Syntheses and Electrochemical and EPR Studies of Porphyrins Functionalized with Bulky Aromatic Amine Donors
Abstract
1. Introduction
2. Results and Discussion
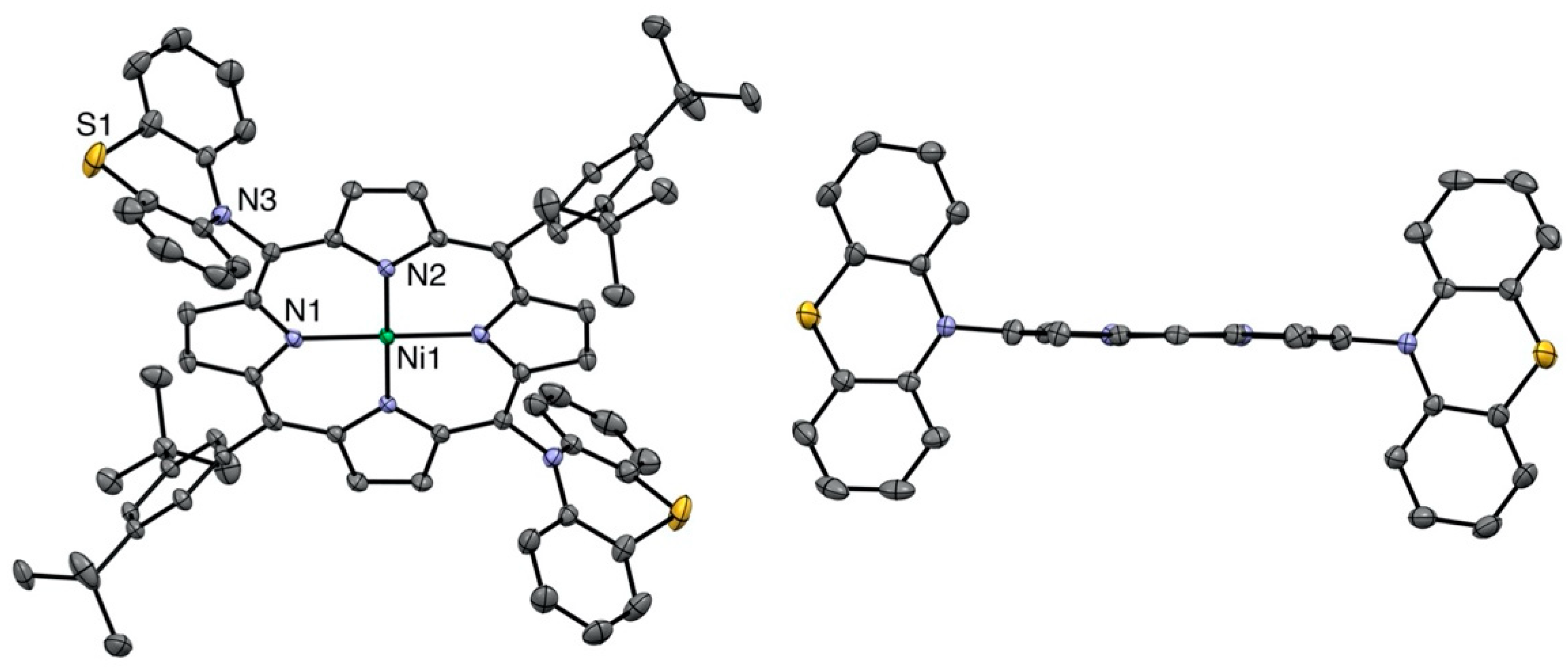
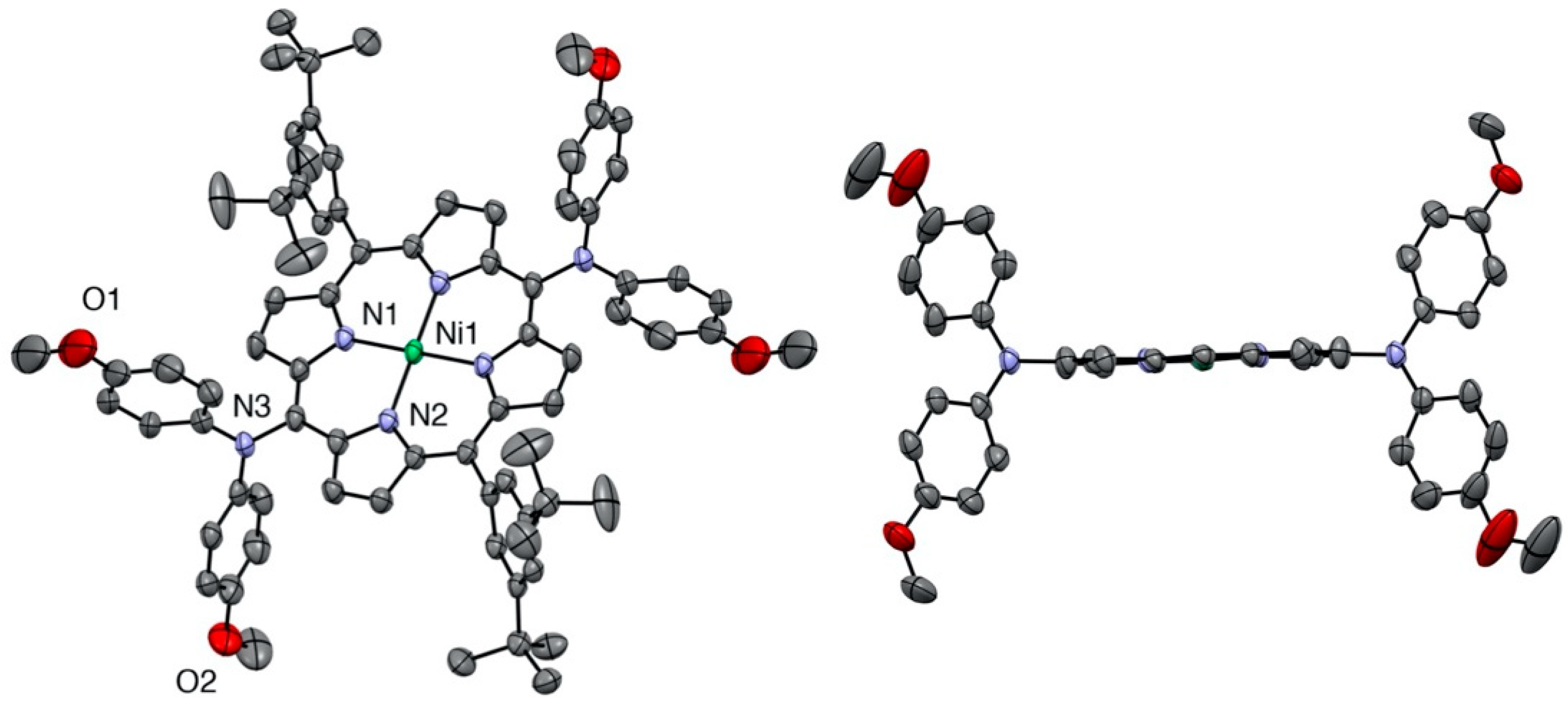
2.1. Syntheses and Characterization
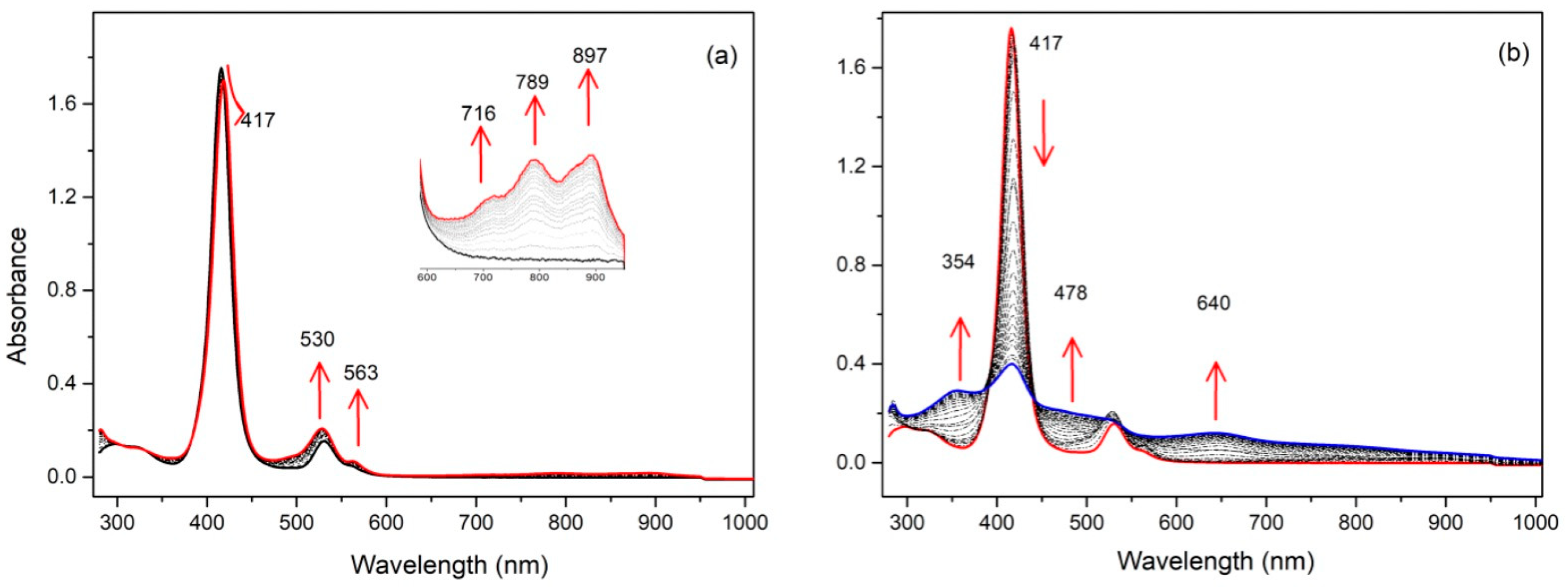
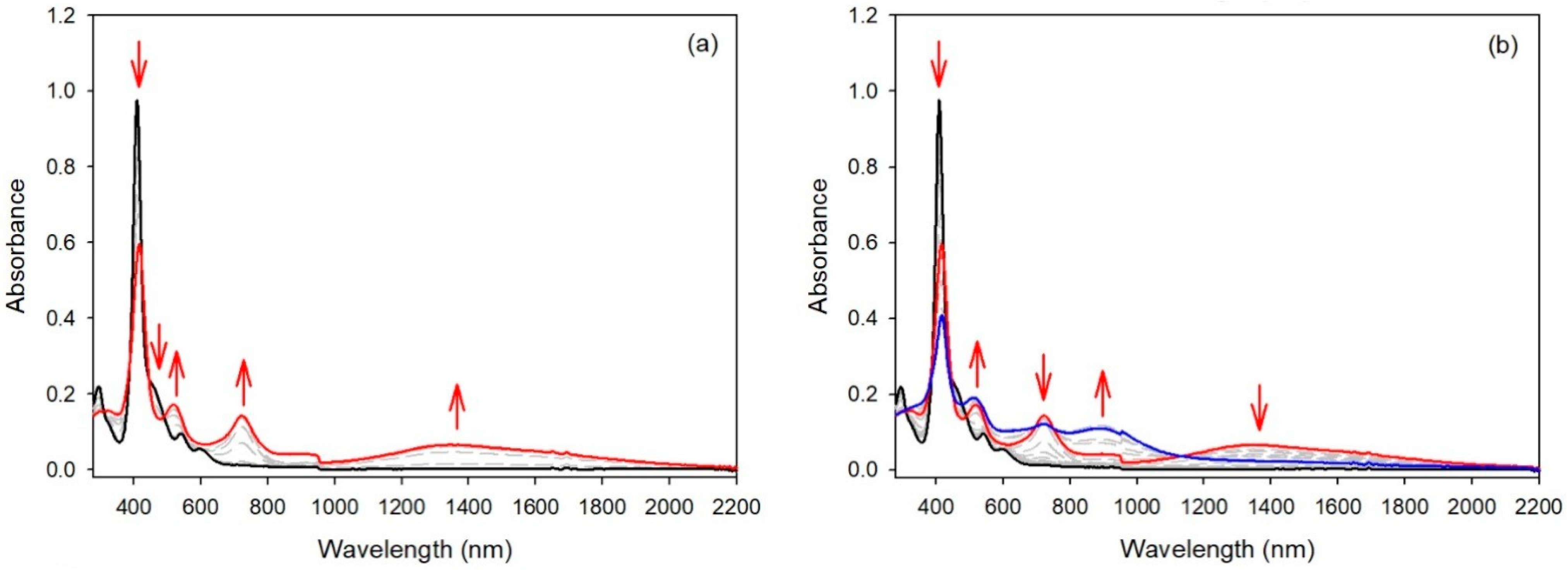
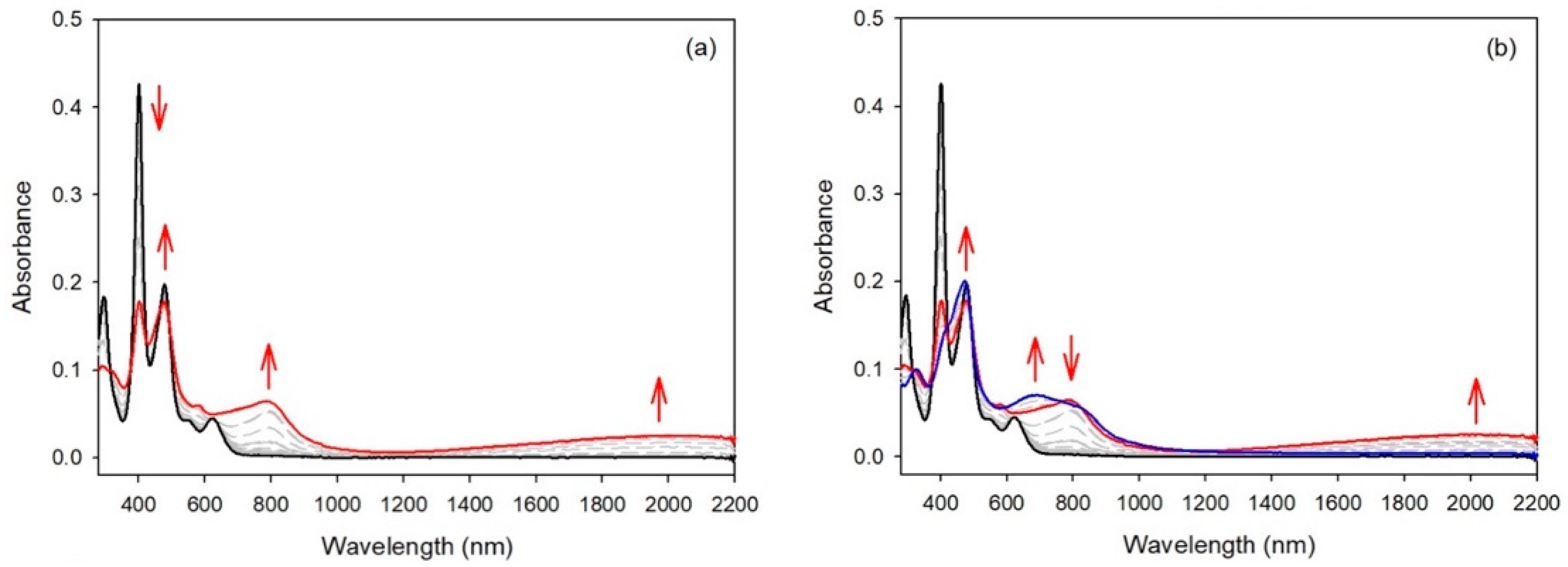
2.2. Electrochemical Studies
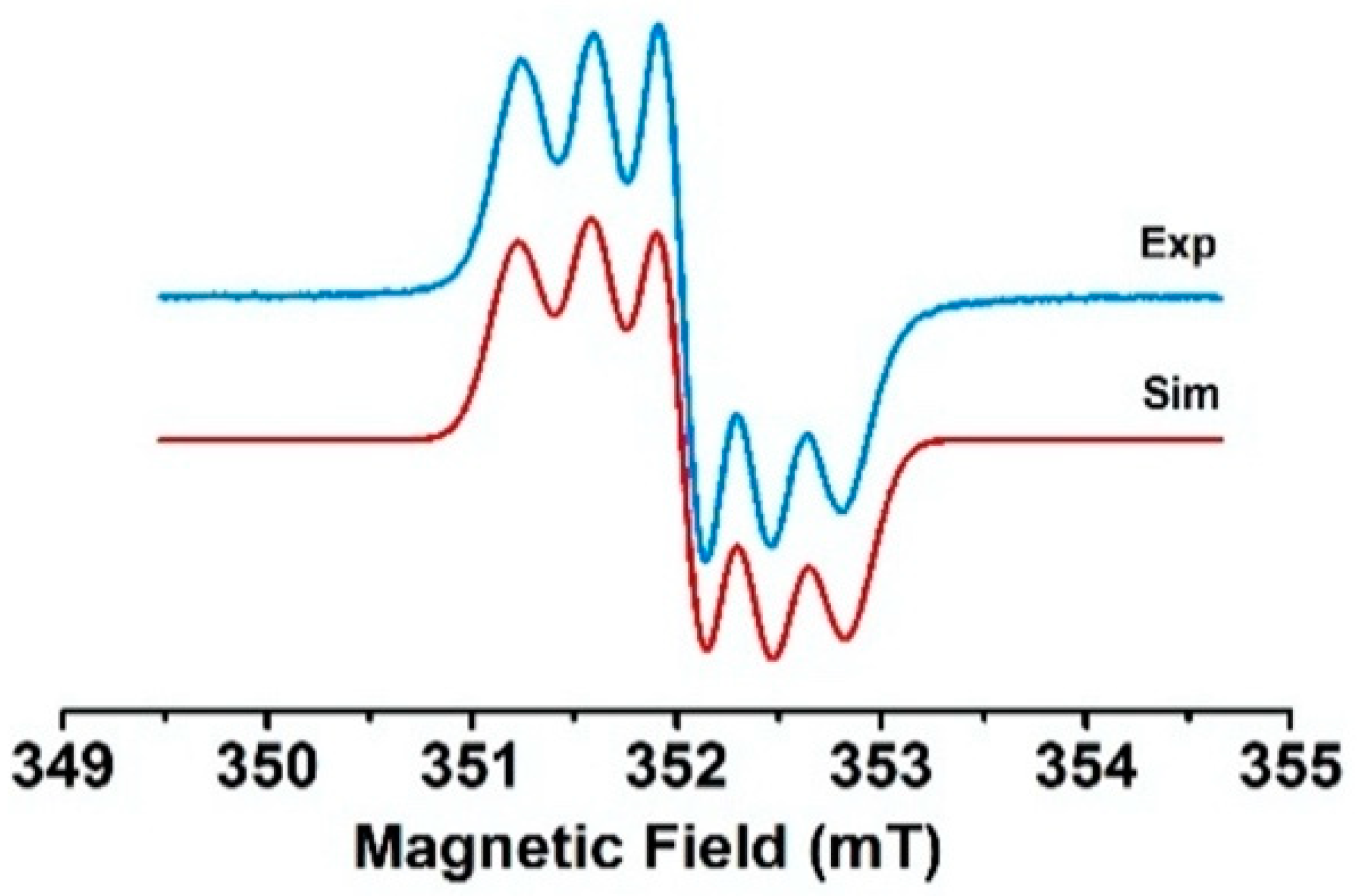
2.3. EPR Studies
2.3.1. CW-EPR/ENDOR Measurements
2.3.2. HYSCORE Results
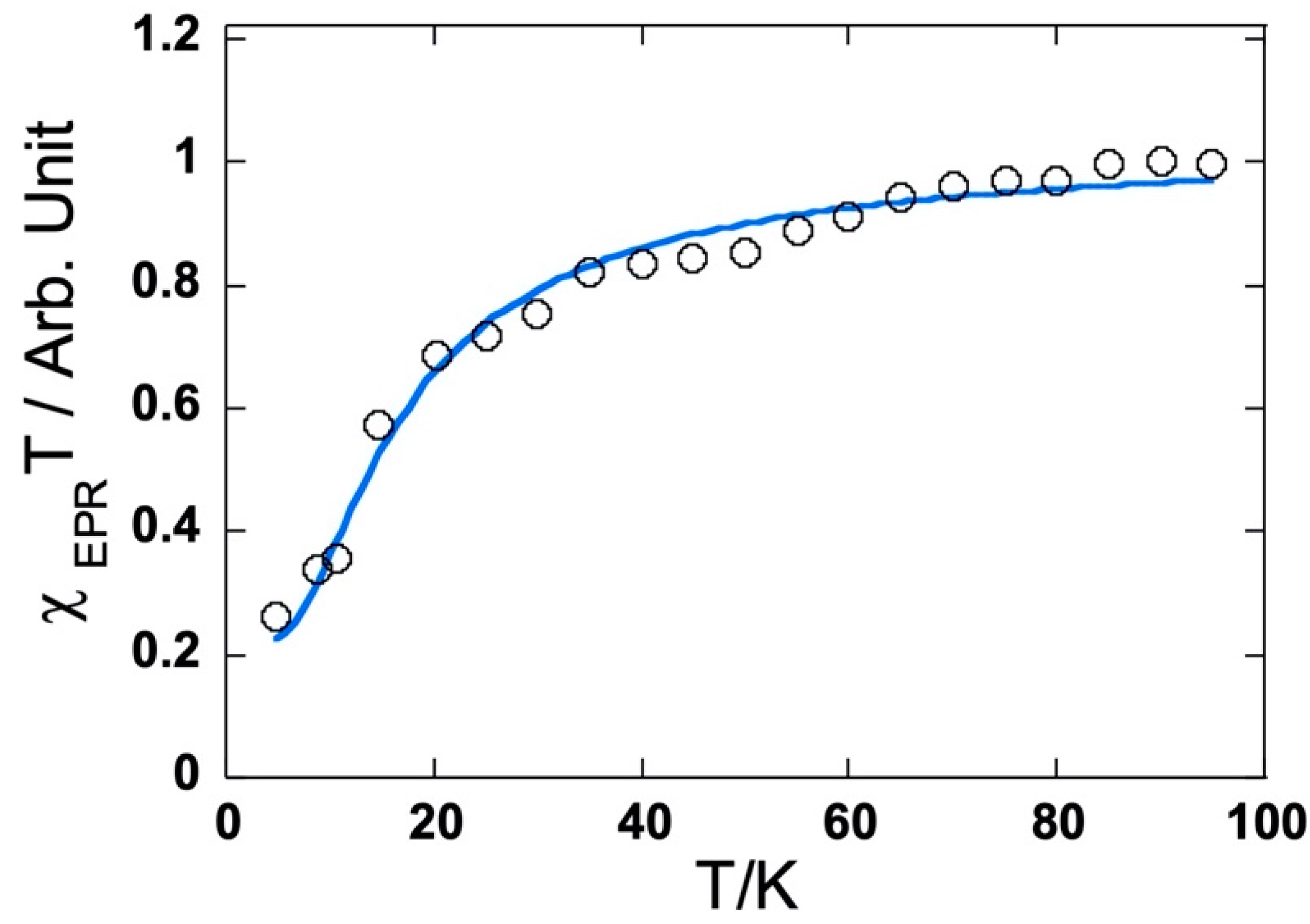
2.3.3. Magnetic Properties
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Compounds
3.3. Electrochemistry
3.4. EPR Measurements
3.5. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kadish, K.M.; Smith, K.M.; Guilard, R. (Eds.) The Porphyrin Handbook; Academic Press: San Diego, CA, USA, 2000; Volume 1, pp. 1–400. [Google Scholar]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and functionalization of porphyrins through organometallic methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef]
- Balaban, M.C.; Chappaz-Gillot, C.; Canard, G.; Fuhr, O.; Roussel, C.; Balaban, T.S. Metal catalyst-free amination of meso-bromoporphrins: An entry to supramolecular porphyrinoid frameworks. Tetrahedron 2009, 65, 3733–3739. [Google Scholar] [CrossRef]
