Comparative Study of Polymer-Grafted BaTiO3 Nanoparticles Synthesized Using Normal ATRP as Well as ATRP and ARGET-ATRP with Sacrificial Initiator with a Focus on Controlling the Polymer Graft Density and Molecular Weight
Abstract
1. Introduction
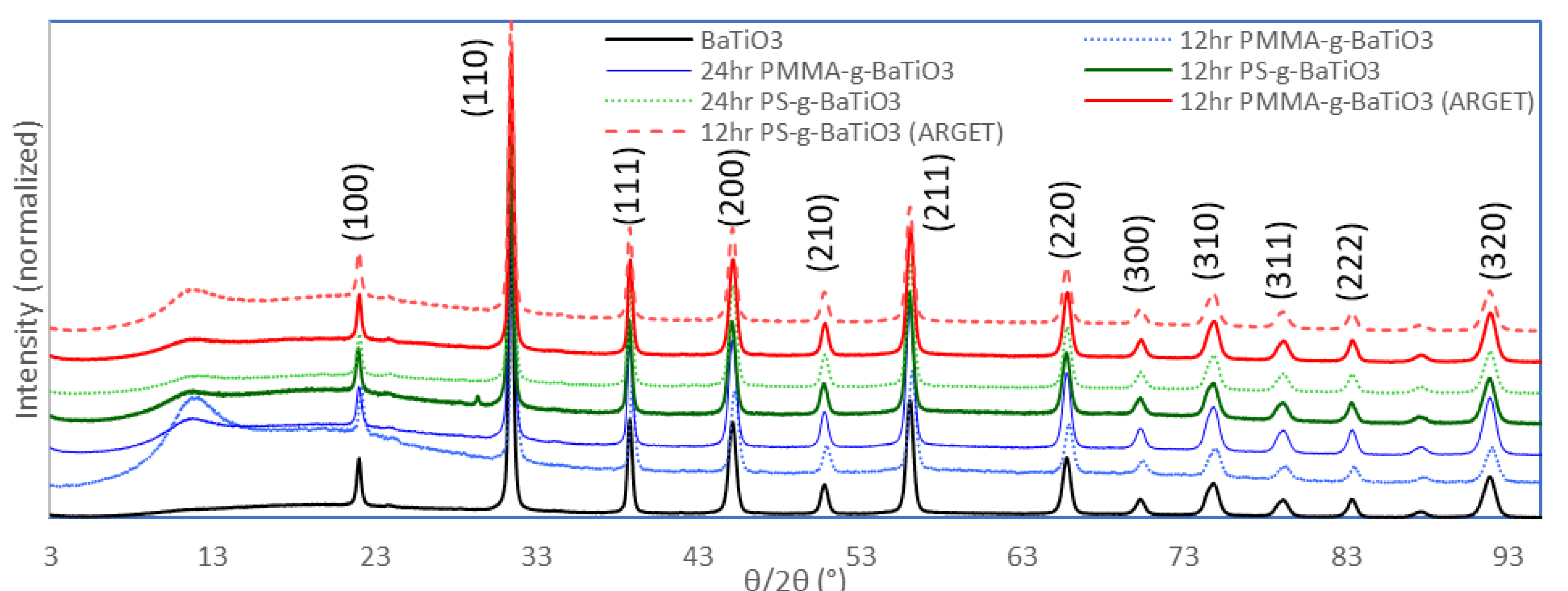
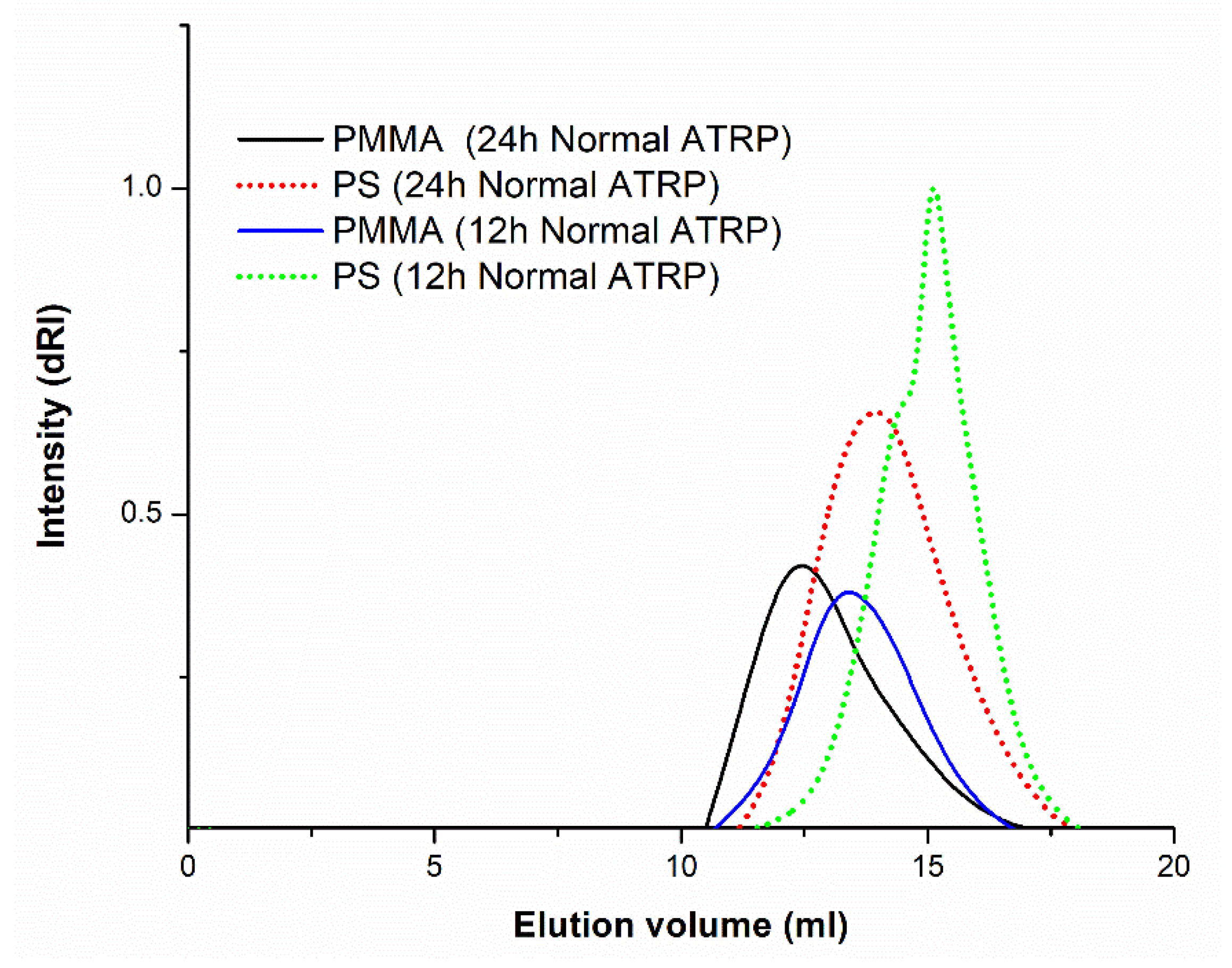
2. Results and Discussion
Effect of Reaction Times on ATRP-Synthesized Polymer-Grafted Nanoparticles
3. Materials and Methods
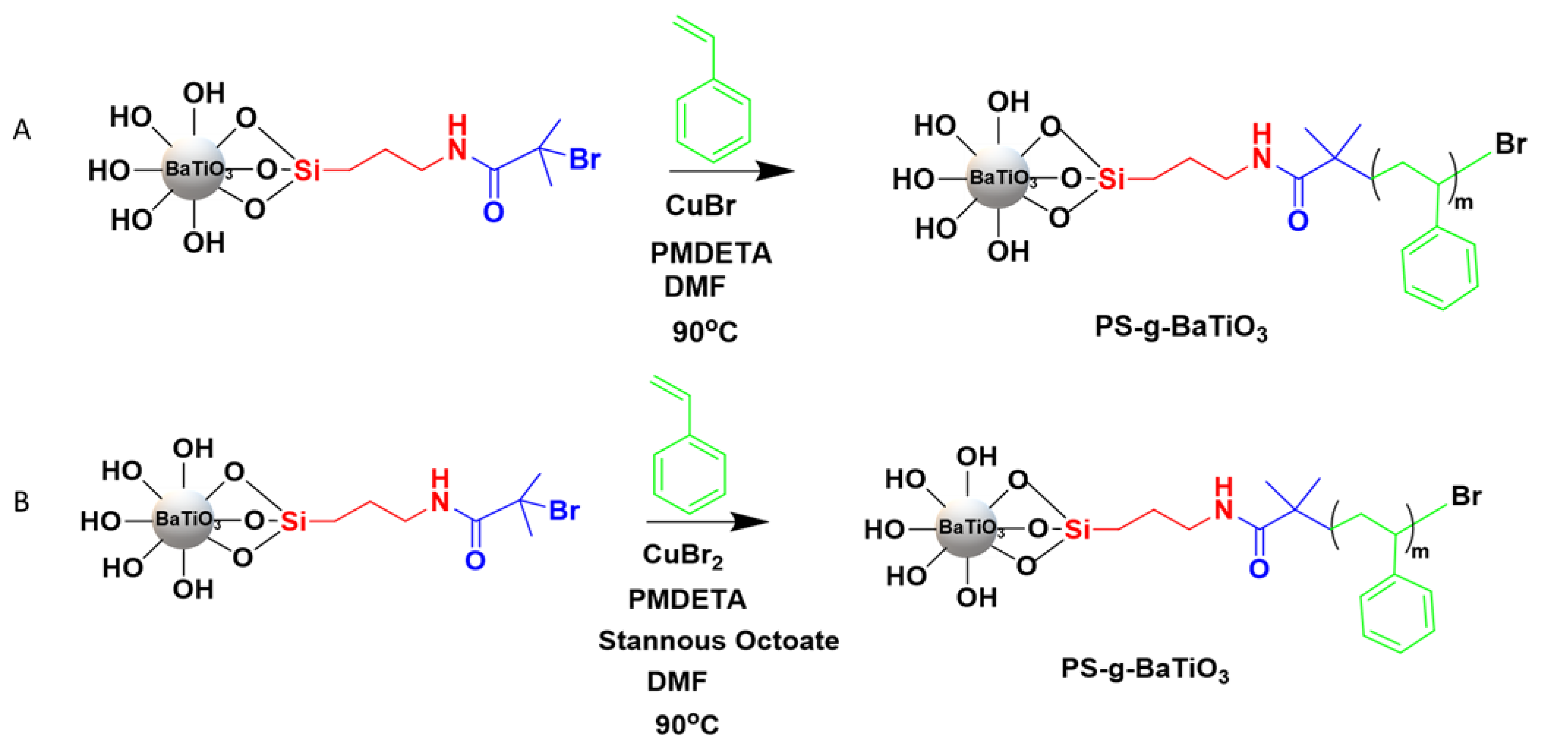
3.1. Synthesis of Core–Shell Nanoparticles
3.1.1. Surface-Initiated Atomic Transfer Radical Polymerization (ATRP)
3.1.2. Activator Regeneration via Electron Transfer (ARGET) ATRP with Sacrificial Initiator for Synthesis of PS- and PMMA-Grafted BaTiO3 Nanoparticles
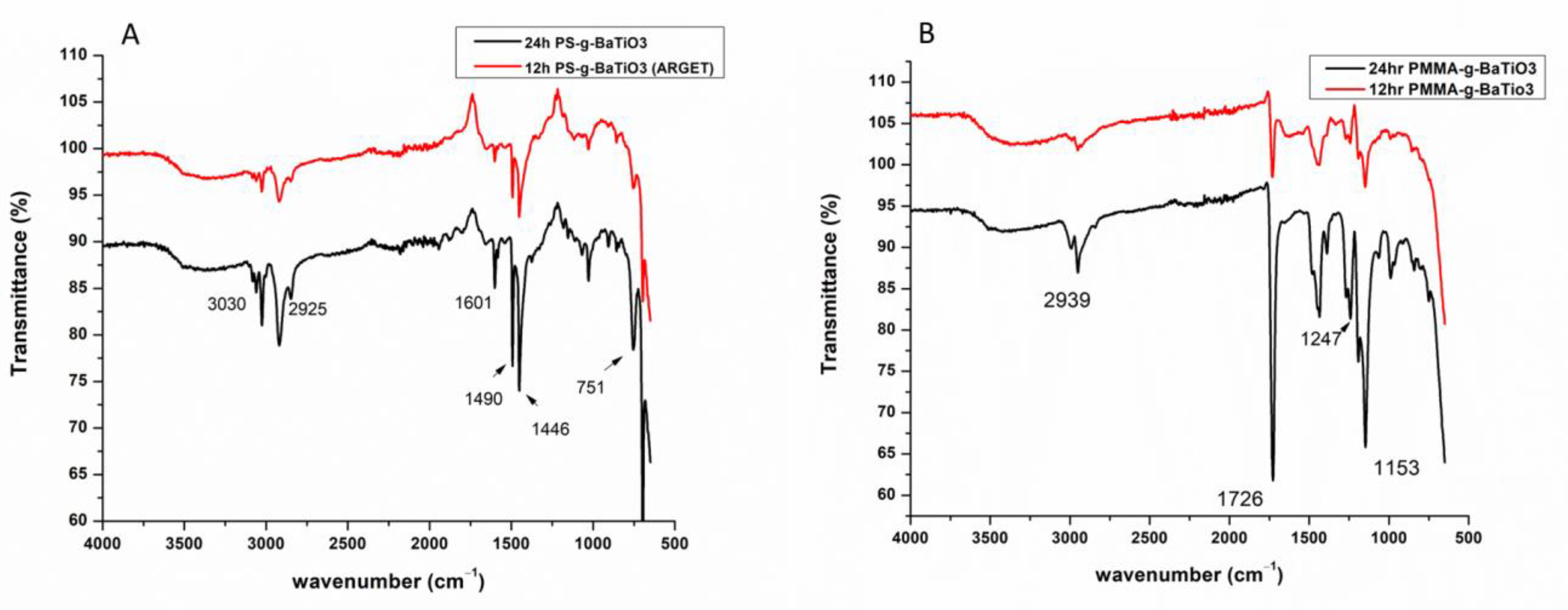
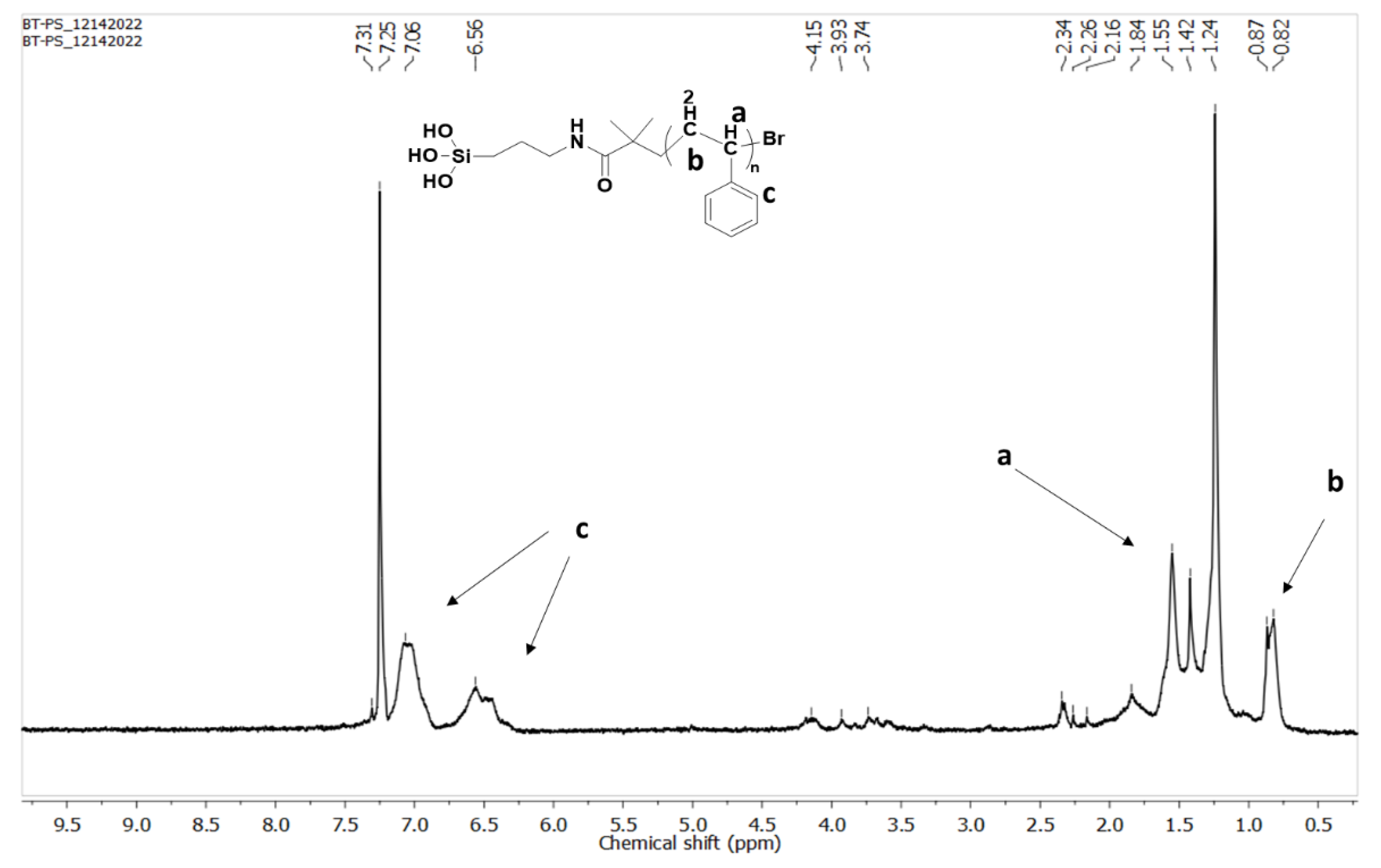
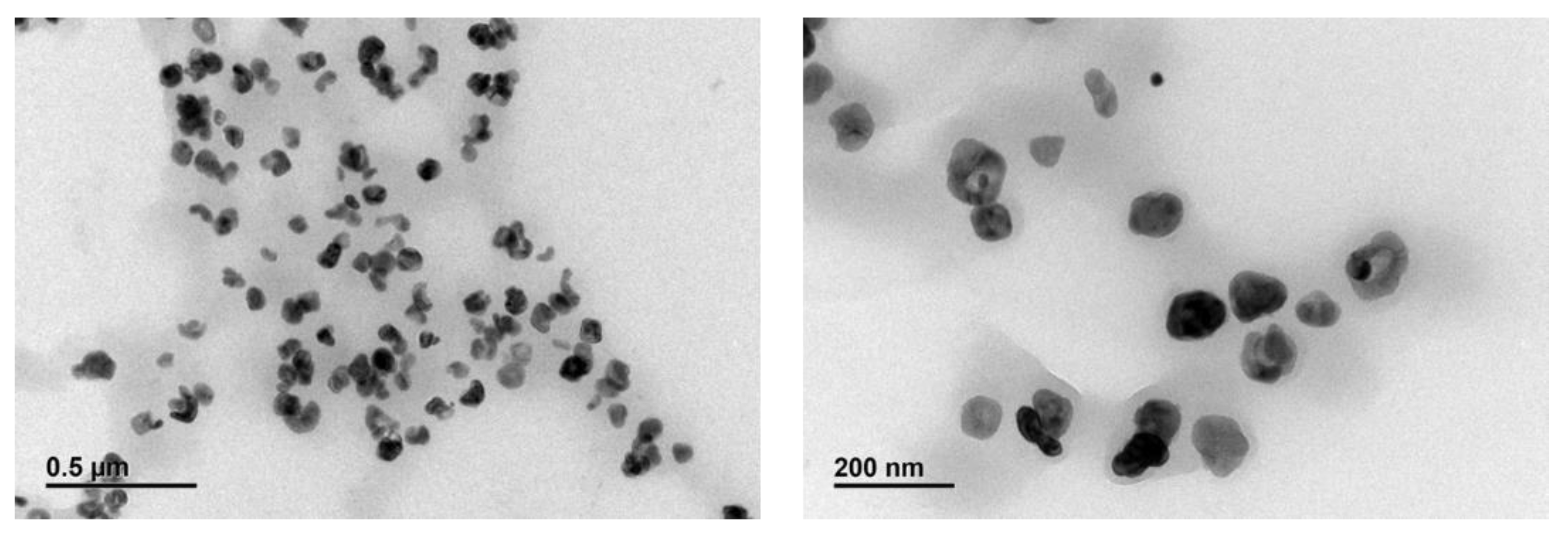
3.2. Characterization of Polymer-Grafted BaTiO3 Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, J.; Bockstaller, M.R.; Matyjaszewski, K. Brush-modified materials: Control of molecular architecture, assembly behavior, properties and applications. Prog. Polym. Sci. 2020, 100, 101180. [Google Scholar] [CrossRef]
- Shameem, M.M.; Sasikanth, S.M.; Annamalai, R.; Raman, R.G. A brief review on polymer nanocomposites and its applications. Mater. Today Proc. 2021, 45, 2536–2539. [Google Scholar] [CrossRef]
- Singh, M.; Apata, I.; Samant, S.; Wu, W.; Tawade, B.; Pradhan, N.; Raghavan, D.; Karim, A. Nanoscale Strategies to Enhance the Energy Storage Capacity of Polymeric Dielectric Capacitors: Review of Recent Advances. Polym. Rev. 2021, 62, 211–260. [Google Scholar] [CrossRef]
- Tawade, B.V.; Apata, I.E.; Singh, M.; Das, P.; Pradhan, N.; Al-Enizi, A.M.; Karim, A.; Raghavan, D. Recent developments in the synthesis of chemically modified nanomaterials for use in dielectric and electronics applications. Nanotechnology 2021, 32, 142004. [Google Scholar] [CrossRef]
