancer Res. Clin. Oncol. 2017, 143, 821–834. [Google Scholar] [CrossRef]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E. Stat3 as an Oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive Stat3 Activity Up-Regulates VEGF Expression and Tumor Angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Refresh Cell Culture. Nat. Biomed. Eng. 2021, 5, 783–784. [CrossRef]
- Dreier, D.A.; Bowden, J.A.; Aristizabal-Henao, J.J.; Denslow, N.D.; Martyniuk, C.J. Ecotoxico-Lipidomics: An Emerging Concept to Understand Chemical-Metabolic Relationships in Comparative Fish Models. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 36, 100742. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Teng, X.; Jin, S.; Chen, Y.; Xue, H.; Xiao, L.; Wu, Y. Endogenous Hydrogen Sulfide Improves Vascular Remodeling through PPARδ/SOCS3 Signaling. J. Adv. Res. 2021, 27, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Manasa, K.L.; Saifi, M.A.; Tangella, Y.; Godugu, C.; Alvala, M. Synthesis of Substituted Cinnamido Linked Quinazolinone Congeners as Potential Anticancer Agents via Mitochondrial Dependent Intrinsic Apoptotic Pathway. Anti-Cancer Agents Med. Chem. 2019, 19, 1935–1948. [Google Scholar] [CrossRef]
- AboulWafa, O.M.; Daabees, H.M.G.; Badawi, W.A. 2-Anilinopyrimidine Derivatives: Design, Synthesis, in Vitro Anti-Proliferative Activity, EGFR and ARO Inhibitory Activity, Cell Cycle Analysis and Molecular Docking Study. Bioorganic Chem. 2020, 99, 103798. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Xu, T.; Guo, M.; Wang, C.; Zhao, M.; Chen, H.; Kuang, J.; Li, W.; Zhang, Y.; et al. Inhibition of Enterovirus 71 by Selenium Nanoparticles Loaded with SiRNA through Bax Signaling Pathways. ACS Omega 2020, 5, 12495–12500. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Ramazanov, I.R.; Makarova, E.K.; Dzhemilev, U.M. Natural Trienoic Acids as Anticancer Agents: First Stereoselective Synthesis, Cell Cycle Analysis, Induction of Apoptosis, Cell Signaling and Mitochondrial Targeting Studies. Cancers 2021, 13, 1808. [Google Scholar] [CrossRef]
- Rieger, A.M. Flow Cytometry and Cell Cycle Analysis: An Overview. Methods Mol. Biol. 2022, 2579, 47–57. [Google Scholar] [CrossRef]
- Yu, J.; Yang, A.; Wang, N.; Ling, H.; Song, J.; Chen, X.; Lian, Y.; Zhang, Z.; Yan, F.; Gu, M. Highly Sensitive Detection of Caspase-3 Activity Based on Peptide-Modified Organic Electrochemical Transistor Biosensors. Nanoscale 2021, 13, 2868–2874. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tjia, J.Y.Y.; Zhou, Y.; Liu, Q.; Fu, C.; Yang, H. Effects of Tocopherol Nanoemulsion Addition on Fish Sausage Properties and Fatty Acid Oxidation. LWT 2020, 118, 108737. [Google Scholar] [CrossRef]
- Fu, L.-H.; Wan, Y.; Qi, C.; He, J.; Li, C.; Yang, C.; Xu, H.; Lin, J.; Huang, P. Nanocatalytic Theranostics with Glutathione Depletion and Enhanced Reactive Oxygen Species Generation for Efficient Cancer Therapy. Adv. Mater. 2021, 33, 2006892. [Google Scholar] [CrossRef]
- Guo, J.; Yu, Z.; Sun, D.; Zou, Y.; Liu, Y.; Huang, L. Two Nanoformulations Induce Reactive Oxygen Species and Immunogenetic Cell Death for Synergistic Chemo-Immunotherapy Eradicating Colorectal Cancer and Hepatocellular Carcinoma. Mol. Cancer 2021, 20, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, X.; Du, J.; Gu, Z.; Zhao, Y. Reactive Oxygen Species-Regulating Strategies Based on Nanomaterials for Disease Treatment. Adv. Sci. 2020, 8, 2002797. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Dinkova-Kostova, A.T.; Abramov, A.Y. Assessment of ROS Production in the Mitochondria of Live Cells. Methods Mol. Biol. 2021, 2202, 33–42. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Yu, H. Aptamer-Based Western Blot for Selective Protein Recognition. Front Chem. 2020, 8, 570528. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Lai, J.; Wu, Q.; Lai, J.; Su, J.; Zhu, B.; Li, Y. Naringenin Induces HepG2 Cell Apoptosis via ROS-Mediated JAK-2/STAT-3 Signaling Pathways. Molecules 2023, 28, 4506. https://doi.org/10.3390/molecules28114506
Zhang M, Lai J, Wu Q, Lai J, Su J, Zhu B, Li Y. Naringenin Induces HepG2 Cell Apoptosis via ROS-Mediated JAK-2/STAT-3 Signaling Pathways. Molecules. 2023; 28(11):4506. https://doi.org/10.3390/molecules28114506
Chicago/Turabian StyleZhang, Ming, Jianmei Lai, Qianlong Wu, Jia Lai, Jingyao Su, Bing Zhu, and Yinghua Li. 2023. "Naringenin Induces HepG2 Cell Apoptosis via ROS-Mediated JAK-2/STAT-3 Signaling Pathways" Molecules 28, no. 11: 4506. https://doi.org/10.3390/molecules28114506
APA StyleZhang, M., Lai, J., Wu, Q., Lai, J., Su, J., Zhu, B., & Li, Y. (2023). Naringenin Induces HepG2 Cell Apoptosis via ROS-Mediated JAK-2/STAT-3 Signaling Pathways. Molecules, 28(11), 4506. https://doi.org/10.3390/molecules28114506