- Devillers, C.H.; Hebié, S.; Lucas, D.; Cattey, H.; Clément, S.; Richeter, S. Aromatic nucleophilic substitution (SNAr) of meso-nitroporphyrin with azide and amines as an alternative metal catalyst free synthetic approach to obtain meso-N-substituted porphyrins. J. Org. Chem. 2014, 79, 6424–6434. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of palladium-catalyzed C-N cross-coupling reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Imahori, H.; Matsubara, Y.; Iijima, H.; Umeyama, T.; Matano, Y.; Ito, S.; Niemi, M.; Tkachenko, N.V.; Lemmetyinen, H. Effects of meso-diarylamino group of porphyrins as sensitizers in dye-sensitized solar cells on optical, electrochemical, and photovoltaic properties. J. Phys. Chem. C 2010, 114, 10656–10665. [Google Scholar] [CrossRef]
- Nowak-Krol, A.; Gryko, D.T. Oxidative aromatic coupling of meso-arylamino-porphyrins. Org. Lett. 2013, 15, 5618–5621. [Google Scholar] [CrossRef] [PubMed]
- Fields, K.B.; Ruppel, J.V.; Snyder, N.L.; Zhang, X.P. Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2010; Volume 3, pp. 367–427. [Google Scholar]
- Kawano, S.; Kawada, S.; Kitagawa, Y.; Teramoto, R.; Nakano, M.; Tanaka, K. Near-infrared absorption by intramolecular charge-transfer transition in 5,10,15,20-tetra-(N-carbazolyl)porphyrin through protonation. Chem. Commun. 2019, 55, 2992–2995. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Kawada, S.; Matsubuchi, A.; Tanaka, K. Metalloporphyrins substituted with N-carbazolyl groups quadruply at meso positions. J. Porphyr. Phthalocyanines 2022, 26, 140–146. [Google Scholar] [CrossRef]