- Cai, Q.J.; Gan, Y.; Chan-Park, M.B.; Yang, H.B.; Lu, Z.S.; Li, C.M.; Guo, J.; Dong, Z.L. Solution-processable barium titanate and strontium titanate nanoparticle dielectrics for low-voltage organic thin-film transistors. Chem. Mater. 2009, 21, 3153–3161. [Google Scholar] [CrossRef]
- Hong, K.; Lee, T.H.; Suh, J.M.; Yoon, S.-H.; Jang, H.W. Perspectives and challenges in multilayer ceramic capacitors for next generation electronics. J. Mater. Chem. C 2019, 7, 9782–9802. [Google Scholar] [CrossRef]
- Tawade, B.V.; Singh, M.; Apata, I.E.; Veerasamy, J.; Pradhan, N.; Karim, A.; Douglas, J.F.; Raghavan, D. Polymer-Grafted Nanoparticles with Variable Grafting Densities for High Energy Density Polymeric Nanocomposite Dielectric Capacitors. JACS Au 2023, 3, 1365–1375. [Google Scholar] [CrossRef]
- Tawade, B.V.; Apata, I.E.; Pradhan, N.; Karim, A.; Raghavan, D. Recent Advances in the Synthesis of Polymer-Grafted Low-K and High-K Nanoparticles for Dielectric and Electronic Applications. Molecules 2021, 26, 2942. [Google Scholar] [CrossRef]
- Chancellor, A.J.; Seymour, B.T.; Zhao, B. Characterizing Polymer-Grafted Nanoparticles: From Basic Defining Parameters to Behavior in Solvents and Self-Assembled Structures. Anal. Chem. 2019, 91, 6391–6402. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, X.; Yu, B.; Zhou, F. Brushing up functional materials. NPG Asia Mater. 2019, 11, 24. [Google Scholar] [CrossRef]
- Francis, R.; Joy, N.; Aparna, E.P.; Vijayan, R. Polymer grafted inorganic nanoparticles, preparation, properties, and applications: A review. Polym. Rev. 2014, 54, 268–347. [Google Scholar] [CrossRef]
- Ehlert, S.; Taheri, S.M.; Pirner, D.; Drechsler, M.; Schmidt, H.W.; Förster, S. Polymer ligand exchange to control stabilization and compatibilization of nanocrystals. ACS Nano 2014, 8, 6114–6122. [Google Scholar] [CrossRef]