- Pawlicki, M.; Hurej, K.; Kwiecinska, K.; Szterenberg, L.; Latos-Grazynski, L. A fused meso-aminoporphyrin: A switchable near-IR chromophore. Chem. Commun. 2015, 51, 11362–11365. [Google Scholar] [CrossRef]
- Fukui, N.; Cho, W.Y.; Lee, S.; Tokuji, S.; Kim, D.; Yorimitsu, H.; Osuka, A. Oxidative fusion reactions of meso-(diarylamino)porphyrins. Angew. Chem. Int. Ed. 2013, 52, 9728–9732. [Google Scholar] [CrossRef]
- Susuki, Y.; Fukui, N.; Murakami, K.; Yorimitsu, H.; Osuka, A. Amination of meso-bromoporphyrins and haloanthracenes with diarylamines catalyzed by a palladium-PEPPSI complex. Asian J. Org. Chem. 2013, 2, 1066–1071. [Google Scholar] [CrossRef]
- Fukui, N.; Lee, S.K.; Kato, K.; Shimizu, D.; Tanaka, T.; Lee, S.; Yorimitsu, H.; Kim, D.; Osuka, A. Regioselective phenylene-fusion reactions of Ni(II)-porphyrins controlled by an electron-withdrawing meso-substituent. Chem. Sci. 2016, 7, 4059–4066. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Osuka, A.; Song, J. Pd-catalyzed cross coupling strategy for functional porphyrin arrays. ACS Cent. Sci. 2020, 6, 2159–2178. [Google Scholar] [CrossRef] [PubMed]
- Haumesser, J.; Gisselbrecht, J.-P.; Weiss, J.; Ruppert, R. Carbene Spacers in bis-porphyrinic scaffolds. Chem. Commun. 2012, 48, 11653–11655. [Google Scholar] [CrossRef] [PubMed]
- Haumesser, J.; Pereira, A.M.V.M.; Gisselbrecht, J.-P.; Merahi, K.; Choua, S.; Weiss, J.; Cavaleiro, J.A.S.; Ruppert, R. Inexpensive and efficient Ullmann methodology to prepare donor-substituted porphyrins. Org. Lett. 2013, 15, 6282–6285. [Google Scholar] [CrossRef]
- Esdaile, L.J.; Senge, M.O.; Arnold, D.P. New palladium catalysed reactions of bromoporphyrins: Synthesis and crystal structures of nickel(II)complexes of primary 5-aminoporphyrin, 5,5′-bis(porphyrinyl) secondary amine, and 5-hydroxyporphyrin. Chem. Commun. 2006, 4192–4194. [Google Scholar] [CrossRef]
- Pereira, A.M.V.M.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Jeandon, C.; Gisselbrecht, J.-P.; Choua, S.; Ruppert, R. Diporphyrinylamines: Synthesis and electrochemistry. Org. Lett. 2011, 13, 4742–4745. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, M.; Sugai, T.; Minoura, M.; Maruyama, Y.; Furukawa, K.; Holstrom, C.; Nemykin, V.N.; Nakano, H.; Matano, Y. Nitrogen-bridged metallodiazaporphyrin dimers: Synergistic effects of nitrogen bridges and meso-nitrogen atoms on structure and properties. Chem. Asian J. 2017, 12, 816–821. [Google Scholar] [CrossRef]