- Shui, Y.; Su, Y.; Kuang, X.; Zhao, W.; Cai, Y.; Wang, D. Facile and controllable synthesis of hybrid silica nanoparticles densely grafted with poly(ethylene glycol). Polym. Int. 2017, 66, 1395–1401. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Miller, P.J.; Shukla, N.; Immaraporn, B.; Gelman, A.; Luokala, B.B.; Siclovan, T.M.; Kickelbick, G.; Vallant, T.; Hoffmann, H.; et al. Polymers at Interfaces: Using Atom Transfer Radical Polymerization in the Controlled Growth of Homopolymers and Block Copolymers from Silicon Surfaces in the Absence of Untethered Sacrificial Initiator. Macromolecules 1999, 32, 8716–8724. [Google Scholar] [CrossRef]
- Cheng, G.; Böker, A.; Zhang, M.; Krausch, G.; Müller, A.H.E. Amphiphilic Cylindrical Core−Shell Brushes via a “Grafting From” Process Using ATRP. Macromolecules 2001, 34, 6883–6888. [Google Scholar] [CrossRef]
- Li, C.; Benicewicz, B.C. Synthesis of well-defined polymer brushes grafted onto silica nanoparticles via surface reversible addition-fragmentation chain transfer polymerization. Macromolecules 2005, 38, 5929–5936. [Google Scholar] [CrossRef]
- Matsuno, R.; Yamamoto, K.; Otsuka, H.; Takahara, A. Polystyrene-Grafted Magnetite Nanoparticles Prepared through Surface-Initiated Nitroxyl-Mediated Radical Polymerization. Chem. Mater. 2003, 15, 3–5. [Google Scholar] [CrossRef]
- Husseman, M.; Malmström, E.E.; McNamara, M.; Mate, M.; Mecerreyes, D.; Benoit, D.G.; Hedrick, J.L.; Mansky, P.; Huang, E.; Russell, T.P.; et al. Controlled Synthesis of Polymer Brushes by “Living” Free Radical Polymerization Techniques. Macromolecules 1999, 32, 1424–1431. [Google Scholar] [CrossRef]
- Min, K.; Gao, H.; Matyjaszewski, K. Preparation of homopolymers and block copolymers in miniemulsion by ATRP using activators generated by electron transfer (AGET). J. Am. Chem. Soc. 2005, 127, 3825–3830. [Google Scholar] [CrossRef]
- Krys, P.; Matyjaszewski, K. Kinetics of Atom Transfer Radical Polymerization. Eur. Polym. J. 2017, 89, 482–523. [Google Scholar] [CrossRef]