- Shimizu, D.; Fujimoto, K.; Osuka, A. Stable diporphyrinylaminyl radical and nitrenium ion. Angew. Chem. Int. Ed. 2018, 57, 9434–9438. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.G.; Yeh, C.Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt(II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Sakamoto, R.; Sasaki, T.; Honda, N.; Yamamura, T. 5,15-Bis(di-p-anisylamino)-10,20-diphenylporphyrin: Distant and intense electronic communication between two amine sites. Chem. Commun. 2009, 45, 5156–5158. [Google Scholar] [CrossRef]
- Sakamoto, R.; Nishikawa, M.; Yamamura, T.; Kume, S.; Nishihira, H. A new special pair model comprising meso-di-p-anisylaminoporphyrin: Enhancement of visible-light absorptivities and quantification of electronic communication in mixed valent cation radical. Chem. Commun. 2010, 46, 2028–2030. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Davis, M. 5,15-dianthracen-9-yl-10,20-dihexylporphynato)nickel(II): A planar nickel(II) porphyrin. Acta Crystallogr. Sect. E Struct. Rep. Online 2010, E66, m790. [Google Scholar] [CrossRef]
- Kadish, K.M.; Royal, G.; Van Caemelbecke, E.; Gueletti, L. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 9, pp. 1–219. [Google Scholar]
- Neugebauer, F.A.; Bamberger, S. Diarylamine radical cations. Angew. Chem. Int. Ed. Engl. 1971, 10, 71–72. [Google Scholar] [CrossRef]
- Kennedy, D.E.; Dalal, N.S.; McDowell, C.A. Endor of protons and determination of chlorine hyperfine couplings in neutral free radicals of biologically interesting compounds. Chem. Phys. Lett. 1974, 29, 521–525. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Csonka, G.I. Proper basis set for quantum mechanical studies of potential energy surfaces of carbohydrates. J. Mol. Struct. THEOCHEM. 2002, 584, 1–4. [Google Scholar] [CrossRef]
- Boese, A.D.; Martin, J.M.L.; Handy, N.C. The role of the basis set: Assessing density functional theory. J. Chem. Phys. 2003, 119, 3005–3014. [Google Scholar] [CrossRef]
- Mackie, I.D.; DiLabio, G.A. Accurate dispersion interactions from standard density-functional theory methods with small basis sets. Phys. Chem. Chem. Phys. 2010, 12, 6092–6098. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 799–805. [Google Scholar] [CrossRef]
- Neese, F. Prediction and interpretation of the 57Fe isomer shift in Mössbauer spectra by density functional theory. Inorg. Chim. Acta 2002, 337, 181–192. [Google Scholar] [CrossRef]
- Barone, V. Recent Advances in Density Functional Methods, Part I; Chong, D.P., Ed.; World Scientific Publ Co.: Singapore, 1995; p. 287. [Google Scholar]
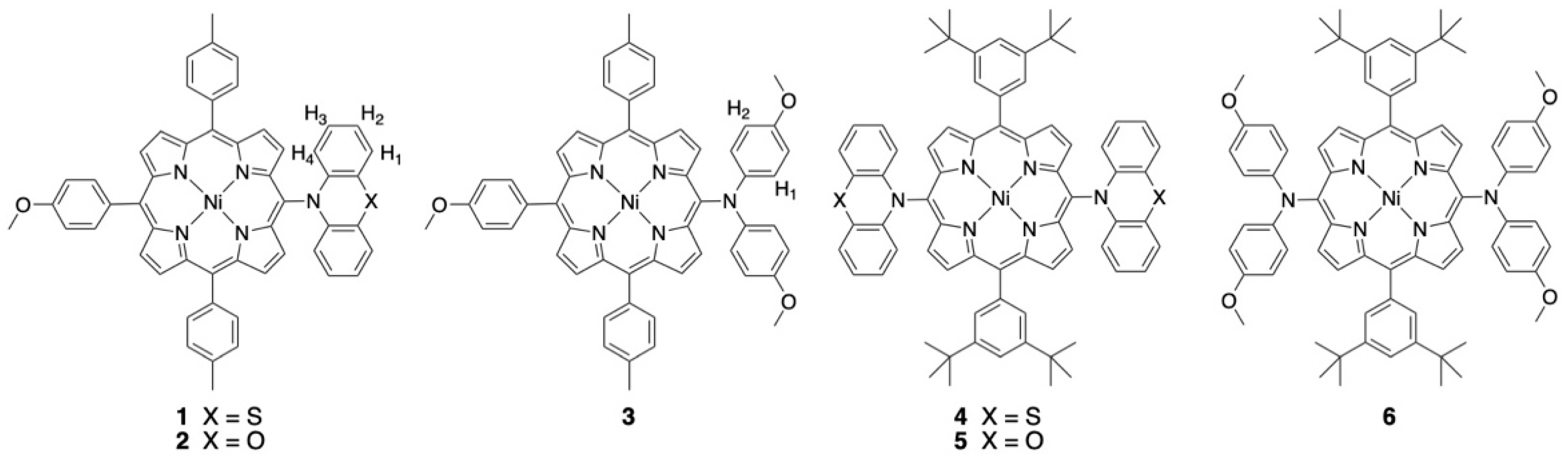
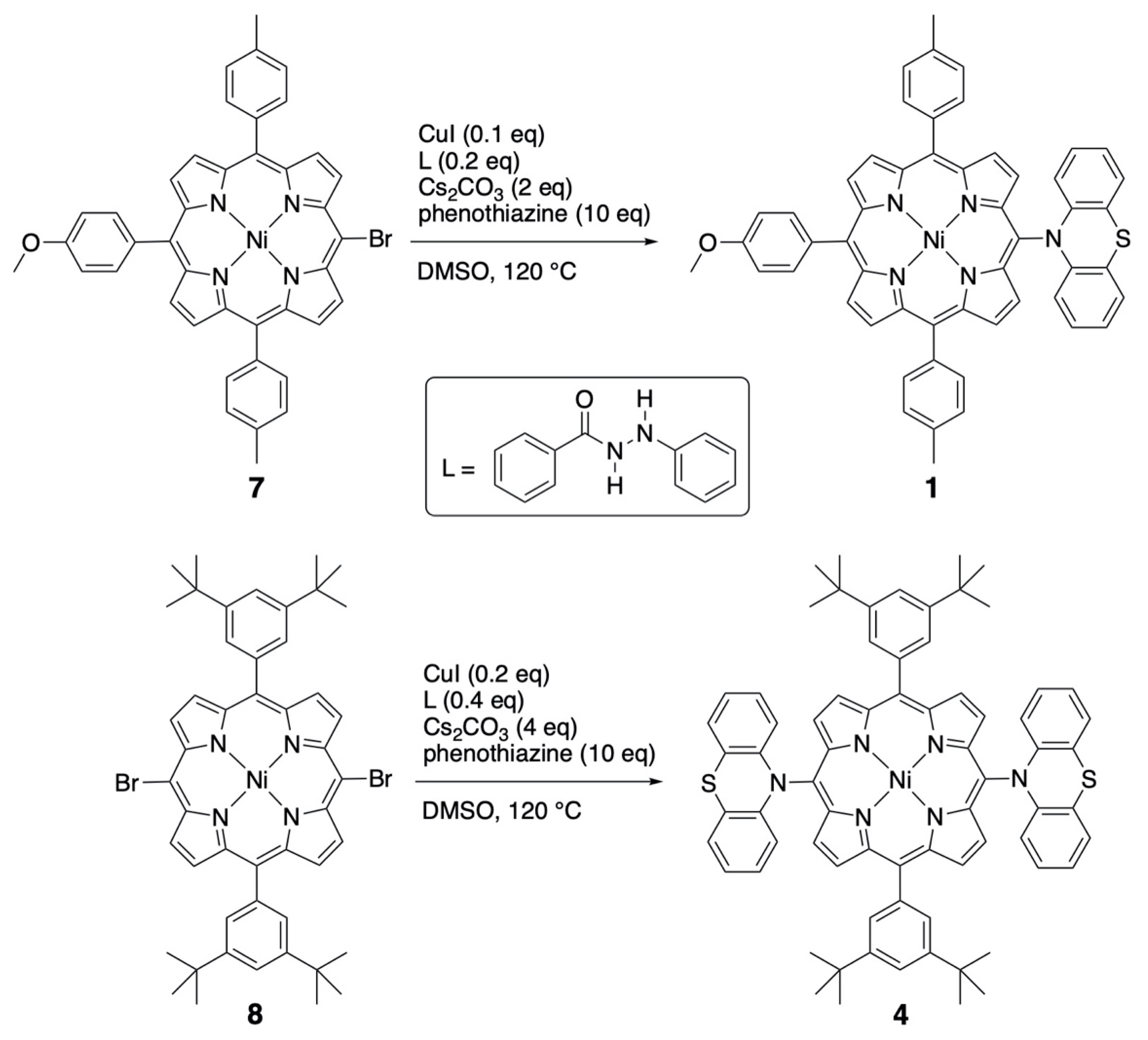




| Compound | Ered2 (Volts) | Ered1 (Volts) | Eox1 (Volts) | ΔEp (mV) | Eox2 (Volts) | ΔEp (mV) | Eox3 (Volts) | Eox4 (Volts) |
|---|---|---|---|---|---|---|---|---|
| 1 | −2.18 b | −1.64 (1) | +0.34 (1) | 60 | +0.85 (2) | 100 | +1.24 b | - |