- Tang, H.; Arulsamy, N.; Radosz, M.; Shen, Y.; Tsarevsky, N.V.; Braunecker, W.A.; Tang, W.; Matyjaszewski, K. Highly active copper-based catalyst for atom transfer radical polymerization. J. Am. Chem. Soc. 2006, 128, 16277–16285. [Google Scholar] [CrossRef] [PubMed]
- Ribelli, T.G.; Lorandi, F.; Fantin, M.; Matyjaszewski, K. Atom Transfer Radical Polymerization: Billion Times More Active Catalysts and New Initiation Systems. Macromol. Rapid Commun. 2019, 40, 1800616. [Google Scholar] [CrossRef] [PubMed]
- Tsubokawa, N. Surface grafting of polymers onto nanoparticles in a solvent-free dry-system and applications of polymer-grafted nanoparticles as novel functional hybrid materials. Polym. J. 2007, 39, 983–1000. [Google Scholar] [CrossRef]
- Dworakowska, S.; Lorandi, F.; Gorczyński, A.; Matyjaszewski, K. Toward Green Atom Transfer Radical Polymerization: Current Status and Future Challenges. Adv. Sci. 2022, 9, 2106076. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, J.; Liu, T.; Wei, Q.; Li, S.; Olszewski, M.; Wu, J.; Sobieski, J.; Fantin, M.; Bockstaller, M.R.; et al. Control of Dispersity and Grafting Density of Particle Brushes by Variation of ATRP Catalyst Concentration. ACS Macro Lett. 2019, 8, 859–864. [Google Scholar] [CrossRef]
- Benetti, E.M.; Kang, C.; Mandal, J.; Divandari, M.; Spencer, N.D. Modulation of Surface-Initiated ATRP by Confinement: Mechanism and Applications. Macromolecules 2017, 50, 5711–5718. [Google Scholar] [CrossRef]
- Mohammad Rabea, A.; Zhu, S. Controlled radical polymerization at high conversion: Bulk ICAR ATRP of methyl methacrylate. Ind. Eng. Chem. Res. 2014, 53, 3472–3477. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, Y.; Hamielec, A.E.; Eaton, D.R. Radical trapping and termination in free-radical polymerization of mma. Macromolecules 1990, 23, 1144–1150. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, J.; Wang, Z.; Zhao, Y.; Yan, J.; Lin, Y.; Li, S.; Liu, T.; Olszewski, M.; Pietrasik, J.; et al. Tunable Assembly of Block Copolymer Tethered Particle Brushes by Surface-Initiated Atom Transfer Radical Polymerization. ACS Macro Lett. 2020, 9, 806–812. [Google Scholar] [CrossRef]
- von Werne, T.; Patten, T.E. Atom transfer radical polymerization from nanoparticles: A tool for the preparation of well-defined hybrid nanostructures and for understanding the chemistry of controlled/”living” radical polymerizations from surfaces. J. Am. Chem. Soc. 2001, 123, 7497–7505. [Google Scholar] [CrossRef]
- Paniagua, S.A.; Kim, Y.; Henry, K.; Kumar, R.; Perry, J.W.; Marder, S.R. Surface-initiated polymerization from barium titanate nanoparticles for hybrid dielectric capacitors. ACS Appl. Mater. Interfaces 2014, 6, 3477–3482. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Wang, F.; Ma, Y.; Wang, L.; Chen, D.; Zhao, C.; Yang, W. Improving dielectric properties of BaTiO3/poly(vinylidene fluoride) composites by employing core-shell structured BaTiO3@Poly(methylmethacrylate) and BaTiO3@Poly(trifluoroethyl methacrylate) nanoparticles. Appl. Surf. Sci. 2017, 403, 71–79. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Ma, Y.; Zhao, C.; Yang, W. Preparation and dielectric properties of core–shell structural composites of poly(1H,1H,2H,2H-perfluorooctyl methacrylate)@BaTiO3 nanoparticles. Appl. Surf. Sci. 2013, 277, 121–127. [Google Scholar] [CrossRef]
- You, N.; Zhang, C.; Liang, Y.; Zhang, Q.; Fu, P.; Liu, M.; Zhao, Q.; Cui, Z.; Pang, X. Facile Fabrication of Size-Tunable Core/Shell Ferroelectric/Polymeric Nanoparticles with Tailorable Dielectric Properties via Organocatalyzed Atom Transfer Radical Polymerization Driven by Visible Light. Sci. Rep. 2019, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Huang, X.; Wu, C.; Jiang, P. Core-shell structured poly(methyl methacrylate)/BaTiO3 nanocomposites prepared by in situ atom transfer radical polymerization: A route to high dielectric constant materials with the inherent low loss of the base polymer. J. Mater. Chem. 2011, 21, 5897–5906. [Google Scholar] [CrossRef]