| 4 | −2.20 b | −1.62 (1) | +0.34 (1) | 60 | +0.40 (1) | 60 | +0.98 (2) | +1.25 b |
| 2 | - | −1.70 (1) | +0.36 (1) | 80 | +0.84 (2) | 100 | - | - |
| 5 | - | −1.64 (1) | +0.37 (1) | 60 | +0.43 (1) | 75 | +0.99 (2) | - |
| 3 | - | −1.09 b | +0.58 (1) | 133 | +0.95 (2) | 122 | - | - |
| 6 | - | −1.09 b | +0.57 (2) | 100 | +1.06 b | - | - | - |
| Compound | N | H1 | H2 | H3 | H4 | giso | |
|---|---|---|---|---|---|---|---|
| 1 | Exp | 19 | - | 5.6 | 2.0 | 2.2 | 2.0034 |
| DFT | 15 | 1.2 | 6.2 | 2.2 | 2.3 | 2.0060 | |
| 2 | Exp | 23 | 1.3 | 8.3 | 2.1 | 4.3 | 2.0035 |
| DFT | 18 | 1.9 | 8.2 | 2.0 | 3.3 | 2.0037 | |
| 3 | Exp | 19 | 4.3 | 1.8 | - | - | 2.0035 |
| DFT | 15 | 4.9 | 1.5 | - | - | 2.0033 |
| Compound 1 | Compound 2 | Compound 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp | DFT | Exp | DFT | Exp | DFT | Exp | DFT | Exp | DFT | Exp | DFT | |
| 14Nporphyrin | 14Nphenothiazine | 14Nporphyrin | 14Nphenoxazine | 14Nporphyrin | 14Nanisyl | |||||||
| T (MHz) ± 0.1 | 0.2 | 0.1 b | 14 a | 14 | 0.1 | 0.1 b | 13 a | 17 a | 0.5 | 1.1 c | - | 12 |
| |Aiso| (MHz) ± 0.1 | 0.6 | 0.35 b | 19 | 15 | 0.8 | 0.5 b | 19 c | 18 | 1.2 | 1.2 c | - | 19 |
| |e2qQ/h| (MHz) ± 0.1 | 1.8 | 2.1 b | 3.5 | 3.2 | 1.8 | 2.2 b | 2.8 | 2.8 | 2.0 | 2.0 c | - | 3.6 |