- Wang, J.; Guan, F.; Cui, L.; Pan, J.; Wang, Q.; Zhu, L. Achieving high electric energy storage in a polymer nanocomposite at low filling ratios using a highly polarizable phthalocyanine interphase. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1669–1680. [Google Scholar] [CrossRef]
- Xie, L.; Huang, X.; Huang, Y.; Yang, K.; Jiang, P. Core@Double-shell structured BaTiO3-polymer nanocomposites with high dielectric constant and low dielectric loss for energy Storage Application. J. Phys. Chem. C 2013, 117, 22525–22537. [Google Scholar] [CrossRef]
- Samant, S.; Hailu, S.; Singh, M.; Pradhan, N.; Yager, K.; Al-Enizi, A.M.; Raghavan, D.; Karim, A. Alignment frustration in block copolymer films with block copolymer grafted TiO2 nanoparticles under soft-shear cold zone annealing. Polym. Adv. Technol. 2021, 32, 2052–2060. [Google Scholar] [CrossRef]
- Kim, K.; Park, M.S.; Na, Y.; Choi, J.; Jenekhe, S.A.; Kim, F.S. Preparation and application of polystyrene-grafted alumina core-shell nanoparticles for dielectric surface passivation in solution-processed polymer thin film transistors. Org. Electron. 2019, 65, 305–310. [Google Scholar] [CrossRef]
- Askar, S.; Li, L.; Torkelson, J.M. Polystyrene-Grafted Silica Nanoparticles: Investigating the Molecular Weight Dependence of Glass Transition and Fragility Behavior. Macromolecules 2017, 50, 1589–1598. [Google Scholar] [CrossRef]
- Turgman-Cohen, S.; Genzer, J. Computer simulation of controlled radical polymerization: Effect of chain confinement due to initiator grafting density and solvent quality in “grafting from” method. Macromolecules 2010, 43, 9567–9577. [Google Scholar] [CrossRef]
- Pasuk, I.; Neațu, F.; Neațu, Ș.; Florea, M.; Istrate, C.M.; Pintilie, I.; Pintilie, L. Structural details of batio3 nano-powders deduced from the anisotropic xrd peak broadening. Nanomaterials 2021, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Sheng, X.; Zhao, B. Environmentally responsive “hairy” nanoparticles: Mixed homopolymer brushes on silica nanoparticles synthesized by living radical polymerization techniques. J. Am. Chem. Soc. 2005, 127, 6248–6256. [Google Scholar] [CrossRef] [PubMed]
- Mastan, E.; Xi, L.; Zhu, S. Factors Affecting Grafting Density in Surface-Initiated ATRP: A Simulation Study. Macromol. Theory Simulations 2016, 25, 220–228. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Lu, Z.Y.; Zhang, Z.G.; Sun, C.C. Influence of Surface-Initiated Polymerization Rate and Initiator Density on the Properties of Polymer Brushes. Macromolecules 2009, 42, 2863–2872. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Patten, T.E.; Xia, J. Controlled/‘living’ radical polymerization. Kinetics of the homogeneous atom transfer radical polymerization of styrene. J. Am. Chem. Soc. 1997, 119, 674–680. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Tam, K.C.; Sèbe, G. A comparative study on grafting polymers from cellulose nanocrystals via surface-initiated atom transfer radical polymerization (ATRP) and activator re-generated by electron transfer ATRP. Carbohydr. Polym. 2019, 205, 322–329. [Google Scholar] [CrossRef]


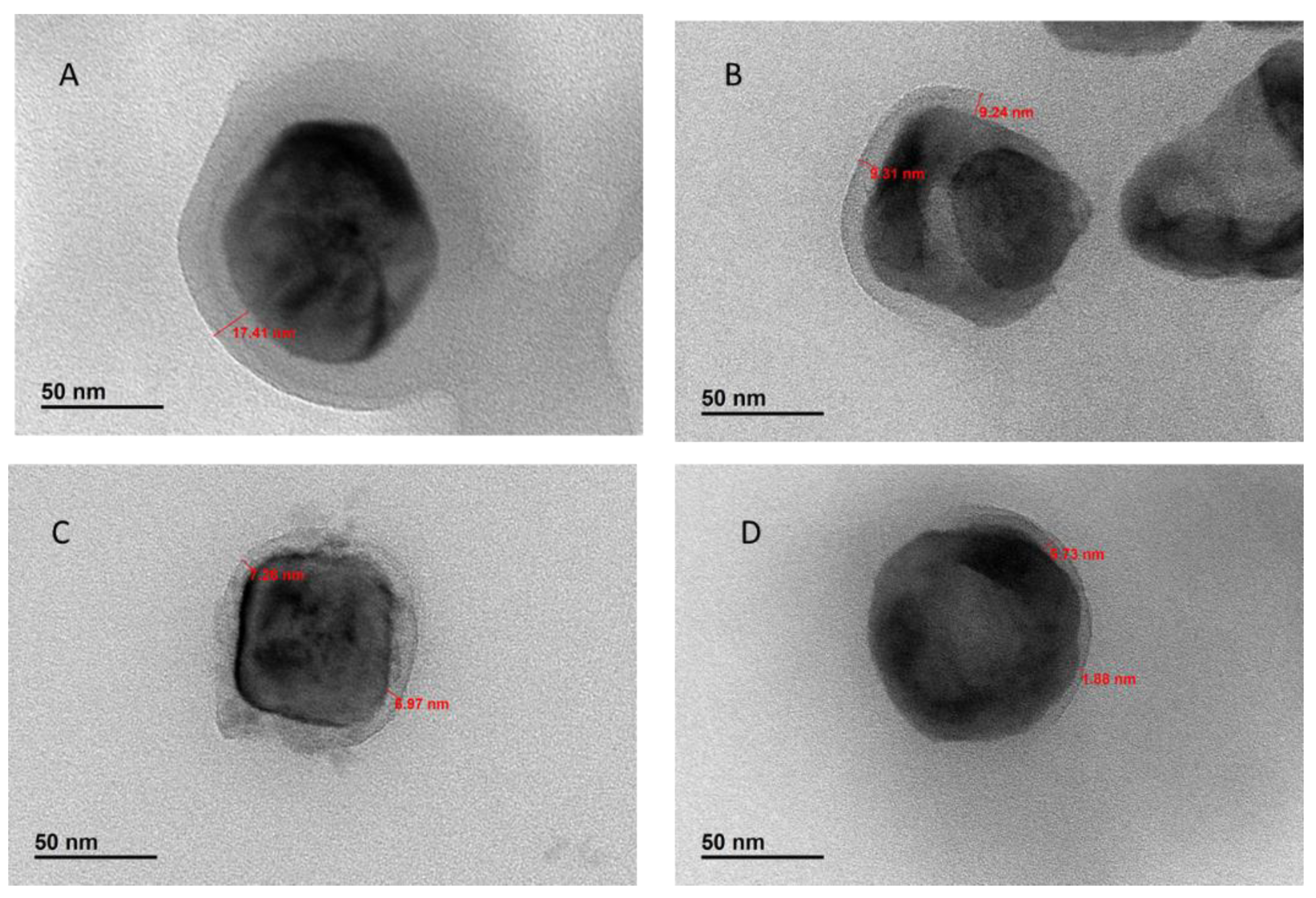

| Samples (Polymerization Time) | TGA Char Yield of PGNPs, (%) | (Char Yield of BT NPs—Char Yield of PGNPs) (%) | Mn (g/mol) a | Mw (g/mol) a | Dispersity a | Graft Density (chain/nm2) | Degree of Polymerization (DP) |
|---|---|---|---|---|---|---|---|
| PS-g-BaTiO3 ATRP (24 h) | 72 | 23.3 | 52,200 | 83.900 | 1.609 | 0.120 | 502 |
| PS-g-BaTiO3 ATRP (12 h) | 85 | 10.6 | 20,600 | 31,100 | 1.514 | 0.122 | 198 |
| PS-g-BaTiO3 ATRP (EBIB) (12 h) | 88.8 | 7.2 | 20,020 | 30,400 | 1.522 | 0.081 | 192 |
| PS-g-BaTiO3 ARGET (EBIB) (12 h) | 88.4 | 7.4 | 30,090 | 37,870 | 1.259 | 0.067 | 289 |