| η ± 0.1 | 0.2 | 0.1 b | 0.1 | 0.1 | 0.2 | 0.13 b | 0.1 | 0.0 | 0.1 | 0.1 c | - | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, M.-A.; Merahi, K.; Haumesser, J.; Pereira, A.M.V.M.; Parizel, N.; Weiss, J.; Orio, M.; Maurel, V.; Ruhlmann, L.; Choua, S.; et al. Syntheses and Electrochemical and EPR Studies of Porphyrins Functionalized with Bulky Aromatic Amine Donors. Molecules 2023, 28, 4405. https://doi.org/10.3390/molecules28114405
Carvalho M-A, Merahi K, Haumesser J, Pereira AMVM, Parizel N, Weiss J, Orio M, Maurel V, Ruhlmann L, Choua S, et al. Syntheses and Electrochemical and EPR Studies of Porphyrins Functionalized with Bulky Aromatic Amine Donors. Molecules. 2023; 28(11):4405. https://doi.org/10.3390/molecules28114405
Chicago/Turabian StyleCarvalho, Mary-Ambre, Khalissa Merahi, Julien Haumesser, Ana Mafalda Vaz Martins Pereira, Nathalie Parizel, Jean Weiss, Maylis Orio, Vincent Maurel, Laurent Ruhlmann, Sylvie Choua, and et al. 2023. "Syntheses and Electrochemical and EPR Studies of Porphyrins Functionalized with Bulky Aromatic Amine Donors" Molecules 28, no. 11: 4405. https://doi.org/10.3390/molecules28114405
APA StyleCarvalho, M.-A., Merahi, K., Haumesser, J., Pereira, A. M. V. M., Parizel, N., Weiss, J., Orio, M., Maurel, V., Ruhlmann, L., Choua, S., & Ruppert, R. (2023). Syntheses and Electrochemical and EPR Studies of Porphyrins Functionalized with Bulky Aromatic Amine Donors. Molecules, 28(11), 4405. https://doi.org/10.3390/molecules28114405