| PMMA-g-BaTiO3 ATRP (24 h) | 68.8 | 20.2 | 152,800 | 230,000 | 1.505 | 0.039 | 1528 |
| PMMA-g-BaTiO3 ATRP (12 h) | 89 | 5.0 | 73,800 | 115,800 | 1.568 | 0.015 | 738 |
| PMMA-g-BaTiO3 ATRP (EBIB) (12 h) | 85.6 | 10.4 | 29,390 | 52,080 | 1.775 | 0.071 | 294 |
| PMMA-g-BaTiO3 ARGET (EBIB) (12 h) | 88.2 | 7.6 | 35,320 | 44,620 | 1.263 | 0.059 | 353 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apata, I.E.; Tawade, B.V.; Cummings, S.P.; Pradhan, N.; Karim, A.; Raghavan, D. Comparative Study of Polymer-Grafted BaTiO3 Nanoparticles Synthesized Using Normal ATRP as Well as ATRP and ARGET-ATRP with Sacrificial Initiator with a Focus on Controlling the Polymer Graft Density and Molecular Weight. Molecules 2023, 28, 4444. https://doi.org/10.3390/molecules28114444
Apata IE, Tawade BV, Cummings SP, Pradhan N, Karim A, Raghavan D. Comparative Study of Polymer-Grafted BaTiO3 Nanoparticles Synthesized Using Normal ATRP as Well as ATRP and ARGET-ATRP with Sacrificial Initiator with a Focus on Controlling the Polymer Graft Density and Molecular Weight. Molecules. 2023; 28(11):4444. https://doi.org/10.3390/molecules28114444
Chicago/Turabian StyleApata, Ikeoluwa E., Bhausaheb V. Tawade, Steven P. Cummings, Nihar Pradhan, Alamgir Karim, and Dharmaraj Raghavan. 2023. "Comparative Study of Polymer-Grafted BaTiO3 Nanoparticles Synthesized Using Normal ATRP as Well as ATRP and ARGET-ATRP with Sacrificial Initiator with a Focus on Controlling the Polymer Graft Density and Molecular Weight" Molecules 28, no. 11: 4444. https://doi.org/10.3390/molecules28114444
APA StyleApata, I. E., Tawade, B. V., Cummings, S. P., Pradhan, N., Karim, A., & Raghavan, D. (2023). Comparative Study of Polymer-Grafted BaTiO3 Nanoparticles Synthesized Using Normal ATRP as Well as ATRP and ARGET-ATRP with Sacrificial Initiator with a Focus on Controlling the Polymer Graft Density and Molecular Weight. Molecules, 28(11), 4444. https://doi.org/10.3390/molecules28114444







